Abstract
Hippocampus volume decreases and verbal memory deficits have been reported in bipolar disorder (BD) as independent observations. We investigated potential associations between these deficits in subjects with BD. Hippocampus volumes were measured on magnetic resonance images of 31 subjects with BD and 32 healthy comparison (HC) subjects. The California Verbal Learning Test-Second Edition (CVLT) assessed verbal memory function in these subjects. Compared to the HC group, the BD group showed both significantly smaller hippocampus volumes and impaired performance on CVLT tests of immediate, short delay and long delay cued and free recall. Further, smaller hippocampus volume correlated with impaired performance in BD. Post-hoc analyses revealed a trend towards improved memory in BD subjects taking antidepressant medications. These results support associations between morphological changes in hippocampus structure in BD and verbal memory impairment. They provide preliminary evidence pharmacotherapy may reverse hippocampus-related memory deficits.
Keywords: CVLT, Memory, Hippocampus, Magnetic Resonance Imaging, Bipolar Disorder
1. INTRODUCTION
Converging evidence implicates the hippocampus in bipolar disorder (BD). Findings from both postmortem histochemical studies and neuroimaging research demonstrate structural hippocampal abnormalities in BD, including decreased cell density, abnormal markers of neuronal function and plasticity, and decreases in hippocampus volume (Bearden et al., 2008; Chepenik et al., 2009; Frazier et al., 2005; Hauser et al., 1989; Noga et al., 2001; Savitz and Drevets, 2010; Strasser et al., 2005; Swayze et al., 1992). Medications used to treat BD are thought to function, at least in part, by stimulating neurogenesis and neuroprotection within hippocampus (Chen et al., 2000; Hao et al., 2004; Manji et al., 2000; Walz et al., 2008).
Consistent with these neurobiological findings, deficits in verbal memory functions that are subserved by the hippocampus are the cognitive abnormalities most consistently identified in individuals with BD (Robinson et al., 2006). These deficits are present across all mood states in BD including euthymia (Cavanagh et al., 2002; Clark et al., 2001; Deckersbach et al., 2004; Glahn et al., 2005; Martinez-Aran et al., 2004a; Martinez-Aran et al., 2004b; Matsuo et al., 2009; Pavuluri et al., 2006; van Gorp et al., 1999; Wolfe et al., 1987). They have been identified in both children and adults with BD (Cavanagh et al., 2002; Clark et al., 2001; Deckersbach et al., 2004; Glahn et al., 2005; Pavuluri et al., 2006; van Gorp et al., 1999; Wolfe et al., 1987) and in their unaffected siblings (Gourovitch et al., 1999; Keri et al., 2001; Kieseppa et al., 2005).
The California Verbal Learning Test (CVLT) is a widely used measure of declarative verbal memory functions, including episodic verbal learning and recall, shown to be associated with hippocampus functioning and disrupted in individuals with hippocampus pathology (Deweer et al., 1995; Kohler et al., 1998; Lepage et al., 1998; Libon et al., 1998; Tischler et al., 2006, Yucel et al., 2007). Although the CVLT also tests various other constructs, subjects with BD tend to demonstrate difficulty on the CVLT measures of immediate and delayed recall (Altshuler et al., 2004; Cavanagh et al., 2002; Glahn et al., 2005; Martinez-Aran et al., 2004; Senturk et al., 2007; Simonsen et al., 2007, van Gorp et al., 1998). Together with the findings described above, these results suggest verbal memory dysfunction is a pervasive problem in BD and may reflect an underlying vulnerability in the disorder for hippocampus disturbances. However, no study has yet demonstrated a direct association between hippocampus volume and verbal memory dysfunction in BD.
The present study tested the hypotheses that BD individuals would demonstrate decreases in hippocampus volume, as well as decreased verbal memory functioning as measured by the CVLT, relative to healthy comparison (HC) subjects. It was further hypothesized there would be an association between the magnitude of hippocampus volume decreases and verbal memory decreases in subjects with BD.
2. MATERIALS AND METHODS
2.1 Subjects
Subjects included 31 adults with BD (58% female, ages 20–58 years) and 32 HC individuals (68% female, ages 19–58 years). Study subjects were recruited through the Veterans Affairs Connecticut Health Care System (West Haven, CT), the Yale School of Medicine (New Haven, CT), clinicians in the community and from advertisement in local newspapers. All subjects provided written, informed consent for participation in this study protocol as approved by the Yale School of Medicine and Department of Veterans Affairs institutional review boards.
Presence or absence of DSM-IV Axis I disorders was confirmed using the Structured Clinical Interview for DSM-IV Axis I Disorders, Version 2.0 (First et al., 1995). None of the participants had an intelligence quotient below 80, a major neurological illness or history of loss of consciousness >5 minutes. Three (10%) individuals with BD had treated thyroid dysfunction. HC subjects also lacked a personal history of a mood, psychotic or substance use disorder.
The mean length of illness duration for the subjects with BD was 19 years (SD = 11 years; range = 1– 38 years). Number of psychiatric hospitalizations included none (11, 35%), 1–5 (16, 52%) and >5 (4, 13%). Eleven (35%) of the BD subjects met criteria for rapid-cycling. Seventeen (55%) of the subjects with BD were euthymic at time of scan, 7 (23%) were depressed and 7 (23%) were in a manic, hypomanic or mixed state. Thirteen (42%) of the BD subjects had experienced past psychotic symptoms that occurred within the context of a mood episode. None of the subjects were psychotic at time of scan.
Among individuals with BD, history of comorbid psychiatric disorder included panic disorder (6, 19%), specific phobia (4, 13%), post-traumatic stress disorder (4, 13%) or generalized anxiety disorder (1, 3%). Eight (26%) BD subjects had a prior history of substance dependence, which included alcohol (2, 3%), cannabis (1, 3%), sedatives (2, 6%), opiates (1, 3%), and polysubstances (3, 10%). Seven subjects with BD (23%) had a prior history of substance abuse. Subjects were in remission from substance dependence for an average period of over 5 years, and from abuse for an average period of over 4 years. Ten (30%) individuals with BD were unmedicated at time of scan. The remaining subjects were prescribed lithium carbonate (8, 26%), an anticonvulsant (15, 48%), an atypical antipsychotic (12, 39%), an antidepressant (8, 26%), a benzodiazepine (6, 19%) or a stimulant (2, 6%).
