Abstract
Although plaques composed of the amyloid β-protein (Aβ) are considered a defining feature of Alzheimer's disease (AD), they are also found in cognitively normal individuals and extensive evidence suggests that non-plaque, water-soluble forms of Aβ may play a role in AD pathogenesis. However, the relationship between the levels of water-soluble Aβ and the clinical severity of disease has never been investigated. Here, we present results of a pilot study designed to examine the levels of water-soluble forms of Aβ in brains of individuals who died at clinically distinct stages of AD. Using a serial extraction method, we also investigated the levels of triton-soluble and formic acid-soluble Aβ. We found that water-soluble and detergent-soluble Aβ monomer and SDS-stable dimer were elevated in AD and that the levels of water soluble Aβ did not increase with plaque pathology. These results support the notion that both water- and detergent-soluble Aβ are important in AD and are not simply released from plaques by mechanical disruption. Moreover, the fact that the levels of water- and triton-soluble Aβ were similar in very mild/mild AD and moderate/severe AD suggests that once a certain level of these species is attained, further accumulation is not necessary for the disease to progress. Consequently, therapeutic targeting of water-soluble Aβ should best benefit individuals in earliest phases of the disease process.
Keywords: Alzheimer's disease, amyloid β-protein, water-soluble Aβ, SDS-stable Aβ dimer, neuritic plaques
1. INTRODUCTION
Alzheimer's Disease (AD) is defined histologically by the presence of filamentous lesions within neurons (neurofibrillary tangles), cerebral blood vessels (congophilic amyloid angiopathy) and brain parenchyma (plaques) (Glenner and Wong, 1984; Masters et al., 1985; Mirra et al., 1991). The primary proteinaceous component of plaques and CAA is the amyloid β–protein (Aβ) (Miller et al., 1993) and the accumulation of fibrillar Aβ resulting in the deposition of plaques, is believed to be a seminal event in AD (Allsop and Hardy, 1991; Selkoe, 1991). In agreement with this hypothesis, a number of studies have demonstrated a strong correlation between plaque number and the degree of cognitive decline (Blessed et al., 1968; Cummings and Cotman, 1995; He et al., 1993; Kanne et al., 1998). However, other studies have failed to find a positive relationship between plaque number and severity of dementia (Arrigada et al., 1992; Mann et al., 1988; McKee et al., 1991; Terry et al., 1987; Terry et al., 1991; Wilcock and Esiri, 1982) and it is well established that certain cognitively normal individuals die with substantial amyloid pathology (Dickson et al., 1991; Katzman et al., 1988), whereas other individuals present a clinical picture typical of AD-type dementia, but have little amyloid pathology (Cairns et al., 2009). Thus, given that extensive data support a central role for Aβ in AD, it is reasonable to ask if forms of Aβ other than those deposited in plaques cause the disease, and if their abundance correlates with the severity of dementia.
Based on the knowledge that plaques (composed of fibrillar Aβ) are insoluble in aqueous solution and sediment as a pellet when centrifuged at high speed (Allsop et al., 1983; Glenner and Wong, 1984; Masters et al., 1985); centrifugation of aqueous extracts of human brain has been used in an attempt to separate non-fibrillar/non-plaque Aβ from plaque Aβ (Kuo et al., 1996; Lue et al., 1999; McLean et al., 1999). Surprisingly, only a small number of studies have sought to investigate the role of water-soluble, non-plaque Aβ in AD (Lue et al., 1999; Mc Donald et al., 2010; McLean et al., 1999). In a study by McLean et al., the levels of soluble Aβ analysed by western blotting were found to correlate significantly with neuritic plaques and neurofibrillary tangles (McLean et al., 1999). Similarly, ELISA-measured levels of soluble Aβ40 readily discriminated AD cases from high pathology controls (non-demented cases with comparable levels of plaques to the AD group) and closely correlated with the extent of synapse loss (Lue et al., 1999).
Moreover, we previously reported that water-soluble Aβ levels are elevated in AD brain, but not in brains from non-demented cases with similar levels of amyloid pathology (Mc Donald et al., 2010). However, due to the small sample size and lack of detailed clinical assessment, the relationship between different forms of Aβ and clinical severity of disease was not investigated. In a study of brains donated by individuals who resided in a nursing home, Naslund et al., found that plaque number did not correlate well with cognitive impairment, but the level of FA-extracted Aβ did (Naslund et al., 2000). The apparent disconnect between the levels of FA-extracted Aβ and plaque number suggests that a form of Aβ undetected by IHC may correlate with the degree of cognitive decline and may have included water-soluble Aβ. However, the relationship between soluble Aβ levels and disease severity was not directly investigated.
Here, we report the results of a pilot study designed to investigate the levels of water-soluble, detergent-soluble and formic acid-soluble forms of Aβ in brains of individuals who died at clinically distinct stages of AD. Using conditions that were qualitatively similar to our previous study (Mc Donald et al., 2010), we confirmed that water-soluble and triton-soluble Aβ-containing fractions were elevated in demented cases compared to non-demented controls, but FA-soluble Aβ did not differentiate moderate/severe dementia cases from the non-demented group. None of the Aβ-containing fractions distinguished early stage dementia from moderate/severe AD, suggesting that while the presence of water- and triton-soluble Aβ is associated with AD, the levels of these species do not steadily increase as the disease progresses.
2. RESULTS
2.1 Immunoprecipitation/western blot (IP/wb) analysis of serially extracted cortical tissue reveals the presence of water-soluble SDS-stable Aβ dimer in 15 out of 19 dementia cases
We have previously shown that the levels of water-soluble and triton-soluble Aβ were elevated in AD brain, but not in brain from non-demented cases with similar levels of amyloid pathology (Mc Donald et al., 2010). However, only 14 AD cases were studied and it was not possible to stratify them by clinical severity. In this study, we sought to investigate if the levels of water-soluble, triton-soluble or FA-soluble Aβ related to the clinical severity of disease. The detergent and formic acid extracts were included in an effort to reveal Aβ species other than those directly soluble in water. For instance, Aβ associated with lipid membranes (TBS-TX) or Aβ species present in amyloid plaques (FA). In addition, we also further optimised our extraction and detection techniques. While our prior protocol produced consistent qualitative results, when two adjacent (0.2 g) tissue samples from the same 0.5 g cube of cortex were serially extracted, we noted considerable variability in the concentration of Aβ detected (Mc Donald et al., 2010). Thus in order to obtain estimates of Aβ concentrations representative of the frontal cortex, we reasoned that the larger the piece of tissue used, the more representative the values would be. Accordingly, we used the largest amount of tissue (1g) available. Secondly, we employed more stringent centrifugation conditions, spinning homogenates longer and at higher speed in an attempt to ensure that the TBS extract represented authentic soluble material. Thirdly, we increased the lower limit for detecting Aβ by using larger volumes of samples for the IP (900 μl) and further optimising western blot detection (Experimental Procedures). Fourthly, in an effort to more accurately determine Aβ concentration, we used four synthetic standards (5, 10, 20 and 50 ng) on each blot instead of the three standards (5, 10 and 20 ng) used previously.
