Abstract
Bone marrow stromal cells (BMSC) and osteoblasts are critical components of the microenvironment that support hematopoietic recovery following bone marrow transplantation. Aggressive chemotherapy not only affects tumor cells, but also influences additional structural and functional components of the microenvironment. Successful reconstitution of hematopoiesis following stem cell or bone marrow transplantation after aggressive chemotherapy is dependent upon components of the microenvironment maintaining their supportive function. This includes secretion of soluble factors and expression of cellular adhesion molecules that impact on development of hematopoietic cells. In the current study, we investigated the effects of chemotherapy treatment on BMSC and human osteoblast (HOB) expression of Interleukin-6 (IL-6) as one regulatory factor.
IL-6 is a pleiotrophic cytokine which has diverse effects on hematopoietic cell development. In the current study we demonstrate that exposure of BMSC or HOB to melphalan leads to decreases in IL-6 protein expression. Decreased IL-6 protein is the most pronounced following melphalan exposure compared to several other chemotherapeutic agents tested. We also observed that melphalan decreased IL-6 mRNA in both BMSC and HOB. Finally, using a model of BMSC or HOB co-cultured with myeloma cells exposed to melphalan, we observed that IL-6 protein was also decreased, consistent with treatment of adherent cells alone. Collectively, these observations are of dual significance. First, suggesting that chemotherapy induced IL-6 deficits in the bone marrow occur which may result in defective hematopoietic support of early progenitor cells. In contrast, the decrease in IL-6 protein may be a beneficial mechanism by which melphalan acts as a valuable therapeutic agent for treatment of multiple myeloma, where IL-6 present in the bone marrow acts as a proliferative factor and contributes to disease progression. Taken together, these data emphasize the responsiveness of the microenvironment to diverse stress that is important to consider in therapeutic settings.
Keywords: Interleukin-6, melphalan, bone marrow microenvironment, osteoblast, bone marrow stromal cell, chemotherapy
1. Introduction
The ability of the supportive cells of the bone marrow microenvironment, including bone marrow stromal cells (BMSC) and osteoblasts that comprise the endosteal niche, to maintain their functional integrity following chemotherapy or irradiation is vital for efficient reconstitution of hematopoiesis. The importance of specialized niches within the marrow environment that support stem cell self-renewal and a supply of mature blood cells has been described in detail[1–4].
Several groups have documented chemotherapy-induced stromal cell damage[5–7]. In addition, BMSCs isolated from patients receiving standard chemotherapy regimens had a reduced capacity to form confluent monolayers[5]. Chemotherapy-induced damage diminishes the ability of the BMSCs to self-repair, ultimately leading to decreased numbers of functional mature blood cells[6]. Galotto et al. demonstrated that the patients receiving allogenic bone marrow transplants have irreversible stromal damage measured using functional assays that showed CFU-F frequencies did not recover to normal levels even after 12 years post-transplant[7]. These investigations emphasize the vulnerability of the components of the endosteal niche.
Interleukin-6 (IL-6) is a pleiotropic cytokine that has important roles in expansion of hematopoietic progenitors, induction of acute-phase proteins for immune and inflammatory responses, and regulation of bone metabolism[8,9]. IL-6 is secreted from BMSCs and osteoblasts, and has both proliferative and anti-proliferative effects. In the bone marrow microenvironment, IL-6 regulates B-cell differentiation and stimulation of T-cells, both necessary to maintain the immune system[10]. An IL-6 deficiency in the microenvironment decreases DNA synthesis in normal hematopoietic progenitor cells[11]. Long-term bone marrow cultures established from IL-6 knockout mice had delayed stromal cell layer development[11]. Additionally, reduced hematopoietic support activity, measured by CFU-GM, BFU-E, and cobblestone areas, which are characteristic of active hematopoietic proliferation was noted in the absence of IL-6 as well [11]. Moreover, IL-6 deficient mice have impaired immune and acute-phase responses[12]. IL-6 deficient mice challenged with diverse viruses and pathogens demonstrated acute-phase inflammatory responses were compromised[12]. Relevant to the current study, Patchen et al. observed that IL-6 administration following radiotherapy accelerated hematopoietic recovery in a murine model[13]. Based on the association of IL-6 deficits with sub-optimal hematopoietic recovery, we undertook investigation to determine whether chemotherapy dysregulates IL-6 expression in BMSC and osteoblasts as one factor which might be involved in the dysregulated hematopoietic support capacity of the bone marrow microenvironment following dose-escalated chemotherapy that has been described by others[5–7].
In the current model of chemotherapy-induced damage we included the chemotherapeutic agent melphalan. Melphalan is extensively used in pre-transplant chemotherapy regimens for autologous and allogeneic stem cell transplantations[14,15]. As such, damage imposed by it on the microenvironment is of pronounced clinical relevance.
Our results indicated that BMSCs and osteoblasts express diminished IL-6 protein following chemotherapy exposure with melphalan. DNA damage accumulation by interruption of repair did not result in the same magnitude of decrease in IL-6 as that observed following melphalan exposure, suggesting that there is some specificity to the melphalan induced change that exceeds general DNA damage effects. In addition, in a co-culture model of BMSC and HOB with myeloma cells, melphalan exposure sustained its ability to decrease IL-6 protein, suggesting melphalan’s effect is sustained in a microenvironment that is typical of tumor and stromal or osteoblast interactions. Collectively, our data suggest that melphalan treatment induces an IL-6 deficit in BMSCs and osteoblasts of the endosteal niche, which may contribute to diminished ability of the bone marrow microenvironment to support reconstitution of hematopoiesis following transplantation. Additionally, melphalan’s effect is maintained even in the presence of myeloma tumor cells, potentially contributing to its efficacy in this disease setting. As such, both the negative and positive effects of this alklyating agent via its influence on the marrow microenvironment are highlighted in the distinct settings of hematopoietic support as well as a tumor sanctuary site.
