Abstract
The liver specific bile salt export pump (BSEP) is crucial for bile-acid dependent bile flow at the apical membrane. BSEP, a member of the family of structurally related ATP-Binding Cassette (ABC) proteins, is composed of 12 transmembrane segments (TMS) and 2 large cytoplasmic nucleotide binding domains (NBD). The regulation of trafficking of BSEP to and from the cell surface is not well understood, but is believed to play an important role in cholestatic liver diseases such as primary familial intrahepatic cholestasis type 2 (PFIC2). To address this issue, BSEP endocytosis was studied by immunofluorescence and a cell surface ELISA endocytosis reporter system using a chimera of the interleukin 2 receptor α (previously referred to as Tac) and the C-terminal tail of BSEP (TacCterm). An autonomous endocytosis motif in the carboxyl cytoplasmic terminus of BSEP was identified. We define this endocytic motif by site-directed mutagenesis as a canonical tyrosine-based motif 1310YYKLV1314 (Yxx∅). When expressed in HEK293T cells TacCterm is constitutively internalized via a dynamin- and clathrin-dependent pathway. Mutation of the Y1310Y1311 amino acids in TacCterm and in full length human BSEP blocks the internalization. Subsequent sequence analysis reveals this motif to be highly conserved between the closely related ABCB subfamily members that mediate ATP-dependent transport of broad substrate specificity.
Conclusion
Our results indicate constitutive internalization of BSEP is clathrin-mediated and dependent on the tyrosine-based endocytic motif at the C-terminal end of BSEP.
Keywords: bile acid transporter, trafficking motif
The bile salt export pump (BSEP, ABCB11) is an ATP-dependent bile salt pump that functions at the liver canalicular membrane. Mutations in BSEP that result in defective trafficking can cause cholestatic disorders including progressive familial intrahepatic cholestasis type 2 (PFIC2), benign recurrent intrahepatic cholestasis and cholestasis of pregnancy (1-3). The amount of BSEP on the canalicular membrane is regulated by postprandial demand for the enterohepatic circulation of bile salts (4). Pulse-chase experiments revealed a large intracellular pool of Bsep in rat liver that is mobilized for targeting and recycling of Bsep to and from the canalicular membrane (5). Furthermore, Bsep constitutively recycles between the canalicular membrane and Rab11a positive endosomes in WIF-B9 cells (6). Thus, the maintenance and retrieval of BSEP on the apical membrane is crucial for its function (4, 6). The retrieval of Bsep also plays an important role in the pathophysiology of rat cholestatic models. Bsep protein is internalized in isolated perfused rat liver and rat hepatocyte couplets after estradiol 17β-D-glucuronide administration, a process blocked by protein kinase inhibitors or dibutyrl cAMP (7, 8). Cholestasis due to lipopolysaccharide administration, as well as oxidative stress in rats results in internalization of Bsep (9). However, the mechanism by which Bsep is retrieved from the canalicular membrane remains largely unknown.
In MDCK cells, dominant negative expression of Eps15 increases the apical membrane expression of rat Bsep suggesting that a clathrin-dependent mechanism may play a role in regulating cell surface Bsep expression (10). Clathrin has previously been shown to be involved in apical endocytosis in rat hepatocytes (11). Targeting and trafficking of membrane proteins depend on sequence motifs and protein-protein interaction with various trafficking machinery (12). Targeting of a number of membrane proteins to coated pits and their traffic through endocytic compartments are generally mediated by endocytic signals located at the cytoplasmic domain of proteins (13-16). Two major types of signals have been described to regulate targeting and trafficking of a number of receptor proteins: 1. a tyrosine-based YXX∅ sequence where ∅ is an amino acid with a bulky hydrophobic group, and 2. dileucine-based LL motifs where the latter leucine may be replaced by a hydrophobic amino acid residue (16-19). Depending on the primary sequence context and the relative position of the motif to the membrane, these motifs serve as plasma membrane targeting signals (20, 21). They are also utilized for efficient sorting to the endosomal system where the protein may be recycled or transported to the lysosome for degradation (22). The adaptor AP2 is a plasma membrane localized clathrin adaptor whose subunits bind directly to tyrosine-based Yxx∅ or NPXY internalization motifs within cytoplasmic or transmembrane regions of proteins to mediate clathrin-dependent endocytosis (23-27). For example, the cytosolic domain of another ABC transporter, Cystic Fibrosis Transmembrane Regulator (CFTR) has numerous consensus endocytic motifs that regulate the clathrin-dependent endocytosis (14, 28). However, the presence of discrete sorting signals in the cytosolic tail of BSEP has not been elucidated.
In this study, we tested whether the C-terminus of human BSEP has a targeting/trafficking signal and whether it contributes to the cell surface expression of BSEP. We demonstrated by domain swapping using the C-terminal region of BSEP attached to Tac (IL2Rα) that an internalization motif is present in BSEP. Tac is a cell surface type 1 transmembrane protein that is targeted to the plasma membrane by default. Tac chimeras have been extensively used for the identification of trafficking signals because of the monomeric nature of the Tac protein and the existence of highly specific N-terminal antibodies to track the chimeric protein (29). We identify by mutagenesis analysis the exact motif in BSEP as a YYKLV sequence. By transferring this YYKLV motif directly to Tac, we further showed that this motif is sufficient for internalization. Finally, we demonstrated that this motif is functional in the full length human BSEP.
EXPERIMENTAL PROCEDURES
Cell Culture
HeLa, HEK293T and COS-7 were maintained in Dulbecco’s modified medium (Invitrogen) supplemented with 10% fetal calf serum (Invitrogen) containing penicillin and streptomycin.
Construction of Tac Chimeras
The Tac backbone construct was kindly provided by Dr. John Marshall (Brown University). The schematic structure of the TacCterm chimeras, consisting of the extracellular and transmembrane domains of Tac and the C-terminal tail of BSEP, is shown in Figure 1. The Tac coding sequence was amplified by PCR insertion of EcoRV and XbaI sites for subcloning into pcDNA3 (Invitrogen, San Diego, CA, U.S.A). TacCterm chimeras were constructed by two-stage PCR method, using two sets of overlapping primers and ligating into the Tac construct using EcoRV and the XbaI site. The C-terminal tail of BSEP encoding residues D1284 to S1321 was amplified using human BSEP (kindly provided by Dr. Bruno Stieger, University Hospital, Zurich). Deletion mutants of TacCterm (del 1298-1316; del1308-1316) and alanine substitutions in the following mutants were generated by site-directed mutagenesis: YY (Y1310A, Y1311A); LM (L1303A, M1304A); LMYY (L1303A, M1303A, Y1310A, Y1311A). A shorter version of the C-terminal BSEP, Tac8A-YY, was also generated by inserting 8 alanines and the corresponding two residues G1308AYYKLV1314). All constructs were confirmed by DNA sequencing.