2.2 Neuropsychological Assessment
The American version of the Nelson Adult Reading Test (AMNART) (Grober and Sliwinski, 1991) and the vocabulary and matrix reasoning subtests of the Wechsler Abbreviated Scale of Intelligence (WASI) (Wechsler, 1999)provided estimates of subjects’ pre-morbid and current Intelligence Quotient (IQ), respectively. Subjects were administered the California Verbal Learning Test-Second Edition (CVLT) (Delis et al., 2000).
2.3 Acquisition and Processing of Magnetic Resonance Images
MRI scans were obtained using a single 3-T Trio MR scanner (Siemens, Erlangen, Germany). Head position was standardized using canthomeatal landmarks and anatomic data collected using a T1-weighted 3-dimensional magnetization prepared rapid acquisition gradient echo pulse sequence (repetition time, 1500 ms; echo time, 2.83 ms; flip angle, 15°; matrix, 256×256; field of view, 256×256 mm2; slice thickness, 1.0 mm without gap; 160 contiguous slices; and 2 excitations).
2.4 Hand-delineation of Hippocampus Region of Interest (ROI)
A single individual blind to subject diagnosis performed the morphometric analyses. Stripping of the skull and brain segmentation were performed as described previously (Chepenik et al., 2009). Total brain volume (TBV) was calculated as the sum of cerebral gray matter volume and white matter volume. Hippocampus tracings were performed in the coronal plane according to methods previously described (Kates et al., 1997; Peterson et al., 2001). Briefly, the slice at which the temporal horn shifts from a lateral to superior position in relation to the hippocampus defined the anterior of the hippocampus. The slice in which the splenium of the corpus callosum begins to join the fornix defined the posterior boundary. Final tracings were confirmed in orthogonal views. Intra-rater reliability was 95% and inter-rater reliability was 96% as determined by delineation of hippocampus from 10 scans not included in the dataset.
2.5 Statistical Analysis
Differences in demographic variables between BD and HC groups (age, sex, years of education, pre-morbid and current IQ) were assessed using t-tests and chi-square analysis where appropriate.
Analysis of CVLT performance was completed using group (BD vs. HC) as a between subjects factor in an ANCOVA model adjusted for AMNART scores. Dependent measures of CVLT performance included subscores for total immediate recall (trials 1–5), short delay cued and free recall and long delay cued and free recall, standardized for age, sex and years of education (Delis et al., 2000).
A linear mixed model was used to evaluate hippocampus volume using group (BD vs. HC) as a between subjects factor, hemisphere as a within-subjects factor and subject as the clustering factor. TBV served as a covariate to account for general scaling effects. All significant (p<0.05) main, 2- and 3-way interactions are reported. Least squares (ls) means were calculated from the mixed models and plotted to interpret significant effects.
ANCOVA was used to investigate potential associations between clinical variables and either CVLT performance or hippocampus volume in subjects with BD, controlling for AMNART and TBV respectively. Clinical variables were tested one at a time and included length of time with symptoms of BD, number of hospitalizations, history of psychosis, rapidcycling, mood state at time of scan and medication status (overall and for lithium carbonate, anticonvulsants, atypical antipsychotics and antidepressants).
Partial correlations were used to assess potential associations between CVLT performance and mean hippocampus volume (mean of left and right volume) within each diagnostic group, after adjustment for TBV and AMNART scores.
All of the above analyses were performed using SAS, version 9.1 (Cary, NC).
Voxel-based morphometry was used to explore additional brain regions that correlated with CVLT performance measures. Images were processed and analyzed using Statistical Parametric Mapping 5 (SPM5) (http://www.fil.ion.ucl.ac.uk/spm) as detailed in our previous publications (Kalmar et al., 2009; Wang et al., 2011). Whole-brain linear regression analysis was performed separately for the BD and HC groups using SPM5 to investigate the potential relationship between gray matter volume and the above CVLT performance measures. Findings were considered significant at a height threshold of p< 0.005 uncorrected for multiple comparisons, and an extent threshold of 20 voxels.
3. RESULTS
3.1 Demographic Variables
The groups did not significantly differ in age, education, sex distribution, pre-morbid or current IQ (Table 1).
Table 1. Demographic Variables for the Bipolar Disorder and Healthy Comparison Groups.
| BD | HC | |||
|---|---|---|---|---|
| Mean (SEM) | Mean (SEM) | Statistic | P-value | |
| Age (years) | 33.2 (2.1) | 30.3 (1.9) | t(61)=1.0 | 0.31 |
| Education (years) | 14.8 (0.3) | 15.6 (0.4) | t(61)=1.7 | 0.10 |
| WASI IQ | 114.1 (2.5) | 115.4 (2.3) | t(56)=0.39 | 0.70 |
| AMNART | 117.2 (1.5) | 115.7 (1.3) | t(60)=0.79 | 0.43 |
| Sex M/F | 13/18 | 10/22 | Χ2(1)=0.78 | 0.38 |
Abbreviations: BD, bipolar disorder; HC, healthy control; SEM, standard error; M, male; F, female; WASI IQ, Wechsler Abbreviated Scale of Intelligence-Intelligence Quotient; AMNART, American Version of the Nelson Adult Reading Test score
3.2 CVLT Assessment
AMNART was significantly associated with total immediate recall [F(1,59)=4.9, p=0.031]. Compared to the HC group, the group with BD demonstrated significantly impaired performance on total immediate recall, short delay cued and free recall and long delay cued recall (Table 2). The group with BD demonstrated a trend towards impaired performance on long delay free recall, relative to the HC group (Table 2). None of the clinical factors present in subjects with BD were significantly associated with CVLT performance, including length of time ill, number of hospitalizations, presence of rapid cycling, mood state, co-morbid substance dependence, abuse or other psychiatric illness, and medication status. However, there were non-significant trends towards better performance on short delay free recall [F(1,27)=3.14, p=0.088], short delay cued recall [F(1,27)=4.17, p=0.051] and long delay free recall [F(1,27)=3.72, p=0.064] in participants prescribed antidepressants.
Table 2. California Verbal Learning Test Measures for the Bipolar Disorder and Healthy Control Groups.
