Abstract
Maternal neglect, including physical and emotional neglect, is a pervasive public health challenge with serious long-term effects on child health and development. The purpose of this paper is to provide an overview of the neurobiological basis of maternal caregiving, in order to better understand how to prevent and respond to maternal neglect. Drawing from both animal and human studies, key biological systems are identified which contribute to maternal caregiving behavior, focusing on the oxytocinergic and dopaminergic systems. Mesocorticolimbic and nigrostriatal dopamine pathways contribute to the processing of infant-related sensory cues leading to a behavioral response. Oxytocin may activate the dopaminergic reward pathways in response to social cues. Human neuroimaging studies are summarized which demonstrate parallels between animal and human maternal caregiving responses in the brain. By comparing different patterns of human adult attachment, we gain a clearer understanding of how differences in maternal brain and endocrine responses may contribute to maternal neglect. For example, in insecure/dismissing attachment, which may be associated with emotional neglect, we see reduced activation of the mesocorticolimbic dopamine reward system in response to infant face cues, as well as decreased peripheral oxytocin response to mother-infant contact. We are currently testing whether administration of intranasal oxytocin, as part of a randomized placebo controlled trial, may reverse some of these neurological differences, and potentially augment psychosocial and behavioral interventions for maternal neglect.
Introduction
Child neglect is the most prevalent and most rapidly increasing form of child maltreatment today (1) with arguably the most adverse long-term effects on child development (2, 3). Each year in the United States, almost 700 000 children are reported as victims of child maltreatment, with around 60% reported as a result of neglect (1). Neglected children are more likely to show a progressive decline in cognitive functioning over time (4, 5), more delayed language development (6, 7), less competent social and academic functioning (8), and are at increased risk of developing childhood aggression (9). Yet neglect is one of the least studied and most poorly understood forms of child maltreatment today (10).
Interestingly, nationwide data from the United States suggests that the most frequent perpetrator of child neglect is the biological mother(1), with maternally perpetrated neglect noted in almost 80% of substantiated episodes (11). In one respect this is not surprising, given that the biological mother is most often the primary caregiver—a role integrally associated with the definition of neglect (12). However, this is despite evidence that biological mothers may be primed, through the neuroendocrine changes associated with pregnancy, parturition and lactation, to provide optimal nurturance and protection to their offspring (11, 13). For example, the hormonal changes associated with pregnancy may induce neural modifications within the hippocampus (14) that facilitate various aspects of maternal caregiving, such as learning, spatial memory (13, 15) and emotion processing of facial cues (16). Likewise, postnatal infant stimuli, including facial expressions, cries, and tactile/suckling stimulation, may also help to re-shape the maternal brain during a period of inherent neural plasticity (13). Maternal experience upregulates oxytocin receptor expression in the brain (17), and oxytocin, released during parturition and lactation, appears to exert long-term anxiolytic and bonding effects through changes in specific brain regions (18–21). As such, oxytocin may mediate the association between breastfeeding and lower rates of maternal neglect (11). Understanding the biological processes underlying maternal caregiving may help us to better understand how a disruption to these processes may contribute to maternal neglect.
The purpose of this review paper is to: 1) describe and define maternal neglect and its impact on human development and attachment; 2) outline some of the biological mechanisms underlying maternal caregiving and neglect, both from animal and human models, focusing specifically on the oxytocinergic and dopaminergic systems; and 3) to give an overview of recent neuroimaging studies on maternal brain and endocrine responses, which may give additional insight into the problem of maternal neglect.