2. Materials and Methods
2.1 Cell culture and reagents
Primary human BMSC were derived from consenting donors with the approval of the West Virginia University Institutional Review Board. These cells are cultured to morphological homogeneity and are characterized by their constitutive expression of VCAM-1 as well as GM-CSF, kit-ligand and other hematopoietic factors. In addition, the BMSC support IL-7 dependent pro-B cells. BMSC were maintained in alpha-modification of Eagle’s medium (α-MEM) supplemented with 2mM L-glutamine, 10% fetal bovine serum (Hyclone, Logan, UT), 100μg/ml streptomycin, 100 IU/ml penicillin and 5×10−5-M 2-β mercaptoethanol at 37°C in 6% CO2. Primary human osteoblasts (HOB) were obtained from PromoCell (Heidelberg, Germany) and maintained in osteoblast growth media with osteoblast supplement as recommended by the manufacturer. HOB are isolated from normal femoral bone tissue from the hip and knee region. Phenotype of the osteoblasts was confirmed by alkaline phosphatase staining and bone mineralization assays. The HOB also express osteocalcin and collagen 1A1 consistent with the typical osteoblast phenotype. In experiments that include chemotherapy exposure, melphalan (Sigma Aldrich, St. Louis, MO) was used at a concentration of [50μg/ml]. Melphalan was dissolved in diluent at a concentration of 50 mg/ml immediately before use. Etoposide (VP-16) (Bristol-Myers Squibb, New York, NY) was stored at a concentration of 33.98 mM and a final concentration of 50 μM was used in all experiments. Methotrexate (Sigma; [50μg/ml]), vincristine (Sigma; [20μg/ml]), docetaxel (Sigma; [50μM]), carboplatin (Sigma; [50μM]) or mechlorethamine hydrochloride (Sigma; [10μM or 25μM]) were included where indicated. KU-55933, an ATM kinase inhibitor (Calbiochem, Philadelphia, PA) was used at a concentration of 10 μM.
2.2 ELISA
BMSC and HOB were cultured in α-MEM complete media or complete osteoblast growth media until confluent, and subsequently treated with melphalan [50μg/ml] for 24 hours. Following treatment, the media was replaced, supernatants collected at 2, 4, 6, 8, 24, and 48 hours post-treatment and the confluent layers of BMSC and HOB were lysed in RIPA buffer to allow quantitation of both supernatant and intracellular IL-6 protein levels. Following collection of samples at all timepoints, an IL-6 ELISA (eBioscience, San Diego, CA) was completed. In additional IL-6 ELISAs, BMSC and HOB were treated for 24 hours with melphalan, VP-16, methotrexate, vincristine, docetaxel, carboplatin or mechlorethamine hydrochloride at the doses indicated previously. Following exposure, the cells were rinsed, media replaced and supernatants collected at 2, 4, 6, 8, 24 or 48 hours post-treatment and an IL-6 ELISA completed. For ATM kinase inhibition experiments, BMSC or HOB were treated with KU-55933 [10 μM] or melphalan [50 μg/ml] for 24 hours, rinsed and supernatants collected and IL-6 ELISA completed as described above. For co-culture experiments, BMSC and HOB were grown to confluence and H929 myeloma cells (ATCC: NCI-H929) were added at a density of 1×106 H929 cells per milliliter for 24 hrs prior to treatment with melphalan [50μg/ml] for 24 hours.
2.3 RNA Isolation and Real Time PCR
Total RNA was isolated using the Qiagen RNeasy kit following the recommendations of the manufacturer (Qiagen Inc., Valencia, CA). RNA concentration was determined by NanoDrop. To determine relative levels of IL-6 expression, real-time PCR was completed. All reactions were performed in triplicate using 50 ng of RNA per reaction and the one-step QuantiTect SYBR Green RT-PCT kit (Applied Biosystems, Foster city, CA). Specific primers for IL-6 (Real Time Primers, Elkins Park, PA) or the housekeeping gene beta-glucuronidase (GusB) (Real Time Primers) were used. Amplifications were completed using a 7500 real-time cycler (Applied Biosystems). The amplification conditions included 50°C/ 30 minutes, 95°C/ 15 minutes and 45 cycles of 94°C/ 15 seconds, 55°C/ 30 seconds, and 72°C/ 45 seconds. The relative changes in gene expression were calculated using the Comparative Ct method[16].
2.4 Statistical Analysis
Analysis of data was completed using SigmaPlot 11.0 graphing and statistical software using One-Way ANOVA, student’s t-test and Tukey post hoc test when appropriate. P< 0.05 is indicated as statistically significant. Data are presented as means +/− standard deviation. Additional statistical analysis was completed using log transformed data prior to ANOVA. Post hoc comparisons were made with Tukey’s HSD (Honestly Significantly Different) procedure. The statistical analysis used for each data set is indicated in the appropriate figure legend.
3. Results
3.1 Melphalan treatment of BMSCs and HOBs results in diminished IL-6 protein.
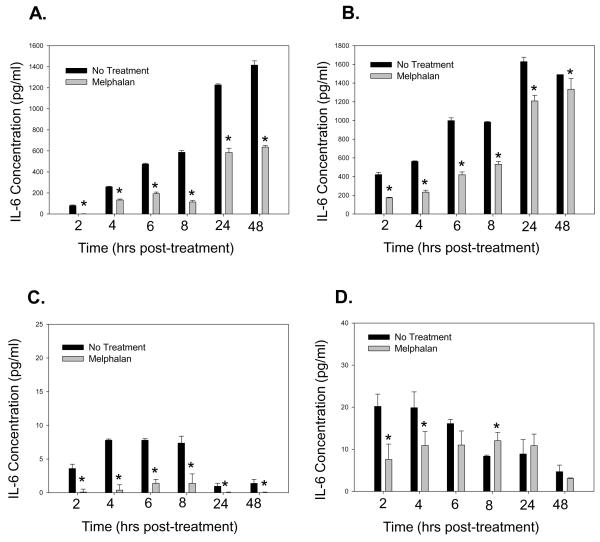
To determine if melphalan disrupted IL-6 protein expression, BMSCs and HOBs were exposed to melphalan and evaluated for IL-6 expression. Melphalan treatment led to decreased IL-6 protein detected in supernatants and lysates of both BMSCs and HOBs (Figure 1 A-D). To determine if IL-6 protein detection was decreased due to expression of the soluble IL-6 receptor (sIL-6R) which might mask antibody recognition, BMSCs and HOBs were exposed to melphalan and a sIL-6R ELISA completed. No detectable levels of sIL-6R were detected in BMSCs or HOBs (data not shown). Finally, to evaluate whether IL-6 protein was being degraded more rapidly in melphalan treated populations of cells, BMSCs and HOBs were exposed to melphalan alone or in combination with cyclohexamide [100ug/ml]. Cycloheximide exposure resulted in the expected decrease in IL-6 protein over 24 hours with addition of melphalan not affecting the rate of degradation of IL-6 protein (data not shown).
