Abstract
We estimate cross-sectional associations of neighborhood level disorder, socioeconomic characteristics and social capital with individual level systemic inflammation, measured as high C-reactive protein (CRP), using Boston Metropolitan Immigrant Health & Legal Status Survey (BM-IHLSS) data – a sample of relatively young, healthy foreign born Brazilian adults. Logistic regression analyses suggest high CRP is positively associated with neighborhood disorder and negatively related to neighborhood social capital. Although we find no significant associations between other neighborhood socioeconomic variables and high CRP; males, those who were born in an urban area and those who had been graduated from high school were less likely to have had high CRP. Unauthorized Brazilian adults, those who smoked cigarettes daily and those who had a higher body mass index were more likely to have had high CRP. Our findings suggest that investigating sociogeographic stressors and social support may be important for understanding physiological dysregulation even among relatively healthy U.S. sub populations.
Keywords: Neighborhood, Immigrant, Biomarkers, C reactive protein, Cardiovascular Disease
1. INTRODUCTION
The observation that newcomers to a socioeconomically disadvantaged urban area may be at greater risk of various diseases and all cause mortality was first recorded by John Graunt in the 17th century, and since then many physical and social risk factors have been implicated in this process (Macintyre and Ellaway, 2003). Indeed, the origins of American public health began by acknowledging the need to protect the working class, many of whom were immigrants residing in impoverished neighborhoods, from both environmental and social toxins associated with early and rapid industrialization and urbanization that is at home and work (Duffy, 1992 [1990]; Melosi, 2000). Residents of lower income areas have been shown to be more susceptible to conditions such as psychological distress and depression (Hill et al., 2005; Ross, 2000) obesity (Mujahid et al., 2008) and chronic disease (Cubbin et al., 2001; Murray et al., 2010). The mechanisms linking disadvantage to disease are varied and not always well understood; however, lower income groups that are also disproportionately composed of ethno racial minorities, including immigrants, are more likely to live in residentially segregated neighborhoods (Acevedo-Garcia and Lochner, 2003; Massey and Denton, 1998), areas of higher crime and with greater alcohol and fast food outlet density (Block et al., 2004; Cohen et al., 2008; Kwate et al., 2009), as well as areas with less access to municipal services like recreational facilities or walkable sidewalks that may promote health (Cubbin et al., 2001; Holmes and Marcelli, 2011; Lovasi et al., 2009a). Most studies demonstrating these links rely on measures of individual income, often aggregated to a “neighborhood” or local area boundary, to define associations between illness and disadvantage; however, those that have instead constructed measures of neighborhood level SES have similarly found material deprivation to be associated with poor health outcomes (Bird et al., 2009; Merkin et al., 2009).
More recently and increasingly, neighborhood socioeconomic disadvantage has been linked to cardiometabolic disease (Augustin et al., 2008; Cubbin et al., 2001; Cubbin et al., 2006; Diez-Roux et al., 2001; Murray et al., 2010) and cumulative biological risk for disease (Bird et al., 2009; Finch et al., 2010; Merkin et al., 2009). However, much less research exists on more specific links between neighborhood sociogeographic factors and physiological mechanisms that may be important for predicting disease onset (Buxton and Marcelli, 2010). Chronic systemic (as opposed to acute) inflammation has emerged as a potentially decisive risk factor for the development of cardiometabolic disease, and validation of high sensitivity assays for inflammatory markers like C reactive protein (CRP) have made it possible to measure inflammation in population based studies (McDade et al., 2004). CRP – an acute phase protein produced in the liver in response to pro inflammatory cytokines – has become a particularly important marker of inflammation as well as an accurate predictor of cardiovascular disease and mortality (McDade et al., 2006; Ridker, 2001; Rifai and Ridker, 2001). Furthermore, recent research has identified psychosocial factors that may be associated with inflammatory processes, such as chronic and acute stressors, social support and poor psychological health (McDade et al., 2006; Ranjit et al., 2007; Uchino, 2006). Yet only a handful of studies have investigated relationships between neighborhood sociogeographic environments, in which stressors may be generated and social support forged, and inflammation (Liang et al., 2008; Nazmi et al., 2010; Petersen et al., 2008; Schootman et al., 2010).
Furthermore, most population based research investigating associations between cardiometabolic risk factors (including systemic inflammation) and neighborhood characteristics has included study populations with mean ages over 50. These populations exhibit a greater variety of risk factors and higher disease prevalence than populations with wider age distributions, and such data limit researchers’ ability to study the etiology of chronic disease onset. In order to better understand how risk factors for cardiometabolic disease develop it is essential to study relatively young, healthy populations when researching sociogeographic environments and physiological health. By doing so it is possible to gain greater insight into: (1) how risk factors accumulate across the life course and lead to disease onset in older populations, and (2) opportunities for prevention earlier in life or later intervention. In this paper we employ data from the Boston Metropolitan Immigrant Health & Legal Status Survey (BM IHLSS) – a random household sample of adult Brazilian migrants who resided in the Boston metropolitan area in 2007 – to estimate linkages between systemic inflammation as measured by CRP, and its relationship to economic, physical and social aspects of the local environment.
While the experience of systemic inflammation among Brazilian migrants in the Northeastern United States may not be fully generalizeable to the U.S. population as a whole, it is particularly compelling to study inflammation in this group for several reasons: first, as is the case with Latin American migrant populations more generally, Brazilian migrants are younger and healthier on average than the U.S. population (Marcelli et al., 2009a) therefore providing an opportunity to potentially discern processes by which risk factors for cardiometabolic disease develop into chronic illness. Second, whereas most research on migrant health focuses on migrants with relatively low socioeconomic status with low proportions of unauthorized residents, Brazilian migrants have notably high levels of educational attainment, earnings and unauthorized residents. Thus, although Brazilians are similar to many other U.S. migrants in terms of being recent arrivals and can be expected to face many of the same barriers or hurdles, this is uncertain due to their unique SES and legal status profile (Marcelli et al., 2009a; Margolis, 1998). Brazilian migrants have also lived in their neighborhoods for shorter amounts of time than their neighbors in many cases, and may have a different experience of these neighborhoods depending on migration related stressors. For example, some of the neighborhoods included in the BM IHLSS contained a multitude of Brazilian owned businesses, conceivably easing settlement for new residents, while others were situated near an Immigration and Customs Enforcement (ICE) office, a potentially threatening environment. This suggests another reason for studying Brazilian migrants – as the migrant population with the largest proportion of unauthorized residents in the United States (71 percent in the BM IHLSS), Brazilians may have particular exposure to migration related stressors, such as deportation fears or cultural differences with neighbors, and this stress may in turn have an effect on physiological regulation, such as inflammatory processes (Marcelli et al., 2009a). Finally, the BM IHLSS is the only area level probabilistic U.S. household survey to date that includes data on legal status, various sociogeographic life domains and bioindicators of health.
In this paper we thus hypothesize that migrants residing in neighborhoods with higher levels of disorder (i.e., violence, theft, property damage) and lower levels of social capital (i.e., neighbors getting along, helping one another, sharing the same values, knowing one another and not being afraid to go out at night) will be more likely to experience systemic chronic inflammation in the form of high C reactive protein (CRP). We propose to test this hypothesis in sociogeographic context, i.e. treating neighborhood as one important domain in the larger social environment in which people are engaged, and with an eye to how neighborhood characteristics interact with individual behavior and characteristics, such as legal status, to influence systemic inflammation. As there is no commonly agreed upon definition of “neighborhood” in either the health literature or more generally, the BM IHLSS relied on subject perceptions of what constituted their neighborhoods for measures of disorder and social capital (Coulton et al., 2001; Sawicki and Flynn, 1996; Weiss et al., 2007), and we have further linked these data to 2000 Census block level data as we describe further in the next section.
2. DATA AND METHODS
The Boston Metropolitan Immigrant Health & Legal Status Survey (BM IHLSS) was a community based biodemographic research (CBBR) project designed and carried out in the Boston Cambridge Quincy, MA NH Metropolitan Statistical Area (BCQ MSA) in 2007 (Marcelli and Buxton, 2010; Marcelli and Heer, 1997; Marcelli et al., 2009a; Marcelli et al., 2009b; Minkler and Wallerstein, 2003). To our knowledge the BM IHLSS is the first random household survey to collect both legal status and biological data from any foreign born population in the United States. It builds on the 1994 Los Angeles County Mexican Immigrant Legal Status Survey (Marcelli and Heer, 1997) and subsequent studies that have either employed legal status information from the 1994 LAC MILSS or adopted a variant of its legal status questions (Brown and Yu, 2002; Goldman et al., 2005; Ortega et al., 2007; Passel and Clark, 1998). Participants were randomly selected from 10 BCQ MSA census tracts in which at least seven percent of the population was born in Brazil. According to 2005 2009 American Community Survey data, the BCQ MSA is home to the second largest population of foreign born Brazilians in the United States (after the New York Northern New Jersey Long Island MSA) and this population has grown by 87 percent between 2000 and 2009 (U.S. Census Bureau, 2001, 2010). Data regarding migration and legal status, socioeconomic status, social capital, neighborhood characteristics and self reported health behavior and conditions were collected from 307 foreign born Brazilian adults and 120 of their U.S. and foreign born children. In addition, 249 of the Brazilian adult subjects (81 percent) provided biological data in the forms of height, weight and blood pressure measurements, and 176 subjects (57 percent) consented to providing blood droplets from which CRP, glycated hemoglobin (HbA1c) and Epstein Barr Virus (EBV) measurements have been obtained.