Definitions: physical vs. emotional neglect
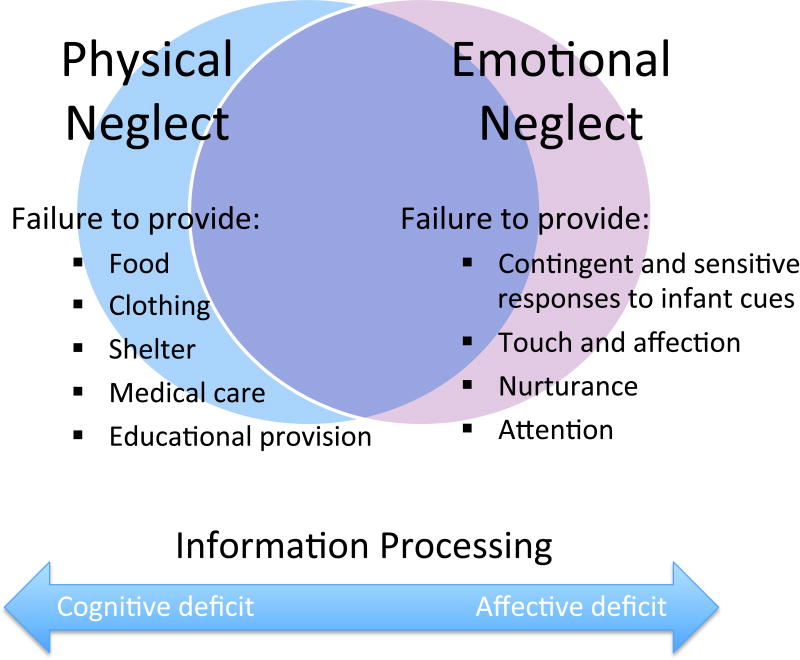
On the most basic level, child neglect is defined as a failure to provide for a child’s intrinsic needs (22), whether physical or emotional (Figure 1). Physical neglect includes a failure to provide adequate nutrition, clothing, hygiene, medical care or educational provision. Emotional (or psychological) neglect involves a lack of emotional warmth, physical affection and nurturance, or ignoring signs of needed comfort or attention. Most neglected children experience a combination of physical and emotional neglect, often manifest in multi-problem, crisis-prone and chaotic families, in which unregulated affect motivates and organizes behavior, and maternal caregiving responses are unpredictable. In other families, parents may be withdrawn, unresponsive and unmotivated, while unable to perceive or respond to their children’s cues for attention (23).
Figure 1.
Maternal neglect. The relationship between physical and emotional neglect and proposed differences in how social information is processed in the brain.
However, some children may experience isolated physical neglect without emotional neglect, such as in the case of extreme poverty or socioeconomic disadvantage (3). Parents may be emotionally responsive to their children, but unable to meet the more “cognitive” demands of organizing and coordinating necessary care, such as in providing regular meals, medical care or meeting educational needs. Emotional neglect without physical neglect is more difficult to define and identify, because of its less tangible and often hidden consequences. For example, economically advantaged parents may provide every physical need for their child (even to excess), while neglecting their just-as-crucial needs for love and emotional nurturance. “Hypervigilant” parents may focus solely on their children’s task performance and compliance, while dismissing their emotional needs or punishing displays of negative affect (24, 25).
Emotional neglect is much less likely to come to the attention of child protection authorities (23), but may result in even more serious long-term consequences for a child’s social and emotional development (8, 26, 27). One longitudinal study of mother-infant dyads examined the effect of differing types of neglect on behavioral and cognitive outcomes (27). At 3 years of age, emotional neglect was found to be the only type associated with externalizing or internalizing behavior problems, independent of socioeconomic status or maternal depression. Similar adverse outcomes were noted in a prospective study of children followed between the ages of 6 and 9 years (28).
While most researchers and clinicians define child neglect in terms of a child’s basic unfulfilled needs, others have conceptualized the problem in terms of how a caregiver’s brain processes sensory information, and associated differences in adult attachment strategies (23, 29). It has been hypothesized that emotional neglect results from deficits in a mother’s affective information processing, with a bias toward cognitive processing. Physical neglect may be the end result of deficits in cognitive information processing (see Figure 1).
Thus, one way of better understanding physical and emotional neglect may be to consider differences in mother-infant attachment and how mothers’ brains process cognitive and affective sensory information.
Maternal neglect: A disorder of mother-infant attachment?
After studying the associations between maternal neglect and juvenile delinquency, John Bowlby first formulated his attachment theory, postulating a universal human need to form close affectional bonds, primarily between mother and infant (30). He strongly argued, from an evolutionary perspective, that attachment was an innate biological system promoting proximity seeking between an infant and a specific attachment figure, in order to increase the likelihood of survival to a reproductive age. As a result of this powerful biological instinct, he hypothesized that all human infants become attached to their caregiver—even if the care is harsh or neglectful—but that these children manifest different patterns of attachment “security”. Infants of caregivers who are available, responsive and sensitive to their emotional and physical needs tend to manifest patterns of “secure attachment”. However, if the care provided is chaotic, unpredictable, rejecting, or neglectful, infants develop self-protective strategies manifest as various “insecure” patterns of attachment.