Figure 1. Treatment of human BMSC and HOB with Melphalan decreases Interleukin-6 protein.
BMSC (A,C) and HOB (B,D) cells were seeded into a 96 well plate in triplicate and left untreated or were treated with melphalan [50 μg/ml] in media for 24 hours. After 24 hours, the media was replaced and supernatants (A,B) or cell lysates in RIPA buffer (C,D) were collected at the time points above. An Interleukin-6 ELISA was performed to quantitate changes in the levels of IL-6 protein secreted following chemotherapy. While IL-6 protein levels in supernatants were consistently reduced, no accumulation of intracellular IL-6 was observed to suggest deregulated secretion. One-way ANOVA and Tukey post hoc tests were used for analysis of data summarized in graphs A and B. One-way ANOVA and Tukey post hoc and Student’s t-tests were used for data presented in graphs C and D. For data included in graph C, Student’s t-test was used for the 24 and 48 hour time points and for data shown in graph D, Student’s t-test was used for the 6, 8, 24 and 48 hour time points, as these points fell out of the range that could be tested appropriately in the ANOVA (* represents p<0.05).
3.2 Melphalan exposure, compared to other chemotherapeutic agents tested, resulted in the most pronounced decrease in IL-6 protein.
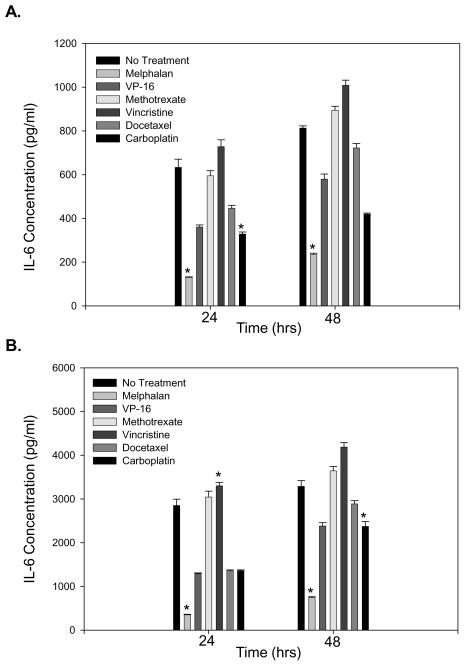
Based on the previous data, we investigated if the melphalan-induced decrease in IL-6 protein was specific or if chemotherapeutic agents, in general, had a comparable effect. While all the different chemotherapeutic agents investigated led to significant changes in IL-6 protein compared to untreated BMSC or HOB, melphalan treatment consistently led to the most pronounced decrease in IL-6 (Figure 2 A and B).
Figure 2. Melphalan exposure led to the most pronounced decrease in IL-6 protein compared to several agents.
BMSC (A) and HOB (B) were exposed to the various chemotherapeutic agents indicated for 24 hours. After 24 hours, the cells were rinsed, the media replaced, supernatants collected at 24 and 48 hours post-treatment and an IL-6 ELISA completed. The concentration of drug used was the highest tolerable dose without a decrease in cell viability (data not shown). Melphalan exposure led to the most pronounced decrease in IL-6 protein in both BMSC and HOB. Log transformed ANOVA and Tukey post hoc statistical analysis were completed (* represents p<0.05).
3.3 Melphalan leads to a more pronounced decrease in IL-6 than that observed following mechlorethamine hydrochloride exposure.
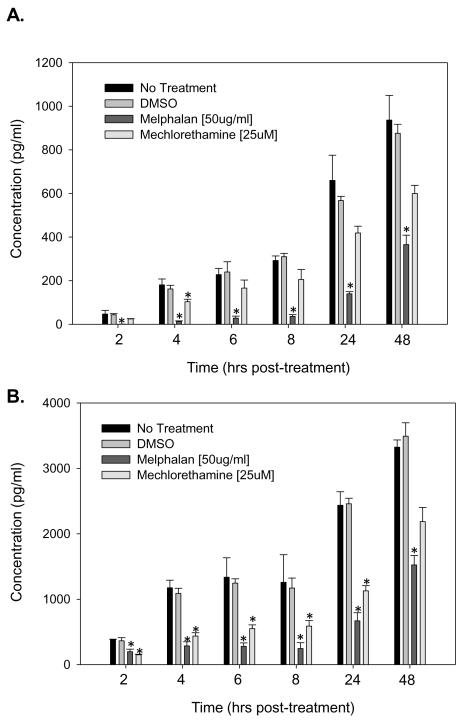
To determine if melphalan’s effects on IL-6 protein expression in BMSC and HOB was drug class specific, an additional alkylating agent, mechlorethamine hydrochloride (nitrogen mustard) was evaluated by treating the cells for 24 hours, removing treatment and completing an IL-6 ELISA as described for melphalan. While both additional alkylating agents elicited decreased IL-6 protein, BMSC and HOB had differential sensitivity to them. Exposure of BMSC to mechlorethamine hydrochloride did not lead to significant decreases in IL-6 protein compared to untreated BMSC. While exposure of HOB to mechlorethamine hydrochloride led to significant decreases in IL-6 up to 8 hours post-treatment, this decrease was not as pronounced as that following melphalan exposure (Figure 3 A and B). Overall, the effect of melphalan was consistent across both BMSC and HOB, suggesting the melphalan-induced decrease in IL-6 protein may be attributed to an additional mechanism of action beyond that of its alkylating effect.
