Abstract
Background
Life expectancy in people with HIV is now estimated to approach that in the general population in some successfully treated subgroups. However, to attain these life expectancies, viral suppression must be maintained for decades.
Methods
We studied the rate of triple class virologic failure (TCVF) in patients within the Collaboration of Observational HIV Epidemiological Research Europe (COHERE) who started antiretroviral therapy (ART) with an NNRTI- or PI/r-containing regimen from 1998 onwards. We also focussed on TCVF in patients who started a PI/r-containing regimen after virologically failing a first-line NNRTI-containing regimen.
Results
Of 45937 patients followed for a median (IQR) 3.0 (1.5-5.0) years, 980 (2.1%) developed TCVF. By 5 and 9 years after starting ART, an estimated 3.4% (95% CI:3.1%-3.6%) and 8.6% (95% CI:7.5%-9.8%) of patients had developed TCVF. The incidence of TCVF rose during the first 3-4 years on ART, but plateaued thereafter. There was no significant difference in the risk of TCVF according to whether the initial regimen was NNRTI- or PI/r-based (p=0.11). By 5 years after starting a PI/r as second-line, 46% of patients had developed TCVF.
Conclusions
The rate of virologic failure of the three original drug classes is low, but not negligible, and does not appear to diminish over time from starting ART. If this trend continues, many patients are likely to need newer drugs to maintain viral suppression. The rate of TCVF from start of a PI/r after NNRTI failure provides a comparator for studies of response to second-line regimens in resource-limited settings.
Keywords: antiretroviral therapy, virologic failure, triple class failure, NNRTI, ritonavir boosted protease inhibitor, adult, HIV, cohort study, Europe
Introduction
Life expectancy and, equivalently, age-specific death rates in people with HIV have been estimated to be approaching that of the uninfected general population in some successfully treated subgroups 1, 2. These promising life expectancies will only be actually realised, however, if death rates associated with HIV do not rise in the future. The main underlying mechanism by which rates have been reduced is complete suppression of viral replication by antiretroviral therapy (ART), and so low death rates are dependant on the duration of continued viral suppression. Most regimens used so far contain drugs from the original three ART classes (nucleos(t)ide reverse transcriptase inhibitors (NRTI), non-nucleoside reverse transcriptase inhibitors (NNRTI), and protease inhibitors (PI)). Current regimens tend to lead to sustained viral suppression in the majority of patients but rates of virologic failure (failure to (continue to) suppress viral load) remain appreciable. Virologic failure is thought to occur as the result of an uncertain mixture between sub-optimal adherence and the development of resistance. The fact that some patients are infected with resistant virus is probably an additional cause. So, while multiple drugs from several new classes are now available (integrase inhibitors, fusion inhibitors and CCR5 antagonists), at least in high-income countries, life-long viral suppression and consequential low death rates cannot be assured indefinitely. Virologic failure of the three original classes represents a key stage in a patient’s failure history and it is important to monitor the rate with which this is occurring in order to anticipate the continuing need for new drugs in the future 3-12. Such prediction of future need is important in the light of the lengthy drug development process.
In high-income countries, regimens containing a ritonavir boosted PI (PI/r) with two NRTIs or an NNRTI with two NRTIs are recommended to be used as first-line treatment 13-16. Short term randomized trials have suggested that those initiating ART with regimens based on the NNRTI efavirenz have at least as low rates of virologic failure as those starting with PI/r-containing regimens 17. Long term follow-up to compare rates of triple class failure according to the drug classes used in the starting regimen have been restricted to non-randomized comparisons in cohort studies and have reported little marked difference between PI/r- and NNRTI-containing regimens, albeit with wide confidence intervals 4. In resource-limited settings, NNRTI-containing regimens are the almost universal choice for initial regimens. Increasingly, second-line regimens containing a PI/r are being introduced in such settings for patients who appear, by whatever means of monitoring available, to be failing first-line regimens 18. Viral load outcomes of first-line regimens in such settings have been reported and, increasingly, virologic responses to second-line PI/r-based regimens will also be reported. However, there is currently little robust data from high-income countries on response to PI/r-based regimens in patients who have previously failed a first-line NNRTI regimen that can be used as a benchmark for comparison.
In this paper, we report on the rate of virologic failure of the three original drug classes (triple class virologic failure: TCVF) in over 45000 patients within the Collaboration of Observational HIV Epidemiological Research Europe (COHERE) who started ART with an NNRTI- or PI/r-containing regimen. We further present the rate of TCVF in the subgroup of patients who, having previously virologically failed an NNRTI-containing regimen, initiate a PI/r-containing regimen. This study forms part of the PLATO II project (Pursuing Later Treatment Options). The original PLATO collaboration reported in 2004 on outcomes in patients who had virologically failed an NRTI, an NNRTI, and a PI 11.
Methods
Patients
COHERE is a collaboration of most HIV observational cohorts in Europe 19. The 28 cohorts participating in the PLATO II project submitted data in a standardized format 20 to one of two regional coordinating centres, where error checks were performed prior to merging the cohort data to form the COHERE database. Patients appearing in more than one cohort were identified and duplicate records removed. This analysis (on data merged in mid-2008) was restricted to antiretroviral-naïve patients aged 16 or over who started ART from 1998 onwards with an initial regimen of two NRTIs and either an NNRTI or a PI/r. Patients were followed from the start of ART to their last viral load. As the definition of virologic failure used in this study requires 4 months of use of a drug, patients were only included if they had at least 4 months (122 days) of follow-up.
Statistical methods
Virologic failure of a drug was defined as one viral load above 500 copies/ml following at least 4 months of continuous use. The main endpoint in this study was TCVF, defined as virologic failure of two NRTIs, one NNRTI, and one PI/r. Modifications to the definition of virologic failure were considered in several sensitivity analyses: excluding those virologic failures that were followed by a viral load <50 copies/ml without any change in treatment; requiring 3 or 6 months of continuous use of a drug instead of 4 months; and defining virologic failure by a viral load above 1000 copies/ml instead of 500 copies/ml. A further sensitivity analysis considered a combined drug stopping and virologic failure endpoint defined as: virologic failure of 2 NRTIs, or using and stopping 5 NRTIs; and virologic failure of a PI/r, or using and stopping 3 PIs; and failure of an NNRTI, or using and stopping both nevirapine and efavirenz.