Specifically, 12 teams of two interviewers who were born in Brazil were trained by an interdisciplinary team of researchers from Harvard University and the University of Massachusetts Boston in collaboration with the Brazilian Immigrant Center. Teams approached more than 4,000 randomly selected households located in 100 randomly chosen census blocks up to three times, and after recording a subject’s self reported answers an interviewer asked whether the subject would agree to have his or her weight, height and blood pressure measured; to provide saliva by inserting a cotton swab into his or her mouth; and to permitting the interviewer to collect five blood droplets using the conventional finger prick technique (Christensen, 2000; Jaszczak et al., 2009; McDade et al., 2007). Individual sample weights were computed using the conventional method of dividing one by the probability of random selection at each level (i.e., census tract, census block, housing unit, individual migrant). More detailed information about the BM IHLSS study design and objectives is available from two peer reviewed research reports (Marcelli et al., 2009a; Marcelli et al., 2009b).
Measures
High-sensitivity C-Reactive Protein (hsCRP)
Blood samples were collected by trained interviewers in the homes of randomly selected subjects using disposable lancets and standardized filter paper. These were stored as dried blood spots (DBS) at 80° C in Brigham and Women’s Hospital Biomarker and Actigraphy Data Coordinating Center. After completion of BM IHLSS fieldwork, the DBS were sent to the Laboratory for Human Biology Research at Northwestern University where high sensitivity enzyme immunoassays for CRP were conducted. This method has been validated; the hsCRP concentrations obtained from DBS were found to be very similar to those obtained from blood serum, and DBS are an effective means of storing blood samples in population based surveys for which laboratory examinations are not feasible (Finch et al., 2000; McDade et al., 2004). A regression equation is employed (plasma = 2.3372xDBS + 0.0778) to convert the DBS values of hsCRP, which are lower than serum values as a result of lysed erythrocytes in the sample, to serum values (McDade, 2010). This is done in order to utilize recommended clinical risk thresholds for hsCRP, based on serum measures, in order to delineate high CRP (>3 mg/L) from moderate (1 to 3 mg/L) and low (<1 mg/L) values (Pearson et al., 2003). Some clinicians have suggested that values of hsCRP exceeding 10 mg/L may indicate nonspecific inflammation, thereby making higher levels less useful for predicting cardiovascular disease; however, Ridker and Cook (2004) have found that even very high levels of hsCRP (>10 mg/L) provide important diagnostic information. Therefore, the outcome variable we employ (high CRP) is a dichotomous variable indicating whether a subject had high CRP (hsCRP levels > 3 mg/L and ≤20 mg/L). We use a cutoff of 20 mg/L in order to exclude active infection and retain as many subjects as possible who may be at cardiovascular risk (Ledue and Rifai, 2001). Because no studies or data of which we are aware include information on the distribution of CRP levels among foreign born Brazilian migrant adults, the BM IHLSS CRP data were compared, in separate analyses, to CRP levels reported in the 2005 2008 National Health and Nutrition Examination Survey (NHANES) data for the total U.S. adult population and foreign born Latin American adults. NHANES utilizes a measure of CRP rather than hsCRP, and CRP levels>1 mg/dL are generally considered to be high (Visser et al., 1999). According to the NHANES data, the frequency of high CRP among U.S. adults ages 18 64 is approximately 18 percent (Centers for Disease Control and Prevention, 2010), which is similar to the 22 percent found in the BM IHLSS Brazilian adult sample.
Neighborhood environment
Neighborhood factors that may be independently associated with high CRP are measured using self reported responses to questions about various neighborhood characteristics, and relying on subjects’ own definitions of “your neighborhood.” The BM IHLSS data are further linked to 2000 Census Summary File 1 (SF1) data to obtain measures of socioeconomic status and population composition. The census data are estimated at the block level as this provides the most proximate local neighborhood geography and encompasses features that residents may encounter as part of their daily activities. The first three neighborhood measures, population density per square mile (POPULATION DENSITY), percent minority residents (% MINORITY) and percent homeownership (% OWNERSHIP), are continuous and measured at the block level using the SF1 data. Each of these variables is often used to assess neighborhood socioeconomic characteristics and stability: population density and the concentration of nonwhite populations have commonly been used as proxies for the neighborhood socioeconomic environment under the assumption that areas higher in density and with larger minority populations are more likely to be lower income, although the opposite relationship has been found in some studies of health behavior and the built environment (Gordon-Larsen et al., 2006; Lovasi et al., 2009b; Rohe and Stewart, 1996). Similarly for homeownership, the greater the rate of homeownership the wealthier and more stable the neighborhood is generally expected to be (Ross and Mirowsky, 2001). Neighborhood disorder (DISORDER) is measured using a dummy variable indicating whether respondents or their neighbors had experienced personal violence, had their homes broken into, had experienced property damage, or had property stolen from them. Neighborhood “social capital” (SOCIAL CAPITAL) is an index (0 5) based on whether subjects agreed that their neighborhood is safe at night, and that their neighbors know each other, get along with one other, share similar values, and are willing to help each other (Sampson and Raudenbush, 1999). Three of the five components constituting our social capital index are clearly suggestive of positive social relations (i.e., safety, getting along, willingness to help), and thus we assumed that higher scores indicated higher subject assessed social capital. In other words, we acknowledge that the remaining two component variables sharing values and knowing one another may represent healthful or harmful social interactions depending on the characteristics of network members (Portes, 1998). Finally, we control for length of residence, in a subject’s current neighborhood.
Individual socioeconomic characteristics include dichotomous variables indicating whether a subject was married (MARRIED), completed high school (HS GRADUATE), was currently employed (EMPLOYED), reported speaking English “very well” (ENGLISH) and was unauthorized to reside in the USA (UNAUTHORIZED). A continuous measure of total individual earnings for 2006 (the year prior to the survey) is also included (EARNINGS). Individual socioeconomic status, most often measured by years of education, income and occupation, has repeatedly been linked to health outcomes, as has marital status. For migrants, English speaking ability is a measure of integration, and poor English skills may be a source of psychosocial stress (Padilla et al., 1998). Furthermore, unauthorized legal status may be an important stressor that is unique to foreign born populations (Marcelli, 2004), and according to estimates from the BM IHLSS, 71 percent of Brazilian migrant adults in New England were unauthorized in 2007 (Marcelli et al., 2009a), a figure nearly 20 percent higher than most estimates of the unauthorized Mexican migrant population in the United States (Passel, 2006).
Health status and behaviors
Markers of health status and behaviors that may be independently associated with inflammation are measured using self reported responses to questions about psychological health and behavior as well as height, weight and blood pressure measurements and two additional biomarkers obtained from DBS assays (HbA1c and Epstein Barr Virus). Serious psychological distress (DISTRESS) is defined as scoring a value of 13 or above using a well known distress scale (the K6 scale) which ranges from 0 24; the scale is based on six questions with a 5 point Likert response, and gauges clinical and subclinical psychological illness in population based studies (Kessler et al., 2002). Psychological distress has been cited as a risk factor for cardiovascular disease and linked to increases in inflammation (Black, 2002; Hamer et al., 2008; Stansfeld et al., 2002). Body mass index (BMI) is a continuous variable calculated from height and weight measurements taken at the time of interview. HbA1c and Epstein Barr Virus (EBV) are continuous measures of glycated hemoglobin and EBV antibodies obtained from DBS assays. Hypertension is a dichotomous variable indicating whether subjects had systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg or had ever been told by a doctor that they had high blood pressure (Marcelli and Holmes, 2012). With respect to health behavior, nutrition was evaluated using a dichotomous variable indicating whether subjects consumed five or more servings of fruits and vegetables each day on average, physical activity was assessed according to whether subjects engaged in moderate (20 minutes) or vigorous (30 minutes) “physical activity” during the preceding week, and smoking was measured according to whether subjects had smoked at least 100 cigarettes in their lifetime and currently smoked every day. Finally, sleep debt is a continuous measure indicating the difference between usual hours of sleep on non work days and work days. Sleep deficiency, nutrition, smoking, obesity, high levels of glycated hemoglobin (Hba1c) and high blood pressure are all demonstrated risk factors for inflammation and cardiovascular disease (Buxton and Marcelli, 2010). Physical activity, alternatively, appears to have protective effects with respect to systemic inflammation (Ford, 2002).
Individual exogenous characteristics were captured by measuring age in years since birth, skin color – self reported according to a scale numbered 1 10 where 1 corresponds with the lightest pigmentation and 10 corresponds with the darkest (Massey and Martin, 2003) – and dichotomous variables for sex and whether the subject was born in an urban area in Brazil. Women, blacks and Latinos have been shown to be more likely to have high CRP than other groups (Albert et al., 2004; Araújo et al., 2004; Petersen et al., 2008; Winkleby et al., 1998; Woloshin and Schwartz, 2005). We also controlled for urbanicity in subject place of birth because more urbanized areas typically include residents who are of a higher socioeconomic status, are less likely to expose children to infection or other pro inflammatory conditions, and offer access to established health care infrastructures (Crimmins and Finch, 2006; Vlahov et al., 2005). Descriptive and multivariate regression results for cross sectional analyses are reported below. STATA 10’s “logit” command was used to perform all logistic regressions. Bias that may occur as a result of multiple respondents living in the same census blocks was controlled using STATA’s “cluster” function (Huber, 1967). Three models were fitted: Model 1 controls for neighborhood characteristics and length of neighborhood residence along with individual exogenous characteristics, Model 2 adjusts for individual socioeconomic characteristics and Model 3 controls for all listed variables in addition to health conditions and behaviors. Logistic regression is employed in this analysis for two related reasons: 1) CRP distributions are not uniform across population groups and have been shown to differ between foreign and U.S. born populations in the United States (Centers for Disease Control and Prevention (CDC), 2010; Crimmins et al., 2007); and 2) it follows that employing standard cutoffs for high, normal and low CRP levels may provide the most useful comparison between Brazilian migrants and the rest of the U.S. population. In this paper we focus on high CRP using the clinical threshold of greater than 3 mg/L with an upper bound of 20 mg/L.