Figure 1.
Identification and analysis of putative tyrosine-based and leucine-based motifs in BSEP. (A) Sequence alignment of BSEP from ten different species. The C-terminal 36-amino acid of BSEP contain a putative tyrosine-based motif (Y1310YKLV) and a leucine-based (THEEL1301M) motif. The latter has been predicted to be a casein kinase II site. (B) Schematic representation of the Tac-chimeras that were constructed as described in Materials and Methods. Tac chimeras (TacCterm and Tac-8AGAYYKLV) are constructs with the arrangement of Tac (open bar) and the C-terminal cytoplasmic tail of BSEP (filled bar) as illustrated. The BSEP C-terminal sequences are indicated with numbers representing the original BSEP sequence. Tac-8AGAYYKLV is the minimal BSEP construct consisting of eight alanines (8A) inserted between the Tac fragment and the short BSEP sequence, YYKLV, to generate Tac-8AGAYYKLV and different mutations as described in the text. TM refers to the transmembrane segment of Tac.
Construction of full length human BSEP
Human full-length BSEP was amplified by PCR and inserted into the EcoRV site of pWAY21-EGFP expression vector provided by Dr. Anton Bennett, Yale University. Mutant GFP-BSEP (Y1310A/Y1311A) was generated by site-directed mutagenesis using the QuickChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA).
Monitoring TAC internalization
HEK293T cells were plated on poly-L-lysine coverslips and transiently transfected using LipofectAMINE 2000 reagent (Invitrogen) for 18 hours. Cells were washed with ice cold PBS (with Mg and Ca) and labeled with mouse anti-Tac antibody (IL2R, 0.5ug/ml, 30min, 4°C) (BD Transduction Laboratories, San Jose, CA) in labeling buffer (PBS/Mg/Ca/0.2%BSA). Internalization was initiated by warming to 37°C and carried out for the indicated time, then stopped by washing repeatedly with ice cold labeling buffer. Cells were fixed in 4% paraformaldehyde and washed with PBS and permeabilized with 0.1% Triton X-100. TacCterm-anti-Tac complexes were detected with Alexa488 or Alexa-568 anti-mouse secondary antibody (1:500; 1 hr) and fluorescent images were acquired on an LSM 510 confocal microscope (Carl Zeiss Inc, Thornwood, NY).
Inhibition of internalization by dominant negative constructs
Internalization of Tac-chimeras was examined after co-transfection with dominant negative construct K44A dynamin (provided by Dr. Pietro de Camilli, Yale University, New Haven, CT) and with wildtype Rab5a-DsRed and dominant negative N133I Rab5a-DsRed kindly provided by Dr. Richard Pagano (Addgene plasmid 13050, 13051, Cambridge, MA).
Cell ELISA
The internalization efficiency of Tac was quantitated by determining the cell surface density of Tac before and after internalization at 37°C for 20 min. After fixation with 2% paraformaldehyde, cells were stained with HRP-goat anti-mouse secondary antibody for 1 hr and developed using 3,3′,5,5′-tetramethylbenzidine liquid substrate for 15 min. Endpoint absorbance measurements were taken at 450nm using a Synergy 2 plate reader (BioTek Instruments Inc, Winooski, VT). The rate of internalization of the TacCterm chimeras was expressed as a percentage of the decrease in the initial surface binding at 4°C. Background of HEK293T transfected with empty vector was subtracted from each absorbance measurement. All experiments were performed in quadruplicate and repeated at least three times.
Surface biotinylation and endocytosis of full length human BSEP
Surface biotinylation of BSEP was performed as previously described, with some modifications (30, 31). HEK293T cells were grown on poly-lysine coated 6-well plates and transiently transfected using LipofectAMINE 2000 reagent for 48 hours. Cells were cooled to 4°C and washed three times with PBS (Ca++, Mg++). The plasma membrane proteins were biotinylated in PBS buffer containing 1 mg/ml sulfo-NHS-SS-biotin (Pierce, Rockford, IL) for one hour. After biotinylation, cells were washed with quenching buffer (100mM glycine in PBS buffer) to remove excess free biotin and then washed twice with PBS. The cells were either lysed immediately with M-PER Mammalian Reagent (Thermo scientific, Rockford, IL) containing protease inhibitor cocktail or warmed to 37°C and incubated for 0, 2.5, 5, 10, or 20 min to allow for endocytosis. After 20 min the cells were quickly cooled to 4°C and biotinylated protein remaining at the cell surface was stripped with three 10-min washes in sodium 2-mercaptoethanesulfonate (MESNA) stripping buffer (50 mM 2-mercaptoethanesulfonic acid, 150 mM NaCl, 1 mM EDTA, 0.2% BSA, and 20 mM Tris, pH 8.6). Excess MESNA was removed with three 5-min washes in iodoacetamide buffer (50 mM iodoacetamide in PBS). Equal amount of protein in the cell lysates was incubated overnight at 4°C with Streptavidin agarose resin (Thermo, Rockford, IL). Biotinylated proteins were eluted in 2x SDS buffer, resolved by SDS-PAGE, transferred to nitrocellulose membrane, and immunoblotted with anti-GFP antibody (Clontech, Mountain View, CA).
