Abstract
TOX is a member of an evolutionarily conserved DNA-binding protein family and is expressed in several immune-relevant cell subsets. Here, we review the key role of TOX in regulating development of CD4 T cells, natural killer cells and lymphoid tissue inducer cells, the latter responsible for the generation of lymph nodes. Although the exact molecular mechanism of action of TOX remains to be elucidated, the role of TOX in establishment of gene programs in the thymus and the potential of TOX as a regulator of E protein activity are discussed.
Introduction
Development of mature cells requires coordinated expression of gene regulatory networks that promote differentiation of precursors, while simultaneously inhibiting alternate cell fates. Many nuclear factors, including transcription factors, cofactors, and chromatin modifiers, have been described to play essential roles in the development of the immune system. In this review, we focus on the specific role of nuclear factor TOX (Thymocyte selection-associated HMG bOX protein) in the immune system. TOX was originally identified by microarray as a thymic transcript that was highly upregulated in CD4+CD8+ double positive (DP) thymocytes activated with pharmacological agents phorbol ester and ionomycin ex vivo, as a mimic of the TCR signaling that initiates thymic positive selection [1]. Subsequent analysis at the level of gene and protein expression confirmed that TOX was expressed in the thymus in a stage-specific regulated manner. However, TOX expression is not limited to the thymus and is detected in other tissues, including liver, where it likely plays additional functions in regulating metabolic processes (J. Kaye, unpublished data). TOX is part of the larger HMG-box superfamily of proteins, but also defines a small subfamily of proteins including TOX2, TOX3 and TOX4, all of which are highly conserved in vertebrate species (Figure 1) [2]. While the in vivo functions of TOX have been best characterized, TOX2 may play a role in reproductive organs [3], TOX3 has been implicated in regulation of neuron cell survival [4] and breast cancer [5,6], and TOX4 is known to interact with a phosphatase complex involved in the control of chromatin structure and cell cycle progression [7,8].
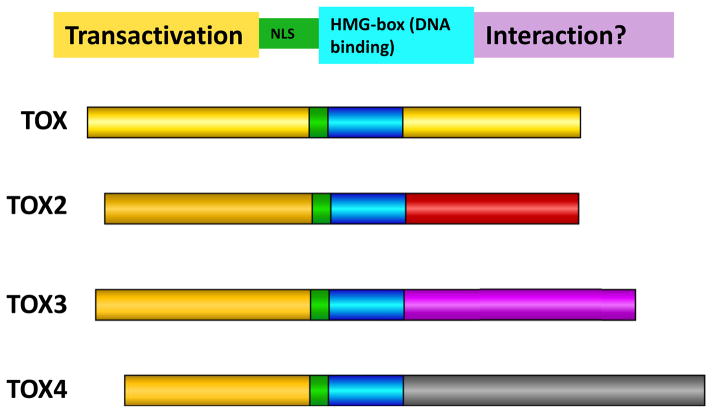
Figure 1.
The TOX subfamily of HMG-box proteins. TOX contains an HMG-box DNA binding domain that is highly conserved in three additional proteins TOX2, TOX3, and TOX4. An adjacent lysine-rich region may serve as the nuclear localization signal (NLS) as well as influence the interaction with DNA. The N-terminal domains of these proteins show approximately 30–40% sequence identity and have transactivation activity, while the C-terminal domains differ greatly between family members.
The sequence of the HMG-box domain of the TOX family of proteins places these factors in the sequence-independent but structure-dependent family of DNA-binding proteins [2]. Like other HMG-box proteins, the DNA binding domain of TOX is comprised of three helices that fold into an L-shaped structure (Protein Data Bank; URL: http://www.pdb.org/pdb/explore/explore.do?structureId=2co9), and can interact with distorted DNA structures including cisplatinated DNA and double stranded circular DNA (J. Kaye, unpublished data). Unlike many HMG-box proteins however, the TOX DNA binding domain cannot induce bending of DNA due to lack of a key hydrophobic wedge residue (J. Kaye, unpublished data). Among TOX family members, the DNA binding domain is near identical (and with shared genomic organization), the N-terminal domain is fairly conserved and has transactivation activity, and the C-terminal domain is family-member specific. Using genetically modified mice we have revealed the first in vivo function for a TOX-family member, in this case as a key regulator of immune system development.