Although measuring and defining attachment in humans is a complex task, several research instruments have been developed to systematically classify attachment strategies, as observed from infancy to adulthood. The Strange Situation Procedure (SSP) (31) and the Adult Attachment Interview (AAI) (25, 32) are two of the most widely used and validated instruments, which assess the processing of attachment information. In the AAI, the transcribed interview is analyzed for specific discourse markers and patterns, which are used to categorize adult attachment into one of 3 basic groups -“secure”, “insecure/dismissing” or “insecure/preoccupied”. Over the past 25 years, over 200 studies have reported on over 10,000 Adult Attachment Interviews (33).
As noted above, one model of attachment proposes that differences in attachment strategies may represent differences in how the brain processes sensory information (29, 34): temporally ordered “cognitive” information and intensity-based “affective” information. Based on thousands of AAI discourse analyses, “dismissing” adults tend to minimize affective information, dismiss their own feelings, intentions and perspectives, and rely more upon rules and learned temporal sequences in predicting future reward (25). Studies have also shown that “dismissing” mothers score much lower on measures of parental warmth and responsivity (35, 36), suggesting that this pattern of attachment may be associated with emotional neglect (23). “Preoccupied” adults, in contrast, organize their behavior around affective information, such as fear, anger, or desire for comfort. They tend to be preoccupied with their own feelings and perspectives while omitting or distorting cognitive or temporally ordered information. Adults with secure patterns of attachment are able to integrate temporally ordered information regarding cause and effect, as well as affective information (emotional states, imaged memory, etc.), in order to form close relationships, make accurate decisions and predict future reward (25).
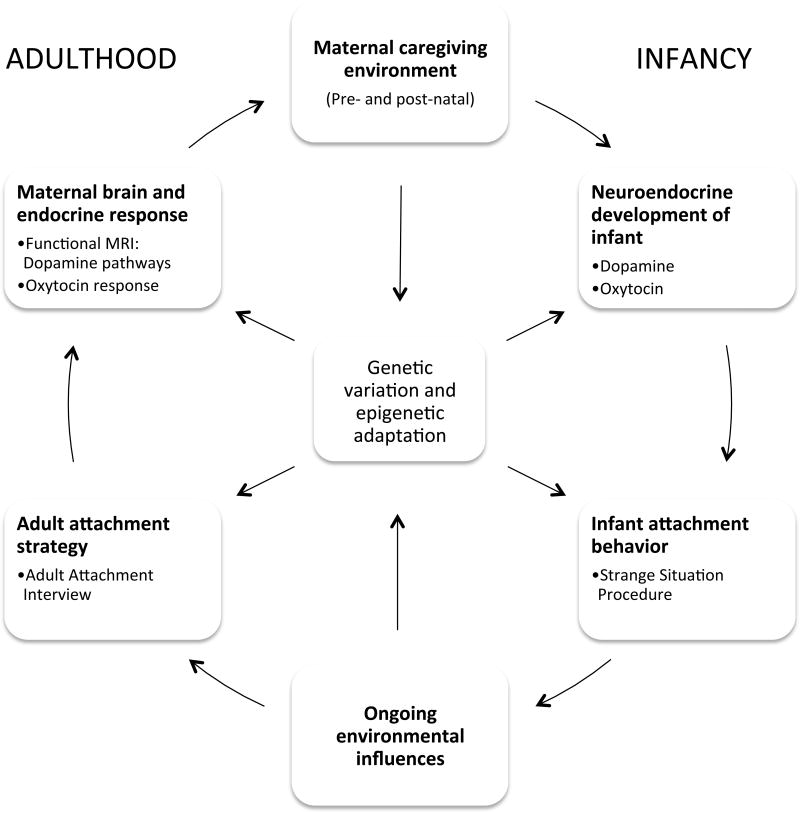
From both cross-sectional and prospective longitudinal studies, adult attachment has been shown to reliably predict maternal behavior patterns, and through this, infant social/emotional development (37) and attachment (38, 39). It is hypothesized that the intergenerational transmission of attachment may be mediated by differences in maternal neuroendocrine responses to infant cues, which translate into differences in maternal caregiving behavior (29, 40) (Figure 2). This, along with individual genetic variation, may help to shape the infant’s neuroendocrine development and subsequent behavioral patterns.