Kaplan-Meier and Cox regression methods were used to investigate the risk of TCVF after starting ART. Potential predictors of TCVF at the time of starting ART were risk group (incorporating gender), year of starting ART, age, initial regimen, pre-ART AIDS diagnosis, CD4 count and viral load.
In further analyses we focussed on a subgroup of patients who had followed a common currently recommended treatment strategy of an NNRTI-regimen followed after virologic failure by a PI/r-based regimen. Patients included in these analyses started ART with an NNRTI-based regimen, virologically failed an NNRTI (not necessarily their original NNRTI) while PI-naïve, and later started a PI/r while still PI-naïve. Follow-up from NNRTI failure to the start of the PI/r was retrospectively broken down by ART use and viral load. These patients were also followed from the start of the PI/r to TCVF, with Kaplan-Meier and Cox regression methods used to investigate the risk of TCVF after starting the PI/r. Potential predictors of TCVF at the start of the PI/r were risk group, age, AIDS diagnosis between the start of ART and the start of the PI/r, CD4 count, viral load, year of starting the PI/r, number of new NRTIs in the regimen at the start of the PI/r, and cumulative time spent on ART with a viral load above 500 copies/ml (after virologically failing an NNRTI and before first starting a PI/r).
The continuous variables (age, CD4 count and viral load) were fitted as categorical variables to allow us to investigate the shape of the relationship between these variables and the risk of TCVF. The Cox regression models were stratified by cohort. All p-values are two sided. Analyses were performed using SAS version 9.1., Cary, North Carolina, United States.
Results
Development of TCVF in patients starting ART
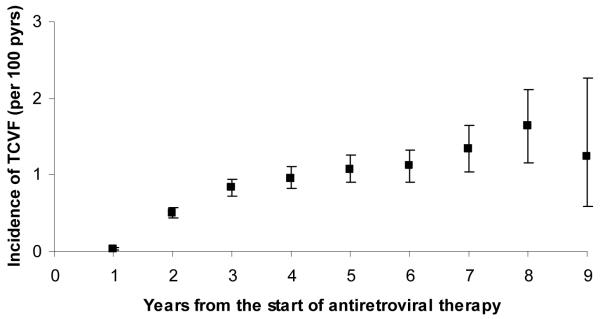
The pre-ART characteristics of the 45937 patients included in the analysis are described in Table 1. Patients were followed for a median (IQR) of 3.0 (1.5-5.0) years (maximum follow-up: 10.2 years), and 980 (2.1%) developed TCVF. The incidence of TCVF after ART initiation rose in the first 3-4 years but appeared to plateau at around 1.0-1.5 per 100 person-years thereafter, up to year 9 (Figure 1). By 5 and 9 years from the start of ART, the estimated cumulative proportions of patients who had developed TCVF were 3.4% (95% CI: 3.1%-3.6%) and 8.6% (95% CI: 7.5%-9.8%). Of the included patients, 1238 (2.7%) died without developing TCVF.
Table 1.
Characteristics at the time of starting antiretroviral therapy for all patients included in the study (n=45937)
| Characteristics at the time of starting ART | n | % | |
|---|---|---|---|
| Combined gender / risk group | Homosexual men | 16649 | 36.2 |
| Heterosexual men | 9122 | 19.9 | |
| Heterosexual women | 10523 | 22.9 | |
| IDU | 5586 | 12.2 | |
| Other / Unknown | 4057 | 8.8 | |
| Age at the time of starting ART years |
16-24 | 2570 | 5.6 |
| 25-34 | 15467 | 33.7 | |
| 35-44 | 17814 | 38.8 | |
| 45-59 | 8516 | 18.5 | |
| 60- | 1570 | 3.4 | |
| Year of starting ART | 1998-2001 | 16509 | 35.9 |
| 2002-2003 | 11474 | 25.0 | |
| 2004-2008 | 17954 | 39.1 | |
| Initial regimen | 2 NRTIs + 1 NNRTI | 29282 | 63.7 |
| Efavirenz | 16978 | 58.0 | |
| Nevirapine | 12304 | 42.0 | |
| 2 NRTIs + 1 PI/r | 16655 | 36.3 | |
| Lopinavir/r | 9638 | 57.9 | |
| Indinavir/r | 2731 | 16.4 | |
| Saquinavir/r | 1804 | 10.8 | |
| Atazanavir/r | 1279 | 7.7 | |
| Fosamprenavir/r | 1016 | 6.1 | |
| Amprenavir/r | 129 | 0.8 | |
| Tipranavir/r | 47 | 0.3 | |
| Darunavir/r | 11 | 0.1 | |
| AIDS diagnoses prior to starting ART | Yes | 8976 | 19.5 |
| CD4 count cells/mm3 |
0-49 | 5937 | 12.9 |
| 50-199 | 13310 | 29.0 | |
| 200-349 | 13478 | 29.3 | |
| 350-499 | 5014 | 10.9 | |
| 500- | 3708 | 8.1 | |
| Unknown | 4490 | 9.8 | |
| Viral load log10 copies/ml |
0-3.99 | 7431 | 16.2 |
| 4.0-4.49 | 5300 | 11.5 | |
| 4.5-4.99 | 9406 | 20.5 | |
| 5.0-5.49 | 10455 | 22.8 | |
| 5.5-5.99 | 6473 | 14.1 | |
| 6.0- | 1528 | 3.3 | |
| Unknown | 5344 | 11.6 | |
| Age at the time of starting ART years | Median (IQR): 37 (32-44) | ||
| CD4 count cells/mm3 | Median (IQR): 211 (100-321) | ||
| Viral load log10 copies/ml | Median (IQR): 4.9 (4.3-5.4) | ||
Figure 1.
Incidence (with 95% confidence interval) of triple class virologic failure (TCVF) during each year after the start of antiretroviral therapy.
Of the 29282 patients starting ART with an NNRTI regimen, 4836 (16.5%) had a pre-ART AIDS diagnosis, compared with 4140 (24.9%) of the 16655 starting ART with a PI/r regimen. The median (IQR) CD4 count and viral load were 228 (129-335) cells/mm3 and 4.8 (4.1-5.3) log10 copies/ml in patients starting an NNRTI and 178 (66-297) cells/mm3 and 5.0 (4.5-5.5) log10 copies/ml in patients starting a PI/r.