Figure 3. Melphalan leads to a more pronounced decrease in IL-6 than that observed following mechlorethamine hydrochloride exposure.
BMSC (A) and HOB (B) were treated with melphalan or mechlorethamine hydrochloride for 24 hours. After 24 hours, the cells were rinsed, the media replaced, supernatants collected at 2, 4, 6, 8, 24 and 48 hours post-treatment and an IL-6 ELISA completed. Melphalan treatment led to the most pronounced decrease in IL-6 protein in both BMSC and HOB. Log transformed ANOVA and Tukey post hoc statistical analysis were completed (* represents p<0.05).
3.4 The G>C174 IL-6 polymorphism does not correlate with BMSC response to melphalan.
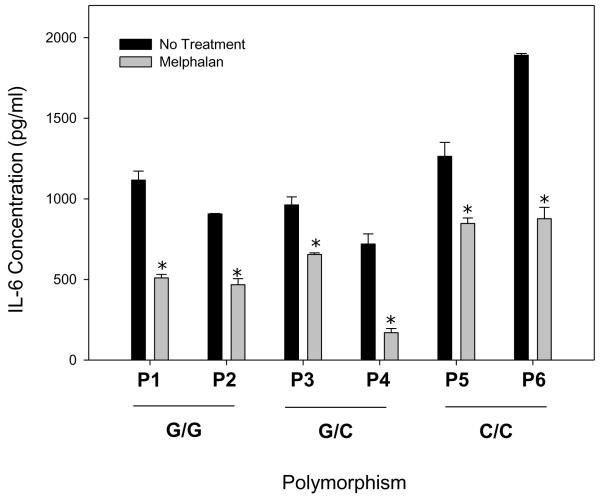
An IL-6 polymorphism has been well described in autoimmune and inflammatory diseases[17–20]. To evaluate a potential correlation between of the G>C174 IL-6 SNP in BMSCs treated with melphalan to drug associated changes in IL-6 expression, primary BMSCs were first genotyped. Two representative cell lines of each SNP were treated with melphalan [50ug/ml] and an IL-6 ELISA completed. Regardless of genotype, all cell lines had decreased detection of IL-6 protein with melphalan treatment (Figure 4).
Figure 4. The IL-6 G>C174 SNP does not affect BMSC response to melphalan.
BMSC isolated from different patients were genotyped for the presence of the IL-6 polymorphism. Two cell lines from each genotype (P1-P6) were treated with melphalan [50ug/ml] for 24 hours. Following treatment, cells were rinsed, supernatants collected 24 hours post-treatment and an IL-6 ELISA completed. All BMSC lines had decreased IL-6 protein, regardless of genotype. One-way ANOVA and Tukey post hoc tests were used for statistical analysis (* represents p<0.001).
3.5 Melphalan treatment decreased IL-6 mRNA.
Based on the observation that melphalan decreases IL-6 protein expression, we investigated changes in IL-6 mRNA. The levels of IL-6 mRNA transcripts increased initially following 4 hours of melphalan exposure, but overall, decreased following longer exposures (Figure 5 A and B). To evaluate if melphalan altered the stability of IL-6 RNA, BMSCs and HOBs were treated with melphalan alone or in combination with Actinomycin-D or α-amanitin. Treatment of cells with Actinomycin-D or α-amanitin in combination with melphalan treatment did not decrease the stability of IL-6 mRNA (data not shown). Additionally, BMSC and HOB were left untreated or treated with melphalan [50ug/ml] and cellular fractionation and western blot completed to determine if there were pronounced changes in the cellular localization of p65, c-jun or C/EBP-β. These three transcription factors have been previously described to be required for optimal IL-6 gene expression[8]. Negligible alterations in localization were noted in the nuclear fraction when melphalan treated cells were compared to untreated cells (data not shown).
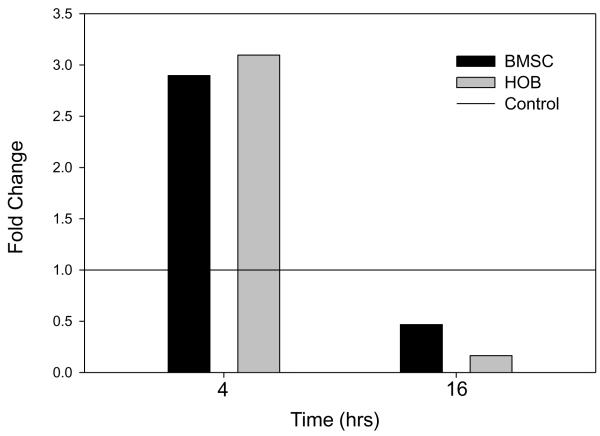
Figure 5. Sustained treatment of BMSC or HOB with melphalan decreases IL-6 mRNA expression.
BMSC (A) or HOB (B) were left untreated or treated with melphalan [50μg/ml] for 4 or 16 hours. Following treatment, RNA was isolated and quantitated and real-time PCR completed to evaluate changes in IL-6 mRNA expression in chemotherapy treated cells as compared to their untreated controls treated controls. Melphalan treatment decreases IL-6 mRNA in both BMSC and HOB after 16 hours of exposure.
