Abstract
Background
Mecamylamine is a nicotine antagonist under investigation in combination with nicotine replacement for smoking treatment.
Methods
A simple, rapid and reliable liquid chromatography tandem mass spectrometry (LCMSMS) method was developed and validated for quantifying nicotine, cotinine, trans-3′-hydroxycotinine, norcotinine and mecamylamine in human urine. Chromatography was performed on a Synergi PolarRP column with a gradient of 0.1% formic acid and 0.1% formic acid in acetonitrile at 0.25 ml/min with an 8-min total runtime. Analytes were monitored by positive mode electrospray ionization and multiple reaction monitoring mass spectrometry.
Results
Linear dynamic ranges were 1–500 ng/ml for nicotine and norcotinine, 0.5–500 ng/ml for trans-3′-hydroxycotinine, 0.2–500 ng/ml for cotinine, and 0.1–100 ng/ml for mecamylamine; correlation coefficients were consistently greater than 0.99, and all calibrator concentrations were within 20% of target Extensive endogenous and exogenous interferences were evaluated. At 3 concentrations spanning the linear dynamic range of the assay, mean extraction efficiencies from urine were 55.1–109.1% with analytical recovery (bias) 82.0–118.7% and total imprecision of 0.7–9.1%. Analytes were stable for 24 h at room temperature, 72h at 4°C, 72h in autosampler at 15°C and after three freeze/thaw cycles.
Conclusion
This method is useful for monitoring mecamylamine, nicotine and nicotine metabolites in smoking cessation and other clinical nicotine research.
Keywords: Mecamylamine, Nicotine, Cotinine, trans-3′-Hydroxycotinine, Norcotinine, Urine, LCMSMS
INTRODUCTION
Developing new and more effective smoking cessation strategies is critical for improving public health. The 2009 National Survey on Drug Use and Health documented decreased smoking; however, 58.7 million Americans aged 12 years or older smoked cigarettes in the past month [1]. Smoking continues to be the single largest preventable cause of death in the U.S. [2]. Nicotine is a highly addictive central and peripheral nervous system stimulant producing short-term adverse health effects, including elevated blood pressure, heart rate, and blood glucose [3]. Besides increased cancer rates, long-term tobacco use is associated with increased incidence of atherosclerotic arterial disease, chronic obstructive pulmonary disease, hypertension and low birth weight of infants born to mothers who smoke [3–5].
Nicotine replacement therapies (NRT; chewing gum, medicated patch, and inhalants) are highly purified nicotine preparations designed to reduce the desire for tobacco products [6]. Silagy et al. performed a meta-analysis of 123 NRT clinical studies finding that all commercially available nicotine replacement therapy (NRT) increased the odds of quitting by a factor of 1.5–2 at a 6–12 month follow-up [7]. Long-term quit rates at this time are typically 10–30% across NRT strategies [8–10]. Mecamylamine is a nicotine antagonist that blocks the rewarding effect of nicotine reducing the urge to smoke [11]. Mecamylamine is being investigated as a nicotine dependence pharmacotherapy [12]. Preliminary studies examining short term mecamylamine effects on smoking behavior showed that low doses tended to increase smoking [13–16], whereas higher doses reduced smoking in 50% of subjects within the first 11 days of treatment [17]. Considering the benefits of NRT and mecamylamine, we are currently conducting clinical studies combining mecamylamine administration with NRT for smoking treatment.
Several methods quantify nicotine and metabolites in human urine, including gas chromatography-mass spectrometry (GCMS) [18], liquid chromatography-mass spectrometry (LCMS) [19, 20] and liquid chromatography tandem mass spectrometry (LCMSMS) assays [21–27]. Only one published method includes quantification of mecamylamine, nicotine and cotinine in human plasma by gas chromatography mass spectrometry (GCMS) achieving limits of quantification (LOQ) of 2, 1 and 2 ng/ml for mecamylamine, nicotine and cotinine, respectively [18]. We report the first, sensitive and specific solid phase extraction (SPE)/LCMSMS method for simultaneous quantification of mecamylamine, nicotine, cotinine, OH-cotinine and norcotinine across a wide range of concentrations in urine. This method will be employed for therapeutic drug monitoring and smoking relapse assessment during our ongoing clinical trials of combined mecamylamine and nicotine administration for the treatment of tobacco addiction.
1. EXPERIMENTAL
2.1. Reagents and supplies
(R, S) OH-cotinine (10 mg powder), OH-cotinine-d3 (1 mg powder), (R,S)-norcotinine (10 mg powder) and (R,S)-norcotinine-d4 (5 mg powder) were purchased from Toronto Research Chemicals (North York, Ontario, Canada). (S)-Cotinine (1 mg/ml in methanol), (S)-cotinine-d3 (100 μg/ml in methanol) and (S)-nicotine-d4 (100 μg/ml in methanol) were acquired from Cerilliant (Austin, TX, USA). Mecamylamine hydrochloride (25 mg powder), (S)–Nicotine (1 mg powder) and formic acid were obtained from Sigma (St. Louis, MO). Mecamylamine-d3 (5 mg powder) was supplied by Peyton Jacob III (University of California, CA) [18]. Water, acetonitrile, sodium phosphate dibasic, sodium phosphate monobasic, hydrochloric acid, dichloromethane, 2-propanol, and ammonium hydroxide were from J.T. Baker (Philipsburg, NJ) and methanol from Fisher Chemical (Pittsburgh, PA). All solvents and reagents were HPLC or ACS grade. CleanScreen SPE columns, part ZSDAU020, were from United Chemical Technologies (Bristol, PA). Analytical chromatography was performed on a Synergi Polar RP HPLC column (100 × 2.1 mm; 2.5 μm particle size) combined with a 4 × 2.0 mm guard column of identical phase were from Phenomenex, Torrance, CA
2.2. Instrumentation
Tandem mass spectrometry analysis was performed on an ABSciex API 3200 QTrap® triple quadrupole/linear ion trap mass spectrometer with a TurboIonSpray source (ABSciex, Foster City, CA). The high performance liquid chromatography (HPLC) system consisted of a DGU-20A3 degasser, LC-20AD pumps, SIL-20AC autosampler and a CTO-20 column oven (Shimadzu Corp, Columbia, MD). Analyst software version 1.4.1 was employed for acquisition and data analysis.