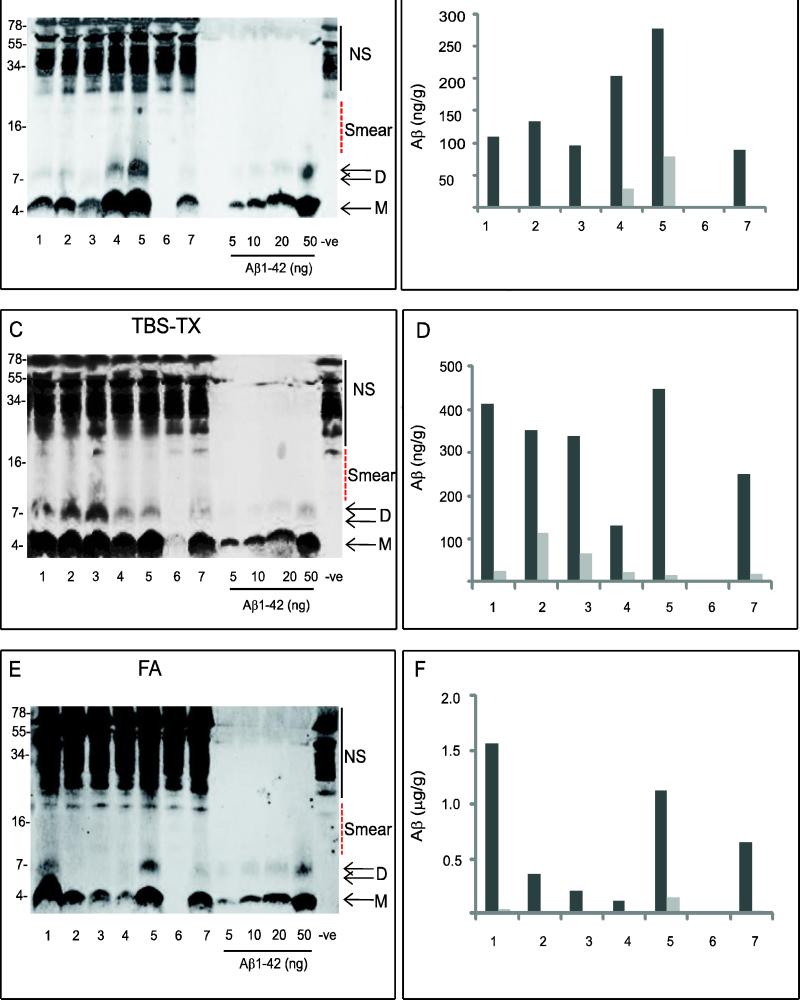
In the example shown, six out of seven TBS extracts contained Aβ migrating at ~ 4 kDa (monomer) (Fig. 1A) and the concentration of monomer in each case was between 89-278 ng/g of wet weight tissue. Interestingly, only 2 of the 6 cases which had quantifiable monomer, (cases 4 and 5) contained quantifiable SDS-stable dimer, and the concentration of dimer was lower than that of monomer (Fig. 1A, B). Moreover, in addition to monomer and SDS-stable dimer, cases # 4 and 5 also contained some higher molecular weight (~ 9-18 kDa) Aβ immunoreactive material (Fig. 1A). In the 28 samples, Aβ-reactive material larger than an ~8 kDa dimer was only detected in 8 cases, and even in these cases the amount was low. Therefore, since Aβ monomer and SDS-dimer were by far the most prominent species we restricted our analysis to these. It should be noted that it was not possible to determine if SDS-stable Aβ species migrating higher than 20 kDa were present, since a ladder of dark bands was detected when buffer alone was IP'd with AW7 and western blotted with 2G3/21F12 (-ve) (Fig. 1A). Since samples were boiled and electrophoresed on SDS-containing gels this procedure precludes assessment of the native assembly forms of Aβ present in TBS extracts. In the case of the TBS-TX and formic acid fractions, even the solvents used for extraction are denaturing, thus preventing assessment of the native state of Aβ prior to extraction. Nonetheless, detection of Aβ which migrates on SDS-PAGE at ~4 and 8 kDa indicates that there are at least two forms of Aβ present. The monomer could represent either real monomer or monomer that is derived from larger SDS-labile assemblies. Similarly, the dimer could be an authentic dimer or a dimer derived from larger assemblies which break down to dimer in SDS.
Figure 1. Water-soluble (TBS), triton-soluble (TBS-TX) and formic acid-soluble (FA) extracts of human brain contain Aβ immunopositive species with molecular weight consistent for monomer and SDS-stable dimer.
Tissue from 28 brain samples were serially extracted and analysed in duplicate by IP with AW7 and wb with a combination of 2G3 and 21F12, antibodies specific for Aβ terminating at 40 and 42, respectively. Representative western blots of TBS (A), TBS-TX (C) and FA (E) extracts from 7 samples are shown. NS indicates non-specific immunoreactive bands detected when buffer only was IP'd (-ve). Smear denotes immunoreactive material from ~ 9 kDa up to ~ 16 kDa. M refers to monomer and D to dimer. Molecular weight markers are shown on the left. The concentration of monomer or SDS-stable dimer in each sample was determined by comparison with synthetic Aβ standards (5, 10, 20 and 50 ng) and estimated using Li-COR software. The calculated values (B, D and F) are averaged results from 2 different IPs of the same extract analysed on two separate western blots. Monomer is shown in black and dimer in grey.
