Abstract
Neutral cues paired with rewards often appear to acquire motivational significance, as if the incentive motivational value of the reward is transferred to the cue. Such cues have been reported to modulate the performance of instrumental action (Pavlovian-instrumental transfer, PIT), serve as conditioned reinforcers in the establishment of new learning, and be the targets of approach and other cue-directed behaviors. Here we examined the effects of lesions of the amygdala central nucleus (CeA) on the acquisition of discriminative autoshaped lever-pressing. Insertion of one lever into the experimental chamber was reinforced by sucrose delivery, but insertion of another lever was not reinforced. Although sucrose delivery was not contingent on lever pressing, both CeA- and sham-lesioned rats rapidly came to press the reinforced but not the nonreinforced lever. Despite their showing little evidence of impairments in autoshaped lever pressing, these same CeA-lesioned rats showed significant deficits in the expression of PIT in a subsequent phase of the experiment. The lack of impaired autoshaping in CeA-lesioned rats contrasts with effects previously reported for conditioned orienting responses (ORs) and for other putative measures of incentive learning including PIT and conditioned approach to visual cues.
Keywords: amygdala, incentive salience, autoshaping, Pavlovian instrumental transfer, orienting
1. Introduction
Localizable visual cues paired with food delivery often come to elicit conditioned approach or orienting responses (ORs) directed toward those cues. For example, Holland (1977, 1980) found that rats reared on their hind legs and oriented towards a punctate light source whose illumination was reliably followed by food delivery. Similarly, Everitt, Robbins and colleagues (e.g., Cardinal et al., 2002) found that rats approached illuminated rectangles presented on video monitors when those cues were paired with food delivery, even when that response competed with approaching the food delivery site. Interestingly, lesions of the amygdala central nucleus (CeA) profoundly impair acquisition of each of these learned responses (e.g., Gallagher et al., 1990; McDannald et al., 2004; Parkinson et al. 2000). Importantly, those deficits were not attributable to general deficits in learning or motor performance: acquisition of anticipatory approach to the food delivery site was unaffected by the lesions.
Different accounts for both the emergence of these cue-directed behaviors and the role of CeA in their acquisition were offered. Gallagher et al. (1990) suggested that these behaviors reflected a conditioning-dependent enhancement of initially-unconditioned ORs, which depended on circuitry including CeA, the substantia nigra pars compacta (El Amamy & Holland, 2006, 2007; Lee et al., 2005) and dorsolateral striatum (Han et al., 1997). By contrast, Cardinal et al. (2002) and Parkinson et al. (2000) suggested that their approach responses reflected the acquisition of incentive value, such that the incentive motivational value of the food reinforcer was transferred to the cue (Berridge, 2001, 2004), through operation of circuitry including the CeA, the ventral tegmental area, and the nucleus accumbens (Acb).
Recently, considerable attention has been directed to another example of cue-directed responding in rats, autoshaped lever pressing, which has also been suggested to indicate acquisition of incentive value. Rats will approach and contact a lever whose insertion into the chamber signals food delivery (e.g., Boakes, 1977; Flagel et al., 2008, 2009; Kearns & Weiss, 2004; Tomie, 1995), often showing protracted biting and manipulating of the lever despite no contingent relation between any of those behaviors and the delivery of food. Several investigators have asserted that this learned behavior provides a good model for compulsive or addictive behavior in contexts as diverse as eating, smoking, and seeking of alcohol or drugs (e.g., Flagel et al., 2008, 2009; Kearns & Weiss, 2004; Tomie, 1995, 1996; Tomie et al., 2008). As such, determining the physiological substrates of this behavior might inform the understanding of those disease states.
Here, we examined the effects of CeA lesions on autoshaping. If this cue-directed behavior reflects the acquisition of either incentive properties or enhanced orienting, one would expect it to be disrupted in rats with lesions of CeA. Surprisingly, we found no such effect. Nevertheless, consistent with several prior studies (e.g. Corbit & Balleine, 2005; Gallagher et al., 1990; Hall et al., 2001; Holland & Gallagher, 2003), we subsequently found these same lesioned rats to show abnormal Pavlovian-instrumental transfer (PIT), another phenomenon attributed to the acquisition of incentive value to cues for food.