2.3. Calibrators, quality control (QC) and internal standards
Blank urine from ten volunteers was evaluated with the methodology detailed in this manuscript to ensure absence of detectable mecamylamine, nicotine or metabolites prior to fortification with working stock solutions to prepare calibrators and quality control samples. Individual primary stock solutions (1 mg/ml) of mecamylamine, nicotine, OH-cotinine, cotinine, and norcotinine were prepared in methanol. Working solutions from 1–5,000 ng/ml were prepared by mixing primary stock solutions and diluting in methanol. Calibrators for mecamylamine (0.1, 0.2, 0.5, 1, 5, 10, 50, 100 ng/ml), cotinine (0.2, 0.5, 1, 5, 10, 50, 100, 500 ng/ml), OH-cotinine (0.5, 1, 5, 10, 50, 100, 500 ng/ml) and nicotine and norcotinine (1, 5, 10, 50, 100, 500 ng/ml) were freshly prepared each day by addition of working stock solutions to 1 ml blank urine.
QC samples were prepared with different lot numbers of reference standard solutions than calibrators. Five mixed QC working solutions, ranging from 5–3,000 ng/ml, were prepared in methanol. QC samples were prepared by adding working solutions to 1 ml blank urine to yield 0.6, 3, and 30 ng/ml mecamylamine, 0.6, 30, and 300 ng/ml cotinine, 1.5, 30, and 300 ng/ml OH-cotinine and 3, 30, and 300 nicotine and norcotinine (low, medium, and high QC, respectively).
Primary stock solutions of mecamylamine-d3, nicotine-d4, cotinine-d3, OH-cotinine-d3, and norcotinine-d4 were diluted in methanol producing a mixed internal standard solution of 250 ng/ml mecamylamine-d3 and cotinine-d3, and 500 ng/ml for OH-cotinine-d3, nicotine-d4 and norcotinine-d4. All primary and working solutions were stored at −20°C in amber glass vials.
2.4. Specimen preparation and SPE
Twenty microliters of internal standard working solution was added to 1 ml participant urine or blank urine fortified with calibrator or QC working solutions. Two ml 0.1 mol/l sodium phosphate buffer, pH 6.0 was added to each sample prior to SPE. CleanScreen DAU SPE columns were conditioned with 3 ml methanol, 3 ml water and 1 ml 0.1 mol/l sodium phosphate buffer, pH 6. Samples were loaded onto columns with gravity flow. Columns were washed with 3 ml water, 1.5 ml 100 mmol/l HCl and 3 ml methanol. Columns were vacuum dried for 1, 5 and 5 min after water, hydrochloric acid and methanol washes, respectively. Analytes were eluted with freshly prepared 5 ml dichloromethane:isopropanol:ammonium hydroxide (78:20:2, v/v/v). Eluates were dried under nitrogen at 40°C after addition of 100 μl 1% hydrochloric acid in methanol (v/v). Samples were reconstituted in 200 μl 0.1% formic acid (v/v) and transferred to glass autosampler vials. Twenty microliters was injected onto the LCMSMS instrument.
2.5. LCMSMS
Chromatographic separation was performed on a Synergi Polar RP column protected by a guard column with identical packing material. Gradient elution was performed with (A) 0.1% formic acid in water and (B) 0.1% formic acid in acetonitrile at a flow rate of 0.25 ml/min. The gradient program was from 5% to 50% B over 3 min, held for 2 min, ramped from 50% to 5% B in one min and re-equilibrated at 5% B for 2 min (total runtime of 8 min). The column oven and auto-injector sample tray were maintained at 30 and 15°C, respectively. Mass spectrometric data were collected via positive mode electrospray ionization, with optimized instrument settings shown in Table 1. Optimized source parameters were: 35 psi curtain nitrogen gas, 40 psi auxiliary nitrogen gas, 70 psi nebulizer nitrogen gas, medium collision nitrogen gas, 3.0 μA nebulizer current, and 450°C source temperature. Quadrupoles one and three were set to unit resolution. The following transitions were monitored: m/z 163.2 to 132.2 and 84.2 for nicotine, m/z 167.2 to 136.1 and 121.0 for nicotine-d4, m/z 177.2 to 80.1 and 98.1 for cotinine, m/z 180.2 to 101.2 and 80.2 for cotinine-d3, m/z 193.2 to 80.2 and 134.0 for OH-cotinine, m/z 196.2 to 134.1 and 79.9 for OH-cotinine-d3, m/z 163.2 to 80.2 and 118.2 for norcotinine, m/z 167.2 to 139.2 and 84.2 for norcotinine-d4, m/z 168.2 to 81.2 and 137.2 for mecamylamine, and m/z 171.0 to 81.0 and 137.0 for mecamylamine-d3. The most abundant transition for each analyte was used for quantification (underlined transition); the second transition served as a qualifier (Table 1).
Table 1.