3.6 ATM kinase inhibition is less effective than melphalan in reducing IL-6.
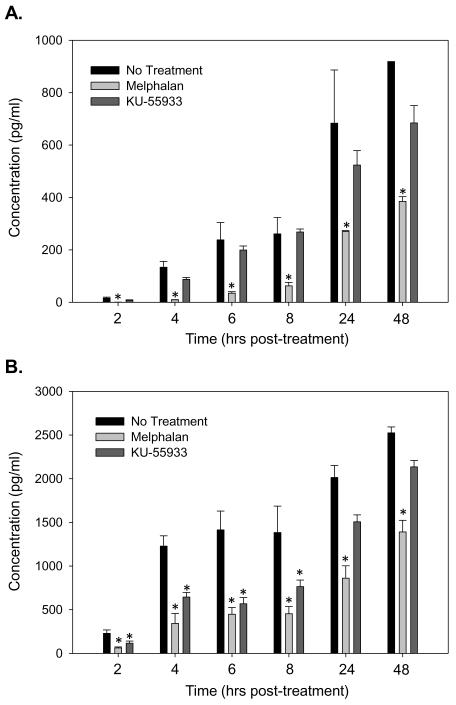
To evaluate an additional mechanism by which melphalan decreases IL-6 protein, KU-55933, an ATM kinase inhibitor, was used to inhibit the repair of generic DNA damage. While melphalan significantly decreased IL-6 at every time point in BMSC and HOB, KU-55933 did not significantly decrease IL-6 protein in BMSC, and only ledto significant decreases in IL-6 protein for the first 8 hours post-treatment (Figure 6 A and B). These data suggest that an additional mechanism other than accumulation of DNA damage that you would expect after either ATM inhibition or melphalan exposure may be responsible for the melphalan-induced decrease in IL-6 protein in both BMSC and HOB.
Figure 6. ATM kinase inhibition is less effective than melphalan in reducing IL-6.
BMSC (A) and HOB (B) were treated with melphalan [50 μg/ml] or KU-55933 [10 μM] for 24 hours. After 24 hours, the cells were rinsed, the media replaced, supernatants collected at 2, 4, 6, 8, 24 and 48 hours post-treatment and an IL-6 ELISA completed. Melphalan treatment led to the most pronounced decrease in IL-6 protein in both BMSC and HOB. Log transformed ANOVA and Tukey post hoc statistical analysis were completed (* represents p<0.05).
3.7 Melphalan decreases total IL-6 in a co-culture with myeloma cells.
Previous studies have shown that the interaction of myeloma cells with supportive cells bone marrow microenviroment leads to increased IL-6 secretion from the microenvironment that enhances scenario, the osteoblast contribution to the effect may be most relevant early in the disease, prior to a predominance of osteoclast activity in later stages. proliferation of the myeloma cells and can contribute disease progression [21]. When considering this To investigate the effects of melphalan in a co-culture setting that might mimic early stages of the tumor:microenvironment interactions, H929 myeloma cells were co-cultured with BMSC or HOB and exposed to melphalan. Following melphalan exposure, the cells were rinsed, fresh media added and supernatants collected at 2, 4, 6 and 8 hours post-treatment. Melphalan retained its ability to result in an overall decrease in IL-6 protein in this co-culture model of BMSC or HOB with myeloma cells (Figure 7 A and B).
Figure 7. Melphalan decreases IL-6 protein in a co-culture with myeloma cells.
BMSC and H929 (A) and HOB and H929 (B) cells were co-cultured for 24 hours. After 24 hours, co-cultures were left untreated or exposed to melphalan [50ug/ml] for 24 hours. Following treatment, cells were rinsed, media replaced, supernatants collected at 2, 4, 6 and 8 hours post-treatment and an IL-6 ELISA completed. Melphalan exposure significantly decreased IL-6 protein expression. Log transformed ANOVA and Tukey post hoc tests were used for statistical analysis (* represents p<0.05).
4. Discussion
In the current study we investigated the effects of chemotherapy on IL-6 expression in BMSC and HOB as two representative supportive cells of the bone marrow microenvironment that influence stem and hematopoietic progenitor cell development[3,22–24] .While the target of dose-escalated chemotherapy or irradiation is a tumor cell population, it is clear that additional cells are also vulnerable to therapy. Successful stem cell or bone marrow transplantation following immuno-suppressive or myeloablative chemotherapy is dependent on the ability of diverse cellular components of the microenvironment to maintain their functionality, including secretion of soluble factors and expression of cellular adhesion molecules that are critical for the survival, proliferation, and differentiation of stem and immature progenitor cells[25–30].
Previously mentioned was the damage that BMSC are vulnerable to during aggressive treatment. In addition, there has also been diverse literature describing the effects of osteoblast functional deficiencies on hematopoiesis. Work by Visnjic et al. described deficits in hematopoiesis in mice where osteoblast deficiency was induced in a transgenic mouse model with herpes virus thymidine kinase gene under the control of a collagen alpha 1 type I promoter, allowing for lineage specific expression of the gene in osteoblasts[31]. This targeting allowed for the specific ablation of osteoblasts by addition of ganciclovir and subsequent loss of lymphoid, myeloid and erythroid progenitors in the bone and significantly decreased HSCs. When osteoblasts recovered, a coincident recovery in hematopoiesis occurred as well in the bone marrow. Chitteti et al. showed that CFU expansion of colonies of multiple lineages was increased when HSCs were cultured with osteoblasts[32], suggesting that osteoblasts have critical roles in the regulation of hematopoiesis, with disrupted function having potential direct impact on hematopoietic recovery.
In the current study, we have shown that melphalan exposure decreases IL-6 protein detected in cell supernatants in the absence of intracellular accumulation, suggesting melphalan induced changes in BMSC or HOB is not causing dysregulated secretion of IL-6 (Figure 1). IL-6 signals through both its membrane receptor as well as a soluble receptor[33–35]. To confirm that the decrease in IL-6 protein detected was not due to masking of IL-6 by the sIL-6R, we evaluated if BMSC and HOB expressed the sIL-6R in vitro. The sIL-6R was not detected in untreated cells or in cells treated with melphalan, suggesting the sIL-6R is not interfering with our detection of IL-6 protein in cell supernatants (data not shown). To determine whether the effects of chemotherapy on IL-6 were specific to melphalan or if chemotherapeutic agents with diverse mechanisms of action resulted in comparable damage, BMSC and HOB were exposed to a variety of agents. Heterogeneity in the drugs examined is reflected by VP-16 being a topoisomerase II inhibitor, methotrexate an anti-metabolite, vincristine a tubulin inhibitor, docetaxel an anti-microtubule agent, carboplatin a heavy metal DNA alkylating-like agent and an additional alkylating agent, mechlorethamine hydrochloride, a nitrogen mustard. While all these agents led to significant changes in IL-6 protein compared to cells that were untreated, melphalan had the most pronounced and consistent decrease in IL-6 protein in both BMSC and HOB (Figures 2 and 3).