Analysis of all 28 brains revealed that water-soluble monomer was detected in 23/28 (82%) cases analysed (Fig. 2A) with concentrations ranging between 2.5-603 ng/g. Twenty cases (71%) had detectable SDS-stable dimer (Fig. 2B) but the concentrations of SDS-stable dimer were always lower (5-199 ng/g) than those observed for monomer. In this study the concentration of Aβ in TBS extracts (based on the sum of monomer and SDS-stable dimer; 7.5-477 ng/g) tended to be slightly lower in value, but of comparable magnitude to values obtained in our earlier study (14.5-941 ng/g) (Mc Donald et al., 2010).
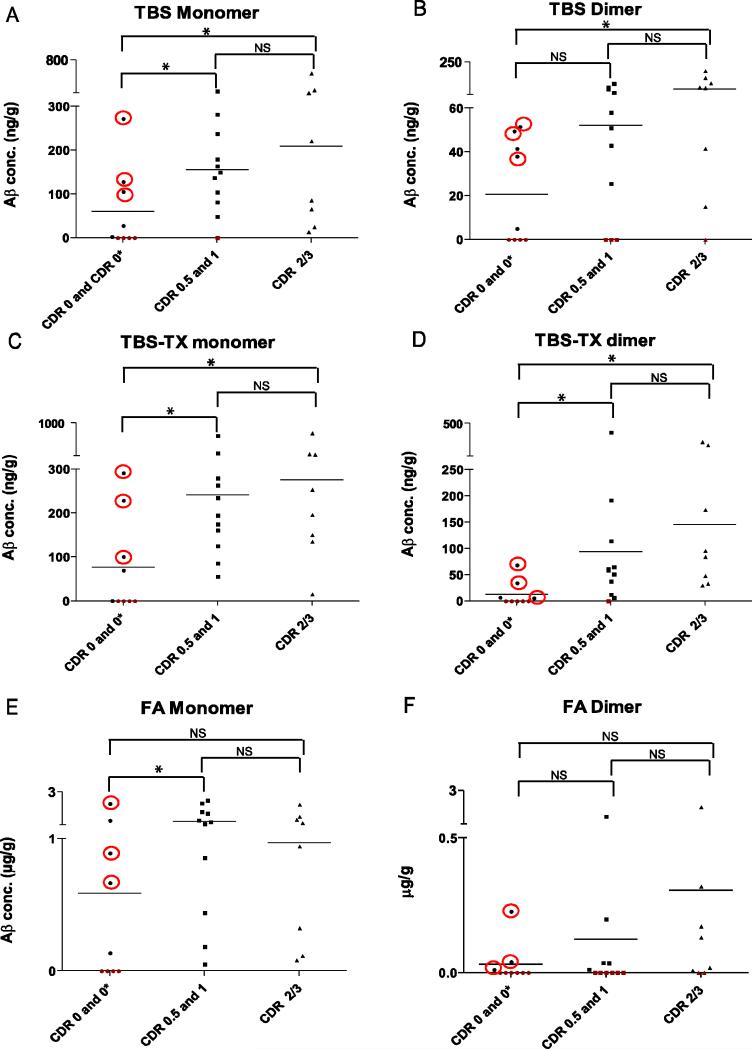
Figure 2. The levels of water-soluble and triton-soluble Aβ are elevated in dementia.
Average quantifiable Aβ monomer (A, C, E) and Aβ dimer (B, D, F) detected in duplicate AW7 IPs from 28 samples analysed by western blot with 2G3/21F12 are shown. Cases are separated into three groups: 1) non-demented (CDR 0 and 0*), 2) mildly demented (CDR 0.5 and 1) and 3) severely demented (CDR 2/3). CDR 0* cases were not demented but had significant amyloid pathology and are highlighted with red circles. The levels of quantifiable Aβ are indicated on the vertical discontinuous y-axis. Horizontal lines indicate the mean value detected for each group. * indicates significant difference (p < 0.05), while NS indicate no significant difference. Detectable levels of Aβ are indicated in black and samples in which Aβ was not detected are shown in red. CDR 0, n=6; CDR 0*, n=3; CDR 0.5, n= 6; CDR 1, n= 5 and CDR 2/3, n= 8.
Similar to TBS extracts, 6 out of 7 cases in the example shown had TBS-TX monomer concentrations between 134-447 ng/g (Fig. 1C). All of these 6 cases also contained detectable SDS-stable dimer, ranging from 19-116 ng/g (Fig. 1D). When all 28 cases were analysed, 24/28 (86%) had monomer, while 22/28 (79%) contained detectable monomer and SDS-stable dimer (Fig. 2C, D). Average total Aβ levels detected in the TBS-TX extract (0.25-257 ng/g) were lower than those observed for TBS extracts (7.5-477 ng/g) and of a similar magnitude to those reported previously (1.7-1493 ng/g) (Mc Donald et al., 2010). Six out of the 7 cases shown had detectable monomer (0.121-1.59 μg/g) in the formic acid extracts (Fig. 1E) and SDS-stable dimer was quantifiable in only two of these 6 cases (cases # 1 with 0.036 μg/g and case # 5 with 1.13 μg/g) (Fig. 1F). When all 28 cases were examined, FA-monomer was discerned in 24/28 (86%) cases (Fig. 2E), whereas, only 15/28 (53%) cases had SDS-stable dimer (Fig. 2F). Average total Aβ levels detected in the FA extract (0.051- 4.04 μg/g) tended to be lower than previously observed (0.03-118.6 μg/g) (Mc Donald et al., 2010), but were on average 12 and 6 times higher than the corresponding TBS and TBS-TX values, respectively.