Liquid chromatography tandem mass spectrometry parameters for mecamylamine, nicotine and nicotine metabolites in urine
| Compounds | Q1 Mass (amu) | Q3 Mass (amu) | Dwell Time (msec) | Declustering Potential (volts) | Entrance Potential (volts) | Collision Entrance Potential (volts) | Collision Energy (volts) | Cell Exit Potential (volts) | Retention Time (± SD) N= 30 |
|---|---|---|---|---|---|---|---|---|---|
| Nicotine | 163.2 | 132.2 | 150 | 35 | 5.5 | 12 | 21 | 4 | 2.59 (± 0.027) |
| 84.2 | 150 | 35 | 4.5 | 12 | 29 | 4 | |||
| Nicotine-d4 | 167.2 | 136.1 | 150 | 35 | 6 | 12 | 21 | 4 | 2.56 (± 0.028) |
| 121.0 | 150 | 35 | 6 | 12 | 29 | 4 | |||
| Cotinine | 177.2 | 98.1 | 150 | 41 | 3 | 12 | 29 | 4 | 2.99 (± 0.030) |
| 80.1 | 150 | 41 | 3 | 12 | 33 | 4 | |||
| Cotinine-d3 | 180.2 | 101.2 | 150 | 36 | 3 | 12 | 31 | 4 | 3.01 (± 0.029) |
| 80.2 | 150 | 36 | 3 | 12 | 33 | 4 | |||
| OH-Cotininea | 193.2 | 134.0 | 150 | 46 | 8 | 12 | 27 | 4 | 2.22 (± 0.021) |
| 80.2 | 150 | 46 | 8 | 12 | 35 | 4 | |||
| OH-Cotinine-d3b | 196.2 | 134.1 | 150 | 46 | 10.5 | 14 | 27 | 4 | 2.22 (± 0.021) |
| 79.9 | 150 | 46 | 10.5 | 14 | 38 | 4 | |||
| Norcotinine | 163.2 | 118.2 | 150 | 46 | 10.5 | 12 | 29 | 4 | 2.33 (± 0.023) |
| 80.2 | 150 | 41 | 10 | 12 | 33 | 4 | |||
| Norcotinine-d4 | 167.2 | 139.2 | 150 | 46 | 10.5 | 12 | 29 | 4 | 2.34 (± 0.023) |
| 84.2 | 150 | 46 | 10.5 | 12 | 33 | 4 | |||
| Mecamylamine | 168.2 | 81.2 | 150 | 20 | 4 | 15 | 28 | 4 | 5.32 (± 0.016) |
| 137.2 | 150 | 20 | 4.5 | 15 | 16 | 4 | |||
| Mecamylamine-d3 | 171.0 | 81.0 | 150 | 25 | 4 | 15 | 30 | 3 | 5.30 (± 0.017) |
| 137.2 | 150 | 25 | 4 | 15 | 16 | 3 | |||
: trans-3′-hydroxycotinine,
: trans-3′-hydroxycotinine-d3
Underlined mass depicts quantification transition.
2.6. Data analysis
Peak area ratios of analytes to corresponding internal standards were calculated for each concentration to construct daily calibration curves via linear least-squares regression with a 1/x weighting factor. Calibration curves were from 0.1 to 100 ng/ml for mecamylamine, 0.2 to 500 ng/ml for cotinine, 0.5 to 500 ng/ml for OH-cotinine, and 1 to 500 ng/ml for nicotine and norcotinine.
2.7. Method validation
Specificity, sensitivity, linearity, imprecision, analytical recovery, extraction efficiency, matrix effect, stability, dilution integrity and carry-over were evaluated during method validation.
2.8. Specificity
Analyte peak identification criteria were relative retention time within ± 0.15 min of the lowest calibrator and qualifier/quantifier transition peak area ratios ±20% of mean calibrator transition ratios. Specificity of the method was assessed by analyzing ten urine specimens from different individuals to evaluate potential endogenous interferences. In addition, potential interferences from commonly used drugs and minor tobacco alkaloids were evaluated by fortifying drugs into low QC samples. Final interferent concentrations were 1 μg/ml of cocaine, benzoylecgonine, norcocaine, norbenzoylecgonine, Δ9-tetrahydrocannabinol (THC), 11-hydroxy-THC, 11-nor-9-carboxy-THC, morphine, normorphine, morphine-3-beta-D-glucuronide, morphine-6-beta-D-glucuronide, codeine, norcodeine, 6-acetylmorphine, 6-acetylcodeine, hydrocodone, hydromorphone, oxycodone, noroxycodone, oxymorphone, noroxymorphone, diazepam, lorazepam, oxazepam, alprazolam, clonidine, ibuprofen, pentazocine, caffeine, diphenhydramine, chlorpheniramine, brompheniramine, aspirin, acetaminophen, phencyclidine, nitrazepam, flunitrazepam, temazepam, nordiazepam, amphetamine, methamphetamine, anabasine and anatabine. No interference was noted if all analytes in the low QC sample quantified within ±20% of target concentrations with acceptable qualifier/quantifier transition ratios.
2.9. Sensitivity and linearity
Limit of detection (LOD) was evaluated in quadruplicate and defined as the lowest concentration producing a peak eluting within ±0.15 min of the analytes’ retention time for the lowest calibrator, a signal-to-noise ratio of at least 3, Gaussian peak shape and qualifier/quantifier transition peak area ratios ±20% of mean calibrator transition ratios. The limit of quantification (LOQ) also was evaluated in quadruplicate and defined as the concentration that met LOD criteria, signal-to-noise of at least 10 and measured concentration within ± 20% of target in four replicates. Performance at the LOQ was confirmed in each batch of specimens.
Preliminary experiments with four sets of calibrators determined the most appropriate calibration model comparing goodness-of-fit for unweighted linear least squares and linear least squares employing 1/x weighting. Calibration curves were fit by linear least squares regression with at least 6 concentrations across the linear dynamic range for each analyte. Calibrators were required to quantify within ±20% for the LOQ and correlation coefficients (R2) were required to exceed 0.995.
2.10. Analytical recovery and imprecision
Intra-day and inter-day analytical recovery (bias) and imprecision were determined from five replicates at three different QC concentrations. Analytical recovery was determined by comparing the mean result for all analyses to the nominal concentration value (i.e., mean % of expected concentration). Inter-day imprecision and analytical recovery were evaluated on four different runs with five replicates in each run, analyzed on four separate days (n=20). Imprecision was expressed as %CV of the calculated concentrations. The guidelines given by Krouwer and Rabinowitz [28] were employed to calculate pooled intra-day, inter-day and total imprecision.
2.11. Extraction efficiency and matrix effect
Extraction efficiency and matrix effect were evaluated on five different urine lots via three sets of samples as described by Matuszewski et al. (n=5 for each set) [29]. In the first set, urine samples were fortified with analytes and internal standards prior to SPE. In set 2, urine samples were fortified with analytes and internal standards after SPE, and the third set contained “neat” analytes and internal standards in mobile phase. Extraction efficiency, expressed as a percentage, was calculated by dividing analyte mean peak areas of set 1 by set 2. Absolute matrix effect was calculated by dividing the mean peak area of the analyte in set 2 by the mean analyte area in set 3. The value was converted to a percentage and subtracted from 100 to represent the amount of signal suppressed by the presence of matrix.
2.12. Stability
Stability was evaluated with blank human urine fortified with analytes of interest at three QC concentrations (n = 5). Short-term temperature stability was evaluated for fortified human urine stored for 24 h at room temperature, 72 h at 4°C, 72 h on the autosampler (15°C), and after three freeze-thaw cycles at −20°C. On the day of analysis, internal standard was added to each specimen and analyzed as described. Calculated concentrations of stability specimens were compared to QC sample concentrations on the day of preparation. Autosampler stability was assessed by re-injecting QC specimens after 72 h and comparing calculated concentrations to values obtained against the original calibration curve.