3. RESULTS
Of the 176 DBS samples collected from adult Brazilian BM IHLSS subjects, CRP data are available for 157 (89 percent). The analyses are further restricted to the 151 subjects who had CRP readings less than or equal to 20 mg/L to exclude those who may be experiencing active infection. Subjects resided in 52 census blocks across the BCQ MSA, with a mean of three per block. Seventy-three percent of these participants were estimated to have been unauthorized migrants, which is the same as the estimated 73 percent of unauthorized migrants found in the entire Brazilian adult sample. Descriptive statistics are detailed in Table 1 below.
Table 1.
Descriptive Statistics (Weighted)
| All Adults μ (S.D.) |
CRP≤3mg/L μ (S.D.) |
CRP>3mg/L & ≤20mg/L μ (S.D.) |
||
|---|---|---|---|---|
| OUTCOME VARIABLE | ||||
| High CRP | High CRP=1 if hsCRP< 3.0mg/L & hsCRP<=20mg/L (converted from DBS to serum values) | 0.22 | 0.00 | 1.00 |
| - | - | - | ||
| hsCRP (mg/L) | Levels of hsCRP (converted from DBS to serum values) | 2.46 | 1.11 | 7.39 |
| 3.38 | 0.66 | 4.55 | ||
| NEIGHBORHOOD CHARACTERISTICS | ||||
| Length of residence (−) | Number of years and months subject has resided in neighborhood | 2.46 | 2.26 | 3.18 |
| 2.49 | 2.07 | 3.60 | ||
| Population density (+/−) | Number of residents per square mile by census block (mean in 10,000s) | 23287.70 | 24235.28 | 19833.97 |
| 14843.61 | 14181.56 | 16834.99 | ||
| % Minority (+) | Percent of non-white residents by census block | 0.40 | 0.41 | 0.36 |
| 0.20 | 0.20 | 0.20 | ||
| % Ownership (−) | Percent of residents who own their homes by census block | 0.35 | 0.32 | 0.43 |
| 0.20 | 0.18 | 0.24 | ||
| Disorder (+) | Disorder=1 if subject or neighbors experienced personal violence, had their homes broken into, had anything stolen from their property or experienced damage to their personal property in the neighborhood |
0.29 | 0.26 | 0.41 |
| - | - | - | ||
| Social capital (−) | Index from 0-5 indicating to what extent the subject agrees or strongly agrees that the neighborhood is 1) safe at night, and neighbors 2) know each other, 3) get along, 4) share values and 5) help each other |
3.00 | 2.96 | 3.15 |
| 1.49 | 1.49 | 1.50 | ||
| INDIVIDUAL EXOGENOUS CHARACTERISTICS | ||||
| Age (+) | Subject age in years | 33.55 | 33.03 | 35.48 |
| 9.56 | 9.21 | 10.70 | ||
| Male (−) | Sex=1 if subject reported sex as male | 0.59 | 0.65 | 0.35 |
| - | - | - | ||
| Skin color (+) | Self-reported subject skin color, measured from lightest (1) to darkest (10) | 2.18 | 2.29 | 1.81 |
| 1.36 | 1.44 | 0.92 | ||
| Urban born (+/−) | Urban born=1 if subject was born in an urban area in Brazil | 0.68 | 0.71 | 0.55 |
| - | - | - | ||
| INDIVIDUAL SOCIOECONOMIC CHARACTERISTICS | ||||
| Married (−) | Married=1 if subject was married at time of survey | 0.54 | 0.51 | 0.65 |
| - | - | - | ||
| HS graduate (−) | HS graduate=1 if subject graduated high school | 0.80 | 0.85 | 0.64 |
| - | - | - | ||
| Employed (−) | Employed=1 if subject worked last week | 0.91 | 0.91 | 0.88 |
| - | - | - | ||
| Earnings (−) | Subject earnings from all jobs in 2006 (Thousands of dollars) | 33.83 | 35.40 | 28.08 |
| 24.41 | 25.40 | 19.66 | ||
| English (−) | English=1 if subject speaks English “very well” | 0.30 | 0.32 | 0.23 |
| - | - | - | ||
| Unauthorized (+) | Unauthorized=1 if subject is unauthorized to reside in the USA | 0.73 | 0.75 | 0.66 |
| - | - | - | ||
| INDIVIDUAL HEALTH STATUS & BEHAVIOR | ||||
| Distress (+) | Distress=1 if subject’s K6 score>12, indicating serious psychological distress | 0.08 | 0.05 | 0.18 |
| - | - | - | ||
| BMI (+) | Body mass index | 25.82 | 25.53 | 26.86 |
| 3.72 | 3.41 | 4.61 | ||
| HbA1c (+) | Glycated hemoglobin level (continuous) | 4.80 | 4.76 | 4.94 |
| 0.63 | 0.53 | 0.90 | ||
| Hypertension (+/−) | Hypertension=1 if subject has measured hypertension or has ever been diagnosed with hypertension |
0.07 | 0.08 | 0.03 |
| - | - | - | ||
| EBV (+) | Epstein-Barr Virus antibody level (continuous) | 90.50 | 88.80 | 96.71 |
| 66.11 | 65.14 | 70.23 | ||
| Nutrition (−) | Nutrition=1 if subject consumes an average of five or more fruits/vegetables per day | 0.20 | 0.19 | 0.27 |
| - | - | - | ||
| Physical activity (−) | Physical activity=1 if subject engaged in moderate (20 min) or vigorous (30 min) exercise at least one day during the previous week |
0.27 | 0.30 | 0.16 |
| - | - | - | ||
| Smoking (+) | Smoking=1 if subject smokes every day | 0.17 | 0.14 | 0.27 |
| - | - | - | ||
| Sleep (+) | Difference between self-reported non-workday and workday sleep hours | 1.23 | 1.17 | 1.44 |
| 1.69 | 1.62 | 1.92 | ||
| N (Weighted) | 29,708 | 23,312 | 6,396 | |
| N (Unweighted) | 151 | 119 | 32 | |
Difference in means is statistically significant, p<0.05
As the continuous measure of hsCRP (CRP2) in Table 1 illustrates, the mean level of hsCRP among Brazilian migrant adults residing in the BCQ MSA at the time of the BM IHLSS was 2.5 mg/L – within the moderate risk range. Altogether, approximately one in five (22 percent) Brazilian migrant adults residing in the BCQ MSA at the time of the BM IHLSS are estimated to have had high CRP. Consistent with past research, those with high CRP were slightly older than those with lower CRP levels (35 versus 33 years of age) and a higher proportion were women (approximately two thirds versus 35 percent). Surprisingly, Brazilians with high CRP had lighter skin color on average (1.8 versus 2.3), but this may simply be an artifact of Brazilian migrants residing in the BCQ MSA having relatively light skin pigmentation (2.2 on a scale of 1 to 10). In terms of individual socioeconomic characteristics, a higher proportion of migrants among those with high CRP were married (65 versus 51 percent); and lower proportions were employed (88 versus 91 percent), completed high school (64 versus 85 percent), reported speaking English “very well” (23 versus 32 percent) and surprisingly unauthorized to reside in the USA (66 versus 75 percent).
Higher proportions of Brazilian migrants with high CRP also smoked (27 versus 14 percent), appear to have been distressed (18 versus five percent), experienced sleep debt (1.4 versus 1.2 hours/week), and had higher BMI (26.9 versus 25.5), HbA1c (4.9 versus 4.8) and EBV (97 versus 89) levels. Conversely, a lower proportion of those with high CRP engaged in regular physical activity (16 versus 30 percent) and a higher proportion were likely to consume five servings of fruits and vegetables each day on average (27 versus 19 percent). Migrants with high CRP were also less likely to have had high blood pressure (three versus eight percent), which may seem antithetical as both CRP and hypertension are risk factors for cardiovascular disease. Research has demonstrated, however, that blood pressure and CRP are independent markers of disease (Blake et al., 2003) and do not necessarily have a causal relationship (Smith et al., 2005).