2.2 Water-soluble and triton-soluble Aβ monomer and dimer are elevated in AD cases compared to non-demented cases
When average results from duplicate homogenates were stratified by clinical dementia rating (CDR) there was a clear discrimination between non-demented, very mild/mild AD and moderate/severe AD. In the 9 non-demented cases, 5/9 had water-soluble Aβ, including 2/6 cases which lacked amyloid pathology (CDR 0) and 3/3 cases which had significant amyloid pathology (CDR 0*) (Fig. 2A). The levels of TBS soluble Aβ monomer in the non-demented group were significantly lower than in the very mild/mild dementia group (CDR 0.5 and 1) (p < 0.05) (Fig. 2A). Similarly, there was a significant difference in the levels of Aβ monomer detected in the non-demented and the moderate/severe dementia group (CDR 2/3) (p < 0.05) (Fig. 2A). In the very mild/mild dementia group (CDR 0.5/1), all but one case had detectable monomer (CDR 1 case), whereas water-soluble monomer was detectable in all moderate/severe (CDR 2/3) cases. Although on average the level of Aβ monomer was higher in the moderate/severe group than in the very mild/mild group, this was not significant. Similar to TBS monomer, 5/9 non-demented cases contained TBS dimer; three cases which had neuritic plaque pathology (CDR 0*) and two which lacked pathology (Fig. 2B). However, the levels of water-soluble SDS-stable dimer did not differ significantly between the non-demented group and the very mild/mild group (p > 0.05). In comparison, the levels of SDS-stable dimer in the non-demented group were significantly lower than those in the moderate/severe dementia group (p < 0.05) (Fig. 2B). Water-soluble SDS-stable dimer was detected in 15/19 (79%) cases with dementia including all but three of the very mild/mild dementia group (CDR 0.5, 1) and in all but one of the moderate/severe group (CDR 2/3).
The difference between groups with respect to TBS-TX monomer followed a pattern similar to that seen for TBS monomer; 5/9 non-demented samples had detectable monomer, 3 of which had significant amyloid pathology. Again, there was a statistical difference between non-demented cases and early-stage dementia cases (p < 0.05) and between the non-demented group and moderate/severe cases (p < 0.05) (Fig. 2C). TBS-TX SDS-stable dimer exhibited a similar trend to TBS-TX monomer, 18/19 dementia cases (CDR 0.5, 1 and 2/3) had detectable SDS-stable dimer. The one case which did not have detectable TBS-TX dimer was from the very mild/mild group. As with the TBS dimer, the levels of TBS-TX dimer within the very mild/mild group did not differ significantly from the levels of TBS-TX dimer in the moderate/severe AD group (p > 0.05).
In the FA extract, 5/9 cases from the non-demented group had detectable monomer (Fig. 2E), 3/3 cases had amyloid pathology, while 2/6 did not. FA monomer distinguished non-demented cases from very mild/mild cases (p < 0.05) (Fig. 2E) but not the non-demented group and the moderate/severe AD group (p > 0.05). In agreement with immnunohistochemical (IHC) analysis that confirmed the presence of substantial amyloid pathology in the dementia cases, all had detectable levels of FA-monomer, but the levels of FA-monomer were not different between the very mild/mild and moderate/severe dementia groups (p > 0.05). Only 3/9 donors from the non-demented group had FA-dimer, (Fig. 2F) and all three were CDR 0*, i.e. they had significant amyloid pathology. In contrast to FA monomer, the levels of FA SDS-stable dimer did not distinguish non-demented cases from very mild/mild (p > 0.05) nor moderate/severe dementia cases (p > 0.05), although a trend was apparent. In contrast to the TBS and TBS-TX dimer, FA dimer was only detected in 11/16 dementia cases; five of the very mild/mild group and 6 from the moderate/severe group had FA dimer. Not surprisingly, there was no distinction between the two groups based on the level of FA dimer present (p > 0.05).
CDR 0 cases were clinically well before death and lacked significant amyloid pathology, but two of these cases had detectable monomer in TBS, TBS-TX and FA extracts (Fig. 2A, C, E). One case had barely detectable levels (TBS = 2.5 ng/g, TBS-TX = 0.5 ng and FA = 136 ng/g) but the other case had relatively high levels in all fractions studied (TBS= 28 ng/g, TBS-TX = 70 ng/g and FA = 1.283 μg/g). Interestingly, neither case had neuritic plaques or tangles in the frontal cortex, thus demonstrating that IHC does not detect all Aβ deposits detected with formic acid. Consistent with this finding, a previous study of transgenic mouse brain revealed that water- and triton-insoluble Aβ was detected in the brains of young mice that lacked deposits discernible by IHC (Shankar et al., 2009). Importantly, the mean concentration of TBS and TBS-TX Aβ was similar for both the very mild/mild and moderate/severe groups, suggesting that the clinical severity of AD does not strongly correlate with the concentration of Aβ present, but rather that there may be a threshold effect.
2.3 Levels of water-soluble Aβ are elevated in cases with neuritic plaques
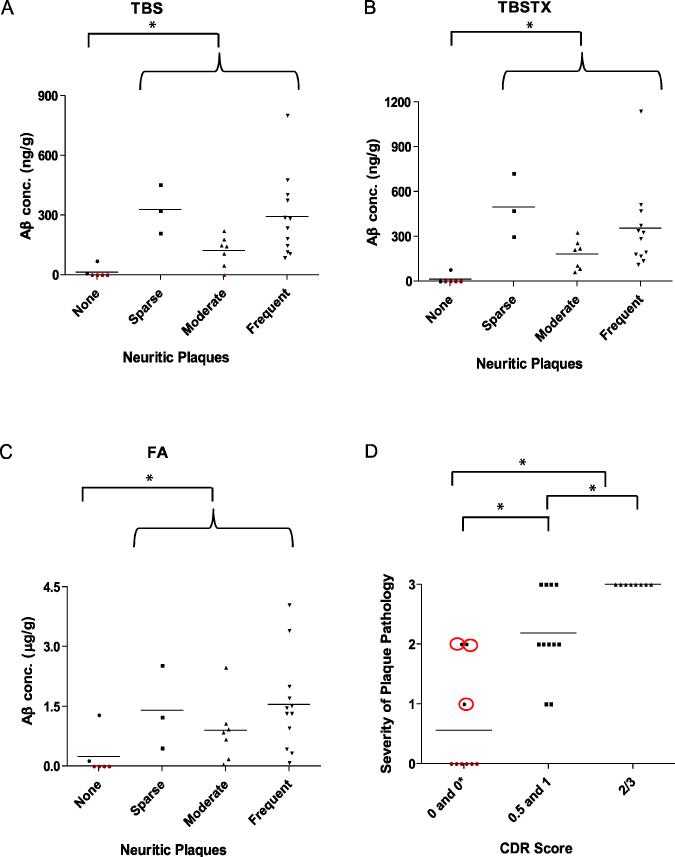
Next, we investigated the relationship between the severity of neuritic plaque pathology and levels of total Aβ (monomer plus dimer) in water-soluble, triton-soluble and FA-soluble fractions. Neuritic plaques were detected in 22/28 (82%) of the cases analysed. When brains were stratified based on the severity of neuritic plaque pathology, brains which had no neuritic plaque pathology also had no/very low levels of water-soluble Aβ (0-69 ng/g) (Fig. 3A). In brains that contained ‘sparse’, ‘moderate’ or ‘frequent’ numbers of neuritic plaques, the concentration of water-soluble Aβ ranged from 0-802 ng/g (Fig. 3A) and the levels of water-soluble Aβ in cases with neuritic plaques was significantly higher than cases without plaques (p < 0.05); but the amount of water-soluble Aβ did not exhibit a simple relationship with the severity of plaque pathology. A similar pattern was seen in TBS-TX extracts (Fig. 3B). Here again, only 2/6 cases that lacked plaques had TBS-TX Aβ (0.5 and 77 ng/g, respectively). Similar to the