RESULTS
C-terminal tail of BSEP contains an endocytic sorting motif
A sequence alignment of the C-terminal cytoplasmic tail of BSEP from ten different species showed the presence of highly conserved consensus Tyr- and Leu-based endocytic sorting signals (Figure 1A). The C-terminal cytoplasmic tail encompassing residues 1284-1321 contains a putative leucine-based signal (Leu1298-Met) and a tyrosine-based signal (Tyr1310-Try-Lys-Leu-Val). To assess whether targeting information is present in the C-terminal cytoplasmic tail of BSEP, we constructed a Tac chimera consisting of the last 38 amino acids (residue 1284-1321) of BSEP (TacCterm, Fig 1B). The localization of the TacCterm was followed by live cell labeling at 4°C with an antibody specific for the extracellular domain of Tac, followed by shifting to 37°C. After 10 min, TacCterm showed plasma membrane localization with small amounts localized to peripheral vesicles (Figure 2 bottom). Internalization from the plasma membrane continued over the 60 min with an increase in the punctuate vesicular fluorescent pattern and, in addition, some shifting to a perinuclear location resembling a recycling endosomal compartment. Minimal internalization from the plasma membrane was seen in the cells transfected with the Tac reporter alone (Figure 2 top). These data were confirmed in COS-7 and HeLa (data not shown). These observations suggest that endocytic sorting signals in the C-terminus of BSEP are functional. Immunofluorescence experiments suggested that the internalized TacCterm was localized to the endosomal compartments (data not shown).
Figure 2.
Internalization of TacCterm in HEK293T cells. (A) HEK293T cells were transiently transfected with TacCterm and internalization was detected after antibody labeling at 4°C and shifting to 37°C. Whereas only a small number of punctuate structures were detected in control Tac transfected cells, cytoplasmic puncta were readily evident in TacCterm, indicating internalization of the construct. Internalized TacCterm first presents (5–10 min) in a number of small vesicles and later (40 min) appears in larger, perinuclear vesicles.
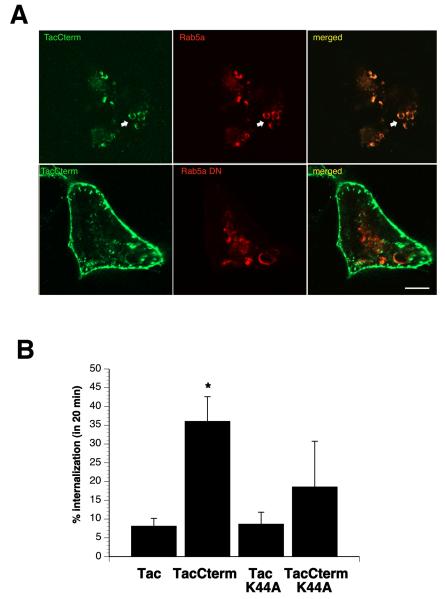
BSEP is internalized through a Rab5a and dynamin-dependent process
In the early endocytic pathway, Rab5 regulates clathrin-coated vesicle mediated transport from the plasma membrane to the early endosomes as well as homotypic early endosome fusion (32, 33). Therefore we compared the effect of co-transfection with Rab5a and Rab5a dominant negative construct (Rab5a DN, I133N) on the internalization of TacCterm in order to determine if these vesicles were internalized via a clathrin dependent pathway. TacCterm co-localized with Rab5a-DsRed in swollen endosomes in co-transfected cells (Figure 3A top). In contrast, when Rab5a DN was co-transfected, there appeared to be less internalization of TacCterm into the endocytic compartment (Figure 3A bottom). This Rab5a DN mutant has reduced GTPase activity and is a potent stimulator of homotypic fusion between early endosomes (34). Western blotting and cell ELISA experiments demonstrated that co-transfection with Rab5a resulted in slightly, but not significantly, less total and surface TacCterm (Supplemental Fig 1A and B). Internalization of TacCterm was slightly higher in cells transfected with Rab5a and slightly lower in the presence of Rab5a DN compared to TacCterm alone, although neither were statistically different (Supplemental Fig 1C). These results suggest that TacCterm most probably enters the early endosomal vesicles following a clathrin-dependent pathway.
Figure 3.
Inhibition of internalization by dominant negative (DN) Rab5a-DSRed, N133I, and DN dynamin, K44A. (A) HEK293T cells were co-transfected with Rab5a (top) or Rab5aDN (bottom) and TacCterm construct. After 18 hrs of transient expression, the cell surface was labeled with anti-Tac antibody at 4° C, and then allowed to internalize at 37°C for 20 min. Endocytosed TacCterm (green) is colocalized with Rab5a-DSRed on swollen endosomes (top). However, expression of Rab5aDN-DSRed, N131I, partially prevented the internalization of surface TacCterm, as shown by accumulation of vesicles and small tubules at the periphery of the cell (bottom) Bar=5μm. (B) Quantitation of internalization of TacCterm after co-transfection with DN dynamin, K44A, was performed using a cell ELISA, as described in Material and Methods. TacCterm is internalized approximately 5 fold more than control Tac and this internalization of TacCterm was partially reduced after co-transfection with K44A dynamin. Tac control transfected with K44A dynamin was not significantly different than control Tac alone. N=3. Astericks indicate significant difference in percent internalization between Tac and TacCterm with p<0.05 using student two tailed t-test.
Clathrin-dependent and a subset of clathrin-independent endocytosis require the activity of dynamin, an ATPase responsible for pinching vesicles from the plasma membrane and therefore driving cargo internalization into carrier vesicles (35, 36). To determine if TacCterm internalization was dynamin-dependent, a dominant-negative dynamin mutant (K44A-GFP) was transfected with TacCterm into HEK293T cells. Cell ELISA experiments revealed approximately 40% loss of surface TacCterm after 20-min internalization and this internalization was partially blocked by the expression of K44A dynamin (Figure 3C). These observations suggest that BSEP is internalized through a Rab5a and dynamin-dependent process.
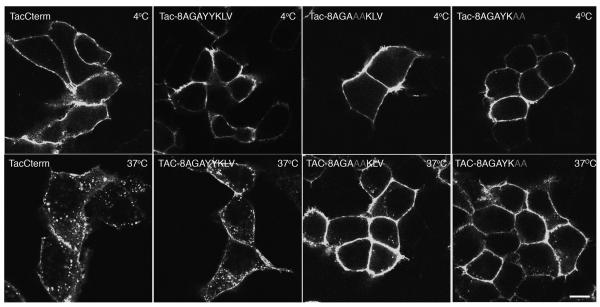
Minimal tyrosine-based sequence (GAYYKLV) is sufficient for Tac internalization
The C-terminal tail of BSEP encompassing residue 1284-1321 contains a canonical Tyr-based signal (Y1310-Y-K-L-V) that might overlap a leucine-based (Leu1301-M) signal. To assess these signals, we appended the seven amino acids (GAYYKLV) to the Tac molecule with an eight alanine amino acid linker region to investigate the ability of this motif to internalize. We found that Tac-8AGAYYKLV was able to internalize into punctuate structures (Figure 4). Mutations of Y1310Y1311 or L1313V1314 to alanines abolished the internalization of the Tac chimera and caused the protein to be retained at the plasma membrane (Figure 4). These data demonstrated that the Y1310Y1311 and L1313V1314 amino acids are important for internalization.