2. Results
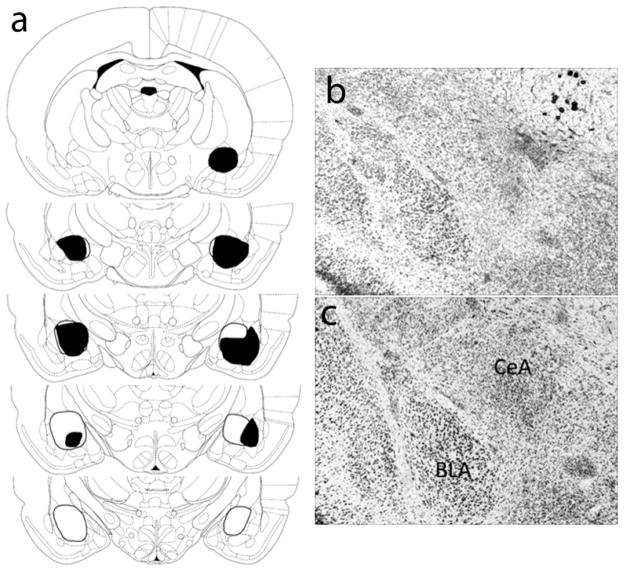
Rats first received either bilateral ibotenic acid or sham lesions of the CeA. Figure 1a presents a schematic representation of neuronal damage in accepted CeA-lesioned rats (n = 9); representative images of neurotoxic- and sham-lesioned brain sections are shown in Figure 1b and 1c, respectively. On average, 78.2 ± 3.0% of CeA was eliminated in neurotoxic-lesioned rats. Many of these brains also had minimal unilateral damage to the basolateral amygdala (BLA.) Data of 24 rats judged as having unacceptable lesions (less than 50% damage to CeA, or substantial damage to BLA) were discarded. Sham-lesioned rats (n = 15) had no observable damage other than near the needle track
Figure 1.
Histological results (A) Schematic representation of bilateral amygdala central nucleus (CeA) lesions showing the minimum (black) and maximum (white) amount of neuronal damage. Coronal sections are −1.60 mm to −3.30 mm relative to bregma (Paxinos & Watson, 1998; used by Permission of Elsevier). (B) Photomicrograph of a rat with a representative CeA lesion. (C) Photomicrograph of a rat with a representative sham CeA lesion
2.1. Autoshaping phase
Rats first received training in which the 10-s insertion of a lever (CS+) into the chamber signaled the delivery of liquid sucrose as the lever was withdrawn, and the 10-s insertion of a second lever (CS−) was not reinforced. The reinforcement contingencies were Pavlovian, that is, sucrose was delivered regardless of the rats’ behavior. We analyzed two measures of autoshaping, the rate of lever pressing and the percentage of trials on which at least one lever press occurred, to permit distinguishing between lesion effects on the vigor or persistence of responding and on the simple occurrence of an autoshaped response. We also analyzed the percentage of time each rat spent with its head in the food cup during the CS presentations. Thus, we reported both responding directed to the lever cues (sometimes called “sign tracking”) and responding directed toward the food source (sometimes called “goal tracking”).
Because another example of cue-directed conditioned behavior, the conditioned visual OR, is typically found to be confined to the early portions of the cue-reinforcer interval, while behaviors directed to the food cup predominate in the later portions (e.g. Holland, 1977, 1980), we divided the 10-s lever presentations into two 5-s bins for analysis. If autoshaped lever presses were an example of an OR, then rats might display peak levels of responding to the lever during the first bin. Initially, each measure of conditioning was subjected to a 4-way mixed analysis of variance (ANOVA) with variables of lesion condition (lesion or sham), cue type (CS+ vs. CS−), interval of the CS (first vs. last 5-s interval), and session. Because these analyses revealed few interactions of interval with other variables, and higher levels of autoshaped responding during the second 5-s CS interval, we focused on this last interval in subsequent lesion × cue × sessions ANOVAs. However, the results of comparable ANOVAs of responding in the first 5-s intervals were nearly identical. In all of these analyses, the occurrence of autoshaping is indicated by significant effects of cue, and the effects of lesions on autoshaping would be revealed as significant interactions of lesion with cue. Significant lesion × cue interactions were followed by planned individual comparisons of responding of lesioned and sham-lesioned rats during CS+ and during CS− trials.
2.1.1. Lever press responses during autoshaping
In contrast to conditioned ORs, lever press responding peaked during the last 5-s period of CS presentations, in both CeA-lesioned and sham-lesioned rats. Preliminary ANOVAs of both lever presses/min and percentage of trials with a lever press confirmed a main effect of interval (Fs1,22 > 18.03, ps < .001) and no lesion × interval interaction (Fs1,22 ≤ 1.46, ps > .239). Thus, we focused our subsequent analysis on responding during the last 5-s periods of CS presentations, when responding was at its peak. Table 1 shows the mean lever press rate and percentage trials with a lever press during the first and second 5-s intervals of CS+ and CS− presentations, averaged over the entire training period.
Table 1.
| Measure | CeA lesion | CeA sham |
|---|---|---|
| Rate CS+ 1st 5s | 16.3 ± 2.9 | 15.0 ± 2.1 |
| Rate CS+ 2nd 5s | 21.7 ± 3.4 | 18.3 ±1.8 |
| Rate CS− 1st 5s | 1.6 ± 0.2 | 1.7 ± 0.2 |
| Rate CS− 2nd 5s | 3.2 ± 0.4 | 2.8 ± 0.2 |
| % trials CS+ 1st 5s | 41.1 ± 5.7 | 50.1 ± 5.5 |
| % trials CS+ 2nd 5s | 52.6 ± 5.6 | 59.5 ± 4.8 |
| % trials CS− 1st 5s | 6.8 ± 0.8 | 7.7 ± 0.8 |
| % trials CS− 2nd 5s | 11.8 ± 1.4 | 11.0 ± 0.9 |
Notes. Rate = lever presses/min; % trials = percentage of trials on which at least one lever press response occurred; CeA = amygdala central nucleus. Entries are mean ± sem, averaged over all acquisition sessions. The measures for each of the first and second 5-s periods of the 10-s lever presentations are independent, for example, the probability of a response occurring in the 2nd interval includes both trials on which a response occurred during the first half and those on which no such response occurred.