Least square means adjusted for American version of the Nelson Adult Reading Test (AMNART) scores
| BD | HC | |||
|---|---|---|---|---|
| Mean (SEM) | Mean (SEM) | F(1, 59)*= | P-value | |
| Immediate Recall | 50 (2.1) | 57.2 (2.0) | 6.11 | 0.016 |
| Short Delayed Free Recall | −0.25 (0.25) | 0.52 (0.24) | 4.93 | 0.03 |
| Short Delayed Cued Recall | −0.32 (0.22) | 0.49 (0.21) | 7.19 | 0.01 |
| Long Delayed Free Recall | −0.18 (0.19) | 0.34 (0.18) | 3.76 | 0.057 |
| Long Delayed Cued Recall | −0.30 (0.20) | 0.26 (0.20) | 3.96 | 0.051 |
adjusted for AMNART scores
Abbreviations: CVLT, California Verbal Learning Test; BD, bipolar disorder; HC, healthy control; SEM, standard error
3.3 Hippocampus Volume
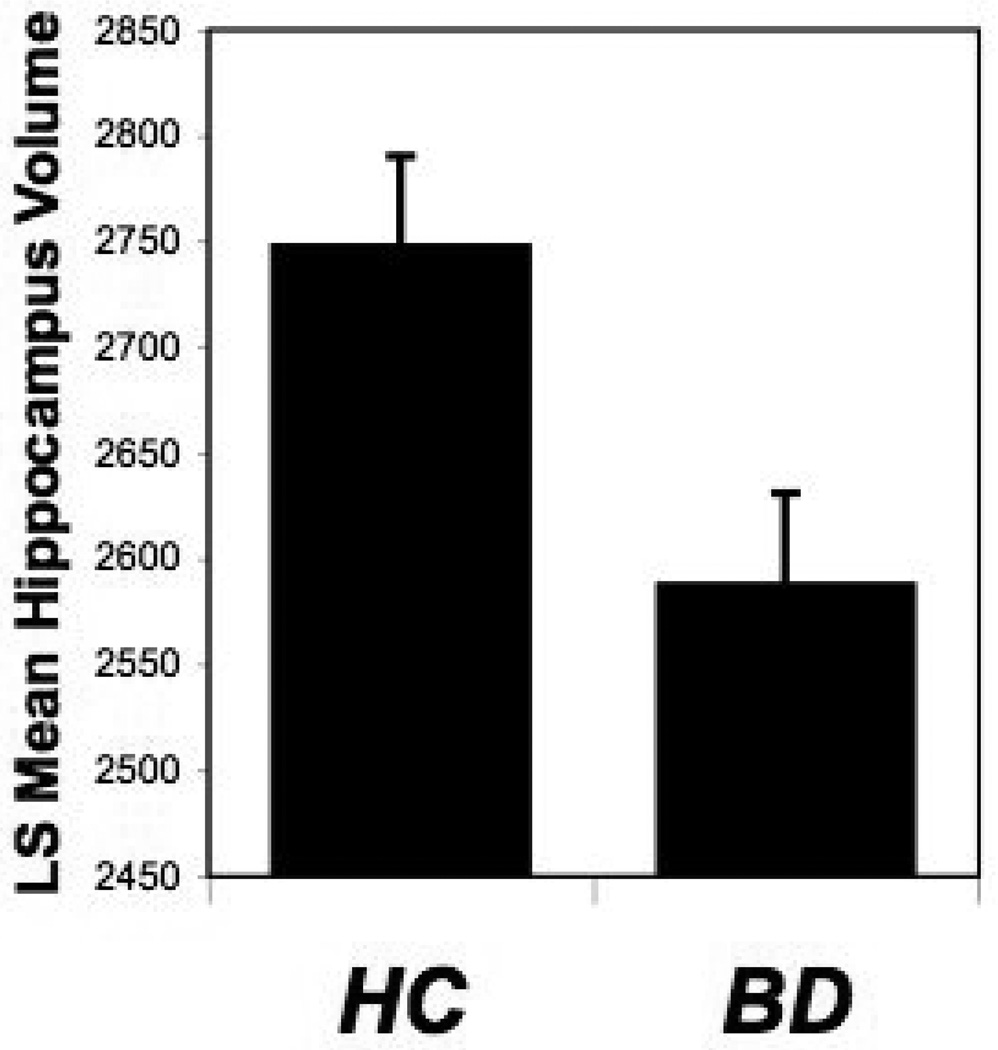
TBV was significantly associated with hippocampus volumes [F(1,60)=27.9, p<0.0001]. As depicted in Figure 1, hippocampus volumes were significantly smaller in BD subjects (ls mean 2588 ± SE 43) compared to HC subjects (ls mean 2748 ± 42) [F(1,60)=7.0, p=0.010]. No hemispheric differences were observed [F(1,61)=1.73, p=0.19]. Rapid-cycling BD participants demonstrated significantly larger hippocampus volumes (rapid-cycling BD ls mean=2737 ± SE 62, non-rapid cycling BD ls mean=2525 ± 45), [F(1,28)=7.3, p=0.012]. None of the other clinical factors present in subjects with BD were significantly associated with hippocampus volume, including length of time ill, number of hospitalizations, mood state, co-morbid substance dependence, abuse or other psychiatric illness, and medication status.
Figure 1. Hippocampus Volume in the Bipolar Disorder and Healthy Control Groups.
The graph displays ls means and standard errors for hippocampus volumes (mm3) by diagnosis, adjusted for total brain volume. The BD group had significantly smaller hippocampus volumes compared to the HC group (p=0.01).
Abbreviations: BD, bipolar disorder; HC, healthy control; ls, least square
3.4 Correlations
After adjustment for TBV and AMNART scores, subjects with BD demonstrated positive associations between hippocampus volume and CVLT performance for total immediate recall (r=0.52, p=0.005), short delay cued recall (r=0.39, p=0.04), long delay free recall (r=0.40, p=0.036) and long delay cued recall (r=0.44, p=0.019). There were no significant correlations between hippocampus volume and CVLT performance measures in the HC group. The whole brain voxel-based exploratory analyses did not reveal additional brain regions for which volume was significantly associated with CVLT measures.
4. DISCUSSION
This study demonstrated a positive correlation between hippocampus volume and CVLT performance in individuals with BD. These findings bring together for the first time the separate observations that hippocampus size is reduced and its function is reduced in adults with BD, compared to healthy individuals. The presence of a correlation between these findings suggests morphological differences in hippocampus in BD may be associated with measurable verbal memory dysfunction.