Figure 4.
Tac-8A-GAYYKLV is sufficient for the internalization in HEK293T cells. The Tac-8AGAYYKLV was constructed with eight alanines (8A) followed by the tyrosine-based motif (GAYYKLV) fused to the C-terminal end of the Tac protein. After 20 min at 37°C Tac-GAYYKLV has been internalized into punctuate structures similar to those seen with TacCterm construct. The internalization was abolished in the mutant TAC-8AGAAAKLV, where both tyrosine residues were mutated to alanine residues or in the mutant TAC-8AGAYKAA, where the lysine and valine residue was mutated to alanine residues. Bar=5μm
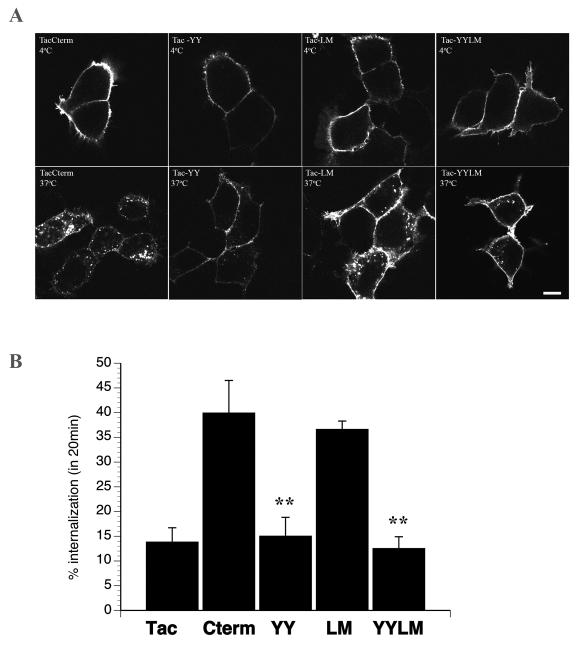
Internalization efficiency of Tac-BSEP chimeras
In order to investigate the relative contribution of the tyrosine-based motif compared to the leucine-based motif within the 38-amino acid C-terminal end of BSEP, we mutated Y1310Y1311 (Tac-YY), L1303M1304 (Tac-LM), or both (Tac-YYLM) to alanine residues and observed their internalization by immunofluorescence and cell ELISA. Compared to TacCterm, Tac-YY remained on the cell surface after internalization for 20 min at 37°C (Figure 5A). However, the Tac-LM internalized to a similar extent as TacCterm indicating that these two residues do not contribute to internalization of this construct (Figure 5A). In addition, mutating both putative motifs resulted in loss of internalization compared to TacCterm, similar to the Tac-YY (Figure 5A). Quantitation of internalization of these Tac chimeras was then determined using a cell ELISA internalization assay. In HEK293T cells, 40% of cell surface TacCterm was internalized in 20 min, resulting in a rate of ~2%/min (Figure 5B). In contrast, Tac alone was internalized at a rate of ~0.5% /min (Figure 5B). The efficiency of internalization was completely abolished in Y1310Y1311 mutant. Mutation of L1303M1304 did not lead to a significant decrease in internalization confirming that the leucine-based motif does not contribute significantly to endocytosis. Therefore, the Y1310Y1311 is the predominant signal for internalization.
Figure 5.
The tyrosine-containing motif is essential for the internalization of TacCterm. (A) To investigate the role of the tyrosine-based (YYKLV) or leucine-based (LM) motif within the C-terminal end of BSEP, alanine substitutions were made in these amino acids and constructs were designated as follows: 1. Tac-YY (Y1310Y1311 ), 2. Tac-LM (L1303M1304) and 3. Tac-YYLM. Anti-Tac labeling at 4°C and internalization for 20 min was performed as previously described. Note the lack of internalization for the Tac-YY and Tac-YYLM mutants. Bar=5μm. (B) The amount of internalization from the cell surface was quantitated by cell ELISA as described in the Materials and Methods. Tac was internalized 13.1+3.1% and TacCterm was internalized 41.1+7.6% after 20 min at 37°C. Mutating Y1310Y1311 to alanine residues (Tac-YY) inhibited internalization completely (15.3+4.5%). Mutating L1303M1304 to alanine residues (Tac-LM) did not affect internalization with 36.3+2.3% internalized after 20 min. Mutating all four residues (Tac-YYLM) resulted in complete inhibition of internalization (12.7+3.0%). Results are means ± SD for three separate experiments performed in quadruplicate. Astericks indicate significant difference in percent internalization between TacCterm and Tac-YY or Tac-YYLM with p<0.05 using student two tailed t-test.