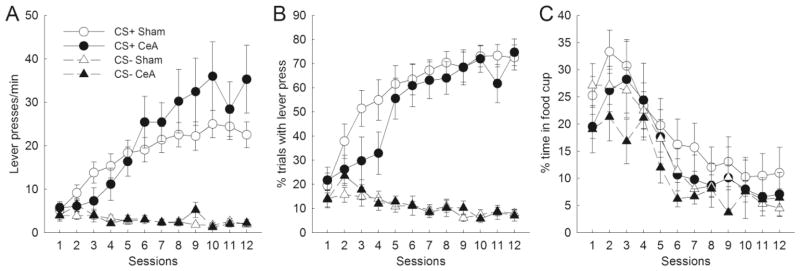
Both CeA- and sham-lesioned rats quickly acquired sign-tracking behavior during CS+ presentations, in terms of both measures of lever pressing. Conversely, both CeA- and sham-lesioned rats responded at minimal levels to the CS− lever. Figures 2a and 2b present lever press rate and the percentage of trials with a lever press during the last 5 s of CS presentations, respectively. In terms of lever press rate, CeA-lesioned rats showed nonsignificant deficits in initial levels of responding to the CS+ compared to sham controls. However, CeA-lesioned rats soon reached levels of responding comparable to sham-lesioned controls, eventually surpassing them (nonsignificantly) over the second half of training. The percentage of trials with a lever press measure showed a similar nonsignificant deficit in initial levels of CS+ responding in CeA-lesioned rats, but CeA-lesioned rats soon reached control levels, and both groups responded at similar levels throughout the rest of training. For both sign-tracking measures, CeA-lesioned and sham-lesioned rats responded minimally to the CS− lever. ANOVAs confirmed main effects of cue (Fs1,22 > 75.08, ps < .001) and session (Fs11,242 > 13.77, ps < .001) for both measures. Importantly, there were no main effects of lesion (Fs1,22 < 0.64, ps > 0.434) or lesion × cue interactions (Fs1,22 < 1.36, ps > 0.257). Although ANOVAs revealed lesion × cue × session interactions (Fs11,242 ≥ 1.83, ps < 0.05), these differences were apparently not systematic. For example, separate ANOVAs over the first and second halves of training (days 1–6 and days 7–12) revealed no significant lesion × cue interactions (Fs1,22 < 1.98, ps > 0.173).
Figure 2.
Effects of amygdala central nucleus (CeA) lesions on autoshaped responding during the last 5 s of CS presentations. Compared to sham-lesioned controls, CeA-lesioned rats showed no deficits in lever presses/min (A), percentage of trials with a lever press (B), or the percent of time spent in the food cup (C). Error bars represent ±SEM.
2.1.2. Food cup responses during autoshaping
CeA lesions had no effect on food cup responding (“goal-tracking”) during CS presentations. CeA-lesioned rats and their sham controls spent comparable amounts of time in the food cup during the first and last 5 s of CS presentations. Neither the effect of interval (F1,22 = 0.083, p = 0.78) nor the lesion × interval interaction (F1,22 = 0.33, p = 0.57) was significant. Figure 2c shows the amount of time spent in the food cup during the last 5 s of CS presentations. In contrast to autoshaped lever-pressing, food cup behavior during the CS+ decreased through the course of training for both CeA-lesioned rats and their sham controls. Additionally, neither CeA-lesioned nor sham-lesioned rats showed greater amounts of food cup behavior during CS+ trials than during CS− trials. ANOVA confirmed a main effect of session (F11,242 = 26.07, p < 0.01), but no effect of cue (F1,22 = 2.18, p = 0.15), lesion (F1,22 = 0.91, p = 0.35), or lesion × cue interaction (F1,22 = 0.004, p = 0.95).
2.2 Pavlovian auditory discrimination training
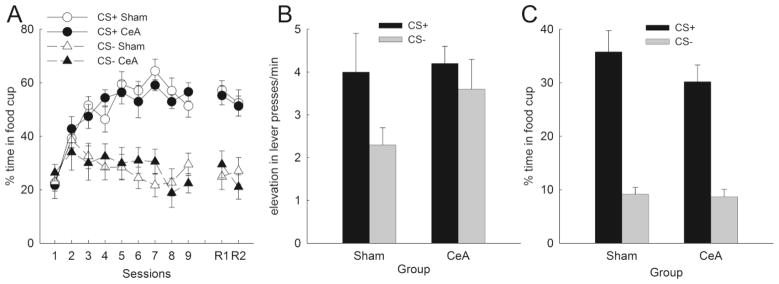
In preparation for PIT testing, the rats first received Pavlovian auditory discrimination training, in which one 2-min auditory cue (CS+) was paired with sucrose and the other (C−) was not. As in previous experiments (e.g., Gallagher et al., 1990; Holland & Gallagher, 2003), acquisition of conditioned food cup responding (Figure 3A) was unaffected by the lesion. ANOVA showed more food cup responding to CS+ than to C− (F1,22 = 139.39, p < .001), but neither the effect of lesion (F1,22 = 0.04, p = .840) nor the lesion × cue interaction (F1,22 = 0.80, p = .380) was significant.
