Summary
Background
Upon activation, neutrophils can release nuclear material known as neutrophil extracellular traps (NETs), which were initially described as a part of antimicrobial defense. Extracellular chromatin was recently reported to be pro-thrombotic in vitro and to accumulate in plasma and thrombi of baboons with experimental deep vein thrombosis (DVT).
Objective
To explore the source and role of extracellular chromatin in DVT.
Methods
We used an established murine model of DVT induced by flow restriction (stenosis) in the inferior vena cava (IVC).
Results
We demonstrate that the levels of extracellular DNA increase in plasma after 6 h IVC stenosis, compared to sham-operated mice. Immunohistochemical staining revealed the presence of Gr-1-positive neutrophils in both red (RBC-rich) and white (platelet-rich) parts of thrombi. Citrullinated histone H3 (CitH3), an element of NETs’ structure, was present only in the red part of thrombi and was frequently associated with the Gr-1 antigen. Immunofluorescent staining of thrombi showed proximity of extracellular CitH3 and von Willebrand factor (VWF), a platelet adhesion molecule crucial for thrombus development in this model. Infusion of Deoxyribonuclease 1 (DNase 1) protected mice from DVT after 6 h and also 48 h IVC stenosis. Infusion of an unfractionated mixture of calf thymus histones increased plasma VWF and promoted DVT early after stenosis application.
Conclusions
Extracellular chromatin, likely originating from neutrophils, is a structural part of a venous thrombus and both the DNA scaffold and histones appear to contribute to the pathogenesis of DVT in mice. NETs may provide new targets for DVT drug development.
Keywords: citrullination, DVT, histones, NETs, VWF
Introduction
Neutrophils stimulated with microbes or pro-inflammatory agents, reactive oxygen species (ROS) or activated platelets, release their nuclear material, forming a web-like extracellular network. These webs, formed by DNA, histones, and neutrophil granule constituents [1], are designated as neutrophil extracellular traps (NETs) and are implicated in anti-microbial defense [2]. NETs are present in blood under septic conditions and platelets play a role in NETs production in a process involving binding of LPS to platelet Toll-like receptor-4 (TLR4) [3]. In in vitro studies, NETs were shown to be pro-thrombotic and pro-coagulant [4,5]. Histones have also been reported to stimulate platelet aggregation [4,6] and promote platelet-dependent thrombin generation [7].
In addition to neutrophils, mast cells, monocytes and eosinophils have also been reported to liberate such extracellular DNA traps [8–10]. NETs can result not only from the presence of microbes but also accompany sterile inflammation, for example, in small-vessel vasculitis or pre-eclampsia [11,12]. Formation of NETs is a step-wise process characterized by nuclear membrane dissolution, chromatin decondensation and cytolysis [13]. Histone citrullination is a hallmark of chromatin decondensation in neutrophils [14]. It is a posttranslational modification (conversion of arginine to a non-conventional amino acid citrulline) mediated by peptidylarginine deiminase 4 (PAD4), an enzyme expressed highly in granulocytes [15,16]. Inactivation of PAD4 results in suppression of NETs formation [16]. PAD4−/− neutrophils have a profound defect in their ability to kill bacteria and PAD4−/− mice are less protected against bacterial infection than wild-type mice [16].
Deep vein thrombosis (DVT) is diagnosed in approximately 900,000 cases in the US annually [17]. We have recently demonstrated that extracellular DNA accumulates in plasma in experimental DVT in baboons and the presence of DNA and histones in venous thrombi [4]. The ability of histones to upregulate thrombin generation in plasma by suppressing protein C activation [18] may have direct implications on DVT as deficiency in protein C or resistance to activated protein C, due to Factor V Leiden mutation, are risk factors for DVT in humans [19–21]. In this study, we explore the role of extracellular chromatin and histones in a murine model of flow restriction-induced DVT [22]. Venous thrombi developed in this model are structurally similar to human thrombi with white (platelet-rich) and red (RBC-rich) parts [22,23]. We show that venous thrombi contain large amounts of extracellular citrullinated histone H3 (CitH3), often in proximity to von Willebrand factor (VWF), an important molecule in the initiation of DVT [22]. We also demonstrate that intravenously administered histones exacerbate DVT, whereas Deoxyribonuclease 1 (DNase 1), which degrades NETs [4], protects mice from venous thrombosis.
Material and Methods
Mice
Wild-type C57BL/6J (WT) mice were from Jackson Laboratory (Bar Harbor, ME, USA). All mice were 7–9-week-old males weighing 22 – 26 g. All experimental procedures involving mice were approved by the Animal Care and Use Committee of the Immune Disease Institute.