All of the variables in Table 1 discussed thus far are controls in our model factors that may be independently associated with high CRP and confound any estimated relationship between neighborhood environment and high CRP if ignored. Among our main variables of interest, those individuals who had high CRP resided in their neighborhoods a slightly longer period of time on average (3.2 versus 2.3 years), and resided in less densely populated neighborhoods (approximately 20,000 versus 24,000 people per square mile) with smaller proportions of ethno racial minorities (36 versus 41 percent) but higher proportions of homeowners (43 versus 32 percent). Lastly, although migrants with high CRP also ranked their apparently higher socioeconomic status neighborhoods as having had more social capital on average (3.2 versus 3.0 on 0-5 index), they also were more likely to report neighborhood disorder (41 versus 26 percent). Table 2 shows the parameter coefficients estimated from logistically regressing high CRP on all the variables listed in Table 1. Hypothesized directional associations between high CRP and each explanatory variable are indicated by a plus (+) or minus (−) sign following each variable name. A two tailed hypothesis employed when the relationship between an explanatory variable and high CRP is theoretically ambiguous (e.g., population density, being born in an urban area) is demarcated by a both positive and a negative sign (+/−). We report parameter coefficients and convert these into changes in the probability that a migrant had high CRP as a result of a one unit (for dichotomous explanatory variables) or one standard deviation (for continuous explanatory variables) change for two straightforward reasons. First, as is customary in economics, we prefer to show estimated associations explicitly rather asking readers to infer them from odds ratios. And second, regardless of how estimated relationships are reported, most researchers eventually employ the language of probability when discussing regression results (Buxton and Marcelli, 2010; Studenmund, 2001).
Table 2.
Logistic Regression of High CRP on Neighborhood and Other Factors
| Model 1 |
Model 2 |
Model 3 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ß | S.E. | Prob. | ß | S.E. | Prob. | ß | S.E. | Prob. | O.R. | |
| NEIGHBORHOOD CHARACTERISTICS | ||||||||||
| Length of residence (−) | 0.023 | (0.089) | 0.97% | 0.066 | (0.097) | 2.77% | 0.076 | (0.103) | 3.20% | 1.079 |
| Pop. density (+/−) | −0.00002 | (0.000) | −4.21% | −0.000004 | (0.000) | −1.08% | −0.00003 | (0.000) | −7.80% | 1.000 |
| % Minority (+) | −0.504 | (1.459) | −1.69% | −0.672 | (1.719) | −2.25% | −0.645 | (1.606) | −2.16% | 0.525 |
| % Ownership (−) | 2.268 | (1.381) | 7.52% | 3.097 | (1.564) | 10.27% ** | 1.775 | (1.614) | 5.89% | 5.901 |
| Disorder (+) | 0.666 | (0.544) | 11.26% | 0.735 | (0.559) | 12.41% * | 1.122 | (0.532) | 18.96% | 3.072 ** |
| Social capital (−) | −0.037 | (0.185) | −0.93% | −0.133 | (0.205) | −3.34% | −0.292 | (0.221) | −7.35% | 0.746 * |
| INDIVIDUAL CHARACTERISTICS | ||||||||||
| Age (+) | 0.013 | (0.024) | 2.05% | 0.026 | (0.025) | 4.12% | −0.013 | (0.032) | −2.10% | 0.987 |
| Male (−) | −1.284 | (0.563) | −21.69% ** | −1.173 | (0.710) | −19.82% ** | −1.839 | (0.801) | −31.07% | 0.159 ** |
| Skin color (+) | −1.294 | (0.220) | −6.74% | −0.409 | (0.231) | −9.38% | −0.549 | (0.239) | −12.60% | 0.577 |
| Urban born (+/−) | −1.015 | (0.454) | −17.15% ** | −1.248 | (0.570) | −21.08% ** | −1.691 | (0.572) | −28.56% | 0.184 *** |
| INDIVIDUAL SOCIOECONOMIC CHARACTERISTICS | ||||||||||
| Married (−) | 0.103 | (0.543) | 1.74% | 0.351 | (0.562) | 5.93% | 1.421 | |||
| HS graduate (−) | −1.709 | (0.555) | −28.88% *** | −2.313 | (0.678) | −39.07% | 0.099 *** | |||
| Employed (−) | −0.448 | (0.819) | −7.57% | −1.392 | (1.078) | −23.52% | 0.249 * | |||
| Earnings (−) | −0.033 | (0.018) | −13.51% ** | −0.017 | (0.019) | −7.07% | 0.983 | |||
| English (−) | −0.917 | (0.557) | −15.49% ** | −1.316 | (0.531) | −22.23% | 0.268 *** | |||
| Unauthorized (+) | 1.044 | (0.543) | 17.64% ** | 1.265 | (0.595) | 21.38% | 3.544 *** | |||
| HEALTH STATUS & BEHAVIOR | ||||||||||
| Distress (+) | 2.145 | (1.016) | 36.24% | 8.544 ** | ||||||
| BMI (+) | 0.161 | (0.077) | 10.16% | 1.175 ** | ||||||
| HbA1c (+) | 0.225 | (0.416) | 2.39% | 1.253 | ||||||
| Hypertension (+/−) | −0.757 | (1.449) | −12.79% | 0.469 | ||||||
| Epstein-Barr Virus (+) | −0.003 | (0.006) | −2.90% | 0.997 | ||||||
| Nutrition (−) | −0.022 | (0.723) | −0.38% | 0.978 | ||||||
| Physical activity (−) | 0.357 | (0.758) | 6.04% | 1.429 | ||||||
| Smoking (+) | 1.570 | (0.626) | 26.53% | 4.809 *** | ||||||
| Sleep debt (+) | 0.030 | (0.175) | 0.85% | 1.030 | ||||||
| Constant term (+/−) | −0.330 | (1.719) | 1.294 | (2.047) | −0.172 | (2.556) | ||||
| Concordant Pairs | 0.804 | 0.794 | 0.813 | |||||||
| Prob > chi2 | 0.012 | 0.000 | 0.000 | |||||||
| Pseudo R2 | 0.170 | 0.255 | 0.362 | |||||||
p≤.10
p≤.05
p≤.01
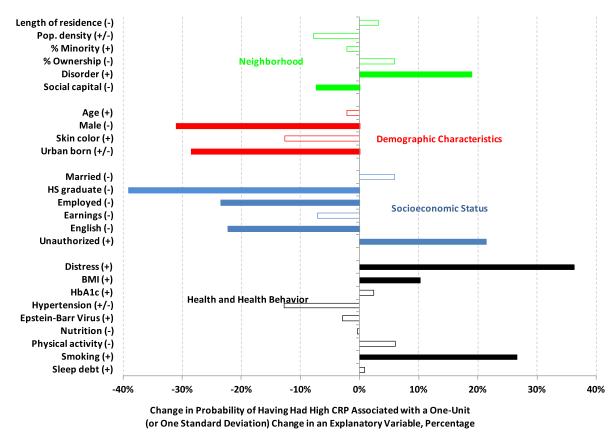
In our final model, which controls for both individual socioeconomic status and health, we estimate that two neighborhood level factors were significantly associated with high CRP among adult Brazilian migrants in metropolitan Boston during the summer of 2007. First, those residing in a neighborhood characterized as disordered were 19 percent more likely to have had high CRP than those who did not. Second, those who reported higher neighborhood social capital were seven percent less likely to have had high CRP. Three more conventional explanatory variables for area level socioeconomic status – minority composition, population density and the rate of homeownership – do not appear to have been significantly related to high CRP. Statistically significant changes in the probability of having had high CRP are illustrated in Figure 1 by the filled bars, and the empty bars represent variables that were included in our final model but are not significant.
Figure 1.
Change in the probability of having high CRP
Consistent with the literature, Brazilian migrant men are significantly and substantially (31 percent) less likely to have high CRP than Brazilian migrant women. Age and skin color, on the other hand, do not appear to be independently associated with high CRP. This is not surprising, however, given that this population is relatively young and white. Additionally, in separate analyses employing categorical measures of race akin to those used by the U.S. Census Bureau, no significant associations were found. Alternatively, subjects who were born in an urban area of Brazil, what we consider a crude proxy for having grown up in an environment with relatively more resources, were almost 30 percent less likely to have high CRP.
We also find that those who were graduated from high school were 39 percent less likely to have had high CRP, those who spoke English very well were 22% less likely and those who were residing in the United States illegally were 21 percent more likely. These relationships remain statistically significant in our third model, which controls for health behaviors and status, having been employed also becomes significant and only one of our remaining four individual level socioeconomic status variables (being married) had a different association with CRP than hypothesized, i.e. it was positively associated. However, little is yet known about the effects of spousal relationships on inflammation specifically, and the quality of marital relationships is likely as important for predicting outcomes such as high CRP as the state of being married itself (Kiecolt-Glaser et al., 2010).
Finally, three of our nine health behavior and health status variables are estimated to be positively and independently associated with high CRP (psychological distress, a higher height adjusted body weight and smoking), and only two are not signed as anticipated; physical activity had a positive association with high CRP while Epstein Barr Virus had a negative association. Brazilian migrant adults whose BMI was measured to be about four points higher had about a 10 percent higher probability of having had high CRP, those who smoked cigarettes daily were 27 percent more likely and those experiencing serious psychological distress were 36% more likely.
4. DISCUSSION
Only a few existing studies have examined relationships between inflammation and neighborhood factors (Petersen et al., 2008; Schootman et al., 2010), and only one of which we are aware has gone beyond conventional neighborhood socioeconomic (SES) characteristics to investigate other sociogeographic aspects of local environments that may be linked to high CRP (Nazmi et al., 2010). We report new evidence from 2007 Boston Metropolitan Immigrant Health & Legal Status (BM IHLSS) data that neighborhood level disorder and social capital are associated with high CRP among Brazilian migrant adults residing in the Boston Cambridge Quincy metropolitan statistical area. These two factors, furthermore, are estimated to be particularly salient predictors of high CRP even though more conventional area level SES indicators such as home ownership and ethnoracial diversity are included in our models. And they are estimated to be increasingly important once individual level SES and health variables were included in our model.