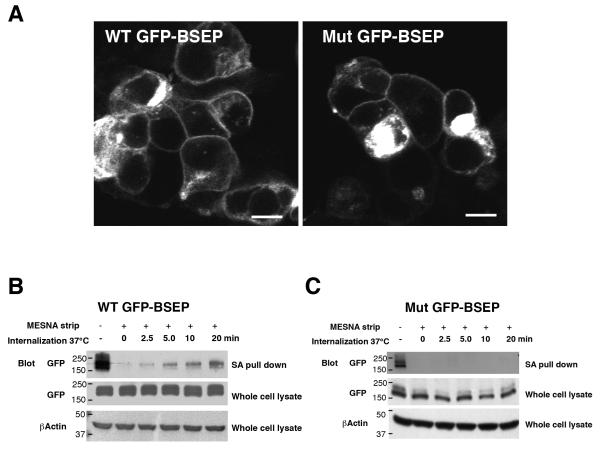
Mutation of the tyrosine-based motif in full length BSEP prevents endocytosis
Transfection of HEK293T cells with full length BSEP with or without the tyrosine mutations was then carried out in order to confirm the importance of this motif in endocytosis. Immunofluorescence showed that GFP-BSEP was expressed on the cell surface in both the WT and mutant transfected cells (Fig 6A). Cell surface biotinylation was used to label the BSEP prior to internalization and membrane stripping. Strepavidin bead pull down demonstrated that full length WT GFP-BSEP that had been expressed on the plasma membrane could be efficiently endocytosed (Figure 6B). Densitometry of the blots revealed that this internalization occurred at a rate similar to that demonstrated with TacCterm (Supplemental Fig 2). Mutation of Y1310Y1311 to alanines did not prevent the cell surface expression of BSEP (Fig. 6A), although there tended to be lower expression of the mutant protein on the cell surface in this transient cell system. Despite this, no endocytosis was seen with the mutant protein, even upon prolonged exposure of the blots (Figure 6C). These data confirm that the tyrosine motif in the C-terminus of BSEP is essential to normal endocytosis.
Figure 6.
Mutation of the tyrosine-based motif in full length BSEP prevents endocytosis (A) HEK293T cells were transiently transfected with full length WT GFP-BSEP or Mut (A1310A1311) GFP-BSEP, fixed with 4% paraformaldehyde, and GFP fluorescence observed on a confocal microscope. Both cells showed cell surface expression of the GFP constructs. Bar=10 μm. (B and C) GFP-BSEP expressed on the cell surface was biotinylated as described in Methods and the cells were warmed to 37°C to follow the endocytic internalization. At the specified time points GFP-BSEP remaining on the cell surface was stripped with MESNA and the cells were lysed. The biotinylated protein internalized from the cell surface was subjected to streptavidin agarose (SA) pull down. Total cell lysates and SA bound proteins were separated by PAGE, transferred to nitrocellulose membrane and blotted with antibody to GFP or βactin. (B) WT GFP-BSEP was endocytosed in a time dependent manner. (C) No internalized Mut GFP-BSEP was detected even after prolonged exposure of the blot, confirming that the C-terminal tyrosine motif is important in BSEP endocytosis. Whole cell lysates indicate that the expression of the GFP-BSEP constructs was similar in all dishes and blotting with antibody to βactin showed equal loading in all lanes.
DISCUSSION
BSEP is the essential determinant of bile salt dependent bile formation. Loss of BSEP expression and function is associated with severe cholestatic disorders and hepatocellular carcinoma (1, 2, 37). Despite this crucial role in liver biology, we still know very little of the cellular mechanisms controlling BSEP expression on the plasma membrane or its functional activity. In this study, we examined the cellular mechanisms and the specific motif in the human BSEP molecule that is responsible for its internalization. We have demonstrated that the amino acids YYKLV in the cytoplasmic tail are sufficient to internalize BSEP and to sort it to the endosomal pathway. Mutation of both tyrosine residues in this motif is sufficient to completely block internalization suggesting that within the C-terminal 38 amino acids, this motif provides the predominant endocytic signal.
In this study, we are also able to follow the TacCterm internalization into early endosomes and to partially block internalization by dominant negative K44A dynamin and dominant negative I133N Rab5a constructs, supporting their contribution to this process. We estimated that BSEP is internalized at a constitutive rate of ~2%/min, consistent with prior photobleaching recovery experiments of rat canalicular membrane Bsep (6). Taken together, these results indicate that a clathrin-dependent pathway is involved in the endocytosis of BSEP from the cell surface. Previously, Ortiz (10) has shown that rat Bsep levels are increased in the apical membranes of MDCK cells co-transfected with dominant negative Eps15, a component of clathrin-dependent endocytic machinery. In addition, Bsep has been found in a clathrin-coated vesicle fraction after membrane fractionation of rat hepatocytes (10). This is the first study to identify a tyrosine-based YYKLV motif in the carboxyl-terminus of BSEP that can be internalized through a dynamin-dependent endocytic pathway.
Sequence alignment of the C-terminal region (amino acid 1284-1321) of human BSEP containing the endocytic motif reveals that this tyrosine-based motif is highly conserved within the ABCB subfamily (Supplemental Fig 3). This suggests that the mechanisms controlling the constitutive endocytosis of this ABC subfamily of proteins may also be mediated by a clathrin-dependent mechanism. A comparison of the carboxyl tail of other ABC transporters indicates the presence of conserved leucine and tyrosine-based motifs. Trafficking studies that rely on transposing putative endocytic signals such as the one described in this study have not been carried out for these other ABC proteins except CFTR (14, 28, 39). Hu et al demonstrated that appending tyrosine or dileucine based motifs of CFTR to a Tac reporter allows for rapid internalization, indicating that the carboxyl-terminus of CFTR contains endocytic signals (14). However, identifying endocytic signals in a full length polytopic protein is often difficult because creating mutations in the putative sequence by alanine scanning or sequential deletion may lead to misprocessing of the full length protein and hamper its trafficking to the plasma membrane. For example, in full-length MDR1, mutations of analogous leucine or tyrosine residues led to misprocessing and ER retention, precluding the evaluation of its targeting function (40). However, we were able to successfully mutate the tyrosines in the carboxyl-terminus of full length BSEP and observe the same defect in endocytosis that we had demonstrated in TacCterm.
To date, there are no known disease producing point mutations of human BSEP in the identified endocytic signal region, however, there are premature stop codons that lead to the deletion of the tyrosine-based motif (3). Deletion of a major portion of the carboxyl-terminus in a human disease-causing Bsep mutant in the rat (R1050X) showed proper targeting to the apical membrane of MDCK cells indicating that a large portion of the C-terminal nucleotide binding domain is not necessary for biosynthetic processing and apical targeting (41). However, we and others have identified a number of BSEP mutations that cause a reduction of Bsep on the cell surface through increased rate of internalization in heterologous expression systems (30, 41). This loss of Bsep protein from the canalicular membrane is characteristic of some forms of experimental cholestatic liver injury, as well as human cholestatic liver diseases. Cholestasis induced by estradiol-17β-D-glucuronide, taurolithocholic acid, cyclosporine A and lipopolycharide all result in redistribution of Bsep to the subapical cytoplasm (7, 8, 42, 43). Efforts have been made to compensate for the loss of cell surface BSEP with the administration of chemical or pharmacological agents such as MG-132 or sodium phenylbutyrate (30, 41, 44). Although the mechanisms of action are not clearly defined for these agents, one possible explanation for the increase of BSEP cell surface expression is that these compounds limit the extent of ubiquitinylation of BSEP (45). Ubiquitinylation of membrane proteins and endocytic adaptor proteins attenuates signaling of ligand-dependent activation of receptors by targeting these receptors to the endolysosomal pathway for degradation. Hayashi et al (45) showed that attaching short chain ubiquitin to BSEP shortens the half-life of cell surface BSEP. Thus, the effects of reduced cell surface expression of BSEP in the absence of a defect in biosynthesis may be explained by enhanced endocytosis as a result of posttranslational modification, such as ubiquitinylation or phosphorylation, of the protein.