2.13. Dilution Integrity
Dilution integrity was evaluated by diluting a urine sample (n=3) containing 500 ng/ml of mecamylamine and 1000 ng/ml of nicotine, cotinine, OH-cotinine, and norcotinine with blank urine to achieve a 1:10 dilution. Internal standards were added and samples extracted as described. Dilution integrity was maintained if specimens quantified within ±15% of 50 ng/ml mecamylamine, and 100 ng/ml of nicotine, cotinine, OH-cotinine, and norcotinine.
2.14. Carry-over
Carry-over was investigated in triplicate by injecting extracted blank urine samples containing internal standards immediately after samples containing target analytes at twice the upper LOQ. The blank urine samples could not fulfill LOQ criteria for any analyte to document the absence of carryover.
2.15. Clinical study
Urine specimens were collected from self-reported smokers, nonsmokers not environmentally exposed to smoking and nonsmokers environmentally exposed to smoking participating in a National Institute on Drug Abuse (NIDA) Institutional Review Board approved protocol. Participants provided written informed consent.
3. RESULTS
3.1. Specificity
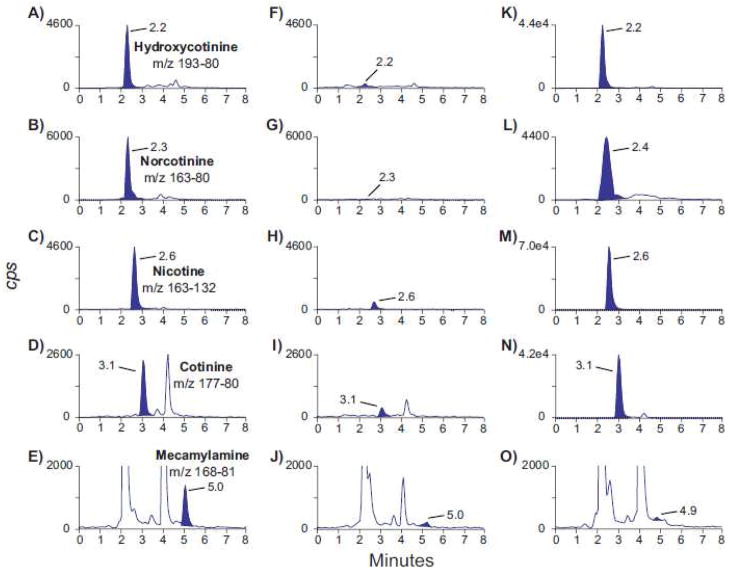
No urine from ten nicotine-abstinent individuals contained interfering compounds with any peaks of interest. None of the 43 potential exogenous interferences fortified at 1 μg/ml into low QC samples caused transition ratio or quantification criteria to fail. Multiple reaction monitoring (MRM) ion chromatograms from a blank urine sample fortified with analytes at the LOQ and a urine specimen from a study participant who self-reported not smoking and not being exposed to smoking at home or work are shown in panels A–J of Figure 1. Panels K–O of Figure 1 are MRM ion chromatograms from a self-reported nonsmoker who was exposed to smoking at home.
Figure 1.
Multiple reaction monitoring ion chromatograms for quantification transitions: m/z 193.2–80.2 (trans-3′-hydroxycotinine), m/z 163.2–80.2 (norcotinine), m/z 163.2–132.2 (nicotine), m/z 177.2–80.1 (cotinine) and m/z 168.2–81.2 (mecamylamine). Panels A–E are blank urine fortified with analytes at the limit of quantification, panels F–J are from an authentic urine specimen collected from a self-reported nonsmoker who is not exposed to smoking at home or work and panels K–O are from an authentic urine specimen collected from a self-reported nonsmoker exposed to smoking at home. Limits of quantification are 0.5, 1, 1, 0.2 and 0.1 ng/ml for trans-3′-hydroxycotinine, norcotinine, nicotine, cotinine and mecamylamine, respectively. Concentrations for all analytes shown in panels F–J were less than limits of quantification. Specimen urine concentrations in panels K–O are 45.0, 3.6, 35.2, 17.4 and less than limit of quantification for trans-3′-hydroxycotinine, norcotinine, nicotine, cotinine and mecamylamine, respectively.
3.2. Sensitivity and linearity
Initial experiments were conducted with four sets of calibration curves fit via both unweighted linear least squares and linear least squares with 1/x weighting factor to identify the most appropriate calibration model. Inspection of residuals obtained from unweighted vs. 1/x weighted models clearly indicated linear least squares with 1/x weighting factor produced a better fit for the calibration data (data not shown). All correlation coefficients exceeded 0.995 (Table 2). Table 2 details LOD, LOQ, linearity and mean calibration results. LOD were between 0.05 and 0.5 ng/ml; LOQ between 0.1 and 1.0 ng/ml. Assays were linear to 500 ng/ml for all analytes except mecamylamine (100 ng/ml).
Table 2.
Mecamylamine, nicotine and nicotine metabolites in urine by liquid chromatography tandem mass spectrometry: limits of detection (LOD), limits of quantification (LOQ) linear ranges and calibration results (N=4)
| Analyte | LOD (ng/ml) | LOQ (ng/ml) | Linear range (ng/ml) | Y-intercept Mean ± SD | Slope Mean ± SD | R2 ± SD |
|---|---|---|---|---|---|---|
| Nicotine | 0.5 | 1 | 1–500 | 0.01 ± 0.01 | 0.92 ± 0.08 | 0.998 ± 0.001 |
| Cotinine | 0.1 | 0.2 | 0.2–500 | 0.03 ± 0.01 | 1.04 ± 0.05 | 0.999 ± 0.001 |
| OH-Cotinineb | 0.2 | 0.5 | 0.5–500 | 0.02 ± 0.02 | 0.77 ± 0.03 | 0.999 ± 0.0002 |
| Norcotinine | 0.5 | 1 | 1–500 | 0.003 ± 0.007 | 0.87 ± 0.02 | 0.999 ± 0.0002 |
| Mecamylamine | 0.05 | 0.1 | 0.1–100 | 0.01 ± 0.07 | 0.69 ± 0.07 | 0.999 ± 0.0003 |
Upper limit of quantification,
trans-3′-hydroxycotinine
3.3. Analytical recovery and imprecision
Analytical recovery and imprecision were evaluated at three concentrations across the linear dynamic range. Analytical recovery in urine ranged from 82.0–118.7% of expected concentration for both intra-day and inter-day analytical recovery (Table 3). Pooled intra-day, inter-day and total CVs were 1.7–7.4, 0–6.4, and 0.7–9.1, respectively (Table 3).