The mechanisms that underlie structural abnormalities in hippocampus and associated dysfunction are not known. Abnormalities in hippocampus plasticity have been implicated in disease pathology in mood disorders (Czeh et al., 2001; Duman et al., 1997). In BD, postmortem molecular studies demonstrate abnormal hippocampus levels of markers associated with neuronal sprouting and plasticity (Dowlatshahi et al., 2000; Fatemi et al., 2001; MacDonald et al., 2006). In addition, magnetic resonance spectroscopy studies demonstrate abnormalities in markers of cell density are present in hippocampus early in BD disease onset (Atmaca et al., 2006; Blasi et al., 2004) and could potentially reflect early dysfunction in cell growth and plasticity. Decreased regional blood flow in hippocampus during verbal memory encoding in subjects with BD, compared to HC subjects, suggests possible functional effects of these cellular changes (Deckersbach et al., 2006).
The observation antidepressants and mood-stabilizing medications have neurotrophic effects in hippocampus (Atmaca et al., 2007; Bearden et al., 2008; Chen et al., 2000; Czeh et al., 2001; Duman et al., 1997; Madsen et al., 2000; Malberg et al., 2000; Manev et al., 2001; Santarelli et al., 2003) suggests these treatments might induce morphological changes in hippocampus in BD which could also lead to improved cognitive function. This hypothesis is supported by the longitudinal study of twelve individuals with BD treated with lithium carbonate who demonstrated increased hippocampus volume in association with improved performance on the CVLT (Yucel et al., 2007). Since this research lacked a comparison group without BD, the magnitude of difference from healthy individuals could not be determined. In the present study, there was no association between treatment with lithium and CVLT performance. Subjects prescribed antidepressants did demonstrate a non-significant trend towards improved memory performance, an observation also described in subjects with major depressive disorder (Deuschle et al., 2004). Antidepressants were not associated with hippocampus volume, however. Possible explanations might include greater CVLT sensitivity to neurotrophic changes in hippocampus than gross volume measures. It is also possible an alternate mechanism underlies the observed memory effects. These results should be considered very preliminary given the level of significance, small sample size, lack of systematic treatment assignment and regimens of more than one medication for many subjects. Further studies with systematic medication assignment in larger sample sizes might reveal salutary effects of medication on hippocampus-related impairments.
The CVLT measures a spectrum of cognitive constructs (Elwood, 1995), including repetition learning and semantic organization. Performance on these various measures has been reported to also engage brain regions other than hippocampus, including the putamen, thalamus and prefrontal cortex (Albuquerque et al., 2008; Baldo et al., 2002; DiStefano et al., 2000; Savage et al., 2001; Ystad et al., 2010). In this study, whole brain voxel-based exploratory analysis did not demonstrate any significant associations between additional brain regions and CVLT measures of recall. However, it is possible these methods were not sensitive to subtle differences in additional brain regions.
The post-hoc exploratory analyses showed a correlation between rapid-cycling in BD and increased hippocampus size. The mechanisms to explain rapid-cycling in BD are not understood, but may include genetic pathways related to stress and plasticity (Muller et al., 2006; Serretti et al., 2006). The serotonin transporter polymorphism (5-HTTLPR, locus SLC6A4) has been associated with rapid-cycling and antidepressant induced mania in BD (Cusin et al., 2001; Ferreira Ade et al., 2009; Mundo et al., 2001; Rousseva et al., 2003), as well as altered hippocampus volume in major depressive disorder (Frodl et al., 2004; Taylor et al., 2005). Whereas homozygosity for the val allele of the Val66Met brain derived neurotrophic growth factor gene is associated with susceptibility to BD and rapid-cycling, the met allele is associated with decreases in hippocampus volume (Chepenik et al., 2009; Geller et al., 2004; Matsuo et al., 2009; Muller et al., 2006; Sklar et al., 2002). This suggests that the val allele may be associated with vulnerability to cycling, while BD carriers of the met allele may be vulnerable to cognitive dysfunction. Future studies that explore potential interactions amongst these genetic factors, clinical course and hippocampus structure and function are warranted.
In sum, this work demonstrates a positive correlation between smaller hippocampus volume and performance deficits on the CVLT in individuals with BD. Preliminary evidence suggests antidepressant treatment may reverse hippocampus-related memory deficits. However, the association between rapid-cycling and higher hippocampus volumes suggests optimized treatment might target a relatively narrow range of hippocampus structure and function to both minimize cognitive dysfunction, as well as tendency towards mood cycling. Future research that includes systematic study of medication and clinical course, as well as exploration of the genetic mechanisms that underlie the hippocampus morphological changes in BD, are needed.
Highlights.
California Verbal Learning Test (CVLT) performance is decreased in bipolar disorder
MRI showed hippocampus volumes are decreased in bipolar disorder
Hippocampus volume was associated with CVLT performance in bipolar disorder
ACKNOWLEDGEMENTS
We thank Kathleen Colonese, Susan Quatrano, Philip Markovich and Allison McDonough for their work with the study participants and database management, Hedy Sarofin, Karen Martin, and Terry Hickey for their technical expertise, and the research subjects for their participation.
We also thank Dr. Brenda Milner for her expert scientific consultation.