That neighborhood level environmental factors remain significantly associated with high CRP in our model even after controlling for individual health and SES variables may provide further evidence that psychosocial and environmental stressors have a particular impact on inflammation. As noted above, neighborhood disorder has repeatedly been found in public health literature to be associated with increased psychological distress, and distress - which is highly significant in our analyses - has been linked to high CRP and inflammation more generally. Lack of social support has additionally been linked to compromised physiological function, and our finding regarding the relationship between low neighborhood social capital and high CRP may offer more evidence of this phenomenon (Kiecolt-Glaser et al., 2010; Uchino, 2006). As these are cross sectional data; however, the true relationships between these measures and chronic inflammation may be obscured.
We should like to note from Table 2 that higher neighborhood disorder and social capital become significant only after controlling for individual level socioeconomic status and health related factors. One might expect, for instance, that individual SES and health behaviors would mediate any observed associations between neighborhood factors and inflammation, but they instead appear to clarify these relationships. In other words, Model 1 likely suffers from omitted variable (specification) bias as systemic inflammation is not an isolated biological process. Incorporating individual socioeconomic and health related variables into our model not only provides an estimate of how these are directly associated with high CRP, but also elucidates how neighborhood environment and inflammation are related by placing the latter in context of individual risk factors. For example, obese individuals have been found to exhibit chronic systemic inflammation; thus, controlling for BMI should allow for a clearer picture of whether neighborhood environment influences CRP directly or through pathways not proxied in our model (Visser et al., 1999). Likewise, inflammation is a process tied to infection; thus, accounting for EBV levels as an indicator of generalized infection likely crystallizes any relationships between CRP and other explanatory variables.
The negative association between being born in an urban area in Brazil (and although insignificant, block level population density) and high CRP is contrary to past findings indicating negative effects of high residential density and urban living on health; however, more recent evidence has suggested that higher levels of urbanization may be an advantage for health as more densely populated urban areas are home to greater social and material resources, such as health care services and opportunities for civic participation (Kim et al., 2006; Vlahov et al., 2005). Furthermore, those residing in more urban areas may be less likely to face a variety of infectious or other inflammatory exposures in childhood, thereby potentially lowering their risk of systemic inflammation over the life course (Finch and Crimmins, 2004; Gurven et al., 2008). It would be ideal to examine the effects of early living conditions and ongoing exposure to urban environments using panel data to better understand the effects of these environments on inflammation, immune function and overall health. Little is known about the pathways from neighborhoods or other urban areas to physiological health in general, and the current study, though employing cross sectional data, is only the second of which we are aware that specifically evaluates both socioeconomic and other neighborhood characteristics in relation to inflammation.
With respect to our findings on the relationships between high CRP and health status and behavior, existing evidence intimates that health behaviors including sleep and physical activity influence inflammation, and CRP specifically (Ford, 2002; Frey et al., 2007; Meier-Ewert et al., 2004). That these variables were not found to be significant as reported in Table 2 and Figure 1, nor in separate analyses controlling simply for exogenous characteristics and these variables, only makes our findings regarding BMI, smoking and distress among Brazilian migrants more compelling. More generally, as the BM IHLSS data represent a young and generally healthy population, it is possible that smoking and BMI are indicative of pathology earlier in life whereas the effects of the other behaviors have more cumulative or palliative effects as disease sets in later in life. It is possible that high blood pressure was not estimated to be statistically associated with high CRP because the BM IHLSS instrument did not include questions about anti inflammatory medication. It is also worth commenting on the estimated positive relationship between being female or unauthorized to reside in the USA and high CRP among adult Brazilian migrants. The 2005 2008 NHANES data indicate that women aged 18 64 in the United States are approximately 1.5 times as likely as men to have high CRP, and among foreign born Mexicans – the only foreign born group that may be identified – this gap is greater (Centers for Disease Control and Prevention, 2010). We also found that Brazilian men are 31 percent less likely to exhibit high CRP than women, and at least two studies conducted among Brazilians in Brazil similarly identified this gender gap in young and otherwise healthy populations (Araújo et al., 2004; Ribeiro, 1997). It will be worthwhile in future studies to clearly articulate hypotheses regarding the environmental and psychosocial factors that may help explain this disparity, in addition to potential hormonal differences that may influence CRP levels.
No previous study has analyzed the relationship between legal status and physiological function among any foreign born population residing in the United States. We estimated that unauthorized adult Brazilian migrants – the majority in the BM IHLSS data – were 21 percent more likely to have had high CRP than were their legal compatriots. This is a striking finding given the relatively young mean age (35 years among those with high CRP) and the near absence of diagnosed cardiometabolic disease among adult Brazilian migrants residing in metropolitan Boston. Some research in economics and sociology suggests that a legal status penalty may exist for earnings (Marcelli, 2004) and social capital formation (Granberry and Marcelli, 2007) among Mexican migrants residing in the USA, and findings reported in this article imply this penalty may extend to physiological regulation for Latin American migrants. This will of course require further study.
What lessons regarding interventions or policies derive from applying our sociogeographic model of biological health to the 2007 BM IHLSS data? The few studies reporting that sociogeographic environment and physiological dysregulation are related have focused largely on social support exchanged between individuals with close personal ties (Uchino, 2006). Our results complement these findings by suggesting that broader social processes such as less intimate interactions occurring within neighborhoods or other relatively small local areas are also important for influencing biological and thus long term health. In particular, collective efficacy and other social capital theorists argue that civic group participation among neighborhood residents (e.g. sports clubs, neighborhood associations) may increase residents’ attachment to their neighborhoods and thus their stake in maintaining neighborhood well being (Browning and Cagney, 2002; Putnam, 2000). Furthermore, a neighborhood’s capacity to organize around common goals plays a role in determining how effective residents are in negotiating with public institutions (e.g. police, city council) to obtain services (Sampson, 2003). This capacity in turn may influence neighborhood disorder; for example, previous research indicates that neighborhoods higher in collective efficacy tend to have lower crime rates (Sampson et al., 1997). The social and material resources thus afforded to neighborhoods exhibiting greater collective efficacy may influence health, though it remains to be seen what kinds of interventions may be most effective in supporting such civic organization (Cohen et al., 2006), especially among socioeconomically vulnerable populations.
Perhaps a first step toward fostering health among U.S. migrants would be to promote awareness of local community based organizations (CBOs) dedicated to assisting them. Fewer than 50 percent of all foreign born Brazilian adults residing in metropolitan Boston, for instance, were familiar with community organizations committed to providing employment, legal and other services according to the 2007 BM IHLSS data. Furthermore, given that a plurality of Brazilians, regardless of gender or legal status, appear to have been actively engaged in their church or a social media group – two of several “civic” organizations about which BM IHLSS respondents were asked to detail their participation – social capital accumulation among Brazilian migrants may be fostered by CBOs by reaching out in these domains. Neither churches nor the internet necessarily include one’s neighbors, of course, but they might, and even if they do not they may help connect individuals who might benefit from knowing one another. Such efforts may be especially important for Brazilian migrants residing in metropolitan Boston because more than two thirds are estimated to be residing in the USA illegally and unauthorized Brazilians were found to be 22 percent more likely to have had high CRP compared to their legal compatriots. Fear of exposure or unwanted attention has the potential to prevent unauthorized as well as legal migrants from fully engaging in existing civic organizations or even reporting incidents of crime to the authorities. Although reducing such disincentives to civic engagement will require national level immigrant policy reforms that help migrants integrate more quickly and thoroughly, local entities such as the cities of Cambridge and Somerville in the BCQ MSA have adopted sanctuary policies preventing city employees from inquiring about legal status when providing services. It remains to be seen, of course, whether such progressive policies (Sullivan, 2009) or increased CBO outreach efforts will result in more active civic engagement among migrants, and whether this would improve migrant health. The foregoing analyses have several limitations. First, the BM IHLSS data are cross sectional and representative of a group of foreign born migrants from Brazil who were residing in New England in 2007. It is difficult to conclude whether our findings can be generalized to geographically and ethnoracially diverse populations, and it is not possible to infer causality regardless of how robust the estimated parameters. Second, the CRP concentrations are derived from DBS samples rather than blood serum and converted to serum equivalent values using a regression equation, which although a validated technique, may slightly skew the CRP results (McDade, 2010; McDade et al., 2004). Third, the BM IHLSS survey did not include questions regarding medication currently being taken for management of cardiovascular risk factors; however, no one in the sample reported having been diagnosed with cardiovascular disease. Lastly, of necessity due to the concentration of foreign born Brazilians, the local area analyzed in this study consisted of a relatively small number of census blocks (52) in a circumscribed area (Eastern Massachusetts). This gives rise to the modifiable areal unit problem (Openshaw, 1984), or the propensity for substantial variation to occur in evaluating an outcome depending on the geography in which it is studied. However, recent neighborhood health studies have suggested that this variation may be minimal and small areal units may be best for capturing relationships between neighborhood characteristics and health outcomes (Tarkiainen et al., 2010; Weiss et al., 2007).