Table 3.
Analytical recovery and imprecision data for mecamylamine, nicotine and nicotine metabolites in urine
| Analytical recovery (% of expected concentration) | Imprecision (% relative standard deviation, N= 20) | |||||||
|---|---|---|---|---|---|---|---|---|
| Intra-day, N=5 | Inter-day, N=20 | |||||||
| Expected concentration (ng/ml) | Mean | Range | Mean | Range | Pooled Intra-day | Inter-day | Total | |
| Nicotine | 3 | 112.7 | 110.7–114.3 | 113.2 | 109.0–118.3 | 1.9 | 2.4 | 3.0 |
| 30 | 102.8 | 97.0–112.3 | 104.9 | 92.7–113.0 | 4.7 | 3.4 | 5.8 | |
| 300 | 100.6 | 97.0–106.0 | 101.0 | 94.3–109.7 | 3.8 | 1.7 | 4.2 | |
|
| ||||||||
| Cotinine | 0.3 | 93.1 | 86.0–102.0 | 92.2 | 82.0–110.3 | 7.2 | 5.5 | 9.1 |
| 30 | 97.5 | 91.0–102.3 | 96.2 | 85.3–103.0 | 5.5 | 0.8 | 5.6 | |
| 300 | 96.8 | 92.7–99.7 | 92.6 | 82.3–102.7 | 3.2 | 4.8 | 5.8 | |
|
| ||||||||
| OH-cotinine a | 1.5 | 108.8 | 97.3–118.7 | 110.6 | 97.3–118.7 | 7.4 | 0 | 7.4 |
| 30 | 104.7 | 97.0–111.3 | 104.1 | 93.3–113.3 | 5.2 | 0 | 5.2 | |
| 300 | 105.7 | 98.7–114.0 | 102.2 | 94.7–114.0 | 5.2 | 0.6 | 5.2 | |
|
| ||||||||
| Norcotinine | 3 | 105.7 | 95.3–109.0 | 102.2 | 89.0–114.3 | 5.0 | 5.3 | 7.3 |
| 30 | 97.7 | 90.7–104.3 | 95.5 | 87.0–105.0 | 4.8 | 2.0 | 5.2 | |
| 300 | 92.7 | 89.3–98.7 | 89.9 | 84.3–98.7 | 3.2 | 1.9 | 3.7 | |
|
| ||||||||
| Mecamylamine | 0.3 | 86.3 | 82.7–91.7 | 86.3 | 82.0–98.3 | 5.0 | 0 | 5.0 |
| 3.0 | 117.6 | 117.0–118.3 | 113.5 | 99.3–118.3 | 1.7 | 0.7 | 0.7 | |
| 30 | 117.0 | 116.3–118.0 | 111.6 | 98.0–118.0 | 2.4 | 6.4 | 6.9 | |
trans-3′-hydroxycotinine
3.4. Extraction efficiency and matrix effect
Extraction efficiencies and matrix effects for mecamylamine, nicotine and metabolites from urine are presented in Table 4. Mean extraction efficiencies were 55.1–109.1% (n=5). Mean matrix effects, expressed as % suppressed signal, were 0.4–48.2% (n=5).
Table 4.
Mean extraction efficiency and matrix effect for mecamylamine, nicotine and nicotine metabolites extracted from urine
| Analyte | Extraction efficiency (%, N = 5) | Matrix effect (% of signal suppressed, N = 5) | ||||
|---|---|---|---|---|---|---|
| Low | Medium | High | Low | Medium | High | |
| Nicotine | 73.3 | 78.5 | 83.7 | 23.6 | 10.3 | 18.4 |
| Nicotine-d4 | 83.4 | 80.8 | 87.7 | 22.8 | 38.9 | 48.2 |
|
| ||||||
| Cotinine | 109.1 | 94.5 | 92.7 | 10.1 | 5.3 | 7.1 |
| Cotinine-d3 | 80.0 | 98.0 | 95.3 | 15.0 | 4.9 | 8.5 |
|
| ||||||
| trans-3′-Hydroxycotinine | 63.7 | 58.1 | 55.1 | 16.7 | 13.1 | 14.9 |
| trans-3′-Hydroxycotinine-d3 | 63.7 | 59.0 | 57.3 | 4.3 | 15.4 | 21.7 |
|
| ||||||
| Norcotinine | 96.7 | 94.0 | 91.5 | 5.9 | 9.9 | 8.4 |
| Norcotinine-d4 | 90.4 | 94.9 | 94.7 | 7.2 | 8.0 | 3.9 |
|
| ||||||
| Mecamylamine | 61.2 | 74.7 | 95.4 | 3.4 | 5.2 | 11.6 |
| Mecamylamine-d3 | 86.7 | 75.9 | 97.2 | 7.5 | 0.4 | 15.9 |
3.5. Stability, dilution integrity and carryover
Analytes at three QC concentrations in urine samples were stable at room temperature for 24 h, 72 h at 4°C, 72 h on the autosampler at 15°C, and after 3 freeze-thaw cycles (Table 5). Mean percent analyte loss for all analytes was <14.3%.
Table 5.