FUNDING SOURCES:
Grant Sponsor: the Department of Veterans Affairs Research Enhancement Award Program, Grant Sponsor: the National Institute of Mental Health; Grant numbers: R01MH69747, R01MH070902, K01MH086621, UL1 RR0249139, T32MH14276, Grant Sponsor: National Institute of Health National Center for Research Resources; CTSA Grant Number: UL1 RR0249139, Grant Sponsors: the National Alliance for Research on Schizophrenia and Depression, The Attias Family Foundation, Marcia Simon Kaplan, Women' Investigator Program – The Ethel F. Donaghue Women’s Health Investigator Program at Yale, and the Klingenstein Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Albuquerque L, Loureiro C, Martins IP. Effect of lesion site on serial position during list learning: a study with the CVLT. International Journal of Neuroscience. 2008;118(7):917–933. doi: 10.1080/00207450701591081. [DOI] [PubMed] [Google Scholar]
- Altshuler LL, Bartzokis G, Grieder T, Curran J, Jimenez T, Leight K, Wilkins J, Gerner R, Mintz J. An MRI study of temporal lobe structures in men with bipolar disorder or schizophrenia. Biol Psychiatry. 2000;48(2):147–162. doi: 10.1016/s0006-3223(00)00836-2. [DOI] [PubMed] [Google Scholar]
- Atmaca M, Yildirim H, Ozdemir H, Ogur E, Tezcan E. Hippocampal 1H MRS in patients with bipolar disorder taking valproate versus valproate plus quetiapine. Psychological Medicine. 2007;37(1):121–129. doi: 10.1017/S0033291706008968. [DOI] [PubMed] [Google Scholar]
- Atmaca M, Yildirim H, Ozdemir H, Poyraz AK, Tezcan E, Ogur E. Hippocampal 1H MRS in first-episode bipolar I patients. Progress in Neuropsychopharmacology and Biological Psychiatry. 2006;30(7):1235–1239. doi: 10.1016/j.pnpbp.2006.03.032. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Delis A, Kramer J, Shimamura AP. Memory performance on the California Verbal Learning Test–II: Findings from patients with focal frontal lesions. Journal of the International Neuropsychological Society. 2002;8:539–546. doi: 10.1017/s135561770281428x. [DOI] [PubMed] [Google Scholar]
- Bearden CE, Thompson PM, Dutton RA, Frey BN, Peluso MA, Nicoletti M, Dierschke N, Hayashi KM, Klunder AD, Glahn DC, et al. Three-dimensional mapping of hippocampal anatomy in unmedicated and lithium-treated patients with bipolar disorder. Neuropsychopharmacology. 2008;33(6):1229–1238. doi: 10.1038/sj.npp.1301507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi G, Bertolino A, Brudaglio F, Sciota D, Altamura M, Antonucci N, Scarabino T, Weinberger DR, Nardini M. Hippocampal neurochemical pathology in patients at first episode of affective psychosis: a proton magnetic resonance spectroscopic imaging study. Psychiatry Research. 2004;131(2):95–105. doi: 10.1016/j.pscychresns.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Cavanagh JT, Van Beck M, Muir W, Blackwood DH. Case-control study of neurocognitive function in euthymic patients with bipolar disorder: an association with mania. British Journal of Psychiatry. 2002;180:320–326. doi: 10.1192/bjp.180.4.320. [DOI] [PubMed] [Google Scholar]
- Chang K, Karchemskiy A, Barnea-Goraly N, Garrett A, Simeonova DI, Reiss A. Reduced amygdalar gray matter volume in familial pediatric bipolar disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44(6):565–573. doi: 10.1097/01.chi.0000159948.75136.0d. [DOI] [PubMed] [Google Scholar]
- Chen BK, Sassi R, Axelson D, Hatch JP, Sanches M, Nicoletti M, Brambilla P, Keshavan MS, Ryan ND, Birmaher B, et al. Cross-sectional study of abnormal amygdala development in adolescents and young adults with bipolar disorder. Biological Psychiatry. 2004;56(6):399–405. doi: 10.1016/j.biopsych.2004.06.024. [DOI] [PubMed] [Google Scholar]
- Chen G, Rajkowska G, Du F, Seraji-Bozorgzad N, Manji HK. Enhancement of hippocampal neurogenesis by lithium. Journal of Neurochemistry. 2000;75(4):1729–1734. doi: 10.1046/j.1471-4159.2000.0751729.x. [DOI] [PubMed] [Google Scholar]
- Chepenik LG, Fredericks C, Papademetris X, Spencer L, Lacadie C, Wang F, Pittman B, Duncan JS, Staib LH, Duman RS, et al. Effects of the brain-derived neurotrophic growth factor val66met variation on hippocampus morphology in bipolar disorder. Neuropsychopharmacology. 2009;34(4):944–951. doi: 10.1038/npp.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L, Iversen SD, Goodwin GM. A neuropsychological investigation of prefrontal cortex involvement in acute mania. American Journal of Psychiatry. 2001;158(10):1605–1611. doi: 10.1176/appi.ajp.158.10.1605. [DOI] [PubMed] [Google Scholar]
- Cusin C, Serretti A, Lattuada E, Lilli R, Lorenzi C, Mandelli L, Pisati E, Smeraldi E. Influence of 5-HTTLPR and TPH variants on illness time course in mood disorders. Journal of Psychiatric Research. 2001;35(4):217–223. doi: 10.1016/s0022-3956(01)00026-7. [DOI] [PubMed] [Google Scholar]
- Czeh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, Bartolomucci A, Fuchs E. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proceedings of the National Academy of Science U S A. 2001;98(22):12796–12801. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckersbach T, Dougherty DD, Savage C, McMurrich S, Fischman AJ, Nierenberg A, Sachs G, Rauch SL. Impaired recruitment of the dorsolateral prefrontal cortex and hippocampus during encoding in bipolar disorder. Biological Psychiatry. 2006;59(2):138–146. doi: 10.1016/j.biopsych.2005.06.030. [DOI] [PubMed] [Google Scholar]
- Deckersbach T, Savage CR, Reilly-Harrington N, Clark L, Sachs G, Rauch SL. Episodic memory impairment in bipolar disorder and obsessive-compulsive disorder: the role of memory strategies. Bipolar Disorders. 2004;6(3):233–244. doi: 10.1111/j.1399-5618.2004.00118.x. [DOI] [PubMed] [Google Scholar]