Figure 2.
The cycle of maternal caregiving and neuroendocrine development across the lifespan.
Biological mechanisms underlying maternal caregiving and neglect
Maternal neglect represents a fundamental breakdown in the most primal of human relationships, defying multiple biological mechanisms designed to ensure the optimal development of the offspring (41). Bowlby first proposed that attachment between a mother and her child was a biologically driven process which could, however, like most biological systems, be adapted or modified by experience (30). Since that time, numerous studies have confirmed that social and parenting behaviors are dependent on genetically programmed biological mechanisms—such as the oxytocinergic and dopaminergic neuroendocrine systems (42, 43)—but are also influenced by environmental factors, such as stress during pregnancy, early caregiving experience and relationships throughout the lifespan (37, 44–46).
From the emerging field of epigenetics, we are beginning to understand how the caregiving environment may influence the development of biological systems and behavioral phenotypes, via stable changes in the regulation of gene expression (47) (Figure 2). For example, from rodent models we learn that lower levels of maternal licking and grooming (LG) of pups results in increased DNA methylation of the estrogen receptor-α (ERα) gene promoter, which inhibits the development of the oxytocin system (48, 49). Furthermore, stress during pregnancy may reduce oxytocin receptor binding in key brain areas involved in maternal behavior, with associated increases in maternal anxiety and decreased maternal LG behavior postnatally (50). This may then result in decreased oxytocin receptor binding in the offspring (44). Environmental enrichment later in life may compensate for, but not reverse, some of these stress-related effects in the offspring (51, 52). Human research has also demonstrated that a mother’s own attachment history, as well as psychosocial stress, may influence the development of secure attachment in her own infant, as assessed by the Strange Situation Procedure (39, 53).
Oxytocinergic and dopaminergic systems
Two neuroendocrine systems critically involved in maternal caregiving behavior are the oxytocinergic and dopaminergic systems (see review, 54). The oxytocinergic system is important in the formation of social and spatial memories, affiliative behavior and emotion regulation (55). The dopaminergic system is involved in reinforcement stimulus-reward learning, and in decision-making based on future predicted reward (56).
a) Oxytocinergic System
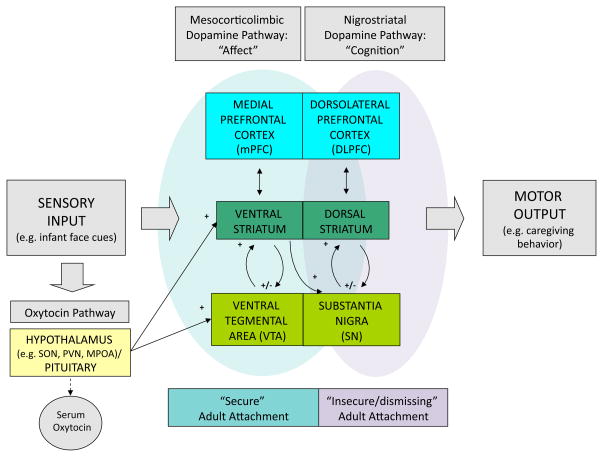
The neuropeptide hormone, oxytocin, is synthesized in the paraventricular and supraoptic nuclei of the hypothalamus that project to the posterior pituitary gland, where it is released into the blood stream. This accounts for its well-described peripheral actions, including uterine contraction at childbirth and milk ejection during lactation. In addition, oxytocin neurons project centrally to brain regions important in the manifestation of social and maternal behaviors, including the medial preoptic area (MPOA), bed nucleus of the stria terminalis, ventral striatum (VS) (including the nucleus accumbens), and the ventral tegmental area (VTA) (57, 58)(Figure 3).
Figure 3.
Model of maternal brain responses to infant cues: proposed dopaminergic and oxytocinergic pathways relating to adult attachment patterns (secure and insecure/dismissing). SON, supraoptic nucleus; PVN, paraventricular nucleus; MPOA, medial preoptic area.