Mean ±SD stability of mecamylamine, nicotine and nicotine metabolites in urine under different specimen storage conditions
| Storage condition N = 5 |
% Analyte decrease | ||||
|---|---|---|---|---|---|
| Nicotine | Cotinine | OH-Cotininea | Norcotinine Mecamylamine | ||
| 24 h RTb | |||||
| Low | 4.0 ± 6.3 | 2.0 ± 10.2 | 9.2 ± 6.3 | 11.5 ± 3.7 | 1.1 ± 9.4 |
| Medium | 12.3 ± 8.1 | 0.8 ± 6.4 | 0.6 ± 8.8 | 8.9 ± 8.6 | 0.5 ± 1.9 |
| High
|
4.5 ± 3.3 | 3.3 ± 7.7 | 0.5 ± 3.2 | 7.7 ± 5.2 | 0.8 ± 2.8 |
| 72 h 4°C | |||||
| Low | 3.8 ± 7.8 | 14.3 ± 23.5 | 2.2 ± 12.2 | 3.5 ± 4.3 | 2.9 ± 21.1 |
| Medium | 0.9 ± 7.1 | 0.6 ± 5.4 | 1.9 ± 4.4 | 6.4 ± 5.1 | 5.5 ± 14.4 |
| High
|
4.6 ± 6.4 | 1.0 ± 4.9 | 2.0 ± 3.5 | 2.1 ± 3.1 | 0.1 ± 2.3 |
| 72 h 15°C Autosampler | |||||
| Low | 0.4 ± 5.9 | 7.2 ± 12.3 | 0.6 ± 4.8 | 1.4 ± 8.7 | 3.0 ± 8.2 |
| Medium | 1.5 ± 1.8 | 1.4 ± 4.1 | 2.8 ± 7.7 | 0.5 ± 4.5 | 1.3 ± 2.8 |
| High
|
3.4 ± 5.9 | 0.6 ± 4.8 | 0.7 ± 5.4 | 1.7 ± 4.4 | 0.1 ± 2.7 |
| 3 Freeze thaw cycles | |||||
| Low | 10.8 ± 9.6 | 4.0 ± 11.2 | 5.8 ± 19.7 | 2.5 ± 3.3 | 7.0 ± 13.1 |
| Medium | 7.3 ± 5.3 | 1.6 ± 10.3 | 7.4 ± 1.4 | 0.1 ± 5.2 | 4.0 ± 3.8 |
| High | 8.6 ± 4.3 | 3.5 ± 3.7 | 7.1 ± 6.4 | 1.9 ± 2.4 | 0.2 ± 1.4 |
trans-3′-hydroxycotinine,
room temperature
Dilution integrity was acceptable for samples diluted 1:10 with blank urine. All samples fortified with 500 ng/ml mecamylamine and 1000 ng/ml of nicotine, cotinine OH-cotinine and norcotinine diluted 1:10 with blank urine quantified within ±11% of 100 ng/ml for nicotine and metabolites (50 ng/ml for mecamylamine).
There was no evidence of carryover for mecamylamine, nicotine or metabolites during carryover evaluations. None of the negative specimens injected after samples containing twice the upper limit of linearity contained analyte satisfying assay LOQ criteria (n=3).
3.6. Proof of method
The method was applied to the measurement of nicotine, cotinine, OH-cotinine, and norcotinine in urine specimens collected from 10 self-reported tobacco smokers, non-environmentally exposed nonsmokers and nonsmokers environmentally exposed to smoking. Results from one environmentally exposed nonsmoking participant’s urine specimen are shown in panels K–O of Figure 1. Urine nicotine, cotinine, OH-cotinine, and norcotinine concentrations in these specimens ranged from below LOQ to 2260, below LOQ to 1120, below LOQ to 8650, and below LOQ to 267 ng/ml, respectively.
4. DISCUSSION
Our clinical studies evaluating NRT and mecamylamine for smoking treatment required development of a validated sensitive and specific analytical method for simultaneous quantification of nicotine, cotinine, OH-cotinine, norcotinine and mecamylamine in urine. Many factors alter nicotine metabolic rates including age, weight, sex and genetic polymorphisms in nicotine metabolic enzymes [30]; therefore, monitoring nicotine and metabolites during NRT clinical studies provides valuable information. Mecamylamine also is being evaluated in nicotine cessation treatment [12], and inclusion of this analyte in the nicotine and metabolite assay provides cost and time savings. Additionally, urinary nicotine and metabolite concentrations will be assessed during follow-up visits to assess smoking relapse.
Only one method for mecamylamine, nicotine and cotinine exists in the literature [18]. Jacob et al. published a GCMS method employing liquid-liquid extraction (LLE) of nicotine, cotinine and mecamylamine from plasma achieving LOQs of 1, 2 and 2 ng/ml, respectively [18]. We present the first fully validated urine method that includes mecamylamine, nicotine, cotinine, 3-OH-cotinine and norcotinine. SPE of one ml urine prior to positive electrospray ionization LCMSMS analysis produced excellent sensitivity with LOQs of 1, 0.2, 0.5, 1 and 0.1 ng/ml for nicotine, cotinine, OH-cotinine, norcotinine and mecamylamine. Upper limit of linearity was 500 ng/ml for all analytes with the exception of mecamylamine (100 ng/ml). Accuracy was maintained when samples were diluted with blank urine. This is important as urine specimens from active smokers likely contain nicotine and metabolite concentrations exceeding 500 ng/ml requiring dilution. All a priori method validation criteria were met for specificity, sensitivity, linearity, analytical recovery, imprecision, stability and carryover. This method will be useful for high throughput sample analysis since total runtime is only 8 min.
Analyte extraction efficiencies varied with this SPE protocol but overall were similar to previous reports. Our method includes analytes with varying physicochemical properties requiring us to compromise SPE recoveries across compounds. We optimized extraction for moderately polar analytes, cotinine and norcotinine, which were recovered at >90%. Our SPE method is less optimal for nonpolar analytes with extraction efficiencies of 73–84 and 61–95% for nicotine and mecamylamine, respectively. Extraction efficiencies were 55–64% for the most polar analyte, OH-cotinine. Similar to our method, previously reported extraction efficiencies ranged from 80–100% for nicotine, cotinine and norcotinine via LLE [26, 27], SPE [19, 22, 24, 25, 31] or simple centrifugation prior to injection [21, 23]. Although one report achieved 100% extraction efficiency for OH-cotinine [24], most reports document less efficient extraction efficiencies of 53–87%, similar to our observations when a range of analytes were extracted [21–23, 26, 27]. Mecamylamine extraction efficiency from plasma was >90% by LLE [32], while we observed >60% recovery. Our SPE procedure for analytes with varying physicochemical properties produced similar extraction efficiencies to previous reports and yielded clean extracts exhibiting less than 48% matrix effect while achieving our desired limits of quantification.