Role of TOX in T cell development
TOX is transiently upregulated during β-selection and positive selection of developing thymocytes [1]. In terms of the latter, upregulation of TOX by DP cells is mediated by TCR-mediated calcineurin signaling [9]. We have taken both transgenic and knockout approaches to identify the role of TOX in the thymus. At the CD4−CD8− (double negative, DN) stage, enforced expression of TOX was sufficient to induce upregulation of both CD4 and CD8αβ, although not the cell proliferation normally associated with progression to the DP stage [9]. Interestingly, TOX also induced derepression of CD4 in DN thymocytes, similar to the phenotype caused by deficiency in components of the SWI/SNF-like chromatin-remodeling complex [10]. Whether TOX also plays a role in chromatin remodeling remains to be determined.
Expression of a TOX-transgene in DP cells induced the CD8-lineage commitment and CD4-silencing factor RUNX3 [11,12] in the absence of positive selection signals, resulting in CD4 downregulation and CD8 single positive (CD8SP) cell formation [9]. However, these CD8SP cells failed to fully mature or exit the thymus, indicating that TOX alone was insufficient to substitute for full TCR signaling during positive selection. A similar phenotype has been observed in thymocytes from mice that lack E protein activity in DP thymocytes ([13] and see below).
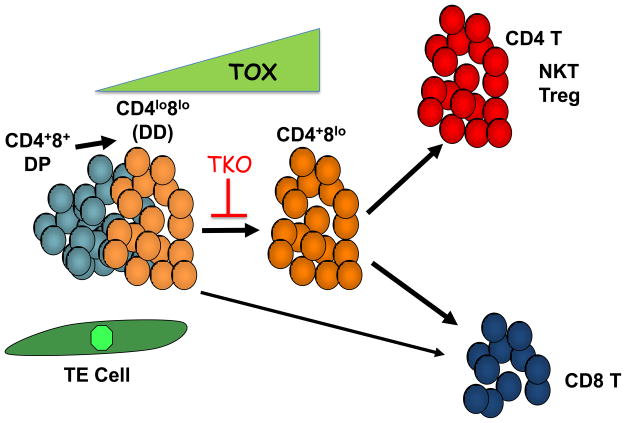
Somewhat surprisingly given these results, mice deficient in TOX (TKO) revealed a requirement for TOX in CD4 T cell lineage development [14] (Figure 2). Specifically, in the absence of TOX developing thymocytes initiated positive selection, including upregulation of CD69, CD5 and GATA3 and downregulation of CD4 and CD8 (CD4loCD8lo, double dull, DD), but failed to progress to the subsequent CD4+CD8lo transitional population, the stage at which lineage fate is established. This developmental block affected all CD4 T lineage cells, including not only production of conventional CD4 T cells, but also development of NKT and FOXP3+ regulatory T cells as well. Interestingly, CD8SP thymocytes developed in TKO mice, populated the spleen and could be activated to have cytolytic effector function [14]. These results emphasize that the CD4+CD8lo transitional stage of development is not obligatory for all CD8 T cell development [14,15]. That there is a pathway for CD8 but not CD4 T cell development directly from the DD stage suggests that the initiation of positive selection by class I and class II MHC are likely distinguishable in some manner, an idea that has fallen out of favor with demonstration of later stage lineage commitment [16], but one that in our view requires reassessment.
Figure 2.
TOX is a key regulator of T cell development in the thymus. TCR signaling in DP thymocytes undergoing positive selection, through interaction with thymic epithelial (TE) cells, causes downregulation of CD4 and CD8 resulting in a double dull (DD) phenotype followed by re-expression of CD4 to yield CD4+CD8lo cells. The latter subpopulation of cells contains the immediate precursors of both CD4+CD8− (CD4SP) and CD4− CD8+ (CD8SP) thymocytes, although some CD8SP can derive directly from DD cells. TOX is induced by TCR signals early in positive selection, first detected in DD cells and further upregulated at the CD4+CD8lo stage, before returning to baseline levels in SP thymocytes. In the absence of TOX (TKO), the DD to CD4+CD8lo progression is severely inhibited, blocking development of CD4 T cells but with a more modest effect on development of CD8 T cells.