In several mammalian species, oxytocin facilitates physical proximity and nurturant care between the mother and infant (41). For example, in estrogen-primed virgin rats that normally exhibit aversive behavior toward rat pups, an intraventricular injection of oxytocin stimulates a broad range of maternal behaviors, including pup grouping, retrieval of separated pups, nest building, and licking (59). Infusion of an oxytocin antagonist into the VTA blocks many of these behaviors in parturient rat dams, who then leave the pups scattered and “neglected” (60). In the amygdala, oxytocin has an anxiolytic effect and is critical for social recognition (61), whereas oxytocin knock-out mice show distinct deficits in social memory (62). Oxytocin is also important in the development of long-term spatial memories via the hippocampus (18), which supports maternal behaviors such as pup retrieval and foraging. In sheep, which are normally aversive toward newborn lambs, vaginocervical stimulation causes a release of central oxytocin, which facilitates proceptive maternal behaviors and minimizes rejection of lambs (63).
While oxytocin plays a role in stimulating the onset and maintenance of maternal behavior, maternal behavior may also program the development of the oxytocin system in female offspring, as well as the quality of maternal behavior in adulthood (see Figure 2) (51, 64, 65). In rodents, natural variation in pup LG behavior is associated with the offspring’s oxytocin receptor (OTR) expression in the hypothalamus, and maternal caregiving behavior in subsequent generations (45, 58, 66). Low levels of naturally occurring LG results in decreased OTR expression in the MPOA. These effects are also seen after experimentally induced changes in maternal behavior (such as following maternal separation) (51, 67), and after cross fostering between dams with “low” and “high” LG (45). Changes in OTR expression in the rodent appear to be mediated via changes in DNA methylation within the ERα gene promoter (48), with low LG associated with increased DNA methylation, down-regulation of ERα gene, estrogen-insensitivity, reduced OTR expression, and diminished LG behavior in the offspring.
In both human and non-human primates, early maternal caregiving has also been associated with the development of the oxytocinergic system. In rhesus monkeys, non-maternal (or nursery) rearing was associated with reduced levels of cerebrospinal fluid (CSF) oxytocin over the first 3 years of life (68). Similarly, women who reported childhood emotional neglect showed significantly reduced levels of CSF oxytocin, as was also seen for other types of maltreatment but not for physical neglect (69). CSF concentrations were inversely correlated with scores for emotional neglect on the Childhood Trauma Questionnaire. Finally, salivary oxytocin levels between mothers and infants were significantly correlated, being moderated by the degree of mother-infant affect synchrony (70).
b) Dopaminergic System
Dopamine is a neurotransmitter associated with motivated behavior in both mother and offspring (19). Dopamine production in the nucleus accumbens of the VS appears to stimulate responsive maternal caregiving in the rat (64, 71). Pharmacologic blocking of dopamine D1 receptors in the nucleus accumbens results in disrupted pup retrieval and LG, and selective pharmacologic destruction of monoamine cells in the VTA also blocks maternal behavior (72, 73). Likewise, maternal behavior is severely impaired in dopamine-transporter knockout mice (74).
Dopaminergic neurons respond to temporally ordered prediction errors signals, facilitating stimulus-reward learning in the brain (75). These signals generally originate in the VTA and substantia nigra (SN) of the midbrain and project to a variety of regions throughout the brain, including the VS, dorsal striatum, prefrontal and anterior cingulate cortex (75) (Figure 3). In humans, unpredictable randomly delivered reward stimuli activate the mesocorticolimbic system (VS and medial prefrontal cortex) (76), whereas temporally ordered prediction errors result in activation of the nigrostriatal pathway (dorsal striatum) (77).
Important anatomic feed-forward loops between the striatum and the VTA/SN region have been demonstrated in primates, suggesting that these striatonigrostriatal circuits funnel information between ventromedial (limbic), central (associative), and dorsolateral (motor) striatal regions (78). Each striatal region is integrally connected to a corresponding region of the midbrain’s VTA and SN via ascending and descending dopaminergic neurons. Likewise, there are corresponding connections between the striatum and the forebrain, including those involved in affect processing (medial prefrontal, anterior cingulate) and cognition (dorsolateral prefrontal). Thus, the striatum is believed to be an important relay station between the limbic and motor systems, integrating affective information from limbic regions with cognitive information from the prefrontal cortex, in shaping motor/behavioral responses (Figure 3).