- Delis D, Kaplan E, Kramer J, Ober B. California Verbal Learning Test-II. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- Di Stafano G, Bachevalier J, Levin HS, Song JX, Scheibel RS, Fletcher JM. Volume of focal brain lesions and hippocampal formation in relation to memory function after closed head injury in children. Journal of Neurology, Neurosurgery and Psychiatry. 2000;69(2):210–216. doi: 10.1136/jnnp.69.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuschle M, Kniest A, Niemann H, Erb-Bies M, Colla N, Hamann B, Heuser I. Impaired declarative memory in depressed patients is slow to recover: clinical experience. Pharmacopsychiatry. 2004;37(4):147–151. doi: 10.1055/s-2004-827168. [DOI] [PubMed] [Google Scholar]
- Deweer B, Lehericy S, Pillon B, Baulac M, Chiras J, Marsault C, Agid Y, Dubois B. Memory disorders in probable Alzheimer's disease: the role of hippocampal atrophy as shown with MRI. Journal of Neurology and Neurosurgical Psychiatry. 1995;58(5):590–597. doi: 10.1136/jnnp.58.5.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlatshahi D, MacQueen G, Wang JF, Chen B, Young LT. Increased hippocampal supragranular Timm staining in subjects with bipolar disorder. Neuroreport. 2000;11(17):3775–3778. doi: 10.1097/00001756-200011270-00036. [DOI] [PubMed] [Google Scholar]
- Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Archives of General Psychiatry. 1997;54(7):597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- Elwood RW. The california verbal learning test: psychometric characteristic and clinical application. Neuropsychology Review. 1995;5(3):173–201. doi: 10.1007/BF02214761. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Earle JA, Stary JM, Lee S, Sedgewick J. Altered levels of the synaptosomal associated protein SNAP-25 in hippocampus of subjects with mood disorders and schizophrenia. Neuroreport. 2001;12(15):3257–3262. doi: 10.1097/00001756-200110290-00023. [DOI] [PubMed] [Google Scholar]
- Ferreira Ade A, Neves FS, da Rocha FF, Silva GS, Romano-Silva MA, Miranda DM, De Marco L, Correa H. The role of 5-HTTLPR polymorphism in antidepressant-associated mania in bipolar disorder. Journal of Affective Disorders. 2009;112(1–3):267–272. doi: 10.1016/j.jad.2008.04.012. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I & II Disorders (Version 2.0) New York, NY: New York State Psychiatric Institute; 1995. [Google Scholar]
- Frazier JA, Chiu S, Breeze JL, Makris N, Lange N, Kennedy DN, Herbert MR, Bent EK, Koneru VK, Dieterich ME, et al. Structural brain magnetic resonance imaging of limbic and thalamic volumes in pediatric bipolar disorder. American Journal of Psychiatry. 2005;162(7):1256–1265. doi: 10.1176/appi.ajp.162.7.1256. [DOI] [PubMed] [Google Scholar]
- Frodl T, Meisenzahl EM, Zill P, Baghai T, Rujescu D, Leinsinger G, Bottlender R, Schule C, Zwanzger P, Engel RR, et al. Reduced hippocampal volumes associated with the long variant of the serotonin transporter polymorphism in major depression. Archives of General Psychiatry. 2004;61(2):177–183. doi: 10.1001/archpsyc.61.2.177. [DOI] [PubMed] [Google Scholar]
- Geller B, Badner JA, Tillman R, Christian SL, Bolhofner K, Cook EH., Jr Linkage disequilibrium of the brain-derived neurotrophic factor Val66Met polymorphism in children with a prepubertal and early adolescent bipolar disorder phenotype. American Journal of Psychiatry. 2004;161(9):1698–1700. doi: 10.1176/appi.ajp.161.9.1698. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Bearden CE, Caetano S, Fonseca M, Najt P, Hunter K, Pliszka SR, Olvera RL, Soares JC. Declarative memory impairment in pediatric bipolar disorder. Bipolar Disord. 2005;7(6):546–554. doi: 10.1111/j.1399-5618.2005.00267.x. [DOI] [PubMed] [Google Scholar]
- Gourovitch ML, Torrey EF, Gold JM, Randolph C, Weinberger DR, Goldberg TE. Neuropsychological performance of monozygotic twins discordant for bipolar disorder. Biol Psychiatry. 1999;45(5):639–646. doi: 10.1016/s0006-3223(98)00148-6. [DOI] [PubMed] [Google Scholar]
- Grober E, Sliwinski M. Development and validation of a model for estimating premorbid verbal intelligence in the elderly. Journal of Clinical and Experimental Neuropsychology. 1991;13(6):933–949. doi: 10.1080/01688639108405109. [DOI] [PubMed] [Google Scholar]
- Hao Y, Creson T, Zhang L, Li P, Du F, Yuan P, Gould tD, Manji HK, Chen G. Mood stabilizer valproate promotes ERK pathway-dependent cortical neuronal growth and neurogenesis. The Journal of Neuroscience. 2004;24(29):6590–6599. doi: 10.1523/JNEUROSCI.5747-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser P, Altshuler LL, Berrettini W, Dauphinais ID, Gelernter J, Post RM. Temporal lobe measurement in primary affective disorder by magnetic resonance imaging. Journal of Neuropsychiatry and Clinical Neuroscience. 1989;1(2):128–134. doi: 10.1176/jnp.1.2.128. [DOI] [PubMed] [Google Scholar]
- Hauser P, Matochik J, Altshuler LL, Denicoff KD, Conrad A, Li X, Post RM. MRI-based measurements of temporal lobe and ventricular structures in patients with bipolar I and bipolar II disorders. Journal of Affective Disorders. 2000;60(1):25–32. doi: 10.1016/s0165-0327(99)00154-8. [DOI] [PubMed] [Google Scholar]
- Kalmar JH, Wang F, Spencer L, Edmiston E, Lacadie CM, Martin A, Constable RT, Duncan JS, Staib LH, Papademetris X, Blumberg HP. Preliminary evidence for progressive prefrontal abnormalities in adolescents and young adults with bipolar disorder. Journal of the International Neuropsychological Society. 2009;15:476–481. doi: 10.1017/S1355617709090584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates WR, Abrams MT, Kaufmann WE, Breiter SN, Reiss AL. Reliability and validity of MRI measurement of the amygdala and hippocampus in children with fragile X syndrome. Psychiatry Research. 1997;75(1):31–48. doi: 10.1016/s0925-4927(97)00019-x. [DOI] [PubMed] [Google Scholar]