RUNX3 and ThPOK (encoded by the Zbtb7b gene) are key nuclear factors for CD8 and CD4 T cell fate decisions in the thymus, respectively, at least in part due to their ability to each antagonize expression of the other [17–20]. Surprisingly, however, CD4 T cells develop in mice that lack both ThPOK and RUNX activity, suggesting that the primary function of ThPOK is in CD8 lineage repression [18]. Thus, this leaves open the question of how the CD4 lineage gene program is established during positive selection in the thymus.
One important clue as to the function of TOX in relation to CD4 T lineage development came from the observation of accumulation of small numbers of ‘lineage confused’ T cells that expressed both CD4 and CD8 molecules in the spleen of older TKO mice. These cells expressed low levels of ThPOK [14,21], pointing to TOX as an upstream regulator of ThPOK. Similar phenotype cells were also reported in mice expressing a hypomorphic ThPOK allele [22]. However, expression of a ThPOK transgene (ThPOK-Tg ) in TKO mice did not result in a complete rescue of the TKO phenotype [21]. While the CD8 lineage was inhibited in these animals, indicating that the alternative fate repression function of ThPOK was not dependent on TOX, the CD4 lineage was still aberrant. In the thymus, ThPOK-Tg/TKO mice contained a population of post-selection CD4loSP cells, suggesting that one function of TOX may be regulation of CD4 itself. When compared to normal CD4SP thymocytes, these CD4loSP cells expressed low levels of Id2 [21], an inhibitor of E protein activity that is normally induced at the CD4+8lo stage. This raised the possibility that lack of TOX might result in a failure to fully repress E protein activity during positive selection. Indeed, we found multiple defects in gene expression that have been linked to loss of E protein activity [13,23,24], most notably FOXO1 and its downstream gene targets KLF2, IL-7Rα, CCR7 and L-selectin [21]. In addition, although CD4 T cells were found in the spleen of ThPOK-Tg/TKO mice, they were poor expressers of FOXO1 and, upon activation, the CD4 lineage marker CD40L, further indicating a failure to implement a normal CD4 lineage gene program in the absence of TOX. Consistent with the role of TOX as an upstream regulator of ThPOK, ThPOK-Tg/TKO cells also failed to upregulate the endogenous ThPOK locus, despite transgene encoded protein expression [21].
Role of TOX in development of lymph nodes and Peyer’s patches
We also observed that TKO mice lacked lymph nodes and had a significant decrease in the frequency and size of Peyer’s patches [25]. This was shown to be independent of the T cell developmental defect in these animals [25]. Lymphoid tissue inducer (LTi) cells are key regulators of lymph node organogenesis [26–28]. These cells are of hematopoietic origin, are found in fetal liver during embryogenesis, and initiate formation of lymph node and Peyer’s patch anlagen through interactions with mesenchymal cells. Transcriptional regulators Ikaros, RORγ(t) and Id2 are required for LTi development, and thus mice deficient in any of these proteins are devoid of lymph nodes and Peyer’s patches[26,29–31]. LTi cells also express TOX and TKO mice lack LTi cells, explaining the absence of normal lymph node development in these animals[25]. Using RorcGFP knock-in reporter mice [26] bred to TKO mice, we failed to observe GFP+ cells in E17 intestines and spleen in the absence of TOX, consistent with the loss of RORγ(t)+ LTi cells. Nevertheless, RORγ(t) expression was maintained in TKO DP thymocytes, indicating that there was no global defect in RORγ(t) expression.
Role of TOX in formation of NK cells
TOX is also expressed in the NK cell lineage, with highest expression in immature NK (iNK, Lin−IL-15Rα+NK1.1+DX5−) and mature NK (mNK, Lin−IL-15Rα+NK1.1+DX5+) cells in the bone marrow [25]. In TKO mice, NK cells were significantly reduced in both the bone marrow and spleen, with a block in the transition of NK progenitor cells (Lin−IL15Rα+NK1.1−DX5−) to the iNK stage. The loss of NK cells was also reflected in reduction in NK cell-mediated cytotoxicity in vivo in TKO mice [25].
Development of NK cells is also dependent on expression of Id2 [30–32]. As was observed in the thymus of ThPOK-Tg/ TKO mice, Id2 expression was significantly reduced in remaining NK cells in the absence of TOX. However, Id2 expression was not able to rescue NK cell development from TKO bone marrow precursors, indicating that loss of Id2 expression cannot fully explain the NK cell developmental block.