A lower risk of TCVF was observed in homosexual men than in the other combined gender/risk groups (Table 2). Older age at the time of starting ART was found to be associated with a lower risk of TCVF (≥60 years compared to 35-44 years: adjusted HR 0.56, 95% CI 0.35-0.92, and 16-24 years compared to 35-44 years: adjusted HR 1.73, 95% CI 1.35-2.22). Lower pre-ART CD4 count and higher pre-ART viral load were associated with an increased risk of TCVF (CD4 350-499 compared to 200-349 cells/mm3: adjusted HR 0.63, 95% CI 0.49-0.82, and viral load ≥6.0 log10 copies/ml compared to <4.0 log10 copies/ml: adjusted HR 1.86, 95% CI 1.30-2.67). There was no significant difference in the risk of TCVF according to whether an NNRTI or a PI/r was used in the initial ART regimen (adjusted HR for initial PI/r versus initial NNRTI: 0.88, 95% CI 0.75-1.03; p=0.11).
Table 2.
Of 45937 patients included in the study, 980 developed triple class failure (TCVF). The table describes the following according to characteristics at the time of starting antiretroviral therapy: Kaplan-Meier estimates (KM) of the cumulative proportions with TCVF by 5 years from the start of ART; hazard ratios (HR) for the risk of TCVF after starting ART from univariable Cox regression models; hazard ratios from a multivariable Cox regression model
| TCVF by 5 years | Univariable analysis | Multivariable analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| %* | 95%CI | HR | 95% CI | p | HR | 95% CI | p | |
| Risk group | ||||||||
| Homosexual men | 2.3 | 1.9-2.6 | 1 | - | <0.0001 | 1 | - | <0.0001 |
| Heterosexual men | 4.3 | 3.6-4.9 | 1.86 | 1.55-2.23 | 1.70 | 1.41-2.05 | ||
| Heterosexual women | 4.2 | 3.6-4.8 | 1.73 | 1.45-2.06 | 1.53 | 1.28-1.83 | ||
| IDU | 3.7 | 3.0-4.5 | 1.96 | 1.58-2.42 | 1.86 | 1.49-2.30 | ||
| Other / Unknown | 3.5 | 2.6-4.4 | 1.62 | 1.26-2.08 | 1.47 | 1.14-1.90 | ||
| Age years |
||||||||
| 16-24 | 5.7 | 4.2-7.1 | 1.53 | 1.20-1.95 | <0.0001 | 1.73 | 1.35-2.22 | <0.0001 |
| 25-34 | 3.6 | 3.1-4.0 | 1.16 | 1.01-1.34 | 1.26 | 1.09-1.46 | ||
| 35-44 | 3.3 | 2.9-3.7 | 1 | - | 1 | - | ||
| 45-59 | 2.5 | 2.0-3.0 | 0.78 | 0.64-0.96 | 0.79 | 0.64-0.97 | ||
| 60- | 1.8 | 0.8-2.8 | 0.59 | 0.36-0.96 | 0.56 | 0.35-0.92 | ||
| Year of starting ART | ||||||||
| 1998-2001 | 3.4 | 3.1-3.7 | 1 | 0.58 | 1 | - | 0.93 | |
| 2002-2003 | 3.4 | 2.9-3.9 | 1.09 | 0.92-1.28 | 1.01 | 0.85-1.20 | ||
| 2004-2008 | - | - | 0.98 | 0.75-1.29 | 0.96 | 0.73-1.27 | ||
| Initial regimen | ||||||||
| 2 NRTIs + 1 NNRTI | 3.3 | 3.0-3.6 | 1 | 0.21 | 1 | 0.11 | ||
| 2 NRTIs + 1 PI/r | 3.5 | 3.0-4.0 | 1.10 | 0.95-1.27 | 0.88 | 0.75-1.03 | ||
| Pre-ART AIDS | ||||||||
| No | 3.0 | 2.8-3.3 | 1 | <0.0001 | 1 | 0.019 | ||
| Yes | 4.7 | 4.1-5.4 | 1.63 | 1.41-1.87 | 1.21 | 1.03-1.41 | ||
| CD4 count cells/mm3 |
||||||||
| 0-49 | 5.8 | 4.9-6.7 | 2.08 | 1.73-2.51 | <0.0001 | 1.70 | 1.38-2.11 | <0.0001 |
| 50-199 | 4.0 | 3.5-4.5 | 1.31 | 1.10-1.56 | 1.22 | 1.02-1.46 | ||
| 200-349 | 2.9 | 2.4-3.3 | 1 | - | 1 | - | ||
| 350-499 | 1.8 | 1.2-2.3 | 0.61 | 0.47-0.80 | 0.63 | 0.49-0.82 | ||
| 500- | 1.7 | 1.1-2.2 | 0.52 | 0.38-0.71 | 0.55 | 0.40-0.74 | ||
| Unknown | 3.4 | 2.6-4.2 | 1.32 | 1.05-1.66 | 1.22 | 0.90-1.65 | ||
| Viral load log10 copies/ml |
||||||||
| 0-3.99 | 2.9 | 2.3-3.5 | 1 | - | <0.0001 | 1 | - | 0.0009 |
| 4.0-4.49 | 2.6 | 2.0-3.2 | 1.03 | 0.79-1.34 | 0.96 | 0.74-1.26 | ||
| 4.5-4.99 | 3.0 | 2.4-3.5 | 1.17 | 0.93-1.46 | 1.03 | 0.82-1.29 | ||
| 5.0-5.49 | 3.5 | 3.0-4.1 | 1.51 | 1.22-1.88 | 1.24 | 0.99-1.55 | ||
| 5.5-5.99 | 4.2 | 3.5-5.0 | 1.88 | 1.50-2.37 | 1.44 | 1.13-1.84 | ||
| 6.0- | 5.0 | 3.2-6.7 | 2.41 | 1.70-3.42 | 1.86 | 1.30-2.67 | ||
| Unknown | 3.7 | 2.9-4.4 | 1.54 | 1.21-1.97 | 1.15 | 0.85-1.56 | ||
Kaplan-Meier estimate
Development of TCVF in patients starting a PI/r as second-line ART
Of the 29282 patients who started ART with an NNRTI, 6568 (22.4%) virologically failed an NNRTI. Of those virologically failing an NNRTI, 6334 (96.4%) did so while PI-naïve. Among these 6334 people, 2429 (38.3%) later started a PI, of whom 2042 (84.1%) started a PI/r. The characteristics of these patients at the time of starting the PI/r are given in Table 3. The median (IQR) time from starting ART to failing the NNRTI was 0.9 (0.5-1.9) years, and the median (IQR) time from failing the NNRTI to starting a PI/r was 0.8 (0.3-2.2) years. Of a total of 2945 person-years of follow-up between failure of the NNRTI and initiation of the PI/r, 353 person-years (12%) were spent off ART, 1928 person-years (65%) were spent on ART with viral load >500 copies/ml, and 664 person-years (23%) were spent on ART with viral load ≤500 copies/ml.