In addition to our studies to evaluate different classes of drugs, we investigated sensitivity to melphalan induced changes in IL-6 associated with an IL-6 polymorphism. The 174 G>C SNP has been well characterized in the setting of autoinflammatory and autoimmune diseases and it remains somewhat controversial regarding whether or not the genotype is a prognostic factor[17–20,36]. In general, individuals with a genotype of G/C are thought to have “normal” levels of IL-6 in their serum and when presented with an immune challenge, IL-6 levels increase and then return to normal. In contrast, individuals with a G/G genotype are described as having high levels of serum IL-6 and hyper-respond when presented with an immune challenge, while individuals with a C/C genotype are described as having very low levels of IL-6 in their serum and have a minimal inflammatory response when presented with an immune challenge[20]. It was of interest to us if the genotype of an individual affected a person’s response to other stresses, including chemotherapy. Our data show that regardless of genotype, all cell lines had decreased IL-6 protein in response to melphalan (Figure 4). Additionally, the amount of IL-6 detected in untreated cells did not correlate with genotype as the BMSC genotyped as C/C had the highest levels of IL-6, compared to relatively low levels in the context of immune challenges reported by others[17–20].
With changes in IL-6 protein in response to chemotherapy, we next investigated whether the decreased IL-6 protein was due to changes in mRNA. Melphalan decreased IL-6 mRNA in both BMSC and HOB (Figure 5), suggesting melphalan may be effecting IL-6 expression at a transcriptional level. We evaluated if melphalan treatment was decreasing the stability of mRNA through experiments using actinomycin-D and α-amanitin and determined that melphalan treatment did not affect the stability of IL-6 mRNA (data not shown). We also investigated whether three of the transcription factors known to positively regulate IL-6 transcription were affected by melphalan exposure. BMSC and HOB were left untreated or exposed to melphalan and cellular fractionation and western blot analysis completed evaluating NF-κB (p65), AP-1 (c-jun) and C/EBP-β (data not shown). It was not evident that any of these transcription factors were completely excluded from the nuclear fraction, which would be expected if this was the mechanism of action by which melphalan decreased IL-6 mRNA and protein. However, this does not address binding efficiently to the DNA during melphalan treatment, which still needs to be investigated to fully evaluate IL-6 promoter activity. We also evaluated if accumulation of DNA damage would elicit the same decrease in IL-6 protein as melphalan exposure. KU-55933, an ATM kinase inhibitor was used to inhibit DNA damage repair, allowing for an accumulation of damage. KU-55933 did not significantly decrease IL-6 protein in BMSC and was only able to significantly decrease IL-6 protein in HOB for 8 hours post-treatment, however the effect was still not as pronounced as the decrease induced by melphalan exposure, suggesting an additional mechanism other than DNA damage (Figure 6).
We next determined if melphalan effectively reduced microenvironment derived IL-6 in a co-culture model of myeloma cells with BMSC or HOB. Work by Gupta et al. and many others has shown that MM cells in contact with BMSC or osteoblasts increase the secretion of IL-6 from both of these microenvironment cell types [21,37,38]. Importantly, in the setting of MM, IL-6 is a potent proliferative and survival factor [39] and many attempts have been made to decrease IL-6 in the bone marrow microenvironment through the use of proteosome inhibitors and anti-IL-6 neutralizing antibodies as part of the therapeutic strategy[40]. For treatment of MM, melphalan is commonly used as first-line therapy but the connection between melphalan and IL-6 in the current study has not been previously described. In an effort to evaluate the effects of melphalan in a biologically relevant setting, co-cultures of BMSC or HOB with H929 were exposed to melphalan (Figure 7). We observed that even in this co-culture setting, melphalan was able to sustain the effects of decreased IL-6 protein that were previously observed in cultures of the adherent, supportive cells only.
5. Conclusions
In conclusion, we have demonstrated that melphalan, more so than other chemotherapeutic agents tested in the current study, decreases BMSC and HOB IL-6 mRNA and protein with decreases not due to instability of message or protein. As previously mentioned, murine IL-6 knock-out models have been described as having hematopoietic deficits, suggesting that the IL-6 family members are not completely redundant and that optimal IL-6 levels are required to sustain hematopoiesis. Based on its documented role in regulation of hematopoietic development, it is important to investigate the sensitivity of the bone marrow microenvironment to stress induced changes in IL-6 production, including those imposed by aggressive chemotherapy prior to bone marrow or stem cell transplantation. Previously, administration rIL-6 to patients following bone marrow transplant was attempted to augment hematopoietic recovery, however, the toxicity associated with rIL-6 precluded its sustained use in a clinical setting[41]. This study demonstrates how decreased IL-6 in the bone marrow microenvironment may lead to two different outcomes. In the setting of hematopoietic recovery, decreased IL-6 may be detrimental while in the setting of MM, the decrease in IL-6 induced by agents, such as melphalan, may be beneficial in the early stages of treatment of the disease. Further investigations are needed in both of these contexts to fully understand the implications of decreased IL-6 in the bone marrow microenvironment in diverse therapeutic settings.
*Highlights.
Bone marrow stromal cells and osteoblasts demonstrate reduced IL-6 expression in response to chemotherapy exposure.
Melphalan more dramatically induces IL-6 reduction than a variety of other agents evaluated.
IL-6 remains effectively reduced by Melphalan even in the setting of myeloma and ostoeblast or stromal cell co-culture.
Previously characterized IL-6 polymorphisms do not correlate with stromal cell response to chemotherapy.