Flow restriction model
The murine model of DVT was performed as described [22]. In brief, mice were anesthetized by isoflurane-oxygen, intestines were exteriorized and the IVC was diligently separated from aorta. A suture was placed on the IVC just below the renal veins over a spacer (diameter of 0.26 mm) and then the spacer was removed. This procedure has been shown to decrease vascular lumen by about 90% and avoid endothelial injury. All visible IVC side branches were also sutured. Thereafter, peritoneum and skin were closed, mice were sacrificed after 1 – 48 h and thrombi formed in the IVC were harvested. Sham-operated mice were opened and IVC sutured similarly to the experimental mice, but the suture was removed immediately after ligation.
Histone infusion
A mixture of all histones isolated from calf thymus (Worthington, Lakewood, NJ, USA) was dissolved in sterile saline and infused intravenously in mice immediately before IVC stenosis application. The dose used (10 mg/kg) is known to produce a 25% decrease in platelet count 10 min after infusion [6]. The infused solution of histone mix was essentially endotoxin-free (less than 0.025 EU/ml; measured using the endotoxin detection kit (Lonza, Walkersville, MD) according to the manufacturer’s instructions). Control mice received infusion of sterile saline. Mice were sacrificed 1 h after surgery and thrombus formation was examined.
DNase 1 infusion
DNase 1 (Pulmozyme®, Genentech, San Francisco, CA, USA) was diluted in sterile saline and injected immediately after surgery (50 μg intraperitoneally and 10 μg intravenously). In experiments with 48 h IVC stenosis, injections were repeated 3 more times after every 12 h. Control mice were injected with the Pulmozyme vehicle buffer (8.77 mg/ml sodium chloride and 0.15 mg/ml calcium chloride) diluted in sterile saline.
Determination of extracellular DNA in plasma
Blood (100 μl) was drawn from the periorbital eye plexus and stabilized with 5 μl of 0.5 M EDTA. Time points for blood drawing were: 24 h before (all mice) and then either 6, 24 and 48 h after DVT (3 mice) or sham surgery (4 mice), or 6 and 48 h after DVT (3 mice) or sham surgery (4 mice). Plasma was obtained by centrifugation at 2300 g, diluted 50-fold with PBS containing 0.1% BSA, mixed with an equal volume of 1 μM of the fluorescent DNA dye SytoxGreen (Invitrogen) and fluorescence of dye bound to DNA was immediately determined by a fluorescence microplate reader (Fluoroskan, Thermo Scientific, Waltham, MA, USA) as described [4]. Background fluorescence of PBS-plasma mixture (without SytoxGreen) was subtracted from all samples.
Frozen sections
Thrombi with or without the surrounding IVC wall or sham IVC (IVC fragment of 6 –8 mm ligated at both ends with blood remaining inside) were harvested, embedded in Optimal Cutting Temperature (OCT) compound (Sakura Finetek, Torrance, CA, USA) and then cryosectioned into 10 μm sections.
Immunohistochemistry
Immunohistochemistry was performed as previously described [24]. Briefly, anti-mouse Gr-1 antibody (clone RB6-8C5; BD Pharmingen (Franklin Lakes, NJ, USA); dilution 1:500) and rabbit polyclonal [CitH3] antibody to citrullinated histone H3 (citrulline 2 + 8 + 17; ab5103, Abcam (Cambridge, MA, USA); dilution 1:300) were used as first antibodies. Histofine Simple Stain Mouse MAX PO for rat (414311F) and rabbit (414351F), respectively, purchased from Nichirei Corporation (Tokyo, Japan), were used as secondary antibodies. Diaminobenzidine (DAB) substrate kit (Vector Laboratories, Burlingame, CA, USA), containing DAB and DAB-Ni, was used for visualization of staining. Finally, sections were counterstained with Nuclear Fast Red (Sigma-Aldrich, St. Louis, MO, USA). No first antibodies were applied in control sections.
Immunofluorescent staining
Sections were incubated with zinc fixative for 15 min, washed in PBS, and permeabilized with 0.1% Triton X-100, 0.1% sodium citrate on ice. After washing and blocking with 3% bovine serum albumin (BSA, Sigma), the sections were incubated for 16 h at 4°C in 0.3% BSA in PBS with 0.3 μg/mL rabbit polyclonal anti-CitH3 (citrulline 2 + 8 + 17; ab5103, Abcam), and sheep polyclonal anti-VWF (1:50 dilution of IgG fraction, ab11713, Abcam). For longitudinal sections of isolated thrombi, anti-CitH3 Ab was applied overnight and anti-VWF Ab for 90 min. After washing, sections were incubated with the following Alexa Fluor-conjugated secondary antibodies (all from Invitrogen, Carlsbad, CA, USA) in 0.3% BSA in PBS: Alexa Fluor 488 donkey anti–rabbit IgG and Alexa Fluor 568 donkey anti–sheep IgG (2 μg/ml for all Abs) for 2 h at room temperature. DNA was labeled with 1 μg/mL Hoechst 33342 (Invitrogen). Fluorescent images for cross-sections were acquired using an Axiovert 200 inverted widefield fluorescence microscope (Zeiss, Thornwood, NY, USA) in conjunction with a Zeiss Axiocam MRm monochromatic CCD camera. Mosaic reconstruction of entire cross-sections was performed with MosaicJ [25] for ImageJ software and consists of 2 to 6 fields of view per image shown. Images for longitudinal sections were obtained by a widefield fluorescence microscope using an Axioplan microscope (Zeiss) with color HRc Zeiss camera. Images were analyzed with Axiovision software (Zeiss).