Previous studies have suggested that BSEP is mobilized from an apical recycling pool for insertion into the canalicular membrane to increase its transport capacity when needed. Once on the membrane, BSEP resides in caveolin-1, “lubrol-X-resistant” microdomains (46). In this study, TacCterm internalization is diminished in the presence of dominant negative K44A dynamin suggesting that caveolar-dependent endocytosis may also be involved since the latter is dependent on the activity of dynamin (47). However, mice infected with recombinant caveolin-1 and caveolin-2 have significant increases in the bile acid (taurocholate) secretory maximum (x 2.5) with no detectable changes in Bsep levels (48). Disrupting cholesterol content of the canalicular membrane also did not affect the levels of Bsep at the canalicular membrane but instead affected its functional activity (49). Taken together with the results presented in this study, clathrin-dependent endocytosis would appear to be the key pathway for regulating BSEP internalization.
In summary, we have identified a signaling motif for endocytosis of BSEP within the 36-amino acids C-terminal end of human BSEP to the exclusion of other signals. Based on this study, the YYKLV sequence is the predominant signal for the internalization of BSEP into early endosomes. We also suggest that this is a clathrin dependent process. We anticipitate that further studies of the mechanisms that regulate BSEP endocytosis will help us to understand how BSEP is retrieved from the cell surface in cholestasis.
Supplementary Material
Acknowledgments
This study was supported by American Liver Foundation Liver Scholar Award (PL), and USPHS grants R37 DK25636 (JLB) and DK P30-34989 (Yale Liver Center)
Abbreviations
- BSEP/Bsep
Bile salt export pump
- ABC
ATP-Binding Cassette
- TMS
transmembrane segments
- NBD
nucleotide binding domains
- PFIC2
primary familial intrahepatic cholestasis type 2
- TacCterm
Tac-C-terminal tail of BSEP chimera
- AP
adaptin
- CFTR
Cystic Fibrosis Transmembrane Regulator
- Tyr
tyrosine
- Leu
leucine
REFERENCES
- 1.Jansen PL, Strautnieks SS, Jacquemin E, Hadchouel M, Sokal EM, Hooiveld GJ, Koning JH, et al. Hepatocanalicular bile salt export pump deficiency in patients with progressive familial intrahepatic cholestasis. Gastroenterology. 1999;117:1370–1379. doi: 10.1016/s0016-5085(99)70287-8. [DOI] [PubMed] [Google Scholar]
- 2.Strautnieks SS, Bull LN, Knisely AS, Kocoshis SA, Dahl N, Arnell H, Sokal E, et al. A gene encoding a liver-specific ABC transporter is mutated in progressive familial intrahepatic cholestasis. Nat Genet. 1998;20:233–238. doi: 10.1038/3034. [DOI] [PubMed] [Google Scholar]
- 3.Strautnieks SS, Byrne JA, Pawlikowska L, Cebecauerova D, Rayner A, Dutton L, Meier Y, et al. Severe bile salt export pump deficiency: 82 different ABCB11 mutations in 109 families. Gastroenterology. 2008;134:1203–1214. doi: 10.1053/j.gastro.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 4.Kipp H, Pichetshote N, Arias IM. Transporters on demand: intrahepatic pools of canalicular ATP binding cassette transporters in rat liver. Journal of Biological Chemistry. 2001;276:7218–7224. doi: 10.1074/jbc.M007794200. [DOI] [PubMed] [Google Scholar]
- 5.Kipp H, Arias IM. Newly synthesized canalicular ABC transporters are directly targeted from the Golgi to the hepatocyte apical domain in rat liver. Journal of Biological Chemistry. 2000;275:15917–15925. doi: 10.1074/jbc.M909875199. [DOI] [PubMed] [Google Scholar]
- 6.Wakabayashi Y, Lippincott-Schwartz J, Arias IM. Intracellular trafficking of bile salt export pump (ABCB11) in polarized hepatic cells: constitutive cycling between the canalicular membrane and rab11-positive endosomes. Mol Biol Cell. 2004;15:3485–3496. doi: 10.1091/mbc.E03-10-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crocenzi FA, Mottino AD, Cao J, Veggi LM, Pozzi EJ, Vore M, Coleman R, et al. Estradiol-17beta-D-glucuronide induces endocytic internalization of Bsep in rats. Am J Physiol Gastrointest Liver Physiol. 2003;285:G449–459. doi: 10.1152/ajpgi.00508.2002. [DOI] [PubMed] [Google Scholar]
- 8.Crocenzi FA, Pozzi EJ Sanchez, Ruiz ML, Zucchetti AE, Roma MG, Mottino AD, Vore M. Ca(2+)-dependent protein kinase C isoforms are critical to estradiol 17beta-D-glucuronide-induced cholestasis in the rat. Hepatology. 2008;48:1885–1895. doi: 10.1002/hep.22532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elferink MG, Olinga P, Draaisma AL, Merema MT, Faber KN, Slooff MJ, Meijer DK, et al. LPS-induced downregulation of MRP2 and BSEP in human liver is due to a posttranscriptional process. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1008–1016. doi: 10.1152/ajpgi.00071.2004. [DOI] [PubMed] [Google Scholar]
- 10.Ortiz DF, Moseley J, Calderon G, Swift AL, Li S, Arias IM. Identification of HAX-1 as a protein that binds bile salt export protein and regulates its abundance in the apical membrane of Madin-Darby canine kidney cells. Journal of Biological Chemistry. 2004;279:32761–32770. doi: 10.1074/jbc.M404337200. [DOI] [PubMed] [Google Scholar]
- 11.Rahner C, Stieger B, Landmann L. Apical Endocytosis in Rat Hepatocytes In Situ Involves Clathrin, Traverses a Subapical Compartment, and Leads to Lysosomes. Gastroenterology. 2000;119:1692–1707. doi: 10.1053/gast.2000.20233. * * [DOI] [PubMed] [Google Scholar]