Table 3.
Characteristics at the time of starting a PI/r as second-line antiretroviral therapy (n=2042)
| Characteristics at the time of first starting a PI/r | n | % | |
|---|---|---|---|
| Combined gender / risk group | Homosexual men | 631 | 30.9 |
| Heterosexual men | 465 | 22.8 | |
| Heterosexual women | 484 | 23.7 | |
| IDU | 303 | 14.8 | |
| Other / Unknown | 159 | 7.8 | |
| Age at the time of first starting a PI/r years |
16-24 | 62 | 3.0 |
| 25-34 | 587 | 28.8 | |
| 35-44 | 947 | 46.4 | |
| 45-59 | 382 | 18.7 | |
| 60- | 64 | 3.1 | |
| Year of first starting a PI/r | 1998-2003 | 750 | 36.7 |
| 2004-2005 | 851 | 41.7 | |
| 2006-2008 | 441 | 21.6 | |
| AIDS diagnosis between start of ART and starting a PI/r |
Yes | 258 | 12.6 |
| CD4 count at the time of first starting a PI/r cells/mm3 |
0-49 | 200 | 9.8 |
| 50-199 | 579 | 28.4 | |
| 200-349 | 650 | 31.8 | |
| 350-499 | 351 | 17.2 | |
| 500- | 256 | 12.5 | |
| Unknown | 6 | 0.3 | |
| Viral load at the time of first starting a PI/r log10 copies/ml |
0-2.99 | 353 | 17.3 |
| 3.0-3.99 | 495 | 24.2 | |
| 4.0-4.99 | 703 | 34.4 | |
| 5.0- | 426 | 20.9 | |
| Unknown | 65 | 3.2 | |
| New NRTIs in regimen at time of first starting a PI/r |
Yes | 1501 | 73.5 |
| Cumulative time on ART with viral load > 500 copies/ml, after virologically failing an NNRTI and before first starting a PI/r months |
0-3 | 536 | 26.3 |
| 3-6 | 429 | 21.0 | |
| 6-18 | 601 | 29.4 | |
| 18- | 476 | 23.3 | |
| Initial PI (or PI pair) | lopinavir/r | 1081 | 52.9 |
| atazanavir/r | 509 | 24.9 | |
| other single PI/r | 368 | 18.0 | |
| double PI/r | 84 | 4.1 | |
| Age at the time of first starting a PI/r years |
Median (IQR): 38 (33-44) | ||
| CD4 count at the time of first starting a PI/r cells/mm3 |
Median (IQR): 244 (140-380) | ||
| Viral load at the time of first starting a PI/r log10 copies/ml |
Median (IQR): 4.2 (3.3-4.9) | ||
Of the 2042 patients in this analysis, 575 (28.2%) developed TCVF, in all cases on the date of virologic failure of a PI/r. The estimated cumulative proportions (95% CI) with TCVF 1 and 5 years after starting a PI/r were 20.4% (18.4%-22.3%) and 46.3% (42.8%-49.7%) respectively. In the heterosexual risk groups, 56.3% (95% CI: 49.3%-60.0%) developed TCVF after 5 years. Predictors of TCVF after the start of the PI/r in this group of patients are shown in Table 4. Higher viral load and lower CD4 count at the time of starting a PI/r were associated with a higher risk of TCVF, and the risk of TCVF was lower in homosexual men than in the other risk groups. Patients who, between failing an NNRTI and starting a PI/r, had spent less time on ART with a viral load above 500 copies/ml had a lower risk of TCVF after starting the PI/r (3-6 months compared to 0-3 months, adjusted HR 1.43, 95% CI 1.11-1.84). Inclusion of new NRTIs in the regimen at the time of starting the PI/r was found to be of borderline significance for predicting TCVF (at least one new NRTI compared to no new NRTIs, adjusted HR 0.85, 95% CI 0.70-1.03).
Table 4.
Of 2042 patients who started a PI/r as second-line antiretroviral therapy, 575 developed triple class virologic failure (TCVF). The table describes the following according to characteristics at the time of first starting a PI/r: Kaplan-Meier estimates (KM) of the cumulative proportions with TCVF by 1 year from first starting a PI/r; hazard ratios (HR) for the risk of TCVF after first starting a PI/r as second-line ART from univariable Cox regression models; hazard ratios from a multivariable Cox regression model
| Characteristic | TCVF by 1 year | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|---|
| %* | 95% CI | HR | 95% CI | p | HR | 95% CI | P | |
| Risk group | ||||||||
| Homosexual men | 13.4 | 8.8-18.0 | 1 | - | <0.0001 | 1 | - | 0.0002 |
| Heterosexual men | 25.5 | 21.0-29.9 | 1.86 | 1.46-2.37 | 1.64 | 1.27-2.10 | ||
| Heterosexual women | 22.3 | 18.1-26.5 | 1.84 | 1.46-2.34 | 1.70 | 1.33-2.18 | ||
| IDU | 22.9 | 17.6-28.2 | 1.74 | 1.30-2.33 | 1.56 | 1.16-2.11 | ||
| Other / Unknown | 22.2 | 14.9-29.5 | 1.58 | 1.12-2.23 | 1.36 | 0.96-1.93 | ||
| Age at the time of first starting a PI/r years |
||||||||
| 16-24 | 29.8 | 17.5-42.2 | 1.60 | 1.06-2.41 | 0.27 | 1.41 | 0.93-2.15 | 0.51 |
| 25-34 | 19.9 | 16.3-23.4 | 1.03 | 0.84-1.25 | 1.05 | 0.86-1.28 | ||
| 35-44 | 19.1 | 16.3-21.9 | 1 | - | 1 | - | ||
| 45-59 | 21.8 | 16.8-26.8 | 1.03 | 0.81-1.31 | 1.04 | 0.82-1.33 | ||
| 60- | 26.4 | 14.5-38.3 | 1.12 | 0.70-1.80 | 1.25 | 0.77-2.03 | ||
| Year of first starting a PI/r |
||||||||
| 1998-2003 | 20.4 | 17.4-23.3 | 1 | - | 0.0029 | 1 | - | 0.0007 |
| 2004-2005 | 18.8 | 15.9-21.7 | 1.24 | 1.03-1.50 | 1.30 | 1.07-1.57 | ||
| 2006-2008 | 26.6 | 19.8-33.3 | 1.71 | 1.23-2.37 | 1.82 | 1.30-2.54 | ||
| AIDS diagnosis between start of ART and first starting a PI/r |
0.0030 | |||||||
| No | 19.2 | 17.2-21.3 | 1 | - | 1 | - | 0.24 | |
| Yes | 28.1 | 21.9-34.2 | 1.40 | 1.12-1.76 | 1.15 | 0.91-1.45 | ||
| CD4 count at the time of first starting a PI/r cells/mm3 |
||||||||
| 0-49 | 40.6 | 33.1-48.2 | 2.39 | 1.86-3.08 | <0.0001 | 1.93 | 1.47-2.53 | <0.0001 |
| 50-199 | 25.0 | 21.0-28.9 | 1.38 | 1.12-1.69 | 1.25 | 1.00-1.54 | ||
| 200-349 | 18.5 | 15.1-21.8 | 1 | - | 1 | - | ||
| 350-499 | 12.5 | 8.6-16.5 | 0.72 | 0.54-0.96 | 0.83 | 0.62-1.11 | ||