It is difficult to distinguish active smoking from NRT with urine nicotine testing. One of our goals was to evaluate whether urine concentrations of nicotine, cotinine, OH-cotinine and norcotinine or ratios of these analytes could be employed as biomarkers for distinguishing NRT from active smoking. LCMSMS is routinely employed for quantifying drugs and metabolites in biologic matrices as it avoids costly derivatization required for GCMS analysis and achieves high analyte sensitivity and specificity [33]. Most urine nicotine LCMSMS methods do not simultaneously quantify the spectrum of nicotine analytes included in our method, and no published LCMSMS urine methods include the minor metabolite norcotinine. We hypothesize that norcotinine may be a useful marker for differentiating smoking from NRT. Tuomi et al. achieved LOQ of 10, 2 and 5 ng/ml for nicotine, cotinine and OH-cotinine in their LCMSMS method requiring 1.5 ml urine [22]. Meger et al. performed simple filtration of urine prior to LCMSMS analysis achieving 10 ng/ml LOQs for nicotine, cotinine and OH-cotinine [23]. Another LCMSMS urine method employed SPE prior to analysis with 1, 0.1 and 0.2 ng/ml LOQs for nicotine, cotinine and OH-cotinine [24]. Recently Jacob et al. detailed a highly sensitive urine LCMSMS assay for cotinine and trans-3′-hydroxycotinine (OH-cotinine) with limits of quantification of 0.05 and 0.10 ng/ml, respectively, but did not include nicotine or additional metabolites [27]. Kataoka et al. monitored a more complete panel of nicotine analytes including nicotine, cotinine, nornicotine, anabasine and anatabine in urine via solid phase microextraction prior to LCMS selected ion monitoring analysis with 0.5 ng/ml LOQ [19]. Hoofnagle et al. measured nicotine, cotinine, OH-cotinine, nornicotine, and anabasine in urine, employing centrifugation and direct injection onto LCMSMS with 2 ng/ml LOQs for all analytes except 10 ng/ml for OH-cotinine [21] Our current method achieves similar sensitivity for nicotine, cotinine and OH-cotinine as previously published LCMSMS methods while for the first time including norcotinine and mecamylamine.
This LCMSMS method for simultaneous quantification of nicotine, cotinine, OH-cotinine, norcotinine and mecamylamine in urine will be useful for monitoring compliance during our and others combined NRT and mecamylamine administration smoking treatment studies.
Acknowledgments
The authors gratefully acknowledge Peyton Jacob III and his group from division of Clinical Pharmacology, University of California, for providing mecamylamine-d3. This research was supported by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Substance Abuse and Mental Health Services Administration; Office of Applied Studies. NSDUH Series H-38A. Rockville, MD: Department of Health and Human Services (DHHS); 2010. Results from the 2009 National Survey on Drug Use and Health: National Findings. [Google Scholar]
- 2.Centers for Disease Control and Prevention. Fast Facts. Atlanta, GA: Centers for Disease Control and Prevention; 2011. [Accessed February 9, 2012]. Smoking & Tobacco Use. Available at: http://www.cdc.gov/tobacco/data_statistics/fact_sheets/fast_facts/index.htm. [Google Scholar]
- 3.Yildiz D. Nicotine, its metabolism and an overview of its biological effects. Toxicon. 2004;43:619–632. doi: 10.1016/j.toxicon.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 4.Bergen AW, Caporaso N. Cigarette smoking. J Natl Cancer Inst. 1999;91:1365–1375. doi: 10.1093/jnci/91.16.1365. [DOI] [PubMed] [Google Scholar]
- 5.Lackmann GM, Salzberger U, Tollner U, Chen M, Carmella SG, Hecht SS. Metabolites of a tobacco-specific carcinogen in urine from newborns. J Natl Cancer Inst. 1999;91:459–465. doi: 10.1093/jnci/91.5.459. [DOI] [PubMed] [Google Scholar]
- 6.Henningfield JE, Fant RV, Buchhalter AR, Stitzer ML. Pharmacotherapy for nicotine dependence. CA Cancer J Clin. 2005;55:281–299. doi: 10.3322/canjclin.55.5.281. quiz 322–3. [DOI] [PubMed] [Google Scholar]
- 7.Silagy C, Lancaster T, Stead L, Mant D, Fowler G. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2004;(3):Art. No.: CD000146. doi: 10.1002/14651858.CD000146.pub2. 000110.001002/14651858.CD14000146.pub14651852. [DOI] [PubMed] [Google Scholar]
- 8.Palmer KJ, Buckley MM, Faulds D. Transdermal Nicotine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy as an aid to smoking cessation. Drugs. 1992;44:498–529. doi: 10.2165/00003495-199244030-00011. [DOI] [PubMed] [Google Scholar]
- 9.Sachs DP, Sawe U, Leischow SJ. Effectiveness of a 16-hour transdermal nicotine patch in a medical practice setting, without intensive group counseling. Arch Intern Med. 1993;153:1881–1890. [PubMed] [Google Scholar]
- 10.Westman EC, Levin ED, Rose JE. The nicotine patch in smoking cessation. A randomized trial with telephone counseling. Arch Intern Med. 1993;153:1917–1923. [PubMed] [Google Scholar]
- 11.Lancaster T, Stead LF. Mecamylamine (a nicotine antagonist) for smoking cessation. Cochrane Database Syst Rev. 2000;(2):Art. No.: CD001009. doi: 10.1002/14651858.CD001009. 001010.001002/14651858.CD14001009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glover ED, Laflin MT, Schuh KJ, et al. A randomized, controlled trial to assess the efficacy and safety of a transdermal delivery system of nicotine/mecamylamine in cigarette smokers. Addiction. 2007;102:795–802. doi: 10.1111/j.1360-0443.2007.01763.x. [DOI] [PubMed] [Google Scholar]