- 12.Boll W, Rapoport I, Brunner C, Modis Y, Prehn S, Kirchhausen T. The mu2 subunit of the clathrin adaptor AP-2 binds to FDNPVY and YppO sorting signals at distinct sites. Traffic. 2002;3:590–600. doi: 10.1034/j.1600-0854.2002.30808.x. [DOI] [PubMed] [Google Scholar]
- 13.Colgan L, Liu H, Huang SY, Liu YJ. Dileucine motif is sufficient for internalization and synaptic vesicle targeting of vesicular acetylcholine transporter. Traffic. 2007;8:512–522. doi: 10.1111/j.1600-0854.2007.00555.x. [DOI] [PubMed] [Google Scholar]
- 14.Hu W, Howard M, Lukacs GL. Multiple endocytic signals in the C-terminal tail of the cystic fibrosis transmembrane conductance regulator. Biochem J. 2001;354:561–572. doi: 10.1042/0264-6021:3540561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peter K, Varga K, Bebok Z, McNicholas-Bevensee CM, Schwiebert L, Sorscher EJ, Schwiebert EM, et al. Ablation of internalization signals in the carboxyl-terminal tail of the cystic fibrosis transmembrane conductance regulator enhances cell surface expression. Journal of Biological Chemistry. 2002;277:49952–49957. doi: 10.1074/jbc.M209275200. [DOI] [PubMed] [Google Scholar]
- 16.Wu H, Windmiller DA, Wang L, Backer JM. YXXM motifs in the PDGF-beta receptor serve dual roles as phosphoinositide 3-kinase binding motifs and tyrosine-based endocytic sorting signals. Journal of Biological Chemistry. 2003;278:40425–40428. doi: 10.1074/jbc.C300225200. [DOI] [PubMed] [Google Scholar]
- 17.Dittrich E, Haft CR, Muys L, Heinrich PC, Graeve L. A di-leucine motif and an upstream serine in the interleukin-6 (IL-6) signal transducer gp130 mediate ligand-induced endocytosis and down-regulation of the IL-6 receptor. Journal of Biological Chemistry. 1996;271:5487–5494. doi: 10.1074/jbc.271.10.5487. [DOI] [PubMed] [Google Scholar]
- 18.Jockers R, Angers S, Da Silva A, Benaroch P, Strosberg AD, Bouvier M, Marullo S. Beta(2)-adrenergic receptor down-regulation. Evidence for a pathway that does not require endocytosis. Journal of Biological Chemistry. 1999;274:28900–28908. doi: 10.1074/jbc.274.41.28900. [DOI] [PubMed] [Google Scholar]
- 19.Ribeiro FM, Black SA, Cregan SP, Prado VF, Prado MA, Rylett RJ, Ferguson SS. Constitutive high-affinity choline transporter endocytosis is determined by a carboxyl-terminal tail dileucine motif. J Neurochem. 2005;94:86–96. doi: 10.1111/j.1471-4159.2005.03171.x. [DOI] [PubMed] [Google Scholar]
- 20.Harter C, Mellman I. Transport of the lysosomal membrane glycoprotein lgp120 (lgp-A) to lysosomes does not require appearance on the plasma membrane. J Cell Biol. 1992;117:311–325. doi: 10.1083/jcb.117.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yokode M, Pathak RK, Hammer RE, Brown MS, Goldstein JL, Anderson RG. Cytoplasmic sequence required for basolateral targeting of LDL receptor in livers of transgenic mice. J Cell Biol. 1992;117:39–46. doi: 10.1083/jcb.117.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Obermuller S, Kiecke C, von Figura K, Honing S. The tyrosine motifs of Lamp 1 and LAP determine their direct and indirect targetting to lysosomes. J Cell Sci. 2002;115:185–194. doi: 10.1242/jcs.115.1.185. [DOI] [PubMed] [Google Scholar]
- 23.Bairstow SF, Ling K, Su X, Firestone AJ, Carbonara C, Anderson RA. Type Igamma661 phosphatidylinositol phosphate kinase directly interacts with AP2 and regulates endocytosis. Journal of Biological Chemistry. 2006;281:20632–20642. doi: 10.1074/jbc.M601465200. [DOI] [PubMed] [Google Scholar]
- 24.Chen WJ, Goldstein JL, Brown MS. NPXY, a sequence often found in cytoplasmic tails, is required for coated pit-mediated internalization of the low density lipoprotein receptor. Journal of Biological Chemistry. 1990;265:3116–3123. [PubMed] [Google Scholar]
- 25.Collins BM, McCoy AJ, Kent HM, Evans PR, Owen DJ. Molecular architecture and functional model of the endocytic AP2 complex. Cell. 2002;109:523–535. doi: 10.1016/s0092-8674(02)00735-3. [DOI] [PubMed] [Google Scholar]
- 26.Kittler JT, Chen G, Honing S, Bogdanov Y, McAinsh K, Arancibia-Carcamo IL, Jovanovic JN, et al. Phospho-dependent binding of the clathrin AP2 adaptor complex to GABAA receptors regulates the efficacy of inhibitory synaptic transmission. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:14871–14876. doi: 10.1073/pnas.0506653102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Owen DJ, Evans PR. A structural explanation for the recognition of tyrosine-based endocytotic signals. Science. 1998;282:1327–1332. doi: 10.1126/science.282.5392.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prince LS, Peter K, Hatton SR, Zaliauskiene L, Cotlin LF, Clancy JP, Marchase RB, et al. Efficient endocytosis of the cystic fibrosis transmembrane conductance regulator requires a tyrosine-based signal. Journal of Biological Chemistry. 1999;274:3602–3609. doi: 10.1074/jbc.274.6.3602. [DOI] [PubMed] [Google Scholar]