situation in the TBS extracts, all cases with at least some detectable neuritic plaques had TBS-TX Aβ and the levels of TBS-TX Aβ in these groups differed significantly from the concentration of TBS-TX Aβ in cases with no plaques (p < 0.05). A comparable trend was evident in the FA extract and 2/6 samples with no neuritic plaques had FA Aβ (Fig. 3C). All cases with neuritic plaques had FA Aβ, however there was no relationship between the levels of FA Aβ and TBS Aβ (r =0.0318) or TBS-TX Aβ with FA (r= 0.1221). While the levels of water-, triton- and FA-soluble Aβ tended to be elevated in demented cases with neuritic plaques, only 3 non-demented cases had neuritic plaques. Therefore, it is difficult to compare levels of Aβ stratified by both pathology and dementia. But comparison of the extent of amyloid pathology and CDR score revealed a strong correspondence between the extent of plaque pathology and the severity of dementia (rho = 0.838).
Figure 3. Levels of water-soluble and triton-soluble Aβ are increased in cases with neuritic plaques.
Total Aβ (the sum of monomer and SDS-stable dimer values) from water-soluble (A), triton-soluble (B) and FA (C) extracts were stratified based on neuritic plaque pathology. All samples with detectable Aβ are indicated in black, while those lacking Aβ are in red. The extent of neuritic plaque pathology closely correlates with severity of dementia as measured by CDR scores (D). Neuritic plaque pathology is scored from 0-3 were 0, 1, 2 and 3 represent none, sparse, moderate and frequent. All samples with detectable plaques are indicated in black, while those lacking plaques are in red. * indicates significant difference (p < 0.05). Also, Aβ levels in the TBS (A) and TBS-TX (B) extracts were significantly higher (p < 0.05) in the sparse group than in the moderate group, but were similar to the Aβ levels in the frequent group.
Most of the brains studied contained neurofibrillary tangles, 46% (13/28). It is interesting to note that of those that did have tangles, 92% had detectable water-soluble and 100% had triton-soluble Aβ (Supplementary Fig. 2A, B). The pattern in the FA extract was similar and all 13 samples that contained tangles also had appreciable FA-Aβ (Supplementary Fig. 2C).
3. DISCUSSION
Extensive evidence suggests that Aβ plays a central role in AD, but as yet the assembly forms involved remain obscure. Numerous studies have shown that non-fibrillar, water-soluble Aβ from a variety of sources (Cleary et al., 2005; Klyubin et al., 2008; Lambert et al., 1998; Walsh et al., 2002), including Aβ extracted from AD brain (Freir et al., 2011; Shankar et al., 2008), have disease relevant activity and postmortem studies indicate that elevated levels of water-soluble Aβ are specific for AD (Kuo et al., 1996; Lue et al., 1999; Mc Donald et al., 2010; McLean et al., 1999; Tabaton et al., 1994). Here we report that water-soluble Aβ monomer and SDS-stable dimer are significantly elevated in AD cases compared to non-demented controls. TBS-TX monomer and SDS-stable dimer displayed a similar pattern, but levels of FA-soluble Aβ did not distinguish the very mild/mild or moderate/severe dementia groups from non-demented cases. However, while the mean values of Aβ monomer and dimer in both TBS and TBS-TX extracts were higher in the moderate/severe group, they were not statistically different from the very mild/mild group, thus, raising the interesting possibility of a threshold effect. That is, once a certain level of Aβ is attained, the disease process is initiated and further accumulation of water-soluble Aβ does not alter disease progression. The fact that both water-soluble and triton-soluble Aβ are elevated is consistent with the notion that a portion of water-soluble Aβ binds to the membrane either directly, or via one or more receptors and initiates toxicity (Cissé et al., 2011; De Felice et al., 2006; Lauren et al., 2009; Renner et al., 2010; Zhao, 2008), and that the TBS-TX Aβ may represent the fraction of Aβ, actually triggering toxicity.
With regard to the role of plaques, soluble Aβ and the presence and severity of dementia, the results of this study reveal apparently contradictory, but not mutually exclusive findings. On the one hand we demonstrate that certain cases which lack significant amyloid pathology have appreciable levels of TBS-, TBS-TX- and FA-soluble Aβ and that these forms of Aβ and amyloid pathology are increased in AD-type dementia. However, the extent of amyloid pathology and the levels of water- and detergent-soluble forms of Aβ show no obvious link. These results indicate that while amyloid deposition continues to accrue (at least in the cohort studied) the levels of TBS- and TBS-TX-soluble forms of Aβ plateau around the time when the first clinical manifestations of AD emerge.
Linking soluble Aβ and/or amyloid burden with the presence and severity of AD-type dementia is further complicated by the fact that 3 out of 9 cognitively intact cases had appreciable amyloid pathology and relatively high levels of water-, triton- and FA-soluble Aβ. This finding underscores the limitations of our study design, since although it is tempting to speculate that these individuals would in time develop AD, we simply do not know. Thus in future studies it will be critical to acquire antemortem biomarker information to facilitate the study of 2 distinct categories of cognitively normal individuals: those with little risk of developing AD and those likely to develop AD (Jack et al., 2010). Similarly, it will also be necessary to better characterize the amyloid deposits in the brain regions used for biochemical analysis. For instance, it has been reported that differences in plaque morphology are evident between demented cases and non-demented cases (Dickson et al., 1991; Wang et al., 1999) and that dementia can develop in the absence of significant plaque pathology (Cairns et al., 2009). Thus new studies should classify and document the types of amyloid deposits so as to understand if there is a link between the presence of certain types of amyloid deposits, water-/detergent-soluble Aβ and the presence of AD-type dementia.