Plasma VWF measurement
The assay was performed as described [22]. The level of VWF in pooled plasma of 20 C57BL/6J WT mice was used as a reference standard.
Statistics
Results obtained on the same animal at different time points (difference in plasma DNA levels between baseline and 6 h and plasma VWF content) were compared using paired Student’s t-test. Plasma DNA levels in mice with IVC stenosis and sham-operated animals were compared by Mann-Whitney test. Difference in thrombi prevalence between different groups of mice was compared using a contingency table and the chi-square test. Differences were considered significant at P < 0.05.
Results
Flow restriction in the IVC promotes plasma DNA accumulation
We performed IVC stenosis in WT mice. Blood was drawn 24 h before and 6, 24 and 48 h after surgery and plasma DNA levels were measured. Plasma of non-operated mice contained 284 ± 18.9 ng/ml DNA. Stenosis of the IVC led to an increase in plasma DNA levels 6 h post surgery (Fig. 1) to 515 ± 34.7 ng/ml (P < 0.003 vs. baseline and P < 0.03 vs. sham-operated mice). This effect did not result from the surgical procedure because sham-operated mice had plasma DNA levels similar to non-operated animals (326 ± 47.4 ng/ml, P = 0.9). The concentration of DNA in plasma 48 h after surgery returned to baseline and was not different in DVT- and sham-operated mice. Appearance of DNA in plasma 6 h after stenosis application, when about half of the mice form visible thrombi (as described in the text), indicates that chromatin release occurs early in the thrombotic process.
Figure 1. Plasma DNA level is elevated 6 h after IVC stenosis application.
Blood was drawn from WT mice before and 6, 24 and/or 48 h after IVC stenosis application (DVT) or sham surgery. Plasma was prepared and DNA levels determined by SytoxGreen dye; n = 3 – 8 per time point. *, P < 0.003 vs. the same mice at baseline (paired t-test). #, P < 0.03, 6 h DVT vs. sham-operated mice (Mann-Whitney test). Error bars represent SEM.
Venous thrombi contain CitH3 located predominantly in the red part of thrombus
Besides neutrophils, other cell types can release chromatin [8–10]. Histone citrullination by PAD4 occurs in activated granulocytes and is necessary for the formation and release of NETs [14–16,26]. To assess the presence of NETs in venous thrombi, we stained thrombi developed in mice after 48 h IVC stenosis, for CitH3. The white part of the thrombus (the platelet-rich part remote from the suture) contained numerous Gr-1-positive cells but was essentially devoid of CitH3 (Fig. 2A, B and Fig. 3 A). Gr-1 is an antigen present on polymorphonuclear leukocytes and also on plasmacytoid dendritic cells and a small subset of monocytes (reviewed in [27]). The large red part of the thrombus (the RBC-rich part proximal to the suture) contained loci heavily stained for either Gr-1 only (Fig. 2C, yellow arrowhead) or both Gr-1 and CitH3 with substantial overlap of the two antigens (Fig. 2C, red arrowhead). Some Gr-1-positive cells formed extracellular fiber-like structures which strongly stained for CitH3, likely representing NETs (Fig. 2D, red arrowheads; Fig. 3B).
Figure 2. Neutrophils expressing citrullinated histone H3 are present in deep vein thrombi.
Frozen sections of a thrombus formed in the mouse IVC after 48 h IVC stenosis were stained for Gr-1 (brown), CitH3 (grey-black), and counterstained for DNA (pink). (A) Composite of photographs of a stained longitudinal section of the entire thrombus with white (remote from the suture towards the tail) platelet-rich and red (adjacent to the suture) RBC-rich parts designated (dotted green line approximately delineates the white part of the thrombus). (B) High magnification of the white thrombus part. Note abundant Gr-1-positive neutrophil staining and absent CitH3 staining. (C, D) High magnification of the red part of the thrombus. (C) Orange arrowhead indicates single Gr-1 staining; red arrowhead designates double Gr-1/CitH3 staining. (D) Red arrowheads indicate NETs-like CitH3-positive structures. Inset represents high magnification of the circled area with NETs-like structures. (E) Negative control without first antibody from the red (upper panel) and white (lower panel) parts of the thrombus. (A) bar 500 μm; (B – E), bar 50 μm.
Figure 3. Extracellular citrullinated histone H3 is partially associated with VWF in murine deep vein thrombi.