There is evidence to suggest that the development of these dopaminergic circuits is influenced by early developmental stimulation. For example, prolonged maternal separation and isolation rearing of rat pups results in reduced dopamine transporter binding in the VS, elevated baseline dopamine levels and increased dopamine release in response to acute stress in adulthood (79, 80). These animals also show enhanced sensitivity to psychostimulants such as cocaine, which activate dopaminergic neurons, and this may lead to increased vulnerability to addiction (80). A human PET study likewise showed that low self-reported maternal care was associated with an elevated dopamine response to stress in the VS (46). High LG dams (who received high levels of maternal care in infancy (64)) also demonstrate enhanced dopamine release—but in response to infant cues rather than stressors (71), while their physiological stress response is dampened (45).
Thus, early maternal caregiving appears to play an important role in programming both the oxytocinergic and dopaminergic neuroendocrine systems in infancy, which then supports maternal behavior in adulthood (Figure 2). A disruption in these systems at any point in the lifespan may predispose to maternal neglect. Understanding how these two systems interact and communicate is also important in understanding possible etiologies of maternal neglect.
c) How oxytocin and dopamine connect
From animal studies, we learn that oxytocinergic circuits are directly linked with the mesocorticolimbic dopamine pathway, with oxytocinergic neurons projecting from the hypothalamic PVN and MPOA to both the VTA and the VS (see Figure 3). The strength of these connections is associated with levels of maternal caregiving behavior, with high LG rat dams having an increased number of oxytocin neurons in the MPOA that project to the VTA (81). An oxytocin infusion in the VTA results in increased dopamine signal in the VS, which signal is blocked by an oxytocin antagonist (81). Maternal LG responses follow the rise in dopamine signal, suggesting that dopamine may trigger the onset of maternal behavior (71).
A direct oxytocinergic connection between the hypothalamus and the VS has also been demonstrated (82), and oxytocin receptor density in the VS is positively associated with levels of maternal behavior (83).
During pregnancy and lactation, oxytocin gene expression increases in areas associated with maternal behavior, including the dopaminergic SN (84), and both oxytocin and dopamine levels increase in the SN during suckling (85).
Addiction studies have also linked oxytocin with the mesocorticolimbic dopamine system. Chronic administration of drugs of abuse, such as cocaine, substantially reduces oxytocin levels in the hypothalamus, while acute administration decreases oxytocin in the VS (86). Conversely, oxytocin may assist in ameliorating addiction and drug withdrawal effects via connections with mesocorticolimbic pathways (see reviews, 54, 87). These findings are significant in view of the strong association between addiction and maternal neglect (88).
Thus, infant cues, such as suckling, vocalization and tactile stimulation, stimulate oxytocin release in the hypothalamus, which may result in activation of the dopaminergic reward pathway and lead to behavioral reinforcement and long-term conditioned preference to social cues.
Exploring attachment in the human brain using functional MRI
Functional MRI (fMRI) is a non-invasive brain imaging technique that has enabled us to explore maternal brain responses in humans. Over the past decade, several fMRI studies have explored maternal brain responses to infant cues, including infant faces and cries (89). Lorberbaum and colleagues were the first to examine maternal brain response to a standard infant cry, which revealed activation of many of the same regions identified in rodent models of maternal behavior, including the hypothalamic region, SN, striatum and medial prefrontal cortex (90). A more recent study demonstrated that mothers who delivered vaginally compared with those who delivered via cesarean section, showed greater activation of the hypothalamus and striatum in response to hearing own infant cries (91). This suggests that vaginal delivery, which results in an oxytocin surge and increased mother-infant bonding (92), may be associated with increased activation of oxytocin and dopamine pathways in the brain.