- Keri S, Kelemen O, Benedek G, Janka Z. Different trait markers for schizophrenia and bipolar disorder: a neurocognitive approach. Psychol Med. 2001;31(5):915–922. doi: 10.1017/s0033291701004068. [DOI] [PubMed] [Google Scholar]
- Kieseppa T, Tuulio-Henriksson A, Haukka J, Van Erp T, Glahn D, Cannon TD, Partonen T, Kaprio J, Lonnqvist J. Memory and verbal learning functions in twins with bipolar-I disorder, and the role of information-processing speed. Psychological Medicine. 2005;35(2):205–215. doi: 10.1017/s0033291704003125. [DOI] [PubMed] [Google Scholar]
- Kohler S, Black SE, Sinden M, Szekely C, Kidron D, Parker JL, Foster JK, Moscovitch M, Winocour G, Szalai JP, et al. Memory impairments associated with hippocampal versus parahippocampal-gyrus atrophy: an MR volumetry study in Alzheimer's disease. Neuropsychologia. 1998;36(9):901–914. doi: 10.1016/s0028-3932(98)00017-7. [DOI] [PubMed] [Google Scholar]
- Lepage M, Habib R, Tulving E. Hippocampal PET activations of memory encoding and retrieval: The HIPER model. Hippocampus. 1998;8:313–322. doi: 10.1002/(SICI)1098-1063(1998)8:4<313::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Libon DJ, Bogdanoff B, Cloud BS, Skalina S, Giovannetti T, Gitlin HL, Bonavita J. Declarative and procedural learning, quantitative measures of the hippocampus, and subcortical white alterations in Alzheimer's disease and ischaemic vascular dementia. Journal of Clinial and Experimental Neuropsychology. 1998;20(1):30–41. doi: 10.1076/jcen.20.1.30.1490. [DOI] [PubMed] [Google Scholar]
- MacDonald ML, Naydenov A, Chu M, Matzilevich D, Konradi C. Decrease in creatine kinase messenger RNA expression in the hippocampus and dorsolateral prefrontal cortex in bipolar disorder. Bipolar Disorders. 2006;8(3):255–264. doi: 10.1111/j.1399-5618.2006.00302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen TM, Treschow A, Bengzon J, Bolwig TG, Lindvall O, Tingstrom A. Increased neurogenesis in a model of electroconvulsive therapy. Biological Psychiatry. 2000;47(12):1043–1049. doi: 10.1016/s0006-3223(00)00228-6. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. Journal of Neuroscience. 2000;20(24):9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manev H, Uz T, Smalheiser NR, Manev R. Antidepressants alter cell proliferation in the adult brain in vivo and in neural cultures in vitro. European Journal of Pharmacology. 2001;411(1–2):67–70. doi: 10.1016/s0014-2999(00)00904-3. [DOI] [PubMed] [Google Scholar]
- Manji HK, Moore GJ, Chen G. Clinical and preclinical evidence for the neurotrophic effects of mood stabilizers: implications for the pathophysiology and treatment of manic–depressive illness. Biological Society. 2000;48:740–754. doi: 10.1016/s0006-3223(00)00979-3. [DOI] [PubMed] [Google Scholar]
- Martinez-Aran A, Vieta E, Colom F, Torrent C, Sanchez-Moreno J, Reinares M, Benabarre A, Goikolea JM, Brugue E, Daban C, et al. Cognitive impairment in euthymic bipolar patients: implications for clinical and functional outcome. Bipolar Disorders. 2004a;6(3):224–232. doi: 10.1111/j.1399-5618.2004.00111.x. [DOI] [PubMed] [Google Scholar]
- Martinez-Aran A, Vieta E, Reinares M, Colom F, Torrent C, Sanchez-Moreno J, Benabarre A, Goikolea JM, Comes M, Salamero M. Cognitive function across manic or hypomanic, depressed, and euthymic states in bipolar disorder. American Journal of Psychiatry. 2004b;161(2):262–270. doi: 10.1176/appi.ajp.161.2.262. [DOI] [PubMed] [Google Scholar]
- Matsuo K, Walss-Bass C, Nery FG, Nicoletti MA, Hatch JP, Frey BN, Monkul ES, Zunta-Soares GB, Bowden CL, Escamilla MA, et al. Neuronal correlates of brain-derived neurotrophic factor Val66Met polymorphism and morphometric abnormalities in bipolar disorder. Neuropsychopharmacology. 2009;34(8):1904–1913. doi: 10.1038/npp.2009.23. [DOI] [PubMed] [Google Scholar]
- Muller DJ, de Luca V, Sicard T, King N, Strauss J, Kennedy JL. Brain-derived neurotrophic factor (BDNF) gene and rapid-cycling bipolar disorder: family-based association study. British Journal of Psychiatry. 2006;189:317–323. doi: 10.1192/bjp.bp.105.010587. [DOI] [PubMed] [Google Scholar]
- Mundo E, Walker M, Cate T, Macciardi F, Kennedy JL. The role of serotonin transporter protein gene in antidepressant-induced mania in bipolar disorder: preliminary findings. Archives of General Psychiatry. 2001;58(6):539–544. doi: 10.1001/archpsyc.58.6.539. [DOI] [PubMed] [Google Scholar]
- Noga JT, Vladar K, Torrey EF. A volumetric magnetic resonance imaging study of monozygotic twins discordant for bipolar disorder. Psychiatry Research. 2001;106(1):25–34. doi: 10.1016/s0925-4927(00)00084-6. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN, Schenkel LS, Aryal S, Harral EM, Hill SK, Herbener ES, Sweeney JA. Neurocognitive function in unmedicated manic and medicated euthymic pediatric bipolar patients. American Journal of Psychiatry. 2006;163(2):286–293. doi: 10.1176/appi.ajp.163.2.286. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Staib L, Scahill L, Zhang H, Anderson C, Leckman JF, Cohen DJ, Gore JC, Albert J, Webster R. Regional brain and ventricular volumes in Tourette syndrome. Archives of General Psychiatry. 2001;58(5):427–440. doi: 10.1001/archpsyc.58.5.427. [DOI] [PubMed] [Google Scholar]