(A, B) Widefield fluorescence microscopy analysis of longitudinal sections of a thrombus developed in the IVC after 48 h stenosis. Sections were immunostained for VWF (red) and for CitH3 (green) (upper panels). Incubation with only secondary antibodies served as a negative control (lower panels). (A) The “white part” of the thrombus (*) was positive for VWF but negative for citrullinated histones H3 whereas the “red part” of the thrombus was positive for both VWF and CitH3. The red part-associated CitH3 could be observed as a punctiform (intracellular) or diffuse (extracellular) staining. (A, C) Scale bars, 500 μm. (B) Higher magnification images show frequent co-distribution of extracellular CitH3 with VWF within the red part of thrombus as indicated by the arrowheads. (B, D) Scale bars, 50 μm.
(E, F, H) Widefield fluorescence microscopy analysis of cross-sections of the red part of a thrombus developed in the IVC after 48 h stenosis and including vessel wall. (E, F, G) Sections were immunostained for VWF (red), CitH3 (green) and counterstained for DNA (Hoechst 33342, blue); (H) control staining without first antibody. (E, F) Prominent CitH3 staining could be found within the thrombus and was often in proximity to VWF (F, arrowheads). No extracellular CitH3 could be detected in a sham-operated vessel containing only blood (G). (E – H) Scale bars, 100 μm.
We have previously reported an important role of VWF in venous thrombosis initiation [22]. In baboon DVT thrombi, extracellular DNA was shown to co-localize with VWF [4]. Immunofluorescent staining for VWF in murine venous thrombi was often associated with extracellular CitH3 staining in the red part of thrombi (Fig. 3A, B, E, F). Multiple CitH3-positive cells were also revealed by the immunofluorescent staining in the core of the red part of thrombi (Fig. 3A), confirming our immunohistochemical data (Fig. 2B, C). No substantial CitH3 staining was observed in the white part of thrombi (Fig. 3A asterisk), IVC wall (Fig. 3E), sham-operated IVC (Fig. 3G) and in control sections stained without first antibody (Fig. 3C, D, H). Thus, histones present in murine IVC thrombi likely originate from neutrophils releasing NETs predominantly in the red part of thrombi. In mice, similar to baboon [4], thrombi extracellular chromatin frequently co-distributes with VWF.
Histones increase plasma VWF levels and promote DVT
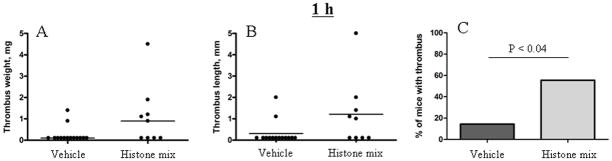
Extracellular histones are cytotoxic to endothelial cells and activate platelets in vitro; histone infusion at a high dose of 75 mg/kg is lethal to mice [4,28]. We therefore used a lower dose (10 mg/kg) of histone mix, a dose which produces only mild thrombocytopenia [6], to test whether it renders mice more prone to venous thrombosis. Only 2 of 14 of vehicle-treated mice (14%) produced thrombi after 1 h IVC stenosis (Fig. 4), while 5 of 9 mice that received histones prior to the surgery (55%) developed a thrombus (P < 0.04).
Figure 4. Histone infusion promotes flow restriction-induced thrombosis in mice.
Histone mix (10 mg/kg) was infused into WT mice immediately before DVT surgery. Mice were sacrificed after 1 h stenosis and thrombi developed in the IVC were examined and harvested. Values for weight (A) and length (B) of the thrombi are shown with medians (horizontal bars). (C) Percentage of mice that developed a thrombus. Vehicle-treated mice, n = 14; Histone-treated mice, n = 9.
Histones have been shown to cause Ca2+ influx in different cell types [6,29,30]. Increase in intracellular Ca2+ level triggers VWF secretion from endothelial cells and platelets with some of the VWF remaining associated with the plasma membrane of these cells [31]. Platelet recruitment mediated by VWF is a key step in the initiation of venous thrombosis in this model of DVT [22]. Therefore stimulation of VWF secretion could be one of the mechanisms responsible for the pro-thrombotic effect of histones. Indeed, histone infusion increased plasma VWF levels compared to baseline (Table 1). In contrast, VWF plasma levels in vehicle-treated mice remained unchanged. Thus, histone infusion increases plasma concentration of VWF, which could contribute to the effect of histones on DVT in the flow restriction setting.
Table 1. Histone infusion elevates plasma VWF levels.