Figure 3.
Effects of amygdala central nucleus (CeA) lesions on (A) acquisition of Pavlovian discriminative conditioning of food cup CRs to auditory stimuli, (B) instrumental responding in the Pavlovian-instrumental transfer (PIT) test, (C) Pavlovian food cup CRs in PIT test. Entries are mean ± SEM.
2.3 Instrumental training and Pavlovian-instrumental transfer testing
After completion of Pavlovian auditory discrimination training, a lever was inserted into the chamber, and the rats were trained to press it for sucrose on an instrumental variable-interval (VI) 1-min schedule of reinforcement. The lever used was the lever that had served as CS+ in the autoshaping phase. All rats rapidly adapted to the VI schedule in the absence of any explicit cues. Over the last two sessions of this training, the mean ± sem rate of lever pressing was 15.9 ± 2.0 responses/min in sham-lesioned rats and 15.9 ± 2.7 responses/min in CeA-lesioned rats. There were no significant (ps > .820) effects of lesion or its interactions over the instrumental response training phase.
However, when the Pavlovian CSs were superimposed on instrumental responding in the transfer test session, only sham-lesioned rats showed differential elevation of baseline instrumental responding by CS+ relative to C− (Figure 3B). Although the lesion × cue interaction was not significant (F1,22 = 0.79, p = 0.38), planned comparisons revealed that while sham-lesioned control rats lever pressed significantly more during CS+ than C− presentations (p = 0.03), CeA-lesioned rats did not show this discrimination (p = 0.51). However, as is apparent in Figure 3B, the lack of differential enhancement by CS+ and C− was primarily (nonsignificantly) greater responding to C−, rather than lower responding to CS+. We have no account for this aspect of our data. Baseline response rates did not differ between CeA- (3.8 ± 0.7 responses/min) and sham-lesioned rats (3.9 ± 0.6 responses/min) (F1,22 = 0.026, p = 0.87).
At the same time, as in the Pavlovian auditory discrimination training phase, the CeA lesion had no effect on food cup responding during CS+ and C− in the PIT test (Figure 3C). Although the main effect of cue was significant (F1,22 = 70.9, p < 0.01), neither the effect of lesion (F1,22 = 0.91, p = 0.35) nor the lesion × cue interaction (F1,22 = 0.79, p = 0.38) was.
3. Discussion
Rats with CeA lesions were not significantly impaired in their acquisition or maintenance of autoshaped lever pressing, but showed abnormal PIT. Thus, CeA is apparently differentially involved in these phenomena, which have both been attributed by some to the acquisition of incentive to food-paired cues (e.g., Flagel et al., 2009; Holland, 1977; Lovibond, 1983; Mackintosh, 1974).
Coupled with the results of other investigations, these results indicate that the brain systems critical to the control of cue-directed conditioned responses depend on the nature of such cues and the behavioral systems they engage. First, the present data show that autoshaped lever pressing, although C− directed, is distinct from conditioned ORs (Holland, 1977). Unlike conditioned ORs, which are most prevalent near CS onset, autoshaped lever presses occurred throughout the CS interval, peaking near the time of US delivery. In addition, whereas in other studies the acquisition of conditioned ORs was blocked by CeA lesions (e.g., Gallagher et al., 1990; Groshek et al., 2005), in this experiment autoshaped lever pressing was not significantly affected. In addition, although conditioned ORs are unaffected by lesions of another amygdala subregion, the BLA (e.g., Hatfield et al., 1996; Setlow et al., 2002), in a recent study that used procedures identical to the ones used here, we found that BLA lesions substantially reduced the asymptotic levels of autoshaped lever pressing (Chang et al., 2011).
Second, autoshaped lever pressing shows a pattern of susceptibility to lesions of amygdala subregions different from that shown by conditioned approach responses to visual cues (illuminated rectangles presented on video monitors) paired with food. Whereas Parkinson et al. (2000) found that such approach responding was disrupted by lesions of CeA but not of BLA, here we found no effects of CeA lesions, and as just noted, Chang et al. (2011) found substantial effects of BLA lesions on autoshaped lever pressing. Thus, the current preparation, which involves consummatory behaviors directed at the cue, requires different aspects of the amygdala compared to situations that involve only approach or orientation toward a cue. Of course, consummatory responding cannot be directed toward the lever without prior approach, so our failure of CeA lesions to affect autoshaped lever pressing remains somewhat puzzling. However, in this regard, an analogy with PIT may be useful. Although CeA lesions disrupt PIT when a single reinforcer is used, they have no effect on PIT when multiple reinforcers are used (Corbit & Balleine, 2005). Typical accounts for this observation suggest that the two procedures themselves force the engagement of different brain systems. Here, we suggest that the use of a CS whose characteristics permit transfer of hedonic properties engages a different kind of learned incentive process (mediated by a different brain circuit) than the use of a “non-consummable” CS.