Gene knockdown and overexpression studies in differentiating cultured human cord blood CD34+ precursor cells have also implicated TOX as a key regulator of NK cell development [33]. In these experiments, a siRNA-mediated decrease in TOX expression was reported to have no effect on Id2 mRNA levels, although there was only a modest increase in Id2 expression detected during differentiation in these cultures. The loss of TOX also had some effect on T-bet expression in this system. However, the phenotype of T-bet knockout mice differs greatly from that of TKO mice with regards to both T cells and NK cells. T-bet-deficient mice do not have an early block in NK cell differentiation but rather a late defect in cell maturation and IFN-γ production [34]. Thus, whether there are any mechanistic differences in the activity of TOX in human compared to murine immune cells remains to be determined. However, the high degree of conservation of the protein in rodents and humans [2] makes this less likely in our view.
Conclusions
TOX has multiple roles in the generation of the immune system, including development of CD4 T cells and NK cells, and lymph node organogenesis, the latter via regulation of LTi cell development. It is not clear if there are overlapping or distinct roles for TOX in each of these cell lineages. The one commonality may be upregulation of Id2, which is associated with each developmental process (Figure 3). Indeed, loss of TOX was associated with decreases in Id2 expression in both thymocytes and NK lineage cells [21,25]. Most strikingly, loss of E protein activity phenocopies overexpression of TOX in DP thymocytes and some genes repressed by E proteins fail to be upregulated in the absence of TOX [13,35]. However, NK and LTi cell development is dependent on Id2 [30–32], while loss of Id2 does not inhibit thymic positive selection [36,37], although compensatory activity by Id3 remains a possible confounding factor. In the thymus, TOX is required for the DD to CD4+8lo transition, and for CD4 reexpression itself [14,21]. In addition, TOX plays a role in ThPOK induction, although not activity, as well as establishment of the CD4 lineage gene program [21]. The specific biological role of TOX in NK and LTi cell development remains to be investigated and will be aided by TOX marker knock-in mice under characterization (J. Kaye, unpublished data). Experiments to identify genomic binding sites of TOX, to understand how this sequence-independent DNA binding factor is targeted to specific regions and what other proteins interact with TOX are ongoing, and will shed light on the mechanism of action of this key regulatory nuclear protein. As TOX is also expressed in some mature T cells (J. Kaye, unpublished data), TOX may play additional roles in the immune system as well as other tissues. Based on our findings for TOX, we think it likely that other members of this protein subfamily will be found to play key roles in other developmental or cell differentiation contexts.
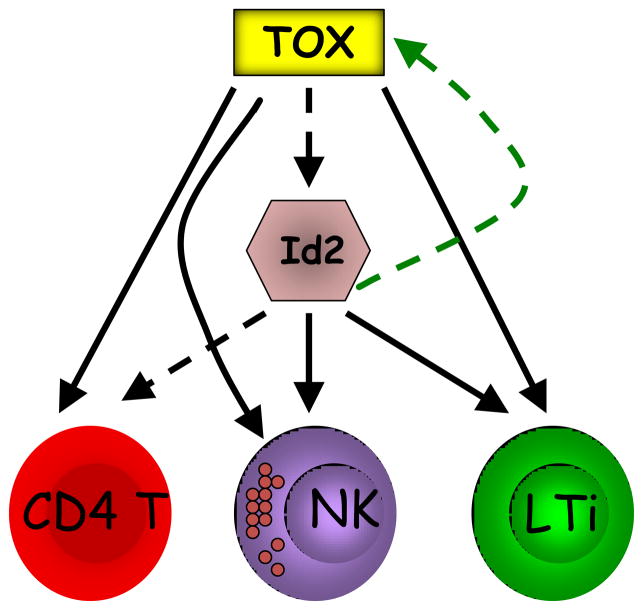
Figure 3.
TOX is required for development of NK, LTi and CD4 T cells, possibly by modulating E protein activity. Both NK and LTi lineages share dependence on expression of Id2 as well as TOX. TKO NK cells (and thymocytes) have reduced expression of Id2, suggesting that TOX may be an upstream regulator of this E protein inhibitor. However, it is likely that TOX also regulates other events required for development of these cell lineages, as reconstitution of Id2 did not rescue NK cell development in TKO bone marrow precursors. Id2 is also expressed in the thymus, but its deletion does not cause a block in T cell development possibly due to compensation by Id3 [36,37]. However, TKO thymocytes express lower levels of Id2, and there is failure to upregulate some genes that are repressed by E proteins [13,21]. Whether this is causally linked remains to be proven. Thus, the role of Id2 during thymocyte development may need to be revisited. Interestingly, as E protein activity has also been shown to have a negative effect on TOX expression [24], a feed forward regulatory circuit might enforce TOX expression in these different cell types. (In this Figure, dashed lines refer to hypotheticals).