| 500- | 9.0 | 5.0-13.0 | 0.70 | 0.51-0.96 | 0.83 | 0.60-1.16 | ||
| Unknown | - | - | 1.66 | 0.39-7.00 | 1.82 | 0.43-7.77 | ||
| Viral load at the time of first starting a PI/r log10 copies/ml |
||||||||
| 0-2.99 | 9.8 | 6.1-13.4 | 1 | - | <0.0001 | 1 | - | 0.0002 |
| 3.0-3.99 | 16.1 | 12.5-19.7 | 1.32 | 0.96-1.82 | 1.34 | 0.96-1.85 | ||
| 4.0-4.99 | 22.1 | 18.6-25.5 | 1.78 | 1.33-2.40 | 1.60 | 1.17-2.19 | ||
| 5.0- | 31.6 | 26.7-36.6 | 2.52 | 1.86-3.42 | 2.07 | 1.48-2.89 | ||
| Unknown | 12.8 | 3.9-21.7 | 1.52 | 0.88-2.62 | 1.30 | 0.74-2.27 | ||
| Number of new NRTIs in regimen at time of first starting a PI/r |
||||||||
| 0 | 24.2 | 20.1-28.4 | 1 | - | 0.061 | 1 | - | 0.098 |
| ≥1 | 19.1 | 16.8-21.3 | 0.84 | 0.69-1.01 | 0.85 | 0.70-1.03 | ||
| Cumulative time on ART with viral load > 500 copies/ml, after virologically failing an NNRTI and before first starting a PI/r months |
||||||||
| 0-3 | 16.1 | 12.8-19.5 | 1 | - | 0.018 | 1 | - | 0.0037 |
| 3-6 | 18.4 | 14.2-22.6 | 1.31 | 1.02-1.70 | 1.43 | 1.11-1.84 | ||
| 6-18 | 24.8 | 20.9-28.6 | 1.44 | 1.14-1.82 | 1.51 | 1.20-1.90 | ||
| 18- | 21.6 | 17.2-26.0 | 1.37 | 1.06-1.78 | 1.28 | 0.99-1.66 | ||
Kaplan-Meier estimate
Sensitivity analyses
In an analysis that excluded those virologic failures which were followed by a viral load <50 copies/ml without any change in treatment, 880 (1.9%) of patients developed TCVF. The estimated cumulative proportions (95% CI) of patients with TCVF by 5 and 9 years after starting ART were 3.0% (2.8%-3.3%) and 7.8% (6.7%-9.0%). The results from the multivariable Cox regression model were consistent with those from the model obtained for the main analysis.
When the definition of virologic failure was modified to require 3 months of continuous use of a drug before failure, the estimated cumulative proportion (95% CI) with TCVF by 9 years from the start of ART was 9.5% (8.3%-10.6%). For 6 months of continuous use, the same estimate was 7.5% (6.3%-8.6%). Where the definition was modified to require 4 months of continuous use and a viral load above 1000 copies/ml, the estimate was 7.6% (6.6%-8.6%).
An endpoint intended to capture exhaustion of the original three antiretroviral classes through use and stopping of drugs as well as through virologic failure was experienced by 1177 (2.6%) patients: the estimated cumulative proportions by 5 and 9 years were 3.8% (3.5%-4.1%) and 12.7% (11.2%-14.2%) respectively.
Discussion
In this large collaborative analysis we found that the rate with which patients experienced virologic failure of the original three drug classes rose over the first 3-4 years from start of ART to around 1% per year, and then stayed around this level thereafter. By 9 years from the start of ART, an estimated 8.6% of patients had developed TCVF. While this confirms the low rate with which the virological benefits of these classes are lost 3-5, 10, from the perspective of life-long maintenance of viral suppression it suggests that many patients are likely to eventually require newer drugs within the coming decades. It is worth noting that development of TCVF may not necessarily mean that all three classes have been exhausted, particularly given the availability of newer PIs and NNRTIs such as darunavir and etravirine, which have been shown to be effective in treatment experienced patients 21. Drugs from new classes are now available in high-income countries 22-24, and encouraging results have been obtained in clinical trials. However, they all have limitations in routine clinical practice: integrase inhibitors are somewhat prone to lose efficacy due to resistance development 25, enfuvirtide requires injection, and CCR5 antagonists are only active against R5 strains of virus. Thus, in patients who have experienced virologic failure of the three original classes it is uncertain whether these new classes will then be able to ensure lifelong viral suppression. Some will eventually require further options and given the length of the drug development process it is important that new compounds continue to be developed.
The rate of development of TCVF was very similar according to whether ART was started with NNRTI-containing regimens or PI/r-containing regimens, consistent with previous observations from cohort studies 4, 10. This supports current guidelines which recommend specific drugs within either class be used in first-line regimens 13-16. Factors in our analysis associated with slower development of TCVF included being in the homosexual male risk group and older age. While the latter observation, which has been identified in previous studies 4, is likely to be explained by a tendency for better adherence in older people 26, 27, the reasons for the former are less clear, although factors such as adherence, socio-economic status, migration status and health-seeking behaviour could also play a role. We also found that those with lower pre-ART viral load and those with higher pre-ART CD4 count tended to develop TCVF more slowly, in common with previous observations 4, 5. This could partly be explained by differences in health seeking behaviour, and hence adherence, between those who present early for therapy and therefore start ART at higher CD4 count.