Acknowledgements
This work was supported, in part, by NIH RO1 HL056888 (LFG), NIH RO1 CA134573 (LFG), NIH P20 RR016440 (LFG), the Alexander B. Osborn Hematopoietic Malignancy and Transplantation Program, and the WV Research Trust Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- 2.Heissig B, Ohki Y, Sato Y, Rafii S, Werb Z, Hattori K. A role for niches in hematopoietic cell development. Hematology. 2005;10:247–253. doi: 10.1080/10245330500067249. [DOI] [PubMed] [Google Scholar]
- 3.Yin T, Li L. The stem cell niches in bone. J Clin Invest. 2006;116:1195–1201. doi: 10.1172/JCI28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Z, Li L. Understanding hematopoietic stem-cell microenvironments. Trends Biochem Sci. 2006;31:589–595. doi: 10.1016/j.tibs.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Spyridonidis A, Kuttler T, Wasch R, Samek E, Waterhouse M, Behringer D, Bertz H, Finke J. Reduced intensity conditioning compared to standard conditioning preserves the in vitro growth capacity of bone marrow stroma, which remains of host origin. Stem Cells Dev. 2005;14:213–222. doi: 10.1089/scd.2005.14.213. [DOI] [PubMed] [Google Scholar]
- 6.Banfi A, Bianchi G, Galotto M, Cancedda R, Quarto R. Bone marrow stromal damage after chemo/radiotherapy: occurrence, consequences and possibilities of treatment. Leuk Lymphoma. 2001;42:863–870. doi: 10.3109/10428190109097705. [DOI] [PubMed] [Google Scholar]
- 7.Galotto M, Berisso G, Delfino L, Podesta M, Ottaggio L, Dallorso S, Dufour C, Ferrara GB, Abbondandolo A, Dini G, Bacigalupo A, Cancedda R, Quarto R. Stromal damage as consequence of high-dose chemo/radiotherapy in bone marrow transplant recipients. Exp Hematol. 1999;27:1460–1466. doi: 10.1016/s0301-472x(99)00076-4. [DOI] [PubMed] [Google Scholar]
- 8.Keller ET, Wanagat J, Ershler WB. Molecular and cellular biology of interleukin-6 and its receptor. Front Biosci. 1996;1:d340–d357. doi: 10.2741/a136. [DOI] [PubMed] [Google Scholar]
- 9.Taga T, Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol. 1997;15:797–819. doi: 10.1146/annurev.immunol.15.1.797. [DOI] [PubMed] [Google Scholar]
- 10.Nemunaitis J, Andrews DF, Mochizuki DY, Lilly MB, Singer JW. Human marrow stromal cells: response to interleukin-6 (IL-6) and control of IL-6 expression. Blood. 1989;74:1929–1935. [PubMed] [Google Scholar]
- 11.Rodriguez MC, Bernad A, Aracil M. Interleukin-6 deficiency affects bone marrow stromal precursors, resulting in defective hematopoietic support. Blood. 2004;103:3349–3354. doi: 10.1182/blood-2003-10-3438. [DOI] [PubMed] [Google Scholar]
- 12.Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, Zinkernagel R, Bluethmann H, Kohler G. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368:339–342. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- 13.Patchen ML, MacVittie TJ, Williams JL, Schwartz GN, Souza LM. Administration of interleukin-6 stimulates multilineage hematopoiesis and accelerates recovery from radiation-induced hematopoietic depression. Blood. 1991;77:472–480. [PubMed] [Google Scholar]
- 14.Kyriakou C, Canals C, Goldstone A, Caballero D, Metzner B, Kobbe G, Kolb HJ, Kienast J, Reimer P, Finke J, Oberg G, Hunter A, Theorin N, Sureda A, Schmitz N. High-dose therapy and autologous stem-cell transplantation in angioimmunoblastic lymphoma: complete remission at transplantation is the major determinant of Outcome-Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2008;26:218–224. doi: 10.1200/JCO.2008.12.6219. [DOI] [PubMed] [Google Scholar]
- 15.Kuruvilla J, Shepherd JD, Sutherland HJ, Nevill TJ, Nitta J, Le A, Forrest DL, Hogge DE, Lavoie JC, Nantel SH, Toze CL, Smith CA, Barnett MJ, Song KW. Long-term outcome of myeloablative allogeneic stem cell transplantation for multiple myeloma. Biol Blood Marrow Transplant. 2007;13:925–931. doi: 10.1016/j.bbmt.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Fishman D, Faulds G, Jeffery R, Mohamed-Ali V, Yudkin JS, Humphries S, Woo P. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J Clin Invest. 1998;102:1369–1376. doi: 10.1172/JCI2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rivera-Chavez FA, Peters-Hybki DL, Barber RC, O’Keefe GE. Interleukin-6 promoter haplotypes and interleukin-6 cytokine responses. Shock. 2003;20:218–223. doi: 10.1097/01.shk.0000079425.52617.db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terry CF, Loukaci V, Green FR. Cooperative influence of genetic polymorphisms on interleukin 6 transcriptional regulation. J Biol Chem. 2000;275:18138–18144. doi: 10.1074/jbc.M000379200. [DOI] [PubMed] [Google Scholar]
- 20.Bennermo M, Held C, Stemme S, Ericsson CG, Silveira A, Green F, Tornvall P. Genetic predisposition of the interleukin-6 response to inflammation: implications for a variety of major diseases. Clin Chem. 2004;50:2136–2140. doi: 10.1373/clinchem.2004.037531. [DOI] [PubMed] [Google Scholar]
- 21.Uchiyama H, Barut BA, Mohrbacher AF, Chauhan D, Anderson KC. Adhesion of human myeloma-derived cell lines to bone marrow stromal cells stimulates interleukin-6 secretion. Blood. 1993;82:3712–3720. [PubMed] [Google Scholar]