Blood was drawn 24 h before and 1 h after infusion of saline or Histone mix (10 mg/kg). P value was calculated by the paired t-test. Saline-injected mice, n = 8; histone-injected mice, n = 6.
| Saline | Histone mix | ||
|---|---|---|---|
| Baseline | 1 h after infusion | Baseline | 1 h after infusion |
| 83 ± 2.7% | 78 ± 5.2% | 78 ± 4.2% | 94 ± 6.7% |
| --- | P = 0.43 | --- | P < 0.003 |
DNase 1 infusion protects mice from DVT
Based on the presence of histones, DNA and NET-like structures in deep vein thrombi in baboons [4] and mice (this report) and on the ability of DNase 1 to disassemble NET-induced thrombi in a flow chamber [4], we hypothesized that DNase 1 might prevent DVT in vivo. To test this possibility, we infused DNase 1 in mice immediately after surgery and examined thrombosis after 6 or 48 h of IVC stenosis (in 48 h DVT experiments, infusions were repeated every 12 h). In the 6 h model, half (7 of 14) of the vehicle-treated mice produced a thrombus (Fig. 5) whereas in mice that received DNase 1, only 1 mouse of 10 formed a thrombus (P < 0.05). In the 48 h IVC stenosis model, mice treated with control buffer developed a thrombus in 63% of cases (5 of 8), whereas thrombus prevalence in mice treated with DNase 1 was 17% (2 of 12, P < 0.04). These data suggest that extracellular chromatin may play a role in flow restriction-induced thrombosis and DNase 1 infusion is protective against thrombosis in this model.
Figure 5. DNase 1 infusion protects mice from flow restriction-induced thrombosis.
Wild-type mice underwent IVC stenosis for 6 h (A – C) or 48 h (D – F). Mice received infusion of either vehicle or DNase 1 (50 μg i.p. and 10 μg i.v.) before the surgery (A – F) and every 12 h thereafter (D – F). (A, D) Thrombus weight and (B, E) thrombus length are presented; horizontal lines represent median. (C, F) Percentage of mice with a thrombus. 6 h vehicle, n = 14; 6 h DNase 1, n = 10; 48 h vehicle. n = 8; 48 h DNase 1, n = 12.
Discussion
Several cell types have been shown to release extracellular chromatin upon activation. The role of the nuclear material originating from neutrophils, NETs, in anti-microbial defense has been convincingly demonstrated [2]. We recently published evidence that extracellular chromatin could be implicated in thrombosis because NETs can form a scaffold able to recruit both platelets and RBCs in vitro [4]. Perfusion of blood over NETs in a flow chamber results in the formation of a red thrombus, which was entirely NET-dependent as DNase 1, which destroys NETs, prevented recruitment of both cell types. This suggested a mechanistic link between NETs and DVT because 1) recruitment of platelets is one of the early events pivotal for thrombus initiation in mice [22] and 2) DVT thrombi are rich in RBCs [23]. Thrombi developed in the murine flow restriction model of DVT also consist of a large RBC-rich red part and a smaller platelet-rich white part with both parts containing fibrin [22]. Thus, thrombi formed in this murine model share close morphological similarity to human DVT thrombi, which also include white and red parts [23].
Thrombi obtained in an experimental DVT model in baboons have been shown to contain extracellular DNA, H3 and DNA/H2A/H2B complex [4]. Here we demonstrate that histone H3 was also abundantly present in murine DVT thrombi. We show that histone H3 was citrullinated, suggesting that it likely originated from neutrophils forming NETs. PAD4, the enzyme responsible for arginine conversion to citrulline, is abundantly expressed in granulocytes [15,16]. Staining for Gr-1, a neutrophil marker, revealed neutrophil presence in both red and white parts of the murine venous thrombi. Neutrophil-specific staining in the red part of thrombi was frequently associated with CitH3, with CitH3 being either confined to nuclei or localized extracellularly. This suggests that here neutrophils are at different stages of NETosis (Figs. 2 and 3). Interestingly, little CitH3-positive staining was observed in the white part of thrombi despite abundant presence of Gr-1-positive cells. As NETosis is an irreversible process, one may speculate that neutrophils in the white part have spent less time in the thrombus compared to the red part neutrophils. This may suggest that the white part, originally adjacent to the stenosis site where thrombus growth begins, plays a role in recruiting neutrophils from the surrounding blood. This is likely through binding to the activated platelets. Later on, these neutrophils may also become activated and form NETs, which in turn would contribute to recruitment of RBCs and the formation of red thrombus.
This model corroborates the reported ability of stimulated platelets to bind neutrophils and induce formation and release of NETs [3,5]. In addition, flow restriction may create hypoxic conditions in the vessel wall and cells buried inside a thrombus are exposed to even more severe hypoxia due to isolation from the blood stream. Hypoxia potentiates the release of ROS [32]. Besides neutrophils, platelets also can generate ROS, such as superoxide [33]. It has been shown that ROS not only directly contribute to thrombosis [34] but also can trigger formation of NETs [13,35]. Therefore, the adherent neutrophils exposed to two major triggers of NETs production, activated platelets and ROS, release extracellular chromatin which then contributes to further thrombus development.