Highlights.
The TOX protein is a member of a small subfamily of HMG-box DNA binding proteins
The TOX protein is predicted to interact with DNA in a sequence-independent fashion
TOX is required for CD4 T cell lineage development in the thymus
TOX regulates NK cell development in the bone marrow and lymph node organogenesis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wilkinson B, Chen JY, Han P, Rufner KM, Goularte OD, Kaye J. TOX: an HMG box protein implicated in the regulation of thymocyte selection. Nat Immunol. 2002;3:272–280. doi: 10.1038/ni767. [DOI] [PubMed] [Google Scholar]
- 2.O’Flaherty E, Kaye J. TOX defines a conserved subfamily of HMG-box proteins. BMC Genomics. 2003;4:13. doi: 10.1186/1471-2164-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kajitani T, Mizutani T, Yamada K, Yazawa T, Sekiguchi T, Yoshino M, Kawata H, Miyamoto K. Cloning and characterization of granulosa cell high-mobility group (HMG)-box protein-1, a novel HMG-box transcriptional regulator strongly expressed in rat ovarian granulosa cells. Endocrinology. 2004;145:2307–2318. doi: 10.1210/en.2003-1343. [DOI] [PubMed] [Google Scholar]
- 4.Dittmer S, Kovacs Z, Yuan SH, Siszler G, Kogl M, Summer H, Geerts A, Golz S, Shioda T, Methner A. TOX3 is a neuronal survival factor that induces transcription depending on the presence of CITED1 or phosphorylated CREB in the transcriptionally active complex. J Cell Sci. 2011;124:252–260. doi: 10.1242/jcs.068759. [DOI] [PubMed] [Google Scholar]
- 5.Easton DF, Pooley KA, Dunning AM, Pharoah PD, Thompson D, Ballinger DG, Struewing JP, Morrison J, Field H, Luben R, et al. Genome-wide association study identifies novel breast cancer susceptibility loci. Nature. 2007;447:1087–1093. doi: 10.1038/nature05887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stacey SN, Manolescu A, Sulem P, Rafnar T, Gudmundsson J, Gudjonsson SA, Masson G, Jakobsdottir M, Thorlacius S, Helgason A, et al. Common variants on chromosomes 2q35 and 16q12 confer susceptibility to estrogen receptor-positive breast cancer. Nat Genet. 2007;39:865–869. doi: 10.1038/ng2064. [DOI] [PubMed] [Google Scholar]
- 7.Lee JH, You J, Dobrota E, Skalnik DG. Identification and characterization of a novel human PP1 phosphatase complex. J Biol Chem. 2010;285:24466–24476. doi: 10.1074/jbc.M110.109801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.du Puch CB, Barbier E, Kraut A, Coute Y, Fuchs J, Buhot A, Livache T, Seve M, Favier A, Douki T, et al. TOX4 and its binding partners recognize DNA adducts generated by platinum anticancer drugs. Arch Biochem Biophys. 2011;507:296–303. doi: 10.1016/j.abb.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 9.Aliahmad P, O’Flaherty E, Han P, Goularte OD, Wilkinson B, Satake M, Molkentin JD, Kaye J. TOX provides a link between calcineurin activation and CD8 lineage commitment. J Exp Med. 2004;199:1089–1099. doi: 10.1084/jem.20040051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chi TH, Wan M, Zhao K, Taniuchi I, Chen L, Littman DR, Crabtree GR. Reciprocal regulation of CD4/CD8 expression by SWI/SNF-like BAF complexes. Nature. 2002;418:195–199. doi: 10.1038/nature00876. [DOI] [PubMed] [Google Scholar]
- 11.Kohu K, Sato T, Ohno S, Hayashi K, Uchino R, Abe N, Nakazato M, Yoshida N, Kikuchi T, Iwakura Y, et al. Overexpression of the Runx3 transcription factor increases the proportion of mature thymocytes of the CD8 single-positive lineage. J Immunol. 2005;174:2627–2636. doi: 10.4049/jimmunol.174.5.2627. [DOI] [PubMed] [Google Scholar]