- 22.Oh IH, Kwon KR. Concise review: multiple niches for hematopoietic stem cell regulations. Stem Cells. 2010;28:1243–1249. doi: 10.1002/stem.453. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura Y, Arai F, Iwasaki H, Hosokawa K, Kobayashi I, Gomei Y, Matsumoto Y, Yoshihara H, Suda T. Isolation and characterization of endosteal niche cell populations that regulate hematopoietic stem cells. Blood. 2010;116:1422–1432. doi: 10.1182/blood-2009-08-239194. [DOI] [PubMed] [Google Scholar]
- 24.Balduino A, Hurtado SP, Frazao P, Takiya CM, Alves LM, Nasciutti LE, El-Cheikh MC, Borojevic R. Bone marrow subendosteal microenvironment harbours functionally distinct haemosupportive stromal cell populations. Cell Tissue Res. 2005;319:255–266. doi: 10.1007/s00441-004-1006-3. [DOI] [PubMed] [Google Scholar]
- 25.Dorshkind K. Regulation of hemopoiesis by bone marrow stromal cells and their products. Annu Rev Immunol. 1990;8:111–137. doi: 10.1146/annurev.iy.08.040190.000551. [DOI] [PubMed] [Google Scholar]
- 26.Kittler EL, McGrath H, Temeles D, Crittenden RB, Kister VK, Quesenberry PJ. Biologic significance of constitutive and subliminal growth factor production by bone marrow stroma. Blood. 1992;79:3168–3178. [PubMed] [Google Scholar]
- 27.Thalmeier K, Meissner P, Reisbach G, Hultner L, Mortensen BT, Brechtel A, Oostendorp RA, Dormer P. Constitutive and modulated cytokine expression in two permanent human bone marrow stromal cell lines. Exp Hematol. 1996;24:1–10. [PubMed] [Google Scholar]
- 28.Jacobsen K, Kravitz J, Kincade PW, Osmond DG. Adhesion receptors on bone marrow stromal cells: in vivo expression of vascular cell adhesion molecule-1 by reticular cells and sinusoidal endothelium in normal and gamma-irradiated mice. Blood. 1996;87:73–82. [PubMed] [Google Scholar]
- 29.Colmone A, Sipkins DA. Beyond angiogenesis: the role of endothelium in the bone marrow vascular niche. Transl Res. 2008;151:1–9. doi: 10.1016/j.trsl.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Rafii S, Mohle R, Shapiro F, Frey BM, Moore MA. Regulation of hematopoiesis by microvascular endothelium. Leuk Lymphoma. 1997;27:375–386. doi: 10.3109/10428199709058305. [DOI] [PubMed] [Google Scholar]
- 31.Visnjic D, Kalajzic Z, Rowe DW, Katavic V, Lorenzo J, Aguila HL. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood. 2004;103:3258–3264. doi: 10.1182/blood-2003-11-4011. [DOI] [PubMed] [Google Scholar]
- 32.Chitteti BR, Cheng YH, Poteat B, Rodriguez-Rodriguez S, Goebel WS, Carlesso N, Kacena MA, Srour EF. Impact of interactions of cellular components of the bone marrow microenvironment on hematopoietic stem and progenitor cell function. Blood. 2010;115:3239–3248. doi: 10.1182/blood-2009-09-246173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simpson RJ, Hammacher A, Smith DK, Matthews JM, Ward LD. Interleukin-6: structure-function relationships. Protein Sci. 1997;6:929–955. doi: 10.1002/pro.5560060501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lust JA, Donovan KA, Kline MP, Greipp PR, Kyle RA, Maihle NJ. Isolation of an mRNA encoding a soluble form of the human interleukin-6 receptor. Cytokine. 1992;4:96–100. doi: 10.1016/1043-4666(92)90043-q. [DOI] [PubMed] [Google Scholar]
- 35.Mullberg J, Durie FH, Otten-Evans C, Alderson MR, Rose-John S, Cosman D, Black RA, Mohler KM. A metalloprotease inhibitor blocks shedding of the IL-6 receptor and the p60 TNF receptor. J Immunol. 1995;155:5198–5205. [PubMed] [Google Scholar]
- 36.Endler G, Marsik C, Joukhadar C, Marculescu R, Mayr F, Mannhalter C, Wagner OF, Jilma B. The interleukin-6 G(-174)C promoter polymorphism does not determine plasma interleukin-6 concentrations in experimental endotoxemia in humans. Clin Chem. 2004;50:195–200. doi: 10.1373/clinchem.2003.022459. [DOI] [PubMed] [Google Scholar]
- 37.Barille S, Collette M, Bataille R, Amiot M. Myeloma cells upregulate interleukin-6 secretion in osteoblastic cells through cell-to-cell contact but downregulate osteocalcin. Blood. 1995;86:3151–3159. [PubMed] [Google Scholar]
- 38.Gupta D, Treon SP, Shima Y, Hideshima T, Podar K, Tai YT, Lin B, Lentzsch S, Davies FE, Chauhan D, Schlossman RL, Richardson P, Ralph P, Wu L, Payvandi F, Muller G, Stirling DI, Anderson KC. Adherence of multiple myeloma cells to bone marrow stromal cells upregulates vascular endothelial growth factor secretion: therapeutic applications. Leukemia. 2001;15:1950–1961. doi: 10.1038/sj.leu.2402295. [DOI] [PubMed] [Google Scholar]
- 39.Kim I, Uchiyama H, Chauhan D, Anderson KC. Cell surface expression and functional significance of adhesion molecules on human myeloma-derived cell lines. Br J Haematol. 1994;87:483–493. doi: 10.1111/j.1365-2141.1994.tb08302.x. [DOI] [PubMed] [Google Scholar]
- 40.Chauhan D, Hideshima T, Anderson KC. Proteasome inhibition in multiple myeloma: therapeutic implication. Annu Rev Pharmacol Toxicol. 2005;45:465–476. doi: 10.1146/annurev.pharmtox.45.120403.100037. [DOI] [PubMed] [Google Scholar]
- 41.Kammuller ME. Recombinant human interleukin-6: safety issues of a pleiotropic growth factor. Toxicology. 1995;105:91–107. doi: 10.1016/0300-483x(95)03128-3. [DOI] [PubMed] [Google Scholar]