We also investigated the rate of development of TCVF in people who had virologically failed an NNRTI regimen and who then started a PI/r containing regimen, as their first PI. Due to their failure history this group will tend to be generally more susceptible to poorer adherence than those who have not previously experienced virological failure. There are relatively few robust data on virological responses in patients using PI/r-containing second-line regimens and our estimate of 46% with TCVF by 5 years (55% in the heterosexual risk groups) provides a benchmark against which comparisons can be made, including studies of second-line regimens in resource-limited settings. Those comparisons will have to consider the factors associated with a slower time to TCVF in our analysis. Again, homosexual men experienced a slower rate of TCVF, as did those with lower viral load and those with higher CD4 count, possibly partially due to these markers reflecting the patients’ adherence. The observed increased risk of TCVF in patients starting the PI/r in later calendar years is difficult to interpret, but a possible explanation is that as avoidance of initial virologic failure improves in later calendar years, in this analysis patients with more serious adherence issues are over-represented in later years of starting a PI/r after NNRTI failure.
For those patients who virologically failed an NNRTI-based first-line regimen, and subsequently started a PI/r as second-line ART, the time to the start of the PI/r was long at a median of 0.8 years, but in line with previous data 28. Lower CD4 count and higher viral load at the time of first virologic failure were associated with shorter time to starting the third antiretroviral class (data not shown). In the present study we observed that TCVF developed more slowly in patients who, between failing an NNRTI and starting the PI/r, had spent less than 3 months on ART with a viral load above 500 copies/ml. This is consistent with the view that earlier detection of failure of NNRTI-containing drug regimens and switch to a PI/r without delay could potentially significantly reduce the rate of TCVF.
We considered the effect of modifications in our definition of triple class failure, but none of these resulted in sizeable changes to our estimates.
There are several limitations to our analysis. First, we only studied virologic failure of drugs and did not directly factor in data on resistance mutations detected. In some cases, virologic failure will occur in the absence of resistance mutations, perhaps largely due to poor adherence. If adherence issues can be overcome then the drugs should remain active and options have not been lost. Second, in our comparison of NNRTI and PI/r-containing regimens patients were not randomized to receive one regimen or the other so there were differences at ART initiation between these two patient groups. While we adjusted for factors measured at the start of ART that were associated with the rate of TCVF, bias in the comparison due to unmeasured confounding may well remain. Thirdly, although the maximum follow-up after starting ART was in excess of ten years, the available follow-up for many patients was substantially less. It may be that, with longer follow-up, the incidence of TCVF would eventually begin to decrease with time from starting ART, both as patients most susceptible to periods of poor adherence are selected out and as the long-term impact on adherence of the more recent easy-to-take combination pills is realised 29, 30. A further limitation is that patients included in COHERE tend to be treated in large clinics with strong research links, and so may not be representative of the population of patients on ART in Europe.
The rates of TCVF that we observed may at first sight seem inconsistent with results from randomized trials in which relatively high proportions of people are observed to fulfil the definition of failure within 1-2 years of the start of therapy. However, most trials tend to use composite definitions of failure so that stopping of some drugs, or the fact that a viral load value is missing, are criteria for defining failure, in the same way as raised viral load itself 31 so it is not possible to directly compare with our findings. Further, most trials consider failure only of one regimen, typically containing two drug classes, while we are considering failure of three classes over at least two separate regimen failure episodes.
In conclusion, the rate of virologic failure of the three original drug classes is low, but not negligible, and does not appear to diminish with time from start of ART. If this trend continues many patients are likely to eventually need newer drugs, possibly other than those currently available, in order to maintain viral suppression for their lifetime. It is important to continue to monitor the development of TCVF over longer periods of follow-up, and to study the incidence of multiple class failure following exposure to the newer antiretroviral classes. The rate of triple class failure from start of a PI/r after previous NNRTI failure is relatively high at around 46% by 5 years and provides a relevant comparator for future studies in resource-limited settings.
Appendix
Acknowledgements
Analysis and Writing Committee (PLATO II Project Team)
Rebecca Lodwick (statistician), Dominique Costagliola (ANRS CO4 FHDH), Peter Reiss (Athena), Carlo Torti (Italian Master), Ramon Teira (VACH), Maria Dorrucci (CASCADE), Bruno Ledergerber (SHCS), Amanda Mocroft (EuroSIDA), Daniel Podzamczer (PISCIS), Alessandro Cozzi-Lepri (ICONA), Niels Obel (Danish HIV Cohort), Bernard Masquelier (ANRS CO3 Aquitaine), Schlomo Staszewski (Frankfurt HIV Cohort), Federico García (Co-RIS), Stephane De Wit (Brussels St Pierre), Antonella Castagna (San Raffaele), Andrea Antinori (ICC), Ali Judd (CHIPS), Jade Ghosn (ANRS CO6 PRIMO), Giota Touloumi (AMACS), Cristina Mussini (Modena), Xavier Duval (ANRS CO8 COPILOTE), Jose Ramos (Madrid Cohort), Laurence Meyer (ANRS CO2 SEROCO), Josiane Warsawski (ANRS CO1 EPF), Claire Thorne (ECS), Joan Masip (HIV-MIP), Santiago Perez-Hoyos (GEMES), Pat Tookey (NSHPC), Deenan Pillay (UK CHIC), Ard van Sighem (Athena), Sergio Lo Caputo (Italian Master), Huldrych Günthard (SHCS), Roger Paredes (EuroSIDA), Andrea De Luca (ICONA), Dimitrios Paraskevis (AMACS), Céline Fabre-Colin (Bordeaux RCC), Jesper Kjaer (Copenhagen RCC), Genevieve Chene (Bordeaux RCC), Jens Lundgren (Copenhagen RCC), Andrew Phillips (PLATO II project leader; UK CHIC).
COHERE Steering Committee
Executive Committee: Ian Weller (Chair, University College London), Dominique Costagliola (Vice-chair, FHDH), Bruno Ledergerber (Vice-chair, SHCS), Jens Lundgren (Head, Copenhagen Regional Co-ordinating Centre), Genevieve Chene (Head, Bordeaux Regional Co-ordinating Centre).