At the early stages of flow restriction, massive recruitment of both platelets and leukocytes to the endothelium occurs simultaneously [22]. It has recently been reported that co-culture of neutrophils with activated endothelial cells can also induce NET formation, which in turn promotes endothelial damage [36]. As the activation state of the endothelium is critical for DVT initiation [22], it is possible that NETs-endothelial interactions could be involved in thrombus initiation. This fits with our observation that NETs biomarkers accumulate in plasma within hours after flow restriction induction and that histones cause an increase in plasma VWF levels, likely from the activated endothelium.
As NETs are generated during the early stage of thrombus initiation and are also abundantly present in mature thrombi, it would be reasonable to hypothesize that NETs degradation might affect thrombosis. DNase 1 has been shown to degrade NETs in vitro [2]; a lesser ability of DNase 1 to dismantle NETs in plasma of patients with systemic lupus erythematosus correlates with severity of kidney dysfunction [37]. Here, infusion of DNase 1 protected mice from flow restriction-induced DVT regardless of the length of stenosis (6 h or 48 h, Fig. 5). As no visible thrombus was detected in most DNase 1-treated mice, DNase 1 apparently cleaves NETs early disrupting the pathways of cellular activation and preventing the cascade of events leading to thrombosis. The anti-thrombotic effect of DNase 1 is likely mediated by removal of NETs generated locally at the site of stenosis, similar to cleavage of endothelium-bound VWF by ADAMTS13. Similar to ADAMTS13, DNase 1 infusion may not reduce the amount of circulating DNA but rather affect its size and local concentration.
It is known that blood coagulation contributes to DVT and hypercoagulable states are considered risk factors for the disease [19,20,21]. The murine DVT model used in our study recapitulates this feature of human DVT because fibrin could be detected throughout the thrombus [22]. Although anticoagulants have not been tested in this model, a pro-coagulant state, such as in mice with high plasma levels of soluble P-selectin [38], is associated with increased DVT induced by stasis in the IVC [39]. It remains unclear whether DNase 1 infusion attenuates, directly or indirectly, blood coagulation and a potential off-target effect of DNase 1 on some components of the coagulation cascade cannot be fully ruled out.
We observed that strings of extracellular CitH3 frequently co-localized with VWF in the thrombi produced by IVC stenosis. This finding corroborates in vitro observations that histone [40] and NETs bind VWF [4]. Secretion of VWF from Weibel-Palade bodies (WPBs) to the surface of endothelial cells appears required for the development of DVT in mice [22].
Interestingly, VWF expression is downregulated in the venous valvular sinus, which experiences stasis and hypoxia, likely to maintain a thromboresistant phenotype at this thrombosis-susceptible site [41]. In thrombi, VWF may originate not only from endothelium but also from platelets, in which it is stored in alpha-granules. It is tempting to speculate that VWF and NETs form a mutually supportive network that contributes to VWF A1 domain activation [31] and to growth and stabilization of a venous thrombus.
Histones also likely participate in the process of thrombus initiation. Infusion of histone mix facilitated thrombosis in mice (Fig. 4). This may result from the observed deleterious effect of histones on endothelium in vitro [28], which at a lower dose in vivo might activate endothelium and stimulate the release of VWF as we observed (Table 1). Another pro-thrombotic effect of histones could result from activation of platelets [4,6]. Activated platelets can stimulate NETs production [3,5] and promote release of WPBs [42] leading to further recruitment of platelets and leukocytes. In either case, histones would contribute to the process of thrombus initiation and propagation.
In conclusion, our study demonstrates an important functional role of NETs in DVT induced by flow restriction and provides possible new targets for drug development.
Acknowledgments
This work was supported by National Heart, Lung, and Blood Institute of the National Institutes of Health grants R01 HL041002, R01 HL095091 and R01 HL102101 (to D.D.W.). S.F.DM. is a postdoctoral fellow of the ‘Fond voor Wetenschappelijk Onderzoek Vlaanderen’ (FWO). We thank Lesley Cowan for help in preparing the manuscript.