- 12.Woolf E, Xiao C, Fainaru O, Lotem J, Rosen D, Negreanu V, Bernstein Y, Goldenberg D, Brenner O, Berke G, et al. Runx3 and Runx1 are required for CD8 T cell development during thymopoiesis. Proc Natl Acad Sci USA. 2003;100:7731–7736. doi: 10.1073/pnas.1232420100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones ME, Zhuang Y. Acquisition of a functional T cell receptor during T lymphocyte development is enforced by HEB and E2A transcription factors. Immunity. 2007;27:860–870. doi: 10.1016/j.immuni.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aliahmad P, Kaye J. Development of all CD4 T lineages requires nuclear factor TOX. J Exp Med. 2008;205:245–256. doi: 10.1084/jem.20071944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Correia-Neves M, Mathis D, Benoist C. A molecular chart of thymocyte positive selection. Eur J Immunol. 2001;31:2583–2592. doi: 10.1002/1521-4141(200109)31:9<2583::aid-immu2583>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 16.Brugnera E, Bhandoola A, Cibotti R, Yu Q, Guinter TI, Yamashita Y, Sharrow SO, Singer A. Coreceptor reversal in the thymus: signaled CD4+8+ thymocytes initially terminate CD8 transcription even when differentiating into CD8+ T cells. Immunity. 2000;13:59–71. doi: 10.1016/s1074-7613(00)00008-x. [DOI] [PubMed] [Google Scholar]
- 17.Egawa T. Runx and ThPOK: a balancing act to regulate thymocyte lineage commitment. J Cell Biochem. 2009;107:1037–1045. doi: 10.1002/jcb.22212. [DOI] [PubMed] [Google Scholar]
- 18**.Egawa T, Littman DR. ThPOK acts late in specification of the helper T cell lineage and suppresses Runx-mediated commitment to the cytotoxic T cell lineage. Nat Immunol. 2008;9:1131–1139. doi: 10.1038/ni.1652. In this paper, the authors showed that thymocyte development was skewed towards the CD4 T lineage in a mouse model where both ThPOK and RUNX activity (RUNX3 and RUNX1, through deletion of their shared binding partner CBFβ) were absent. This suggests that the primary role of ThPOK in CD4 T lineage development is to inhibit the CD8 lineage fate. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Egawa T, Taniuchi I. Antagonistic interplay between ThPOK and Runx in lineage choice of thymocytes. Blood Cells Mol Dis. 2009;43:27–29. doi: 10.1016/j.bcmd.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Setoguchi R, Tachibana M, Naoe Y, Muroi S, Akiyama K, Tezuka C, Okuda T, Taniuchi I. Repression of the transcription factor Th-POK by Runx complexes in cytotoxic T cell development. Science. 2008;319:822–825. doi: 10.1126/science.1151844. [DOI] [PubMed] [Google Scholar]
- 21**.Aliahmad P, Kadavallore A, de la Torre B, Kappes D, Kaye J. TOX Is Required for Development of the CD4 T Cell Lineage Gene Program. J Immunol. 2011;187:5931–5940. doi: 10.4049/jimmunol.1101474. This paper shows that expression of a ThPOK transgene cannot rescue the developmental defect in TOX-deficient mice with regards to CD4 T cell development, while ThPOK-mediated inhibition of CD8 T cell lineage development was unaffected. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang L, Wildt KF, Zhu J, Zhang X, Feigenbaum L, Tessarollo L, Paul WE, Fowlkes BJ, Bosselut R. Distinct functions for the transcription factors GATA-3 and ThPOK during intrathymic differentiation of CD4(+) T cells. Nat Immunol. 2008;9:1122–1130. doi: 10.1038/ni.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin YC, Jhunjhunwala S, Benner C, Heinz S, Welinder E, Mansson R, Sigvardsson M, Hagman J, Espinoza CA, Dutkowski J, et al. A global network of transcription factors, involving E2A, EBF1 and Foxo1, that orchestrates B cell fate. Nat Immunol. 2010;11:635–643. doi: 10.1038/ni.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartz R, Engel I, Fallahi-Sichani M, Petrie HT, Murre C. Gene expression patterns define novel roles for E47 in cell cycle progression, cytokine-mediated signaling, and T lineage development. Proc Natl Acad Sci U S A. 2006;103:9976–9981. doi: 10.1073/pnas.0603728103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25**.Aliahmad P, de la Torre B, Kaye J. Shared dependence on the DNA-binding factor TOX for the development of lymphoid tissue-inducer cell and NK cell lineages. Nat Immunol. 