Despite these limitations, the BM IHLSS data are the only data available with representative information concerning individual and neighborhood level characteristics among foreign born Brazilians in the United States. They are also the only data of which we are aware that have information on both legal status and biological markers of health. Thus, our findings emanate from analysis of the first community based biodemographic research (CBBR) data available and suggest that additional research is required to fully understand the specific pathways between neighborhood characteristics and inflammation. More generally, it may prove useful to investigate the influence of stressors in other sociogeographic domains, such as the household and workplace, to gain a more comprehensive picture of the mechanisms that cut across these domains to influence physiological regulation and disparities in systemic inflammation.
We test whether neighborhood disorder and social capital are associated with C-Reactive Protein
High C-Reactive Protein (CRP) is a biomarker for inflammation and cardiovascular disease
Disorder is estimated to be positively associated with high CRP among Brazilian immigrants
Social capital is estimated to be negatively associated with high CRP among Brazilian immigrants
High CRP is also associated with being male (−), education (−), and unauthorized legal status (+)
ACKNOWLEDGEMENT
We would like to thank Orfeu Buxton for training BM-IHLSS personnel in the proper collection of biological data and further invaluable assistance with respect to assaying and evaluating the biological data, Eileen Crimmins and Caleb Finch for helpful suggestions on an earlier draft, and Thomas McDade for performing the biological assays utilized and providing assistance with measuring our outcome variable. Enrico Marcelli received initial support for this project from the Robert Wood Johnson (RWJ) Foundation’s Health & Society Scholars program, and funding for the 2007 BM IHLSS was provided by the National Cancer Institute (NCI) Dana Farber/Harvard Cancer Center UMASS Boston Partnership Grant #5U56CA118635 03, the University of Massachusetts Boston, and the Blue Cross Blue Shield of Massachusetts Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Louisa M. Holmes, Department of Geography 3620 S. Vermont Avenue, University of Southern California Los Angeles, CA 90027 Louisa.holmes@usc.edu
Enrico A. Marcelli, Department of Sociology 5500 Campanile Drive, San Diego State University San Diego, CA 92182 4423.
References
- Acevedo-Garcia D, Lochner KA. Residential Segregation and Health. In: Kawachi I, Berkman LF, editors. Neighborhoods and Health. Oxford University Press; Oxford: 2003. [Google Scholar]
- Albert MA, Glynn RJ, Buring J, Ridker PM. C Reactive protein levels among women of various ethnic groups living in the United States (from the Women’s Health Study) The American Journal of Cardiology. 2004;93:1238–1242. doi: 10.1016/j.amjcard.2004.01.067. [DOI] [PubMed] [Google Scholar]
- Araújo F, Pereira AC, Latorre M.d.R.D.O., Krieger JE, Mansur AJ. High sensitivity C reactive Protein Concentration in a Healthy Brazilian Population. International Journal of Cardiology. 2004;97:433–438. doi: 10.1016/j.ijcard.2003.10.027. [DOI] [PubMed] [Google Scholar]
- Augustin T, Glass TA, James BD, Schwartz BS. Neighborhood Psychosocial Hazards and Cardiovascular Disease: The Baltimore Memory Study. American Journal of Public Health. 2008;98:1664–1670. doi: 10.2105/AJPH.2007.125138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird CE, Seeman TE, Escarce JJ, Davila R. Basurto, Finch BK, Dubowitz T, Heron M, Hale L, Merkin SS, Weden M, Lurie N. Neighborhood Socioeconomic Status and Biological “Wear & Tear” in a Nationally Representative Sample of U.S. Adults. Journal of Epidemiology & Community Health. 2009 doi: 10.1136/jech.2008.084814. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black PH. Stress and the Inflammatory Response: A Review of Neurogenic Inflammation. Brain, Behavior, and Immunity. 2002;16:622–653. doi: 10.1016/s0889-1591(02)00021-1. [DOI] [PubMed] [Google Scholar]
- Blake GJ, Rifai N, Buring JE, Ridker PM. Blood Pressure, C Reactive Protein, and Risk of Future Cardiovascular Events. Circulation. 2003;108:2993–2999. doi: 10.1161/01.CIR.0000104566.10178.AF. [DOI] [PubMed] [Google Scholar]
- Block JP, Scribner RA, DeSalvo KB. Fast Food, Race/Ethnicity, and Income: A Geographic Analysis. American Journal of Preventive Medicine. 2004;27:211–217. doi: 10.1016/j.amepre.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Brown ER, Yu H. Latinos’ Access to Employment Based Health Insurance. In: Paez M.M.S. O.a.M., editor. Latinos: Remaking America. University of California Press; Berkeley, CA: 2002. pp. 236–253. [Google Scholar]
- Browning CR, Cagney KA. Neighborhood Structural Disadvantage, Collective Efficacy, and Self Rated Physical Health in an Urban Setting. Journal of Health and Social Behavior. 2002;43:383–399. [PubMed] [Google Scholar]
- Buxton OM, Marcelli EA. Short and Long Sleep Are Positively Associated with Obesity, Diabetes, Hypertension, and Cardiovascular Disease among Adults in the United States. Social Science & Medicine. 2010;71:1027–1036. doi: 10.1016/j.socscimed.2010.05.041. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . National Health and Nutrition Examination Survey Data. U.S. Department of Health and Human Services, National Center for Health Statistics; Hyattsville, MD: 2010. [Google Scholar]
- Centers for Disease Control and Prevention (CDC) National Health and Nutrition Examination Survey Data. U.S. Department of Health and Human Services. National Center for Health Statistics (NCHS); Hyattsville, MD: 2010. [Google Scholar]
- Christensen K. Biological Material in Household Surveys: The Interface between Epidemiology and Genetics. In: Finch CE, Vaupel JW, Kinsella K, editors. Cells and Surveys: Should Biological Measures be Included in Social Science Research. National Academy Press; Washington, D.C.: 2000. pp. 43–63. [PubMed] [Google Scholar]
- Cohen DA, Finch BK, Bower A, Sastry N. Collective Efficacy and Obesity: The Potential Influence of Social Factors on Health. Social Science & Medicine. 2006;62:769–778. doi: 10.1016/j.socscimed.2005.06.033. [DOI] [PubMed] [Google Scholar]
- Cohen DA, Inagami S, Finch BK. The Built Environment and Collective Efficacy. Health & Place. 2008;14:198–208. doi: 10.1016/j.healthplace.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulton C, Korbin J, Chan T, Su M. Mapping Residents’ Perceptions of Neighborhood Boundaries: A Methodological Note. American Journal of Community Psychology. 2001;29:371–383. doi: 10.1023/A:1010303419034. [DOI] [PubMed] [Google Scholar]
- Crimmins EM, Finch CE. Infection, Inflammation, Height, and Longevity. Proceedings of the National Academy of Sciences. 2006;103:498–503. doi: 10.1073/pnas.0501470103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins EM, Kim JK, Alley DE, Karlamangla A, Seeman T. Hispanic Paradox in Biological Risk Profiles. American Journal of Public Health. 2007;97:1305–1310. doi: 10.2105/AJPH.2006.091892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubbin C, Hadden W, Winkleby M. Neighborhood Context and Cardiovascular Disease Risk Factors: The Contribution of Material Deprivation. Ethnicity & Disease. 2001;11:687–700. [PubMed] [Google Scholar]
- Cubbin C, Sundquist K, Ahlen H, Johansson SE, Winkleby MA, Sundquist J. Neighborhood Deprivation and Cardiovascular Disease Risk Factors: Protective and Harmful Effects. Scandinavian Journal of Public Health. 2006;34:228–237. doi: 10.1080/14034940500327935. [DOI] [PubMed] [Google Scholar]
- Diez-Roux AV, Merkin SS, Arnett D, Chambless L, Massing M, Nieto FJ, Sorlie P, Szklo M, Tyroler HA, Watson RL. Neighborhood of Residence and Incidence of Coronary Heart Disease. New England Journal of Medicine. 2001;345:99–106. doi: 10.1056/NEJM200107123450205. [DOI] [PubMed] [Google Scholar]
- Duffy J. The Sanitarians: A History of American Public Health. University of Illinois Press; Urbana Champaign, IL: 1992. [1990] [DOI] [PubMed] [Google Scholar]
- Finch BK, Do DP, Heron M, Bird C, Seeman T, Lurie N. Neighborhood Effects on Health: Concentrated Advantage and Disadvantage. Health & Place. 2010;16:1058–1060. doi: 10.1016/j.healthplace.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch CE, Crimmins EM. Inflammatory Exposure and Historical Changes in Human Life Spans. Science. 2004;305:1736–1739. doi: 10.1126/science.1092556. [DOI] [PubMed] [Google Scholar]
- Finch CE, Vaupel JW, Kinsella K. Cells and Surveys: Should Biological Measures Be Included in Social Science Research. National Academy Press; Washington D.C.: 2000. [PubMed] [Google Scholar]