References
- 1.Brinkmann V, Zychlinsky A. Beneficial suicide: why neutrophils die to make NETs. Nat Rev Microbiol. 2007;5:577–82. doi: 10.1038/nrmicro1710. [DOI] [PubMed] [Google Scholar]
- 2.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–5. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 3.Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD, Keys EM, Allen-Vercoe E, Devinney R, Doig CJ, Green FH, Kubes P. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–9. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 4.Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, Jr, Wrobleski SK, Wakefield TW, Hartwig JH, Wagner DD. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A. 2010;107:15880–5. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Massberg S, Grahl L, von Bruehl ML, Manukyan D, Pfeiler S, Goosmann C, Brinkmann V, Lorenz M, Bidzhekov K, Khandagale AB, Konrad I, Kennerknecht E, Reges K, Holdenrieder S, Braun S, Reinhardt C, Spannagl M, Preissner KT, Engelmann B. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med. 2010;16:887–96. doi: 10.1038/nm.2184. [DOI] [PubMed] [Google Scholar]
- 6.Fuchs TA, Bhandari AA, Wagner DD. Histones induce rapid and profound thrombocytopenia in mice. Blood. 2011;118:3708–14. doi: 10.1182/blood-2011-01-332676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Semeraro F, Ammollo CT, Morrissey JH, Dale GL, Friese P, Esmon NL, Esmon CT. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: involvement of platelet TLR2 and TLR4. Blood. 2011;118:1952–61. doi: 10.1182/blood-2011-03-343061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Kockritz-Blickwede M, Goldmann O, Thulin P, Heinemann K, Norrby-Teglund A, Rohde M, Medina E. Phagocytosis-independent antimicrobial activity of mast cells by means of extracellular trap formation. Blood. 2008;111:3070–80. doi: 10.1182/blood-2007-07-104018. [DOI] [PubMed] [Google Scholar]
- 9.Chow OA, von Kockritz-Blickwede M, Bright AT, Hensler ME, Zinkernagel AS, Cogen AL, Gallo RL, Monestier M, Wang Y, Glass CK, Nizet V. Statins enhance formation of phagocyte extracellular traps. Cell Host Microbe. 2010;8:445–54. doi: 10.1016/j.chom.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yousefi S, Gold JA, Andina N, Lee JJ, Kelly AM, Kozlowski E, Schmid I, Straumann A, Reichenbach J, Gleich GJ, Simon HU. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med. 2008;14:949–53. doi: 10.1038/nm.1855. [DOI] [PubMed] [Google Scholar]
- 11.Gupta AK, Hasler P, Holzgreve W, Gebhardt S, Hahn S. Induction of neutrophil extracellular DNA lattices by placental microparticles and IL-8 and their presence in preeclampsia. Hum Immunol. 2005;66:1146–54. doi: 10.1016/j.humimm.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Kessenbrock K, Krumbholz M, Schonermarck U, Back W, Gross WL, Werb Z, Grone HJ, Brinkmann V, Jenne DE. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med. 2009;15:623–5. doi: 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–41. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Li M, Stadler S, Correll S, Li P, Wang D, Hayama R, Leonelli L, Han H, Grigoryev SA, Allis CD, Coonrod SA. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol. 2009;184:205–13. doi: 10.1083/jcb.200806072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakashima K, Hagiwara T, Yamada M. Nuclear localization of peptidylarginine deiminase V and histone deimination in granulocytes. J Biol Chem. 2002;277:49562–8. doi: 10.1074/jbc.M208795200. [DOI] [PubMed] [Google Scholar]
- 16.Li P, Li M, Lindberg MR, Kennett MJ, Xiong N, Wang Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med. 2010;207:1853–62. doi: 10.1084/jem.20100239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol. 2008;28:370–2. doi: 10.1161/ATVBAHA.108.162545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ammollo CT, Semeraro F, Xu J, Esmon NL, Esmon CT. Extracellular histones increase plasma thrombin generation by impairing thrombomodulin-dependent protein C activation. J Thromb Haemost. 2011;9:1795–803. doi: 10.1111/j.1538-7836.2011.04422.x. [DOI] [PubMed] [Google Scholar]
- 19.Griffin JH, Evatt B, Zimmerman TS, Kleiss AJ, Wideman C. Deficiency of protein C in congenital thrombotic disease. J Clin Invest. 1981;68:1370–3. doi: 10.1172/JCI110385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dahlback B, Carlsson M, Svensson PJ. Familial thrombophilia due to a previously unrecognized mechanism characterized by poor anticoagulant response to activated protein C: prediction of a cofactor to activated protein C. Proc Natl Acad Sci U S A. 1993;90:1004–8. doi: 10.1073/pnas.90.3.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bertina RM, Koeleman BP, Koster T, Rosendaal FR, Dirven RJ, de Ronde H, van der Velden PA, Reitsma PH. Mutation in blood coagulation factor V associated with resistance to activated protein C. Nature. 1994;369:64–7. doi: 10.1038/369064a0. [DOI] [PubMed] [Google Scholar]