- Robinson LJ, Thompson JM, Gallagher P, Goswami U, Young AH, Ferrier IN, Moore PB. A meta-analysis of cognitive deficits in euthymic patients with bipolar disorder. Journal of Affective Disorders. 2006;93(1–3):105–115. doi: 10.1016/j.jad.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Rousseva A, Henry C, van den Bulke D, Fournier G, Laplanche JL, Leboyer M, Bellivier F, Aubry JM, Baud P, Boucherie M, et al. Antidepressant-induced mania, rapid cycling and the serotonin transporter gene polymorphism. Pharmacogenomics Journal. 2003;3(2):101–104. doi: 10.1038/sj.tpj.6500156. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301(5634):805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Savage CR, Deckersbach T, Heckers S, Wagner AD, Schacter DL, Alpert NM, Fischman AJ, Rauch SL. Prefrontal regions supporting spontaneous and directed application of verbal learning strategies: Evidence from PET. Brain. 2001;124(1):219–231. doi: 10.1093/brain/124.1.219. [DOI] [PubMed] [Google Scholar]
- Savitz J, Drevets WC. Neuroimaging and Neuropathological Findings in Bipolar Disorder. In: Manji HK, Zarate CA, editors. Behavioral Neurobiology of Bipolar Disorder and its Treatment, Current Topics in Behavioral Neurosciences. Verlag Berlin: Heidelberg; published online Aug 24 2010. [Google Scholar]
- Senturk V, Goker C, Bilgic A, Olmez S, Tugcu H, Oncu B, Atbasoglu EC. Impaired verbal memory and otherwise spared cognition in remitted bipolar patients on monotherapy with lithium or valproate. Bipolar Disorders. 2007;9 suppl s1:136–144. doi: 10.1111/j.1399-5618.2007.00481.x. [DOI] [PubMed] [Google Scholar]
- Serretti A, Calati R, Mandelli L, De Ronchi D. Serotonin transporter gene variants and behavior: a comprehensive review. Current Drug Targets. 2006;7(12):1659–1669. doi: 10.2174/138945006779025419. [DOI] [PubMed] [Google Scholar]
- Sklar P, Gabriel SB, McInnis MG, Bennett P, Lim YM, Tsan G, Schaffner S, Kirov G, Jones I, Owen M, et al. Family-based association study of 76 candidate genes in bipolar disorder: BDNF is a potential risk locus. Brain-derived neutrophic factor. Molecular Psychiatry. 2002;7(6):579–593. doi: 10.1038/sj.mp.4001058. [DOI] [PubMed] [Google Scholar]
- Simonsen C, Sundet K, Vaskinn A, Birkenaes AB, Engh JA, Hansen CF, Jónsdóttir H, Ringen PA, Opjordsmoen S, Friis S, Andreassen OA. Neurocognitive profiles in bipolar I and bipolar II disorder: differences in pattern and magnitude of dysfunction. Bipolar Disorders. 2008;10(2):245–255. doi: 10.1111/j.1399-5618.2007.00492.x. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, DelBello MP, Sax KW, Zimmerman ME, Shear PK, Hawkins JM, Larson ER. Brain magnetic resonance imaging of structural abnormalities in bipolar disorder. Archives of General Psychiatry. 1999;56(3):254–260. doi: 10.1001/archpsyc.56.3.254. [DOI] [PubMed] [Google Scholar]
- Strasser HC, Lilyestrom J, Ashby ER, Honeycutt NA, Schretlen DJ, Pulver AE, Hopkins RO, Depaulo JR, Potash JB, Schweizer B, et al. Hippocampal and ventricular volumes in psychotic and nonpsychotic bipolar patients compared with schizophrenia patients and community control subjects: a pilot study. Biol Psychiatry. 2005;57(6):633–639. doi: 10.1016/j.biopsych.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Swayze VW, 2nd, Andreasen NC, Alliger RJ, Yuh WT, Ehrhardt JC. Subcortical and temporal structures in affective disorder and schizophrenia: a magnetic resonance imaging study. Biological Psychiatry. 1992;31(3):221–240. doi: 10.1016/0006-3223(92)90046-3. [DOI] [PubMed] [Google Scholar]
- Taylor WD, Steffens DC, Payne ME, MacFall JR, Marchuk DA, Svenson IK, Krishnan KR. Influence of serotonin transporter promoter region polymorphisms on hippocampal volumes in late-life depression. Archives of General Psychiatry. 2005;62(5):537–544. doi: 10.1001/archpsyc.62.5.537. [DOI] [PubMed] [Google Scholar]
- Tischler L, Brand SR, Stavitsky K, Labinsky E, Newmark R, Grossman R, Buchsbaum MS, Yehuda R. The relationship between hippocampal volume and declarative memory in a population of combat veterans with and without PTSD. Annals of the N Y Academy of Science. 2006;1071:405–409. doi: 10.1196/annals.1364.031. [DOI] [PubMed] [Google Scholar]
- van Gorp WG, Altshuler LL, Theberge DC, Wilkins J, Dixon W. Cognitive impairment in euthymic bipolar patients with and without prior alcohol dependence. Archives in General Psychiatry. 1998;55:41–46. doi: 10.1001/archpsyc.55.1.41. [DOI] [PubMed] [Google Scholar]
- van Gorp WG, Altshuler L, Theberge DC, Mintz J. Declarative and procedural memory in bipolar disorder. Biological Psychiatry. 1999;46(4):525–531. doi: 10.1016/s0006-3223(98)00336-9. [DOI] [PubMed] [Google Scholar]
- Walz JC, Frey BN, Andreazza AC, Cereser KM, Cacilhas AA, Valvassori SS, Quevedo J, Kapczinski F. Effects of lithium and valproate on serum and hippocampal neurotrophin-3 levels in an animal model of mania. Journal of Psychiatric Research. 2008;42(5):416–421. doi: 10.1016/j.jpsychires.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Wang F, Kalmar JH, Womer FY, Edmiston EE, Chepenik LG, Chen R, Spencer L, Blumberg HP. Olfactocentric paralimbic cortex morphology in adolescents with bipolar disorder. Brain. 2011;134(7):2005–2012. doi: 10.1093/brain/awr124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio, TX: Psychological Corp.; 1999. [Google Scholar]
- Wolfe J, Granholm E, Butters N, Saunders E, Janowsky D. Verbal memory deficits associated with major affective disorders: a comparison of unipolar and bipolar patients. Journal of Affective Disorders. 1987;13(1):83–92. doi: 10.1016/0165-0327(87)90077-2. [DOI] [PubMed] [Google Scholar]
- Ystad M, Eichele T, Lundervold AJ, Lundervold A. Subcortical functional connectivity and verbal episodic memory in healthy elderly-A resting state fMRI study. NeuroImage. 2010;52(1):379–388. doi: 10.1016/j.neuroimage.2010.03.062. [DOI] [PubMed] [Google Scholar]
- Yucel K, McKinnon MC, Taylor VH, Macdonald K, Alda M, Young LT, MacQueen GM. Bilateral hippocampal volume increases after long-term lithium treatment in patients with bipolar disorder: a longitudinal MRI study. Psychopharmacology (Berlin) 2007;195(3):357–367. doi: 10.1007/s00213-007-0906-9. [DOI] [PubMed] [Google Scholar]