- 29.Tsudo M, Kozak RW, Goldman CK, Waldmann TA. Contribution of a p75 interleukin 2 binding peptide to a high-affinity interleukin 2 receptor complex. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:4215–4218. doi: 10.1073/pnas.84.12.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lam P, Pearson CL, Soroka CJ, Xu S, Mennone A, Boyer JL. Levels of plasma membrane expression in progressive and benign mutations of the bile salt export pump (Bsep/Abcb11) correlate with severity of cholestatic diseases. Am J Physiol Cell Physiol. 2007;293:C1709–1716. doi: 10.1152/ajpcell.00327.2007. [DOI] [PubMed] [Google Scholar]
- 31.Nishimura N, Sasaki T. Cell-surface biotinylation to study endocytosis and recycling of occludin. Methods Mol Biol. 2008;440:89–96. doi: 10.1007/978-1-59745-178-9_7. [DOI] [PubMed] [Google Scholar]
- 32.Bucci C, Parton RG, Mather IH, Stunnenberg H, Simons K, Hoflack B, Zerial M. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992;70:715–728. doi: 10.1016/0092-8674(92)90306-w. [DOI] [PubMed] [Google Scholar]
- 33.Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 34.Sharma DK, Choudhury A, Singh RD, Wheatley CL, Marks DL, Pagano RE. Glycosphingolipids internalized via caveolar-related endocytosis rapidly merge with the clathrin pathway in early endosomes and form microdomains for recycling. Journal of Biological Chemistry. 2003;278:7564–7572. doi: 10.1074/jbc.M210457200. [DOI] [PubMed] [Google Scholar]
- 35.Hill E, van Der Kaay J, Downes CP, Smythe E. The role of dynamin and its binding partners in coated pit invagination and scission. J Cell Biol. 2001;152:309–323. doi: 10.1083/jcb.152.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sever S, Damke H, Schmid SL. Dynamin:GTP controls the formation of constricted coated pits, the rate limiting step in clathrin-mediated endocytosis. J Cell Biol. 2000;150:1137–1148. doi: 10.1083/jcb.150.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knisely AS, Strautnieks SS, Meier Y, Stieger B, Byrne JA, Portmann BC, Bull LN, et al. Hepatocellular carcinoma in ten children under five years of age with bile salt export pump deficiency. Hepatology. 2006;44:478–486. doi: 10.1002/hep.21287. [DOI] [PubMed] [Google Scholar]
- 38.Jackson AP, Flett A, Smythe C, Hufton L, Wettey FR, Smythe E. Clathrin promotes incorporation of cargo into coated pits by activation of the AP2 adaptor micro2 kinase. J Cell Biol. 2003;163:231–236. doi: 10.1083/jcb.200304079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weixel KM, Bradbury NA. Mu 2 binding directs the cystic fibrosis transmembrane conductance regulator to the clathrin-mediated endocytic pathway. Journal of Biological Chemistry. 2001;276:46251–46259. doi: 10.1074/jbc.M104545200. [DOI] [PubMed] [Google Scholar]
- 40.Currier SJ, Ueda K, Willingham MC, Pastan I, Gottesman MM. Deletion and insertion mutants of the multidrug transporter. Journal of Biological Chemistry. 1989;264:14376–14381. [PubMed] [Google Scholar]
- 41.Kagawa T, Watanabe N, Mochizuki K, Numari A, Ikeno Y, Itoh J, Tanaka H, et al. Phenotypic differences in PFIC2 and BRIC2 correlate with protein stability of mutant Bsep and impaired taurocholate secretion in MDCK II cells. Am J Physiol Gastrointest Liver Physiol. 2008;294:G58–67. doi: 10.1152/ajpgi.00367.2007. [DOI] [PubMed] [Google Scholar]
- 42.Crocenzi FA, Mottino AD, Pozzi EJ Sanchez, Pellegrino JM, Garay EA Rodriguez, Milkiewicz P, Vore M, et al. Impaired localisation and transport function of canalicular Bsep in taurolithocholate induced cholestasis in the rat. Gut. 2003;52:1170–1177. doi: 10.1136/gut.52.8.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roman ID, Fernandez-Moreno MD, Fueyo JA, Roma MG, Coleman R. Cyclosporin A induced internalization of the bile salt export pump in isolated rat hepatocyte couplets. Toxicological Sciences. 2003;71:276–281. doi: 10.1093/toxsci/71.2.276. [DOI] [PubMed] [Google Scholar]
- 44.Hayashi H, Sugiyama Y. 4-phenylbutyrate enhances the cell surface expression and the transport capacity of wild-type and mutated bile salt export pumps. Hepatology. 2007;45:1506–1516. doi: 10.1002/hep.21630. [DOI] [PubMed] [Google Scholar]
- 45.Hayashi H, Sugiyama Y. Short-chain ubiquitination is associated with the degradation rate of a cell-surface-resident bile salt export pump (BSEP/ABCB11) Mol Pharmacol. 2009;75:143–150. doi: 10.1124/mol.108.049288. [DOI] [PubMed] [Google Scholar]
- 46.Ismair MG, Hausler S, Stuermer CA, Guyot C, Meier PJ, Roth J, Stieger B. ABC-transporters are localized in caveolin-1-positive and reggie-1-negative and reggie-2-negative microdomains of the canalicular membrane in rat hepatocytes. Hepatology. 2009;49:1673–1682. doi: 10.1002/hep.22807. [DOI] [PubMed] [Google Scholar]
- 47.Henley JR, Krueger EW, Oswald BJ, McNiven MA. Dynamin-mediated internalization of caveolae. J Cell Biol. 1998;141:85–99. doi: 10.1083/jcb.141.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moreno M, Molina H, Amigo L, Zanlungo S, Arrese M, Rigotti A, Miquel JF. Hepatic overexpression of caveolins increases bile salt secretion in mice. Hepatology. 2003;38:1477–1488. doi: 10.1016/j.hep.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 49.Paulusma CC, de Waart DR, Kunne C, Mok KS, Elferink RP. Activity of the bile salt export pump (ABCB11) is critically dependent on canalicular membrane cholesterol content. Journal of Biological Chemistry. 2009;284:9947–9954. doi: 10.1074/jbc.M808667200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.