Notwithstanding the limitations detailed above, the present study does provide important information. The data again confirm that both water- and triton-soluble Aβ (monomer and SDS-stable dimer) are elevated in AD and we demonstrate that detection of these species can occur in the absence of immunohistochemically quantifiable amyloid deposition. This latter point obviates concerns that water-soluble Aβ may be a simple artefact of the homogenization process and further highlights the need for additional studies aimed at understanding the role of water-soluble Aβ in AD. The finding that water-soluble Aβ plateaus while amyloid continues to accrue supports a role for soluble Aβ in the initiation of AD pathogenesis and indicates that targeting of these soluble species would best benefit individuals in earliest phases of the disease process. Moreover, the fact that formic acid-extracted Aβ is not directly linked with the extent of amyloid deposition suggests that formic acid can reveal Aβ species beyond those detected by immunostaining.
4. EXPERIMENTAL PROCEDURE
4.1 Reagents and antibodies
Unless stated otherwise, chemicals were from Sigma (Arklow, Co. Wicklow, Republic of Ireland). Aβ1-42 was synthesised and purified by Dr. James I. Elliott at Yale University (New Haven, CT). The mass and purity of the peptide was determined by electrospray ionization/ion trap mass spectrometry and by reverse phase HPLC, respectively. Monoclonal antibodies 2G3 and 21F12, which detect Aβ terminating at 40 and 42, respectively, were kindly provided by Drs Dale Schenk and Peter Seubert (Elan Pharmaceuticals, San Francisco, CA). AW7 was raised against aggregated Aβ1-42 and the immunogen was prepared by suspending synthetic Aβ1-42 in H20 to give a 2 mg/ml solution, then diluted in 2X PBS to produce a 1 mg/ml solution. The solution was vortex mixed and then incubated overnight at 37 °C. At the end of the incubation period the sample was aliquoted in 1.05 ml lots and frozen or used for Congo red binding. Prior to injection into rabbits one of these aliquots was thawed and mixed with an equal volume of Freund's complete adjuvant (FCA, Cambridge Research Biochemicals, UK) so as to form an emulsion. For the initial immunisation, each animal was injected with 1 mg immunogen. Thereafter, at 2-3 week intervals each animal received booster injections of 0.5 mg aggregated Aβ1-42 per animal. Sample or harvest bleeds were collected after each injection, but for this study to ensure that the antibody population was identical for all samples, we restricted serum to one bleed date. Similar to AW8 (Mc Donald et al., 2010), AW7 recognises multiple Aβ assemblies and epitopes, including Aβ40 and Aβ42 (see Supplementary Fig. 1). Fluorochrome-coupled anti-mouse IR800 antibody was from Rockland (Gilbertsville, PA).
4.2 Ethical Approval
Cortical brain tissue was obtained and used in agreement with the UCD Human Research Ethics Committee guidelines (under approval LS-E-10-10-Walsh).
4.3 Clinical Assessment of AD
Brain tissue from individuals with clinical dementia rating (CDR) scores of 0, 0.5, 1 and 2/3, representing normal, very mild, mild and moderate/severe dementia, respectively, were obtained from the Washington University Alzheimer's Disease Research Centre (Table 1). Cases who were clinically normal (CDR 0), but had significant amyloid pathology were designated as CDR 0*. The assignment of these different CDR scores was based on the cognitive status of the individual during the last months before death. Moreover, all scores were determined ante-mortem by a group of senior clinicians, independent of any data obtained post-mortem. Determination of CDR scores was based on information from collateral sources for each subject, usually a spouse or close family member.
Table 1.
Demographics of subjects
| Characteristic | CDR 0 n=9 | CDR 0.5 n=6 | CDR 1 n=5 | CDR 2/3 n=8 | Total n=28 |
|---|---|---|---|---|---|
| Post-mortem interval (SD) | 12.38 (7.2) | 23.2 (33.9) | 13.1(6.6) | 12.6 (7.1) | 14.9 (6.3) |
| Age (SD) | 94.1 (6.8) | 91.8 (7.5) | 91.9 (4.6) | 81.5 (7.6) | 89.6 (7.6) |
| Age range | 84.3-99 | 78.8-99.1 | 86.5-99.2 | 76.2-97.2 | 76.2-99.2 |
| Number of men (mean age) | 2 (87.4) | 3 (96.2) | 3 (92.2) | 5 (82.5) | 13 (88.6) |
| Number of women (mean age) | 7 (96.1) | 3 (87.4) | 2 (91.5) | 3 (79.9) | 15(90.5) |
SD indicates standard deviation. Mean age is indicated in years.
4.4 Pathology
The mean interval between death and post-mortem was 14 hours. The staging of AD pathology was determined based on the use of frontal, temporal, occipital and parietal cortices. The staging used for this study was described by Braak et al. (Braak et al., 2006; Braak and Braak, 1991). The diagnosis of possible AD was based on the presence of sparse neuritic pathology in the absence of NFTs in the frontal cortex and a diagnosis of probable AD was based on the presence of frequently encountered but moderately sized neuritic plaques with no NFTs in the frontal cortex. Definite AD was defined on the basis of frequent plaques and NFTs evident in the frontal cortex (Braak Stage V and VI).