- 22.Brill A, Fuchs TA, Chauhan AK, Yang JJ, De Meyer SF, Kollnberger M, Wakefield TW, Lammle B, Massberg S, Wagner DD. von Willebrand factor-mediated platelet adhesion is critical for deep vein thrombosis in mouse models. Blood. 2011;117:1400–7. doi: 10.1182/blood-2010-05-287623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sevitt S. The structure and growth of valve-pocket thrombi in femoral veins. J Clin Pathol. 1974;27:517–28. doi: 10.1136/jcp.27.7.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Savchenko AS, Hasegawa G, Naito M. Development and maturation of thymic dendritic cells during human ontogeny. Cell Tissue Res. 2006;325:455–60. doi: 10.1007/s00441-006-0202-8. [DOI] [PubMed] [Google Scholar]
- 25.Thevenaz P, Unser M. User-friendly semiautomated assembly of accurate image mosaics in microscopy. Microsc Res Tech. 2007;70:135–46. doi: 10.1002/jemt.20393. [DOI] [PubMed] [Google Scholar]
- 26.Neeli I, Khan SN, Radic M. Histone deimination as a response to inflammatory stimuli in neutrophils. J Immunol. 2008;180:1895–902. doi: 10.4049/jimmunol.180.3.1895. [DOI] [PubMed] [Google Scholar]
- 27.Egan CE, Sukhumavasi W, Bierly AL, Denkers EY. Understanding the multiple functions of Gr-1(+) cell subpopulations during microbial infection. Immunol Res. 2008;40:35–48. doi: 10.1007/s12026-007-0061-8. [DOI] [PubMed] [Google Scholar]
- 28.Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, Taylor FB, Esmon NL, Lupu F, Esmon CT. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15:1318–21. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gamberucci A, Fulceri R, Marcolongo P, Pralong WF, Benedetti A. Histones and basic polypeptides activate Ca2+/cation influx in various cell types. Biochem J. 1998;331 (Pt 2):623–30. doi: 10.1042/bj3310623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kleine TJ, Lewis PN, Lewis SA. Histone-induced damage of a mammalian epithelium: the role of protein and membrane structure. Am J Physiol. 1997;273:C1925–36. doi: 10.1152/ajpcell.1997.273.6.C1925. [DOI] [PubMed] [Google Scholar]
- 31.Wagner DD. Cell biology of von Willebrand factor. Annu Rev Cell Biol. 1990;6:217–46. doi: 10.1146/annurev.cb.06.110190.001245. [DOI] [PubMed] [Google Scholar]
- 32.Guzy RD, Schumacker PT. Oxygen sensing by mitochondria at complex III: the paradox of increased reactive oxygen species during hypoxia. Exp Physiol. 2006;91:807–19. doi: 10.1113/expphysiol.2006.033506. [DOI] [PubMed] [Google Scholar]
- 33.Marcus AJ, Silk ST, Safier LB, Ullman HL. Superoxide production and reducing activity in human platelets. J Clin Invest. 1977;59:149–58. doi: 10.1172/JCI108613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin RC, Mahoney CE, Coleman Anderson L, Ottaviano F, Croce K, Leopold JA, Zhang YY, Tang SS, Handy DE, Loscalzo J. Glutathione peroxidase-3 deficiency promotes platelet-dependent thrombosis in vivo. Circulation. 2011;123:1963–73. doi: 10.1161/CIRCULATIONAHA.110.000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishinaka Y, Arai T, Adachi S, Takaori-Kondo A, Yamashita K. Singlet oxygen is essential for neutrophil extracellular trap formation. Biochem Biophys Res Commun. 2011;413:75–9. doi: 10.1016/j.bbrc.2011.08.052. [DOI] [PubMed] [Google Scholar]
- 36.Gupta AK, Joshi MB, Philippova M, Erne P, Hasler P, Hahn S, Resink TJ. Activated endothelial cells induce neutrophil extracellular traps and are susceptible to NETosis-mediated cell death. FEBS Lett. 2010;584:3193–7. doi: 10.1016/j.febslet.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 37.Hakkim A, Furnrohr BG, Amann K, Laube B, Abed UA, Brinkmann V, Herrmann M, Voll RE, Zychlinsky A. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci U S A. 2010;107:9813–8. doi: 10.1073/pnas.0909927107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andre P, Hartwell D, Hrachovinova I, Saffaripour S, Wagner DD. Pro-coagulant state resulting from high levels of soluble P-selectin in blood. Proc Natl Acad Sci U S A. 2000;97:13835–40. doi: 10.1073/pnas.250475997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Myers DD, Hawley AE, Farris DM, Wrobleski SK, Thanaporn P, Schaub RG, Wagner DD, Kumar A, Wakefield TW. P-selectin and leukocyte microparticles are associated with venous thrombogenesis. J Vasc Surg. 2003;38:1075–89. doi: 10.1016/s0741-5214(03)01033-4. [DOI] [PubMed] [Google Scholar]
- 40.Ward CM, Tetaz TJ, Andrews RK, Berndt MC. Binding of the von Willebrand factor A1 domain to histone. Thromb Res. 1997;86:469–77. doi: 10.1016/s0049-3848(97)00096-0. [DOI] [PubMed] [Google Scholar]
- 41.Brooks EG, Trotman W, Wadsworth MP, Taatjes DJ, Evans MF, Ittleman FP, Callas PW, Esmon CT, Bovill EG. Valves of the deep venous system: an overlooked risk factor. Blood. 2009;114:1276–9. doi: 10.1182/blood-2009-03-209981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dole VS, Bergmeier W, Mitchell HA, Eichenberger SC, Wagner DD. Activated platelets induce Weibel-Palade-body secretion and leukocyte rolling in vivo: role of P-selectin. Blood. 2005;106:2334–9. doi: 10.1182/blood-2005-04-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]