In addition to drawing a distinction between autoshaped lever pressing and conditioned orienting or approach, the present data indicate that these amygdalar subnuclei do not have identical roles in incentive learning processes. Whereas here we found no significant deficit in autoshaped lever pressing in CeA-lesioned rats, these same rats failed to display PIT, consistent with prior PIT data (e.g., Corbit & Balleine, 2005; Holland & Gallagher, 2003; Hall et al., 2001). Conversely, although Chang et al. (2011) found substantial asymptotic effects of BLA lesions on autoshaped lever pressing, such lesions spare PIT when training is conducted with a single reinforcer (Corbit & Balleine, 2005; Holland & Gallagher, 2003). The pattern of lesion effects on autoshaped lever pressing observed here and by Chang et al. (2011) is perhaps most comparable to that observed with conditioned reinforcement, the ability of a food-paired cue to serve as a reinforcer in the acquisition of new learning. As with our autoshaped lever pressing, BLA but not CeA lesions have been found to disrupt conditioned reinforcement in both Pavlovian (Hatfield el al., 1996; Setlow et al., 2002) and instrumental (Burns et al., 1993; Robledo et al., 1996) training procedures.
Given these different patterns of CeA and BLA lesion effects on autoshaped lever pressing, conditioned orienting, conditioned approach, conditioned reinforcement, and PIT, some consideration of the commonalities and differences among these various products of Pavlovian conditioning procedures is in order. Although many of these sequellae have been attributed to acquisition of “incentive motivation”, it is clear that they differ in many ways. It is interesting to speculate that autoshaped lever pressing may differ from some other examples of learned incentive or cue-directed responding because lever-insertion CSs, unlike typical auditory or visual CSs, support the acquisition of consummatory-like behaviors, such as biting, grasping, and handling, which may reflect transfer of hedonic properties of the reinforcer to the CS. In this regard, it is notable that recent investigations of cue-potentiated feeding (e.g., Holland & Petrovich, 2005) show that lesions of BLA, but not of CeA, eliminate the ability of learned food cues to modulate consummatory responding, including the display of hedonic taste reactivity responses (Berridge, 2000). Interestingly, Johnson and Gallagher (2011) reported that effort-induced variations in reinforcer palatability (as measured by patterns of lick microstructure) are correlated with variations in conditioned reinforcement power of cues previously paired with those reinforcers. This observation supports a relation between the transfer of hedonic properties from reinforcer to CS and conditioned reinforcement, consistent with the comparable effects of CeA and BLA lesions on conditioned reinforcement and autoshaped lever pressing noted previously. Taken together, these findings suggest that whereas CeA may be critical for the acquisition of some forms of incentive motivation, it is not crucial for forms that involve the transfer of hedonic properties, as may be the case in autoshaping.
Citing their observations that microinjections of the μ-opioid agonist DAMGO into CeA enhanced, while inactivation of CeA by muscimol microinjections reduced consummatory-like responding to lever or food cup cues associated with food delivery, Mahler and Berridge (2009) suggested that opioid neurotransmission by CeA “magnifies and focuses learned incentive salience onto a specific reward cue”. Although at first glance this assertion seems inconsistent with our failure to find an effect of CeA lesions on autoshaped lever pressing, there is precedent in the literature of conditioned reinforcement: rats with CeA lesions acquire conditioned reinforcement normally but fail to show potentiation of that phenomenon by amphetamine (Robledo et al., 1996). Thus, although function of other brain structures may be sufficient for autoshaping, when available, CeA might still modulate the incentive salience of a CS.
Finally, it is notable that our lever-directed responses were considerably more probable than those observed by other investigators (e.g., Mahler & Berridge, 2009; Flagel et al., 2009). Whereas only a portion of those investigators’ rats acquired the sign-tracking behavior, all of our sham-lesioned rats displayed significant lever pressing. Although there were many differences in procedures between our respective studies, three stand out. First, whereas most recent studies of autoshaped lever pressing have used solid food pellet reinforcers, ours used liquid sucrose. However, although the form of a small portion of lever-directed responses has been shown to differ between sucrose- and food-reinforced lever presentations, the large majority of lever contacts in those studies were comparable across reinforcers (e.g., Davey et al., 1981,1984). Thus it seems unlikely that this difference influenced our results. Second, in our study, a nonreinforced C− lever was inserted and withdrawn on discrete trials in the same manner as the CS+ lever, whereas in Mahler and Berridge (2009) and Flagel et al.’s (2009) studies, a control lever was constantly present. Notably, Boakes (1977) and Davey et al. (1982) found that introduction of a C− lever enhanced the level of autoshaped responding beyond that observed with a CS+ lever alone. Third, it is possible that our rats’ behavior was more focused on lever contacts because lever insertion was virtually silent, unaccompanied by the substantial auditory cues often associated with operation of commercial electromechanical retractable levers used by others. A stimulus with exclusively visual attributes is likely to attract more attention to those attributes than one with both auditory and visual attributes. Furthermore, because of our use of a lever insertion C−, any auditory or other cue produced by lever insertion in general would be a poorer predictor of food than in studies in which all lever insertions are followed by food, as when a static control lever is used. Given that the predominant response to Pavlovian auditory cues for food is food cup entry (Holland, 1977) it is perhaps not surprising that other studies have shown more goal-tracking and less sign-tracking than ours.