- 13.Nemeth-Coslett R, Henningfield JE, O’Keeffe MK, Griffiths RR. Effects of mecamylamine on human cigarette smoking and subjective ratings. Psychopharmacology (Berl) 1986;88:420–425. doi: 10.1007/BF00178502. [DOI] [PubMed] [Google Scholar]
- 14.Stolerman IP, Goldfarb T, Fink R, Jarvik ME. Influencing cigarette smoking with nicotine antagonists. Psychopharmacologia. 1973;28:247–259. doi: 10.1007/BF00429305. [DOI] [PubMed] [Google Scholar]
- 15.Pomerleau CS, Pomerleau OF, Majchrzak MJ. Mecamylamine pretreatment increases subsequent nicotine self-administration as indicated by changes in plasma nicotine level. Psychopharmacology (Berl) 1987;91:391–393. doi: 10.1007/BF00518198. [DOI] [PubMed] [Google Scholar]
- 16.Rose JE, Sampson A, Levin ED, Henningfield JE. Mecamylamine increases nicotine preference and attenuates nicotine discrimination. Pharmacol Biochem Behav. 1989;32:933–938. doi: 10.1016/0091-3057(89)90061-0. [DOI] [PubMed] [Google Scholar]
- 17.Tennant F, Tarver A, Rawson R. Clinical evaluation of mecamylamine for withdrawal from nicotine dependence. Rockville, MD: U.S. Department of Health and Human Services; 1984. [PubMed] [Google Scholar]
- 18.Jacob P, 3rd, Wu S, Yu L, Benowitz NL. Simultaneous determination of mecamylamine, nicotine, and cotinine in plasma by gas chromatography-mass spectrometry. J Pharm Biomed Anal. 2000;23:653–661. doi: 10.1016/s0731-7085(00)00343-5. [DOI] [PubMed] [Google Scholar]
- 19.Kataoka H, Inoue R, Yagi K, Saito K. Determination of nicotine, cotinine, and related alkaloids in human urine and saliva by automated in-tube solid-phase microextraction coupled with liquid chromatography-mass spectrometry. J Pharm Biomed Anal. 2009;49:108–114. doi: 10.1016/j.jpba.2008.09.044. [DOI] [PubMed] [Google Scholar]
- 20.Gabr RQ, Elsherbiny ME, Somayaji V, Pollak PT, Brocks DR. A liquid chromatography-mass spectrometry method for nicotine and cotinine; utility in screening tobacco exposure in patients taking amiodarone. Biomed Chromatogr. 2011;25:1124–1131. doi: 10.1002/bmc.1581. [DOI] [PubMed] [Google Scholar]
- 21.Hoofnagle AN, Laha TJ, Rainey PM, Sadrzadeh SM. Specific detection of anabasine, nicotine, and nicotine metabolites in urine by liquid chromatography-tandem mass spectrometry. Am J Clin Pathol. 2006;126:880–887. doi: 10.1309/LQ8U3UL956ET324X. [DOI] [PubMed] [Google Scholar]
- 22.Tuomi T, Johnsson T, Rejula K. Analysis of nicotine, 3-hydroxycotinine, cotinine, and caffeine in urine of passive smokers by HPLC-tandem mass spectrometry. Clin Chem. 1999;45:2164–2172. [PubMed] [Google Scholar]
- 23.Meger M, Meger-Kossien I, Schuler-Metz A, Janket D, Scherer G. Simultaneous determination of nicotine and eight nicotine metabolites in urine of smokers using liquid chromatography-tandem mass spectrometry. J Chromatogr B. 2002;778:251–261. doi: 10.1016/s0378-4347(01)00451-0. [DOI] [PubMed] [Google Scholar]
- 24.Xu X, Iba MM, Weisel CP. Simultaneous and sensitive measurement of anabasine, nicotine, and nicotine metabolites in human urine by liquid chromatography-tandem mass spectrometry. Clin Chem. 2004;50:2323–2330. doi: 10.1373/clinchem.2004.038489. [DOI] [PubMed] [Google Scholar]
- 25.Hu C-W, Chang Y-Z, Wang H-W, Chao M-R. High-Throughput Simultaneous Analysis of Five Urinary Metabolites of Areca Nut and Tobacco Alkaloids by Isotope-Dilution Liquid Chromatography-Tandem Mass Spectrometry with On-Line Solid-Phase Extraction. Cancer Epidemiol Biomarkers Prev. 2010;19:2570–2581. doi: 10.1158/1055-9965.EPI-10-0483. [DOI] [PubMed] [Google Scholar]
- 26.Marclay F, Saugy M. Determination of nicotine and nicotine metabolites in urine by hydrophilic interaction chromatography–tandem mass spectrometry: Potential use of smokeless tobacco products by ice hockey players. J Chromatogr A. 2010;1217:7528–7538. doi: 10.1016/j.chroma.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Jacob P, III, Yu L, Duan M, Ramos L, Yturralde O, Benowitz NL. Determination of the nicotine metabolites cotinine and trans-3′-hydroxycotinine in biologic fluids of smokers and non-smokers using liquid chromatography–tandem mass spectrometry: Biomarkers for tobacco smoke exposure and for phenotyping cytochrome P450 2A6 activity. Journal Chromatogr B. 2011;879:267–276. doi: 10.1016/j.jchromb.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krouwer JS, Rabinowitz R. How to improve estimates of imprecision. Clin Chem. 1984;30:290–292. [PubMed] [Google Scholar]
- 29.Matuszewski BK, Constanzer ML, Chavez-Eng CM. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal Chem. 2003;75:3019–3030. doi: 10.1021/ac020361s. [DOI] [PubMed] [Google Scholar]
- 30.Hukkanen J, Jacob P, 3rd, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57:79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- 31.Rangiah K, Hwang WT, Mesaros C, Vachani A, Blair IA. Nicotine exposure and metabolizer phenotypes from analysis of urinary nicotine and its 15 metabolites by LC-MS. Bioanalysis. 2011;3:745–761. doi: 10.4155/bio.11.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Debruyne D, Sobrio F, Hinschberger A, Camsonne R, Coquerel A, Barre L. Short-term pharmacokinetics and brain distribution of mecamylamine as a preliminary to carbon-11 labeling for nicotinic receptor investigation. J Pharm Sci. 2003;92:1051–1057. doi: 10.1002/jps.10302. [DOI] [PubMed] [Google Scholar]
- 33.Maurer HH. Advances in analytical toxicology: the current role of liquid chromatography-mass spectrometry in drug quantification in blood and oral fluid. Anal Bioanal Chem. 2005;381:110–118. doi: 10.1007/s00216-004-2774-z. [DOI] [PubMed] [Google Scholar]