- Ford ES. Does Exercise Reduce Inflammation? Physical Activity and C reactive Protein among U.S. Adults. Epidemiology. 2002;13:561–568. doi: 10.1097/00001648-200209000-00012. [DOI] [PubMed] [Google Scholar]
- Frey DJ, Fleshner M, Wright KP., Jr The Effects of 40 Hours of Total Sleep Deprivation on Inflammatory Markers in Healthy Young Adults. Brain, Behavior, and Immunity. 2007;21:1050–1057. doi: 10.1016/j.bbi.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Goldman DP, Smith JP, Sood N. Legal Status And Health Insurance Among Immigrants. Health Affairs. 2005;24:1640–1653. doi: 10.1377/hlthaff.24.6.1640. [DOI] [PubMed] [Google Scholar]
- Gordon-Larsen P, Nelson MC, Page P, Popkin BM. Inequality in the Built Environment Underlies Key Health Disparities in Physical Activity and Obesity. Pediatrics. 2006;117:417–424. doi: 10.1542/peds.2005-0058. [DOI] [PubMed] [Google Scholar]
- Granberry PJ, Marcelli EA. “In the Hood and on the Job”: Social Capital Accumulation among Legal and Unauthorized Mexican Immigrants. Sociological Perspectives. 2007;50:579–595. [Google Scholar]
- Gurven M, Kaplan H, Winking J, Finch CE, Crimmins EM. Aging and Inflammation in Two Epidemiological Worlds. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2008;63:196–199. doi: 10.1093/gerona/63.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer M, Molloy GJ, Stamatakis E. Psychological Distress as a Risk Factor for Cardiovascular Events: Pathophysiological and Behavioral Mechanisms. Journal of the American College of Cardiology. 2008;52:2156–2162. doi: 10.1016/j.jacc.2008.08.057. [DOI] [PubMed] [Google Scholar]
- Hill TD, Ross CE, Angel RJ. Neighborhood Disorder, Psychophysiological Distress, and Health. Journal of Health and Social Behavior. 2005;46:170–186. doi: 10.1177/002214650504600204. [DOI] [PubMed] [Google Scholar]
- Holmes LM, Marcelli EA. A Sociogeography of Cardiovascular Risk: Neighborhood Influences on Blood Pressure and Inflammation. Association of American Geographers; Seattle, WA: 2011. [Google Scholar]
- Huber PJ. The Behavior of Maximum Likelihood Estimates Under Nonstandard Conditions, Fifth Berkeley Symposium on Mathematical Statistics and Probability. University of California Press; Berkeley, CA: 1967. pp. 221–233. [Google Scholar]
- Jaszczak A, Lundeen K, Smith S. Using Nonmedically Trained Interviewers to Collect Biomeasures in a National In home Survey. Field Methods. 2009;21:26–48. doi: 10.1177/1525822X08323988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Andrews G, Colpe LJ, Hiripi E, Mroczek DK, Normand SLT, Walters EE, Zavlasky AM. Short Screening Scales to Monitor Population Prevalences and Trends in Non specific Psychological Distress. Psychological Medicine. 2002;32:950–976. doi: 10.1017/s0033291702006074. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Gouin JP, Hantsoo L. Close Relationships, Inflammation, and Health. Neuroscience & Biobehavioral Reviews. 2010;35:33–38. doi: 10.1016/j.neubiorev.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Subramanian SV, Kawachi I. Bonding versus Bridging Social Capital and Their Associations with Self Rated Health: A Multilevel Analysis of 40 US Communities. Journal of Epidemiology and Community Health. 2006;60:116–122. doi: 10.1136/jech.2005.038281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwate NOA, Yau CY, Loh JM, Williams D. Inequality in Obesigenic Environments: Fast Food Density in New York City. Health & Place. 2009;15:364–373. doi: 10.1016/j.healthplace.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Ledue TB, Rifai N. High Sensitivity Immunoassays for C Reactive Protein: Promises and Pitfalls. Clinical Chemistry and Laboratory Medicine. 2001;39:1171–1176. doi: 10.1515/CCLM.2001.185. [DOI] [PubMed] [Google Scholar]
- Liang H, Tomey K, Chen D, Savar NL, Rimmer JH, Braunschweig CL. Objective Measures of Neighborhood Environment and Self Reported Physical Activity in Spinal Cord Injured Men. Archives of Physical Medicine and Rehabilitation. 2008;89:1468–1473. doi: 10.1016/j.apmr.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Lovasi GS, Hutson MA, Guerra M, Neckerman KM. Built Environments and Obesity in Disadvantaged Populations. Epidemiologic Reviews. 2009a;31:7–20. doi: 10.1093/epirev/mxp005. [DOI] [PubMed] [Google Scholar]
- Lovasi GS, Neckerman KM, Quinn JW, Weiss CC, Rundle A. Effect of Individual or Neighborhood Disadvantage on the Association Between Neighborhood Walkability and Body Mass Index. American Journal of Public Health. 2009b;99:279–284. doi: 10.2105/AJPH.2008.138230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macintyre S, Ellaway A. Neighborhoods and Health: An Overview. In: Kawachi I, Berkman LF, editors. Neighborhoods and Health. Oxford University Press; New York: 2003. [Google Scholar]
- Marcelli EA. Unauthorized Mexican Immigration, Day Labour and Other Lower Wage Informal Employment in California. Regional Studies. 2004;38:1–13. [Google Scholar]
- Marcelli EA, Buxton O. A Sociogeography of Insufficient Sleep: New Evidence from Legal and Unauthorized Migrants in Metropolitan Boston. Annual Meetings of the Population Association of America; Dallas, TX. 2010. [Google Scholar]
- Marcelli EA, Heer DM. Unauthorized Mexican Workers in the 1990 Los Angeles County Labor Force. International Migration. 1997;35:59. doi: 10.1111/1468-2435.00004. [DOI] [PubMed] [Google Scholar]
- Marcelli EA, Holmes LM. Blood Pressure. In: Loue S, Sajatovic M, editors. Encyclopedia of Immigrant Health. Springer; 2012. p. forthcoming. [Google Scholar]
- Marcelli EA, Holmes LM, Estella D, da Rocha F, Granberry P, Buxton O. (In)Visible (Im)Migrants: The Health and Socioeconomic Integration of Brazilians in Metropolitan Boston. Institute for Behavioral and Community Health (iBACH), San Diego State University; San Diego, CA: 2009a. [Google Scholar]
- Marcelli EA, Holmes LM, Troncoso M, Granberry P, Buxton O. Permanently Temporary?: The Health and Socioeconomic Integration of Dominicans in Metropolitan Boston. Center for Behavioral and Community Health Studies (BACH), San Diego State University; San Diego, CA: 2009b. [Google Scholar]
- Margolis M. An Invisible Minority: Brazilians in New York City. Allyn and Bacon; Boston, MA: 1998. [Google Scholar]
- Massey D, Denton N. American Apartheid: Segregation and the Making of the Underclass. Harvard University Press; Cambridge, MA: 1998. [Google Scholar]
- Massey DS, Martin JA. The NIS Skin Color Scale. Office of Population Research, Princeton University; 2003. [Google Scholar]
- McDade TW. In: Boston Immigrant Survey CRP Assays. Holmes LM, editor. Evanston, IL: 2010. [Google Scholar]
- McDade TW, Burhop J, Dohnal J. High Sensitivity Enzyme Immunoassay for C Reactive Protein in Dried Blood Spots. Clinical Chemistry. 2004;50:652–654. doi: 10.1373/clinchem.2003.029488. [DOI] [PubMed] [Google Scholar]
- McDade TW, Hawkley LC, Cacioppo JT. Psychosocial and Behavioral Predictors of Inflammation in Middle Aged and Older Adults: The Chicago Health, Aging, and Social Relations Study. Psychosomatic Medicine. 2006;68:376–381. doi: 10.1097/01.psy.0000221371.43607.64. [DOI] [PubMed] [Google Scholar]
- McDade TW, Williams SA, Snodgrass JJ. What a Drop Can Do: Dried Blood Spots as a Minimally Invasive Method for Integrating Biomarkers Into Population Based Research. Demography. 2007;44:899–925. doi: 10.1353/dem.2007.0038. [DOI] [PubMed] [Google Scholar]
- Meier-Ewert HK, Ridker PM, Rifai N, Regan MM, Price NJ, Dinges DF, Mullington JM. Effect of Sleep Loss on C reactive Protein, An Inflammatory Marker of Cardiovascular Risk. Journal of the American College of Cardiologists. 2004;43:678–683. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- Melosi MV. The Sanitary City: Urban Infrastructure in America from Colonial Times to the Present. The John Hopkins University Press; Baltimore, MD: 2000. [Google Scholar]
- Merkin SS, Basurto-Dávila R, Karlamangla A, Bird CE, Lurie N, Escarce J, Seeman T. Neighborhoods and Cumulative Biological Risk Profiles by Race/Ethnicity in a National Sample of U.S. Adults: NHANES III. Annals of Epidemiology. 2009;19:194–201. doi: 10.1016/j.annepidem.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkler M, Wallerstein N. Introduction to Community Based Participatory Research. In: Minkler M, Wallerstein N, editors. Community Based Participatory Research for Health. Jossey Bass; San Francisco, CA: 2003. pp. 3–26. [Google Scholar]
- Mujahid MS, Roux AV, Shen M, Gowda D, Sanchez B, Shea S, Jacobs DR, Jr., Jackson SA. Relation between Neighborhood Environments and Obesity in the Multi Ethnic Study of Atherosclerosis. Am J Epidemiol. 2008 doi: 10.1093/aje/kwn047. [DOI] [PubMed] [Google Scholar]