4.5 Human Brain Homogenate Preparation and Quantification of Aβ
Frozen frontal cortical samples were carefully dissected and white matter and blood vessels removed. Tissue was thawed for ~ 30 seconds, a 0.9 g cube removed, diced and homogenised in 4.5 ml of ice-cold Tris-buffered saline (TBS) containing 1 mg/ml aprotinin, 1 mg/ml pepstatin A, 1 mM pefabloc, 2 mM 1,10-phenanthroline, 5 mM ethylenediainetetraacetic acid (EDTA), and 5 mM ethylene glycol tetraacetic acid (EGTA) with 25 strokes of a Dounce homogeniser (Fisher, Ottawa, Canada). Homogenates (4.5 ml) were centrifuged in a SW 55 Ti Rotor (Beckman Coultour, Fullerton, CA) at 220,000 g and 4 °C for 78 min. The supernatant referred to as the TBS extract was removed with care taken not to disturb the pellet, mixed and aliquoted into 900 μl lots and stored at – 80 °C. The pellet was homogenised (1:5 w/v) in TBS containing 1% Trition-X-100 (TBS-TX) and centrifuged using the same conditions used to generate the TBS extract. The pellet remaining after the TBS-TX extract was re-suspended in 88% formic acid (FA) (1:0.5 w/v) and incubated overnight at 4°C with gentle agitation. Next day the FA extract was aliquoted and transferred to -80°C pending analysis. The level of Aβ in brain extracts was quantified by IP with AW7 and western blot with a combination of 2G3 and 21F12, essentially as described previously (Mc Donald et al., 2010) but with three modifications designed to improve the lower limit of the Aβ detected. First, the volume of extract used for IP was increased from 300 μl to 900 μl. Second, four synthetic Aβ standards (5, 10, 20 and 50 ng/well), instead of 3, were loaded on each gel. Third, the incubation period for the primary antibody was increased from 1 hour at room temperature to 16 hours at 4 °C. All samples were analysed at least in duplicate.
4.6 Statistical Analysis
Since, the distribution of Aβ levels were skewed, for instance in one analyses (FA-dimer) 14 out of 28 samples had no detectable Aβ, data were analysed using the non-parametric Mann-whitney U test. Due to low numbers, CDR 0 and CDR 0* cases were combined to produce the non-demented group and CDR 0.5 and CDR 1 cases were combined to yield the very mild/mild AD. The moderate/severe AD group consisted of cases with CDR 2/3. For the comparison of neuritic plaque pathology with clinical dementia rating scores, a spearman rank test was employed. All statistical analysis were performed using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA).
Supplementary Material
Highlights.
Water- and detergent-soluble Aβ are elevated in AD compared to controls.
Water- and detergent-soluble Aβ are, in certain cases, detectable in the absence of amyloid plaques; indicating that elevation of these species can occur independently from and precede the formation of amyloid plaques.
The levels of water- and detergent-soluble Aβ are similar in very mild/mild AD and moderate/severe AD suggesting that once a certain level of these species is attained, the disease process is initiated and further accumulation of water-soluble Aβ does not alter disease progression.
ACKNOWLEDGMENTS
We thank Drs. Peter Seubert and Dale Schenk (Elan Pharmaceutical, San Francisco, CA) for providing 2G3 and 21F12; Drs. Richard Albert and George Savva for statistical advice and Dr. Alfred Wetzel and Ms. Veronika Blinder for technical assistance. This research was supported by grants from the NIH (IRO1AGO27443, DMW; P50 AG05681, P01 AG03991, NC), NIH Neuroscience Blueprint Interdisciplinary Center (P30 NS057105, NC), the Charles F. & Joanne Knight Alzheimer's Disease Research Centre and Science foundation Ireland (08/1N.1/B2033, DMW). JMMcD is an IRCSET postgraduate fellow.
ABBREVIATIONS
- AD
Alzheimer's disease
- Aβ
amyloid β-protein
- TBS
Tris-buffered saline
- TBS-TX
TBS containing 1% TX-100
- FA
formic acid
- IP
immunoprecipitation
- CDR
Clinical Dementia Rating
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT
DH receives funding for research grants from the Cure Alzheimer's Fund, the Tau Consortium, Astra Zeneca, Pfizer, Eli Lilly, and C2N Diagnostics. DH is on the Scientific Advisory Board of Pfizer and C2N Diagnostics and has consulted for Bristol Myers Squibb and Innogenetics. DMW is a consultant and a member of the scientific advisory board of Senexis, plc., a consultant for Merck Sharp and Dohme, and Eisai Inc.
REFERENCES
- Allsop D, Hardy J. Amyloid deposition as the central event in the aetiology of Alzheimer's disease. Trends in Pharmacological Science. 1991;12:383–8. doi: 10.1016/0165-6147(91)90609-v. [DOI] [PubMed] [Google Scholar]
- Allsop D, et al. The isolation and amino acid composition of senile plaque core protein. Brain Research. 1983;259:348–352. doi: 10.1016/0006-8993(83)91273-8. [DOI] [PubMed] [Google Scholar]
- Arrigada PV, et al. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992:631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- Blessed G, et al. The association between quantitative measures of dementia and senile change in the cerebral grey matter of elderly subjects. British Journal of Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- Braak H, et al. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathologica. 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathologica. 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Cairns N, et al. Absence of Pittsburgh compound B detection of cerebral amyloid beta in a patient with clinical, cognitive, and cerebrospinal fluid markers of Alzheimer disease: a case report. Archives of Neurology. 2009;66:1557–62. doi: 10.1001/archneurol.2009.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cissé M, et al. Reversing EphB2 depletion rescues cognitive functions in Alzheimer model. Nature. 2011;469:47–52. doi: 10.1038/nature09635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary JP, et al. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nature Neuroscience. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- Cummings BJ, Cotman CW. Image analysis of beta-amyloid load in Alzheimer's disease and relation to dementia severity. Lancet. 1995;346:1524–8. doi: 10.1016/s0140-6736(95)92053-6. [DOI] [PubMed] [Google Scholar]
- De Felice FG, et al. Abeta oligomers induce neuronal oxidative stress through N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the alzheimer drug memantine. Journal of Biological Chemistry. 2006;282:11590–11601. doi: 10.1074/jbc.M607483200. [DOI] [PubMed] [Google Scholar]
- Dickson D, et al. Identification of normal and pathological aging in prospectively studied nondemeneted elderly individuals. Neurobiology of Aging. 1991;13:179–89. doi: 10.1016/0197-4580(92)90027-u. [DOI] [PubMed] [Google Scholar]