Cohort representatives: Giota Touloumi (AMACS), Josiane Warszawski (ANRS CO1 EPF), Laurence Meyer (ANRS CO2 SEROCO), François Dabis (ANRS CO3 AQUITAINE), Murielle Mary Krause (ANRS CO4 FHDH), Cecile Goujard (ANRS CO6 PRIMO), Catherine Leport (ANRS CO8 COPILOTE), Frank de Wolf (ATHENA), Peter Reiss (ATHENA), Kholoud Porter (CASCADE), Maria Dorrucci (CASCADE), Caroline Sabin (UK CHIC), Diana Gibb (CHIPS), Gerd Fätkenheuer (Cologne Bonn), Julia Del Amo (Co-RIS), Niels Obel (Danish HIV Cohort), Claire Thorne (ECS), Amanda Mocroft (EuroSIDA), Ole Kirk (EuroSIDA), Schlomo Staszewski (Frankfurt), Santiago Pérez-Hoyos (GEMES-Haemo), Jesus Almeda (HIV-MIP), Andrea Antinori (ICC), Antonella d’Arminio Monforte (ICONA), Annalisa Ridolfo (IMIT), Pier-Angelo Tovo (ITLR), Maurizio de Martino (ITLR), Norbert H. Brockmeyer (KOMPNET), José Ramos (Madrid Cohort), Manuel Battegay (MoCHIV, SHCS), Cristina Mussini (Modena Cohort), Pat Tookey (NSHPC), Jordi Casabona (PISCIS), Jose M. Miró (PISCIS), Antonella Castagna (San Raffaele), Stephane de Wit (St. Pierre Cohort), Carlo Torti (Italian Master Cohort), Ramon Teira (VACH),Myriam Garrido (VACH).
Project Leads: François Dabis, Matthias Egger, Hansjakob Furrer, Ole Kirk, Charlotte Lewden, Marie-Louise Newell, Andrew Phillips, Caroline Sabin, Jonathan Sterne, Amalio Telenti. Regional Co-ordinating Centres: Fidéline Collin-Filleul, Michelle Ellefson, Céline Fabre-Colin, Jesper Kjaer.
European AIDS Treatment Group: Nikos Dedes.
All members of the PLATO II Project Team participated in discussions on the design of the study, the choice of statistical analyses and interpretation of the findings, and were involved in the preparation and review of the final manuscript for submission. In addition, Rebecca Lodwick and Andrew Phillips are responsible for performing all analyses; Rebecca Lodwick acts as guarantor for the analyses and has full access to the dataset.
The PLATO II project is funded by MRC award G0700832.
The COHERE study group has also received funding from the French Agence Nationale de Recherches sur le Sida et les Hépatites Virales (ANRS), the Dutch HIV Monitoring Foundation and the Danish Augustinus Foundation. COHERE would also like to acknowledge the many different funders of the participating cohorts and these are listed on the Regional Coordinating Centre websites (http://www.cphiv.dk/COHERE/tabid/295/Default.aspx and http://etudes.isped.u-bordeaux2.fr/cohere).
The study sponsors had no role in the study design, analysis, interpretation of data, writing of the report or in the decision to submit the paper for publication.
Footnotes
Conflict of interest statement
No member of the PLATO II Project Team has any financial or personal relationships with people or organizations that could inappropriately influence this work, although most members of the group have, at some stage in the past, received funding from a variety of pharmaceutical companies for research, travel grants, speaking engagements or consultancy fees.
References
- (1).Bhaskaran K, Hamouda O, Sannes M, et al. Changes in the risk of death after HIV seroconversion compared with mortality in the general population. JAMA. 2008;300(1):51–59. doi: 10.1001/jama.300.1.51. [DOI] [PubMed] [Google Scholar]
- (2).Lewden C, Chene G, Morlat P, et al. HIV-infected adults with a CD4 cell count greater than 500 cells/mm(3) on long-term combination antiretroviral therapy reach same mortality rates as the general population. JAIDS. 2007;46(1):72–77. doi: 10.1097/QAI.0b013e318134257a. [DOI] [PubMed] [Google Scholar]
- (3).Lohse N, Obel N, Kronborg G, et al. Declining risk of triple-class antiretroviral drug failure in Danish HIV-infected individuals. AIDS. 2005;19(8):815–822. doi: 10.1097/01.aids.0000168976.51843.9f. [DOI] [PubMed] [Google Scholar]
- (4).Phillips AN, Leen C, Wilson A, et al. Risk of extensive virological failure to the three original antiretroviral drug classes over long-term follow-up from the start of therapy in patients with HIV infection: an observational cohort study. Lancet. 2007;370(9603):1923–1928. doi: 10.1016/S0140-6736(07)61815-7. [DOI] [PubMed] [Google Scholar]
- (5).Mocroft A, Ledergerber B, Viard JP, et al. Time to virological failure of 3 classes of antiretrovirals after initiation of highly active antiretroviral therapy: Results from the EuroSIDA Study Group. Journal of Infectious Diseases. 2004;190(11):1947–1956. doi: 10.1086/425424. [DOI] [PubMed] [Google Scholar]
- (6).Sabin CA, Hill T, Lampe F, et al. Treatment exhaustion of highly active antiretroviral therapy (HAART) among individuals infected with HIV in the United Kingdom: multicentre cohort study. British Medical Journal. 2005;330(7493):695–698B. doi: 10.1136/bmj.38369.669850.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Zaccarelli M, Tozzi V, Lorenzini P, et al. Multiple drug class-wide resistance associated with poorer survival after treatment failure in a cohort of HIV-infected patients. AIDS. 2005;19(10):1081–1089. doi: 10.1097/01.aids.0000174455.01369.ad. [DOI] [PubMed] [Google Scholar]
- (8).Tozzi V, Zaccarelli M, Bonfigli S, et al. Drug-class-wide resistance to antiretrovirals in HIV-infected patients failing therapy: prevalence, risk factors and virological outcome. Antiviral Therapy. 2006;11(5):553–560. [PubMed] [Google Scholar]
- (9).Napravnik S, Keys JR, Quinlivan EB, Wohl DA, Mikeal OV, Eron JJ. Triple-class antiretroviral drug resistance: risk and predictors among HIV-1-infected patients. AIDS. 2007;21(7):825–834. doi: 10.1097/QAD.0b013e32805e8764. [DOI] [PubMed] [Google Scholar]