2010;11:945–952. doi: 10.1038/ni.1930. In this paper, the requirement for TOX in the development of lymph nodes and Peyer’s patches, as well as NK cell development is described. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eberl G, Marmon S, Sunshine MJ, Rennert PD, Choi Y, Littman DR. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5:64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- 27.Cupedo T, Vondenhoff MF, Heeregrave EJ, De Weerd AE, Jansen W, Jackson DG, Kraal G, Mebius RE. Presumptive lymph node organizers are differentially represented in developing mesenteric and peripheral nodes. J Immunol. 2004;173:2968–2975. doi: 10.4049/jimmunol.173.5.2968. [DOI] [PubMed] [Google Scholar]
- 28.Mebius RE, Rennert P, Weissman IL. Developing lymph nodes collect CD4+CD3- LTbeta+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity. 1997;7:493–504. doi: 10.1016/s1074-7613(00)80371-4. [DOI] [PubMed] [Google Scholar]
- 29.Wang JH, Nichogiannopoulou A, Wu L, Sun L, Sharpe AH, Bigby M, Georgopoulos K. Selective defects in the development of the fetal and adult lymphoid system in mice with an Ikaros null mutation. Immunity. 1996;5:537–549. doi: 10.1016/s1074-7613(00)80269-1. [DOI] [PubMed] [Google Scholar]
- 30.Yokota Y, Mansouri A, Mori S, Sugawara S, Adachi S, Nishikawa S, Gruss P. Development of peripheral lymphoid organs and natural killer cells depends on the helix-loop-helix inhibitor Id2. Nature. 1999;397:702–706. doi: 10.1038/17812. [DOI] [PubMed] [Google Scholar]
- 31.Boos MD, Yokota Y, Eberl G, Kee BL. Mature natural killer cell and lymphoid tissue-inducing cell development requires Id2-mediated suppression of E protein activity. J Exp Med. 2007;204:1119–1130. doi: 10.1084/jem.20061959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ikawa T, Fujimoto S, Kawamoto H, Katsura Y, Yokota Y. Commitment to natural killer cells requires the helix-loop-helix inhibitor Id2. Proc Natl Acad Sci USA. 2001;98:5164–5169. doi: 10.1073/pnas.091537598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33*.Yun S, Lee SH, Yoon SR, Kim MS, Piao ZH, Myung PK, Kim TD, Jung H, Choi I. TOX regulates the differentiation of human natural killer cells from hematopoietic stem cells in vitro. Immunol Lett. 2011;136:29–36. doi: 10.1016/j.imlet.2010.11.008. This paper supports a role for TOX in differentiation of cultured CD34+ human cord blood precursor cells into NK cells using knockdown and overexpression methodologies. [DOI] [PubMed] [Google Scholar]
- 34.Townsend MJ, Weinmann AS, Matsuda JL, Salomon R, Farnham PJ, Biron CA, Gapin L, Glimcher LH. T-bet regulates the terminal maturation and homeostasis of NK and Valpha14i NKT cells. Immunity. 2004;20:477–494. doi: 10.1016/s1074-7613(04)00076-7. [DOI] [PubMed] [Google Scholar]
- 35.Morrow MA, Mayer EW, Perez CA, Adlam M, Siu G. Overexpression of the Helix-Loop-Helix protein Id2 blocks T cell development at multiple stages. Mol Immunol. 1999;36:491–503. doi: 10.1016/s0161-5890(99)00071-1. [DOI] [PubMed] [Google Scholar]
- 36.Rivera R, Murre C. The regulation and function of the Id proteins in lymphocyte development. Oncogene. 2001;20:8308–8316. doi: 10.1038/sj.onc.1205091. [DOI] [PubMed] [Google Scholar]
- 37.Ji M, Li H, Suh HC, Klarmann KD, Yokota Y, Keller JR. Id2 intrinsically regulates lymphoid and erythroid development via interaction with different target proteins. Blood. 2008;112:1068–1077. doi: 10.1182/blood-2008-01-133504. [DOI] [PMC free article] [PubMed] [Google Scholar]