In addition to infant cry stimuli, several groups, including our own, are using infant visual stimuli. In our first study, 28 first-time normative mothers were shown 60 novel face images of their own infant, with happy, neutral or sad affect, and a matched unknown infant (93). When mothers viewed their own infant’s face, compared to an unknown infant face, key dopamine-associated reward processing regions of the brain were activated, including mesocorticolimbic pathways (VTA, VS and medial prefrontal cortex) and nigrostriatal pathways (SN, dorsal striatum and dorsolateral prefrontal cortex) (Figure 3). Another study has also shown that VS activation is related to infant facial features, with “cuter” infant faces producing increased activation in nulliparous women (94).
When mothers viewed photographs of familiar and unfamiliar children under 6 years of age, Bartels and colleagues (95) reported activation of the nigrostriatal pathway, including the SN, dorsal striatum and lateral prefrontal cortex (Figure 3). A further study, using video clips of distressed and playful infants, also reported activations in SN and dorsal striatum when mothers viewed their own infant distressed (96). None of the recent studies, however, revealed activation of the mesocorticolimbic dopamine pathway, or looked for differences based on attachment or maternal behavior.
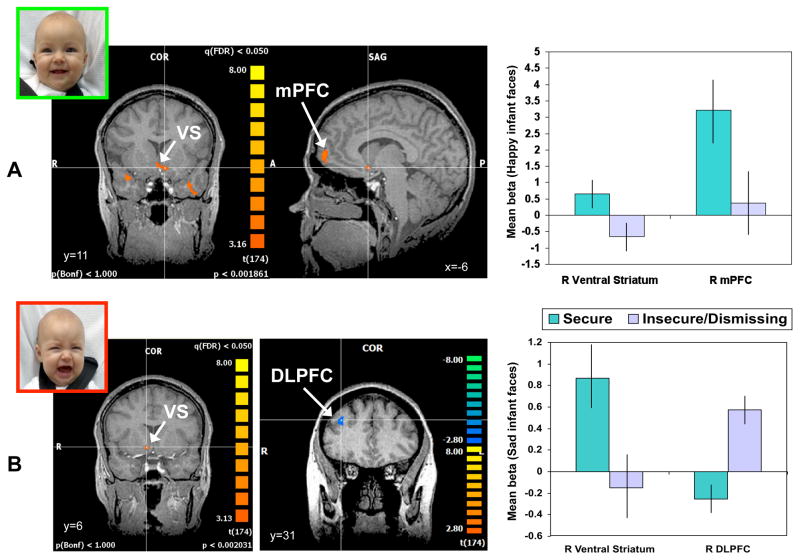
Our next goal, therefore, was to test for individual and group differences in maternal neuroendocrine responses, in order to better understand differences that may occur in maternal neglect. We hypothesized that the mesocorticolimbic and nigrostriatal dopaminergic pathways would be differentially activated depending on the mother’s adult attachment classification (based on the AAI). We also looked for differences in peripheral oxytocin response, during mother-infant interaction. We compared 15 mothers with “secure” attachment and 15 with “insecure/dismissing” patterns, assessing between-group differences in brain activation using a random effects analysis and ANOVA(40). The sample contained an insufficient number of “insecure/preoccupied” mothers (n=4) to conduct a meaningful analysis of this subgroup. Mothers from the two main attachment groups did not differ on measures of socioeconomic status, psychopathology risk, IQ, race, or self-reported parenting stress. However, compared with “secure” mothers, those with an “insecure/dismissing” pattern of adult attachment showed significantly less activation of the VS and medial prefrontal cortex bilaterally, when viewing their own infants’ happy faces (Figure 4A). While “secure” mothers also activated the ventral striatum on viewing their own infant’s crying faces, “insecure/dismissing” mothers showed more activation of the dorsolateral prefrontal cortex (Figure 4B) and the insula (data not shown; see 40). Thus, compared to “secure” mothers, “insecure/dismissing” mothers showed relatively reduced activation of mesocorticolimbic pathways, but increased activation of the nigrostriatal pathway (Figure 3 and 4).
Figure 4.