Appetitive conditioning engages a range of behavioral and brain systems, depending on the choice of reinforcer, CS, and other less well-specified aspects of the conditions of learning. Although characterizing many of these consequences of learning as examples of “incentive learning” has served an important heuristic value in discussing behavioral, cognitive and emotional products of reward-based learning, their determinants and brain bases vary considerably. Progress in the understanding of brain-behavior relations in reward learning may demand replacing such generic constructs with more specific ones.
4. Experimental Procedure
4.1. Subjects
The subjects were 48 male Long-Evans rats (Charles River Laboratories, Raleigh, NC, USA), which weighed 300–325 g upon arrival to the colony room. Rats were individually housed and given ad libitum access to food and water before entering surgery. Following 2 weeks of recovery, rats were food restricted to and maintained at 85% of their ad libitum weights throughout the autoshaping procedure. The colony room was climate controlled and was illuminated from 7:00 A.M. to 7:00 P.M.
4.2. Surgical Procedures
Surgery was performed under aseptic conditions with isoflurane anesthesia, and all infusions were made with a Hamilton 2.0-μl syringe and 25-gauge needle. Bilateral CeA lesions were made with ibotenic acid (10 mg/ml in PBS, pH=7.4; Sigma St. Louis, MO, USA) using the coordinates 2.2 mm posterior of bregma, 4.3 mm from the midline, and 8.1 mm (0.15 μl/site) ventral from the skull surface at the injection site. Rats that received bilateral sham lesions underwent the same surgical procedures, but no infusions were made once the needle was in position.
4.3. Apparatus
The behavioral training apparatus consisted of eight individual chambers (20.5 cm × 22.0 cm × 22.5 cm) with stainless steel front and back walls, clear acrylic sides, and a floor made of 0.48-cm stainless steel rods spaced 1.9 cm apart. An illuminated clear acrylic food cup was recessed in an opening of the front wall, and photocells at the front of the food cup recorded entries and time spent in the cup. Liquid sucrose could be delivered to the bottom of the cup via infusion pumps mounted outside the sound attenuating chambers surrounding the experimental chambers. Locally-fabricated retractable levers, which were operated virtually silently by pneumatic controls, were located on either side of the food cup. Each chamber was enclosed inside a sound attenuating shell. A speaker was mounted inside the shell, directed toward the top front of the chamber, and a relay clicker was mounted on the floor of the shell near the front of the chamber. An infrared motion detector (Coulbourn H24–61; Whitehall, PA) and an infrared light panel were located on the top outside of each chamber. Video cameras mounted within the shell allowed for television viewing.
4.4. Training and testing procedures
4.4.1. Autoshaping
Rats first received two 64-minute sessions in which they were trained to eat from the food cups. In each of these sessions, rats were given sixteen 0.1-ml deliveries of 8% (w/v) sucrose solution, with a mean intertrial interval (ITI) of 240 s. Next, rats underwent 12 sessions of the autoshaping procedure. Within each 64-min session, there were 25 CS+ and 25 CS− trials (mean ITI = 77 s). On CS+ trials, one lever was extended for 10 s and reinforced with 0.1 ml of 8% sucrose upon retraction and on CS− trials, the other lever was extended for 10 s, but no sucrose was delivered. For half the rats, the CS+ lever was the left lever and the CS− lever was the right lever and for the other half, the sides of the CS+ and CS− levers were reversed. Similar to Bussey et al. (1997), trials were ordered so that no more than two of same trial type occurred in sequence.
4.4.2 Pavlovian-Instrumental Transfer (PIT) procedures
Three or four days after autoshaping training was completed, we tested the rats on single-reinforcer PIT. All rats first received one 32-min food cup reminder training session, which included 8 0.1-ml deliveries of 8% sucrose (mean ITI = 240 s). After this session, the rats received either 8 or 9 32-min Pavlovian cue conditioning sessions. In each session, rats received 4 CS+ and 4 CS− trials, each 2 min in duration (mean ITI = 240 s). On average, 4 sucrose reinforcers were delivered semirandomly within each 2-min CS+. During the first 10 s, no reinforcers were delivered, permitting measurement of the acquisition of anticipatory food cup responding. For the remaining 110 s, rats received 0.1 ml deliveries of 8% liquid sucrose on a variable time (VT) 28-s schedule. Sucrose was never delivered on CS− trials. For half the rats, an 80-db white noise was CS+ and a 3-hz clicker was CS−. For the other half, the identities of the CSs were reversed. The levers were never present in Pavlovian training sessions.
After Pavlovian training, rats underwent 10 days of instrumental training in which a single lever (the one used as CS+ in autoshaping) was present in the chamber for the entirety of each session. On the first day, rats received 0.1 ml deliveries of 8% sucrose following each lever-press. This session lasted 32 min or until a rat performed 60 lever presses. For the next 2 days, lever-press responses were reinforced on a variable interval (VI) 30 s schedule. Rats were then reinforced for lever-press responses on a VI-60 s schedule for the remaining 7 sessions. All of these sessions were 32 min in duration. After the completion of instrumental training, rats were given 2 Pavlovian “reminder” sessions, identical to previous Pavlovian sessions. Levers were removed for these sessions.