- (10).Mocroft A, Horban A, Clotet B, et al. Regional differences in the risk of triple class failure in European patients starting combination antiretroviral therapy after 1 January 1999. HIV Medicine. 2008;9(1):41–46. doi: 10.1111/j.1468-1293.2008.00519.x. [DOI] [PubMed] [Google Scholar]
- (11).Ledergerber B, Lundgren JD, Walker AS, et al. Predictors of trend in CD4-positive T-cell count and mortality among HIV-1-infected individuals with virological failure to all three antiretroviral-drug classes. Lancet. 2004;364(9428):51–62. doi: 10.1016/S0140-6736(04)16589-6. [DOI] [PubMed] [Google Scholar]
- (12).Costagliola D, Potard V, Duvivier C, et al. Impact of newly available drugs on clinical progression in patients with virological failure after exposure to three classes of antiretrovirals. Antiviral Therapy. 2005;10(4):563–573. [PubMed] [Google Scholar]
- (13).Hammer SM, Eron JJ, Reiss P, et al. Antiretroviral treatment of adult HIV infection - 2008 recommendations of the International AIDS Society USA panel. JAMA. 2008;300(5):555–570. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- (14).Panel on Antiretroviral Guidelines for Adults and Adolescents . Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; [Accessed December 15, 2008]. Nov 3, 2008. pp. 1–139. Available at http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. [Google Scholar]
- (15).Gazzard BG. British HIV Association guidelines for the treatment of HIV-1-infected adults with antiretroviral therapy 2008. HIV Medicine. 2008;9(8):563–608. doi: 10.1111/j.1468-1293.2008.00636.x. [DOI] [PubMed] [Google Scholar]
- (16).Clumeck N, Pozniak A, Raffi F. European AIDS Clinical Society (EACS) guidelines for the clinical management and treatment of HIV-infected adults. HIV Medicine. 2008;9(2):65–71. doi: 10.1111/j.1468-1293.2007.00533.x. [DOI] [PubMed] [Google Scholar]
- (17).Riddler SA, Haubrich R, DiRienzo AG, et al. Class-sparing regimens for initial treatment of HIV-1 infection. New England Journal of Medicine. 2008;358(20):2095–2106. doi: 10.1056/NEJMoa074609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).World Health Organization . Priority interventions: HIV/AIDS prevention, treatment and care in the health sector. Version 1.2. 2009. [Google Scholar]
- (19).Sabin CA, Smith CJ, Monforte AD, et al. Response to combination antiretroviral therapy: variation by age - The Collaboration of Observational HIV Epidemiological Research Europe (COHERE) study group. AIDS. 2008;22(12):1463–1473. doi: 10.1097/QAD.0b013e3282f88d02. [DOI] [PubMed] [Google Scholar]
- (20).Kjaer J, Ledergerber B. HIV cohort collaborations: proposal for harmonization of data exchange. Antiviral Therapy. 2004;9(4):631–633. [PubMed] [Google Scholar]
- (21).Fagard C, Descamps D, Dubar V, et al. Efficacy and safety of raltegravir plus etravirine and darunavir/ritonavir in treatment-experienced patients with multidrug-resistant virus: 48-week results from the ANRS 139 TRIO trial; 5th IAS Conference on HIV Pathogenesis, Treatment and Prevention; 2009. [Google Scholar]
- (22).Lazzarin A, Clotet B, Cooper D, et al. Efficacy of enfuvirtide in patients infected with drug-resistant HIV-1 in Europe and Australia. New England Journal of Medicine. 2003;348(22):2186–2195. doi: 10.1056/NEJMoa035211. [DOI] [PubMed] [Google Scholar]
- (23).Steigbigel RT, Cooper DA, Kumar PN, et al. Raltegravir with optimized background therapy for resistant HIV-1 infection. New England Journal of Medicine. 2008;359(4):339–354. doi: 10.1056/NEJMoa0708975. [DOI] [PubMed] [Google Scholar]
- (24).Gulick RM, Lalezari J, Goodrich J, et al. Maraviroc for previously treated patients with R5 HIV-1 infection. New England Journal of Medicine. 2008;359(14):1429–U27. doi: 10.1056/NEJMoa0803152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Cooper DA, Steigbigel RT, Gatell JM, et al. Subgroup and resistance analyses of raltegravir for resistant HIV-1 infection. New England Journal of Medicine. 2008;359(4):355–365. doi: 10.1056/NEJMoa0708978. [DOI] [PubMed] [Google Scholar]
- (26).Branas F, Berenguer J, Sanchez-Conde M, et al. The eldest of older adults living with HIV: Response and adherence to highly active antiretroviral therapy. American Journal of Medicine. 2008;121(9):820–824. doi: 10.1016/j.amjmed.2008.05.027. [DOI] [PubMed] [Google Scholar]
- (27).Barclay TR, Hinkin CH, Castellon SA. Age-associated predictors of medication adherence in HIV-positive adults: Health beliefs, self-efficacy, and neurocognitive status. Health Psychology. 2007;26(1):40–49. doi: 10.1037/0278-6133.26.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Sabin CA, Lee KJ, Dunn D, et al. Treatment switches after viral rebound in HIV-infected adults starting antiretroviral therapy: multicentre cohort study. AIDS. 2008;22(15):1943–1950. doi: 10.1097/QAD.0b013e32830e4cf3. [DOI] [PubMed] [Google Scholar]
- (29).Benzie AA, Bansi LK, Sabin CA, et al. Increased duration of viral suppression is associated with lower viral rebound rates in patients with previous treatment failures. AIDS. 2007;21(11):1423–1430. doi: 10.1097/QAD.0b013e3281532ca7. [DOI] [PubMed] [Google Scholar]
- (30).Smith CJ, Phillips AN, Dauer B, et al. Factors associated with viral rebound among highly treatment-experienced HIV-positive patients who have achieved viral suppression. HIV Medicine. 2009;10(1):19–27. doi: 10.1111/j.1468-1293.2008.00650.x. [DOI] [PubMed] [Google Scholar]
- (31).Phillips AN, Walker AS. Drug switching and virologic-based endpoints in trials of antiretroviral drugs for HIV infection. AIDS. 2004;18(3):365–70. doi: 10.1097/00002030-200402200-00001. [DOI] [PubMed] [Google Scholar]