- Murray ET, Diez-Roux AV, Carnethon M, Lutsey PL, Ni H, O’Meara ES. Trajectories of Neighborhood Poverty and Associations with Subclinical Atherosclerosis and Associated Risk Factors: The Multi Ethnic Study of Atherosclerosis. American Journal of Epidemiology. 2010;171:1099–1108. doi: 10.1093/aje/kwq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazmi A, Roux A. Diez, Ranjit N, Seeman TE, Jenny NS. Cross sectional and Longitudinal Associations of Neighborhood Characteristics with Inflammatory Markers: Findings from the Multi ethnic Study of Atherosclerosis. Health & Place. 2010;16:1104–1112. doi: 10.1016/j.healthplace.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Openshaw S. The Modifiable Areal Unit Problem. In: Institute of British Geographers, editor. Concepts and Techniques in Modern Geography. Geo Books; Norwich, UK: 1984. [Google Scholar]
- Ortega AN, Fang H, Perez VH, Rizzo JA, Pokras O. Carter, Wallace SP, Gelberg L. Health Care Access, Use of Services, and Experiences Among Undocumented Mexicans and Other Latinos. Archives of Internal Medicine. 2007;167:2354–2360. doi: 10.1001/archinte.167.21.2354. [DOI] [PubMed] [Google Scholar]
- Padilla AM, Cervantes RC, Maldonado M, Garcia RE. Coping Responses to Psychosocial Stressors among Mexican and Central American Immigrants. In: Organista PB, Chun KM, Marín G, editors. Readings in Ethnic Psychology. Routledge; New York: 1998. pp. 249–259. [Google Scholar]
- Passel JS. The Size and Characteristics of the Unauthorized Migrant Population in the U.S. Estimates Based on the March 2005 Current Population Survey. Pew Hispanic Center; Washington D.C.: 2006. [Google Scholar]
- Passel JS, Clark RL. Immigrant in New York: Their Legal Status, Income, and Taxes. The Urban Institute; Washington, D.C.: 1998. [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon I, Richard O, Criqui MH, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith J, Sidney C, Taubert K, Tracy RP, Vinicor F. Markers of Inflammation and Cardiovascular Disease: Application to Clinical and Public Health Practice: A Statement for Healthcare Professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- Petersen KL, Marsland AL, Flory J, Drzal E. Votruba, Muldoon MF, Manuck SB. Community Socioeconomic Status is Associated With Circulating Interleukin 6 and C Reactive Protein. Psychosomatic Medicine. 2008;70:646–652. doi: 10.1097/PSY.0b013e31817b8ee4. [DOI] [PubMed] [Google Scholar]
- Portes A. Social Capital: Its Origins and Applications in Modern Sociology. Annual Review of Sociology. 1998;24:1–24. [Google Scholar]
- Putnam RD. Bowling Alone: The Collapse and Revival of the American Community. Simon & Schuster; New York: 2000. [Google Scholar]
- Ranjit N, Roux A.V. Diez, Shea S, Cushman M, Seeman T, Jackson SA, Ni H. Psychosocial Factors and Inflammation in the Multi Ethnic Study of Atherosclerosis. Archives of Internal Medicine. 2007;167:174–181. doi: 10.1001/archinte.167.2.174. [DOI] [PubMed] [Google Scholar]
- Ribeiro MA. Levels of C reactive Protein in Serum Samples from Healthy Children and Adults in São Paulo, Brazil. Brazilian Journal of Medical and Biological Research. 1997;30:1055–1059. doi: 10.1590/s0100-879x1997000900002. [DOI] [PubMed] [Google Scholar]
- Ridker PM. High Sensitivity C Reactive Protein : Potential Adjunct for Global Risk Assessment in the Primary Prevention of Cardiovascular Disease. Circulation. 2001;103:1813–1818. doi: 10.1161/01.cir.103.13.1813. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Cook N. Clinical Usefulness of Very High and Very Low Levels of C Reactive Protein Across the Full Range of Framingham Risk Scores. Circulation. 2004;109:1955–1959. doi: 10.1161/01.CIR.0000125690.80303.A8. [DOI] [PubMed] [Google Scholar]
- Rifai N, Ridker PM. High Sensitivity C Reactive Protein: A Novel and Promising Marker of Coronary Heart Disease. Clinical Chemistry. 2001;47:403–411. [PubMed] [Google Scholar]
- Rohe WM, Stewart LS. Homeownership and Neighborhood Stability. Housing Policy Debate. 1996;7:37–81. [Google Scholar]
- Ross C, Mirowsky J. Neighborhood Disorder, Subjective Alienation, and Distress. Journal of Health and Social Behavior. 2009;50:49. doi: 10.1177/002214650905000104. [DOI] [PubMed] [Google Scholar]
- Ross CE. Neighborhood Disadvantage and Adult Depression. Journal of Health and Social Behavior. 2000;41:177. [PubMed] [Google Scholar]
- Ross CE, Mirowsky J. Neighborhood Disadvantage, Disorder, and Health. Journal of Health and Social Behavior. 2001;42:258–276. [PubMed] [Google Scholar]
- Sampson RJ. Neighborhood Level Context and Health: Lessons from Sociology. In: Kawachi I, Berkman LF, editors. Neighborhoods and Health. Oxford University Press; Oxford: 2003. pp. 132–146. [Google Scholar]
- Sampson RJ, Raudenbush S. Systematic Social Observation of Public Spaces: A New Look at Disorder in Urban Neighborhoods. American Journal of Sociology. 1999;105:653–651. [Google Scholar]
- Sampson RJ, Raudenbush S, Earls F. Neighborhoods and Violent Crime: A Multilevel Study of Collective Efficacy. Science. 1997;277:918–924. doi: 10.1126/science.277.5328.918. [DOI] [PubMed] [Google Scholar]
- Sawicki DS, Flynn P. Neighborhood Indicators: A Review of the Literature and an Assessment of Conceptual and Methodological Issues. Journal of the American Planning Association. 1996;62:165–183. [Google Scholar]
- Schootman M, Andresen E, Wolinsky F, Malmstrom T, Morley J, Miller D. Adverse Housing and Neighborhood Conditions and Inflammatory Markers among Middle Aged African Americans. Journal of Urban Health. 2010;87:199–210. doi: 10.1007/s11524-009-9426-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GD, Lawlor DA, Harbord R, Timpson N, Rumley A, Lowe GDO, Day INM, Ebrahim S. Association of C Reactive Protein With Blood Pressure and Hypertension: Life Course Confounding and Mendelian Randomization Tests of Causality. Arteriosclerosis, Thrombosis and Vascular Biology. 2005;25:1051–1056. doi: 10.1161/01.ATV.0000160351.95181.d0. [DOI] [PubMed] [Google Scholar]
- Stansfeld SA, Fuhrer R, Shipley MJ, Marmot MG. Psychological Distress as a Risk Factor for Coronary Heart Disease in the Whitehall II Study. International Journal of Epidemiology. 2002;31:248–255. doi: 10.1093/ije/31.1.248. [DOI] [PubMed] [Google Scholar]
- Studenmund AH. Using Econometrics: A Practical Guide. 4th ed Addison Wesley Longman; Boston, MA: 2001. [Google Scholar]
- Sullivan L. Enforcing Nonenforcement: Countering the Threat Posed to Sanctuary Laws by the Inclusion of Immigration Records in the National Crime Information Center Database. California Law Review. 2009;97:567–600. [Google Scholar]
- Tarkiainen L, Martikainen P, Laaksonen M, Leyland AH. Comparing the Effects of Neighbourhood Characteristics on All cause Mortality Using Two Hierarchical Areal Units in the Capital Region of Helsinki. Health & Place. 2010;16:409–412. doi: 10.1016/j.healthplace.2009.10.008. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau . Table PCT19: Place of Birth for the Foreign born Population, Census 2000 Summary File 3 (SF 3) Sample Data. U.S. Census Bureau; Washington D.C.: 2001. [Google Scholar]
- U.S. Census Bureau . Table B05006: Place of Birth for the Foreign born Population, 2005 2009 American Community Survey 5 Year Estimates. U.S. Census Bureau; Washington D.C.: 2010. [Google Scholar]
- Uchino B. Social Support and Health: A Review of Physiological Processes Potentially Underlying Links to Disease Outcomes. Journal of Behavioral Medicine. 2006;29:377–387. doi: 10.1007/s10865-006-9056-5. [DOI] [PubMed] [Google Scholar]
- Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C Reactive Protein Levels in Overweight and Obese Adults. JAMA: Journal of the American Medical Association. 1999;282:2131–2135. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- Vlahov D, Galea S, Freudenberg N. The Urban Health “Advantage”. Journal of Urban Health. 2005;82:1–4. doi: 10.1093/jurban/jti001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss L, Ompad D, Galea S, Vlahov D. Defining Neighborhood Boundaries for Urban Health Research. American Journal of Preventive Medicine. 2007;32:S154–S159. doi: 10.1016/j.amepre.2007.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkleby MA, Kraemer HC, Ahn DK, Varady AN. Ethnic and Socioeconomic Differences in Cardiovascular Disease Risk Factors: Findings for Women From the Third National Health and Nutrition Examination Survey, 1988 1994. JAMA: Journal of the American Medical Association. 1998;280:356–362. doi: 10.1001/jama.280.4.356. [DOI] [PubMed] [Google Scholar]
- Woloshin S, Schwartz LM. Distribution of C Reactive Protein Values in the United States. New England Journal of Medicine. 2005;352:1611-a–1613. doi: 10.1056/NEJM200504143521525. [DOI] [PubMed] [Google Scholar]