Finally, all rats received a 40-min PIT test session in which the lever was inserted but lever-press responses were never reinforced. During this session, the CS+ and CS− were presented four times each, with the order of presentation counterbalanced. Lever-press responses were recorded during each 2-min CS as well as the 2-min interval prior to each CS presentation.
4.5. Histological Procedures
After behavioral testing, rats were anesthetized with sodium pentobarbital (100 mg/kg) and perfused intracardially with 0.9% saline, followed by 10% (v/v) Formalin in 0.1M PBS. Brains were removed and stored in 0.1M PBS and 20% (w/v) sucrose. 40-μm slices were collected and Nissl stained to verify lesion placements.
Highlights.
Amygdala central nucleus lesions did not affect autoshaped lever pressing in rats
These same lesioned rats showed impairments in PIT
Different measures of incentive are mediated by different brain systems
Acknowledgments
This work was supported by NIH grant MH53667.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Berridge KC. Measuring hedonic impact in animals and infants: Microstructure of affective taste reactivity patterns. Neurosci Biobehav Rev. 2000;24:173–198. doi: 10.1016/s0149-7634(99)00072-x. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Reward learning: Reinforcement, incentives, and expectations. Psychol Learn Motiv Advances Res Theory. 2001;40:223–278. [Google Scholar]
- Berridge KC. Motivation concepts in behavioral neuroscience. Physiol Behavior. 2004;81:179–209. doi: 10.1016/j.physbeh.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Boakes R. Performance on learning to associate a stimulus with positive reinforcement. In: Davis H, Hurwitz H, editors. Operant-Pavlovian interactions. Hillsdale, NJ: Lawrence Eribaum Associates; 1977. pp. 67–97. [Google Scholar]
- Burns LH, Robbins TW, Everitt BJ. Differential-effects of excitotoxic lesions of the basolateral amygdala, ventral subiculum and medial prefrontal cortex on responding with conditioned reinforcement and locomotor-activity potentiated by intraaccumbens infusions of D-amphetamine. Behav Brain Res. 1993;55:167–183. doi: 10.1016/0166-4328(93)90113-5. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Everitt BJ, Robbins TW. Dissociable effects of cingulated and medial frontal cortex lesions on stimulus-reward learning using a novel Pavlovian autoshaping procedure for the rat: Implications for the neurobiology of emotion. Behav Neurosci. 1997;111:908–919. doi: 10.1037//0735-7044.111.5.908. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: The role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Chang SE, Wheeler DS, Holland PC. Roles of nucleus accumbens and basolateral amygdala in autoshaped lever pressing. 2011. (submitted for publication) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW. Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of Pavlovian-instrumental transfer. J Neurosci. 2005;25:962–970. doi: 10.1523/JNEUROSCI.4507-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey GCL, Phillips S, Cleland GG. The topography of signal-centered behavior in the rat: the effects of solid and liquid reinforcers. Behav Analysis Letters. 1981;1:331–337. [Google Scholar]
- Davey GCL, Cleland GG, Oakley DA. Applying Konorski’s model of classical conditioning to signal centered behavior in the rat: Some functional similarities between hunger CRs and sign tracking. Anim Learn Behav. 1982;10:257–262. [Google Scholar]
- Davey GCL, Cleland GG, Oakley DA, Jacobs JL. The effect of early feeding experience on signal-directed response topography in the rat. Physiol Behav. 1984;32:11–15. doi: 10.1016/0031-9384(84)90062-3. [DOI] [PubMed] [Google Scholar]
- El-Amamy H, Holland PC. Substantia nigra pars compacta is critical to both the acquisition and expression of learned orienting of rats. Eur J Neurosci. 2006;24:270–276. doi: 10.1111/j.1460-9568.2006.04896.x. [DOI] [PubMed] [Google Scholar]
- El-Amamy H, Holland PC. Dissociable effects of disconnecting amygdala central nucleus from the ventral tegmental area or substantia nigra on learned orienting and incentive motivation. Eur J Neurosci. 2007;25:1557–1567. doi: 10.1111/j.1460-9568.2007.05402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Watson SJ, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to a reward-related cue: Influence on cocaine sensitization. Behav Brain Res. 2008;186:48–56. doi: 10.1016/j.bbr.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to reward-related cues: Implications for addiction. Neuropharmacology. 2009;56:139–148. doi: 10.1016/j.neuropharm.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, Graham PW, Holland PC. The amygdala central nucleus and appetitive Pavlovian conditioning: Lesions impair one class of conditioned behavior. J Neurosci. 1990;10:1906–1911. doi: 10.1523/JNEUROSCI.10-06-01906.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groshek F, Kerfoot E, McKenna V, Polackwich AS, Gallagher M, Holland PC. Amygdala central nucleus function is necessary for learning, but not expression, of conditioned auditory orienting. Behav Neurosci. 2005;119:202–212. doi: 10.1037/0735-7044.119.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Parkinson JA, Connor TM, Dickinson A, Everitt BJ. Involvement of the central nucleus of the amygdala and nucleus accumbens core in mediating Pavlovian influences on instrumental behaviour. Eur J Neurosci. 2001;13:1984–1992. doi: 10.1046/j.0953-816x.2001.01577.x. [DOI] [PubMed] [Google Scholar]