- Freir D, et al. Interaction between prion protein and toxic amyloid β assemblies can be therapeutically targeted at multiple sites. Nature Communications. 2011;2:1341. doi: 10.1038/ncomms1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenner GG, Wong CW. Alzheimer's disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochemical Biophysical Research Communications. 1984;120:885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- He Y, et al. Two distinct ubiquitin immunoreactive senile plaques in Alzheimer's disease: relationship with the intellectual status in 29 cases. Acta Neuropathologica. 1993;86:109–116. doi: 10.1007/BF00454909. [DOI] [PubMed] [Google Scholar]
- Jack CR, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurology. 2010;9:70299–6. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanne SM, et al. Relating anatomy to function in Alzheimer's disease: neuropsychological profiles predict regional neuropathology 5 years later. Neurology. 1998;50:979–85. doi: 10.1212/wnl.50.4.979. [DOI] [PubMed] [Google Scholar]
- Katzman R, et al. Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Annals of Neurology. 1988;23:138–144. doi: 10.1002/ana.410230206. [DOI] [PubMed] [Google Scholar]
- Klyubin I, et al. Amyloid beta protein dimer-containing human CSF disrupts synaptic plasticity: prevention by systemic passive immunization. Journal of Neuroscience. 2008;28:4231–7. doi: 10.1523/JNEUROSCI.5161-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo Y-M, et al. Water-soluble Aβ (N-40, N-42) oligomers in normal and Alzheimer disease brains. Journal of Biological Chemistry. 1996;271:4077–4081. doi: 10.1074/jbc.271.8.4077. [DOI] [PubMed] [Google Scholar]
- Lambert MP, et al. Diffusible, nonfribrillar ligands derived from Aβ1-42 are potent central nervous system neurotoxins. Proceedings of the National Academy of Sciences. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauren J, et al. Cellular prion protein mediates impairment of synaptic plasticity by amyloid beta oligomers. Nature. 2009;457:1128–1132. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lue LF, et al. Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer's disease. American Journal of Pathology. 1999;155:853–62. doi: 10.1016/s0002-9440(10)65184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann DM, et al. The progression of the pathological changes of Alzheimer's disease in frontal and temoral neorcortex examined both at biopsy and at autopsy. Neuropathology and Applied Neurobiology. 1988;14:177–95. doi: 10.1111/j.1365-2990.1988.tb00880.x. [DOI] [PubMed] [Google Scholar]
- Masters CL, et al. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proceedings of the National Academy of Sciences USA. 1985;82:4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mc Donald J, et al. SDS-stable Aβ dimers extracted in the water- and triton-X100-soluble fractions of brain are specific for Alzheimer-type dementia. Brain. 2010;133:1297–9. [Google Scholar]
- McKee AC, et al. Neuritic pathology and dementia in Alzheimer's disease. Ann. Neurol. 1991;30:156–165. doi: 10.1002/ana.410300206. [DOI] [PubMed] [Google Scholar]
- McLean CA, et al. Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer's disease. Annals of Neurology. 1999;46:860–6. doi: 10.1002/1531-8249(199912)46:6<860::aid-ana8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Miller DL, et al. Peptide compositions of the cerebrovascular and senile plaque core amyloid deposits of Alzheimer's disease. Archives of Biochemistry and Biophysics. 1993;301:41–52. doi: 10.1006/abbi.1993.1112. [DOI] [PubMed] [Google Scholar]
- Mirra S, et al. The Consortium to Establish a Registry for Alzheimer's disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41:479–86. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Naslund J, et al. Correlation between elevated levels of amyloid beta-peptide in the brain and cognitive decline. Journal of the American Medical Association. 2000;283:1571–1577. doi: 10.1001/jama.283.12.1571. [DOI] [PubMed] [Google Scholar]
- Necula M, Kayed R, Milton S, Glabe CG. Small molecular inhibitors of aggregation indicate that amyloid beta oligomerization and fibrillization pathways are independent and distinct. Journal of Biological Chemistry. 2007;282:10311–24. doi: 10.1074/jbc.M608207200. [DOI] [PubMed] [Google Scholar]
- Renner M, et al. Deleterious effects of amyloid beta oligomers acting as an extracellular scaffold for mGluR5. Neuron. 2010;66:739–754. doi: 10.1016/j.neuron.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. The molecular pathology of Alzheimer's disease. Neuron. 1991;6:487–498. doi: 10.1016/0896-6273(91)90052-2. [DOI] [PubMed] [Google Scholar]
- Shankar GM, et al. Biochemical and immunohistochemical analysis of an Alzheimer's disease mouse model reveals the presence of multiple cerebral Abeta assembly forms throughout life. Neurobiology of Disease. 2009;36:293–302. doi: 10.1016/j.nbd.2009.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar GM, et al. Amyloid-beta dimers isolated directly from Alzheimer's disease brains impair synaptic plasticity and memory. Nature Medicine. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabaton M, et al. Soluble amyloid β-protein is a marker of Alzheimer amyloid in brain but not in cerebrospinal fluid. Biochemical and Biophysical Research Communications. 1994;200:1598–1603. doi: 10.1006/bbrc.1994.1634. [DOI] [PubMed] [Google Scholar]
- Terry RD, et al. Senile dementia of the Alzheimer type without neocortical neurofibrillary tangles. Journal of Neuropathology and Experimental Neurology. 1987;46:262–268. doi: 10.1097/00005072-198705000-00003. [DOI] [PubMed] [Google Scholar]
- Terry RD, et al. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Annals of Neurology. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Walsh D, et al. Naturally secreted oligomers of the Alzheimer amyloid β-protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- Walsh DM, et al. Amyloid β-protein fibrillogenesis: Detection of a protofibrillar intermediate. Journal of Biological Chemistry. 1997;272:22364–22374. doi: 10.1074/jbc.272.35.22364. [DOI] [PubMed] [Google Scholar]
- Wang J, et al. The levels of soluble versus insoluble brain Abeta distinguish Alzheimer's disease from normal and pathologic aging. Experimental Neurology. 1999;158:328–37. doi: 10.1006/exnr.1999.7085. [DOI] [PubMed] [Google Scholar]
- Wilcock GK, Esiri MM. Plaques, tangles and dementia. Journal of Neurological Science. 1982;56:343–356. doi: 10.1016/0022-510x(82)90155-1. [DOI] [PubMed] [Google Scholar]
- Zhao W, de Felice FG, Fernandez S, Chen H, Lambert M, Quon MJ, Quon MJ, Krafft GA, Klein WL. Amyloid beta oligomers induce impairment of neuronal insulin receptors. The FASEB Journal. 2008;22:246–260. doi: 10.1096/fj.06-7703com. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.