- Han J-S, McMahan RW, Holland PC, Gallaghe M. The role of an amygdalo-nigrostriatal pathway in associative learning. J Neurosci. 1997;17:3913–3919. doi: 10.1523/JNEUROSCI.17-10-03913.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield T, Han JS, Conley M, Gallagher M, Holland P. Neurotoxic lesions of basolateral, but not central, amygdala interfere with Pavlovian second-order conditioning and reinforcer devaluation effects. J Neurosci. 1996;16:5256–5265. doi: 10.1523/JNEUROSCI.16-16-05256.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC. Conditioned stimulus as a determinant of the form of the Pavlovian conditioned response. J Exp Psychol Anim Behav Proc. 1977;3:77–104. doi: 10.1037//0097-7403.3.1.77. [DOI] [PubMed] [Google Scholar]
- Holland PC. Influence of visual conditioned stimulus characteristics on the form of Pavlovian appetitive conditioned responding in rats. J Exp Psychol Anim Behav Proc. 1980;6:81–97. [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Double dissociation of the effects of lesions of basolateral and central amygdala on conditioned stimulus-potentiated feeding and Pavlovian-instrumental transfer. Eur J Neurosci. 2003;17:1680–1694. doi: 10.1046/j.1460-9568.2003.02585.x. [DOI] [PubMed] [Google Scholar]
- Holland PC, Petrovich GD. A neural systems analysis of the potentiation of feeding by conditioned stimuli. Physiol Behav. 2005;86:747–761. doi: 10.1016/j.physbeh.2005.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AW, Gallagher M. Greater effort boosts the affective taste properties of food. Proc R Soc B. 2011;278:1450–1456. doi: 10.1098/rspb.2010.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns DN, Weiss SJ. Sign-tracking (autoshaping) in rats: A comparison of cocaine and food as unconditioned stimuli. Learn Behav. 2004;32:463–476. doi: 10.3758/bf03196042. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Groshek F, Petrovich GD, Cantalini JP, Gallagher M, Holland PC. Role of amygdalo-nigral circuitry in conditioning of a visual stimulus paired with food. J Neurosci. 2005;25:3881–3888. doi: 10.1523/JNEUROSCI.0416-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovibond PF. Facilitation of instrumental behavior by a Pavlovian appetitive conditioned-stimulus. J Exp Psychol Anim Behav Proc. 1983;9:225–247. [PubMed] [Google Scholar]
- Mackintosh NM. The psychology of animal learning. New York: Academic Press; 1974. [Google Scholar]
- Mahler SV, Berridge KC. Which cue to “want?” Central amygdala opioid activation enhances and focuses incentive salience on a prepotent reward cue. J Neurosci. 2009;29:6500–6513. doi: 10.1523/JNEUROSCI.3875-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDannald M, Kerfoot E, Gallagher M, Holland PC. Amygdala central nucleus function is necessary for learning but not expression of conditioned visual orienting. Eur J Neurosci. 2004;20:240–248. doi: 10.1111/j.0953-816X.2004.03458.x. [DOI] [PubMed] [Google Scholar]
- Parkinson JA, Robbins TW, Everitt BJ. Dissociable roles of the central and basolateral amygdala in appetitive emotional learning. Eur J Neurosci. 2000;12:405–413. doi: 10.1046/j.1460-9568.2000.00960.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4. San Diego, CA, USA: Academic Press; 1998. [Google Scholar]
- Robledo P, Robbins TW, Everitt BJ. Effects of excitotoxic lesions of the central amygdaloid nucleus on the potentiation of reward-related stimuli by intra-accumbens amphetamine. Behav Neurosci. 1996;110:981–990. doi: 10.1037//0735-7044.110.5.981. [DOI] [PubMed] [Google Scholar]
- Setlow B, Gallagher M, Holland PC. The basolateral complex of the amygdala is necessary for acquisition but not expression of CS motivational value in appetitive Pavlovian second-order conditioning. Eur J Neurosci. 2002;15:1841–1853. doi: 10.1046/j.1460-9568.2002.02010.x. [DOI] [PubMed] [Google Scholar]
- Tomie A. CAM: an animal learning model of excessive and compulsive implement-assisted drug-taking in humans. Clin Psychol Rev. 1995;15:145–167. [Google Scholar]
- Tomie A. Locating reward cue at response manipulandum (CAM) induces symptoms of drug abuse. Neurosci Biobehav Rev. 1996;20:505–535. doi: 10.1016/0149-7634(95)00023-2. [DOI] [PubMed] [Google Scholar]
- Tomie A, Grimes KL, Pohorecky LA. Behavioral characteristics and neurobiological substrates shared by Pavlovian sign-tracking and drug abuse. Brain Res Rev. 2008;58:121–135. doi: 10.1016/j.brainresrev.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]