Brain responses to happy and sad own-infant faces, contrasting mothers with insecure/dismissing and secure attachment classifications (mean beta weights ± s.e.m.). Activation patterns between attachment groups were compared using a two-factor random effects ANOVA model. Brain activation maps display areas that exceed the statistical threshold of P<0.05 (false discovery rate corrected). A. “Secure” mothers, compared with “insecure/dismissing” mothers, show greater activation of the ventral striatum (VS; t=3.1, P<0.005) and medial prefrontal cortex (mPFC; t=3.0, P<0.01) in response to happy own-infant faces. B. “Secure” mothers compared with “insecure/dismissing” mothers, show greater activation of the right ventral striatum (t=3.0, P<0.01) in response to sad own-infant faces. “Insecure/dismissing” mothers, compared with “secure” mothers, show greater activation of the right dorsolateral prefrontal cortex (DLPFC; t=−3.8, P<0.001). Adapted from Neuropsychopharmacology (2009) 34:2655–2666.
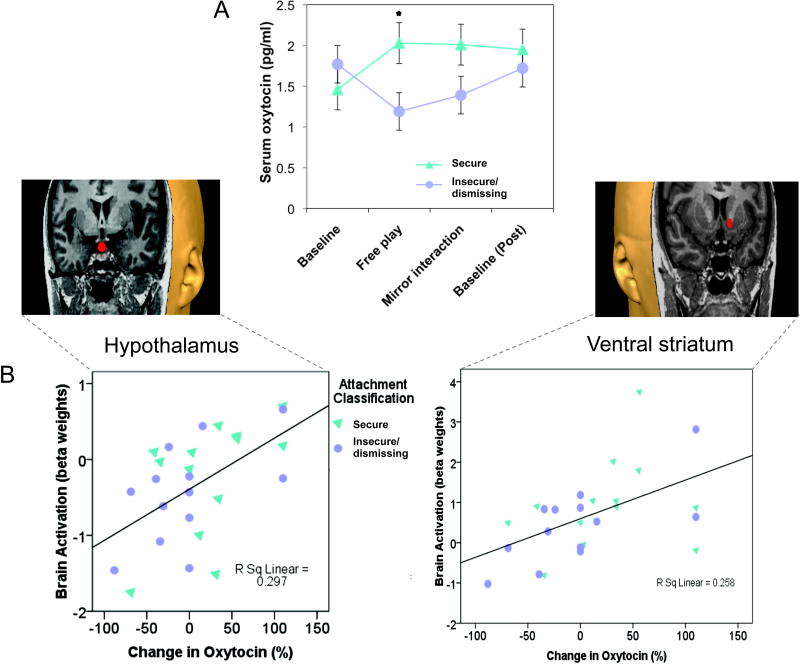
Furthermore, mothers with insecure/dismissing attachment, compared to “secure” mothers, showed reduced peripheral oxytocin production on interacting with their infant (Figure 5A), with oxytocin response correlated with brain activation in the hypothalamus and VS, key oxytocinergic and dopaminergic brain regions (Figure 5B). This suggests that mothers with an insecure/dismissing pattern of attachment may have impaired peripheral and central oxytocin production, which may help account for reduced activation of reward processing regions in the brain when presented with facial cues from their infant (Figure 3).
Figure 5.
Peripheral oxytocin and related brain activation in response to infant cues. A. Mothers with secure attachment patterns show a greater peripheral oxytocin response during an episode of physical interaction with her infant (mean ± s.e.m; *Mann-Whitney U-test, P = 0.03). The first baseline sample was collected 20 minutes after mother-infant separation; the second immediately after a 5-minute “free-play” involving direct physical contact between the mother and infant. The third sample was after a modified still-face procedure, in which the mother was in direct visual and auditory contact with her infant(via a mirror) but was physically separated by a screen divider. The final sample was collected after a further 20-minute period of complete mother-infant separation. B. Peripheral oxytocin response correlates with activation of hypothalamus/pituitary region and the ventral striatum in response to own vs. unknown infant face cues. Adapted from Neuropsychopharmacology (2009) 34:2655–2666.
These findings, although correlational, also suggest that oxytocin may help to drive activation of the mesocorticolimbic pathway in response to social cues in securely attached mothers, just as in high LG rodent dams (81)(Figure 3). Breastfeeding, which results in a surge of endogenous oxytocin, is also associated with increased activation of the striatum when mothers hear their own infant’s cry (97), as is suckling in rodents (21). In a large 15-year longitudinal follow-up study of over 7000 mother-infant dyads, the duration of breastfeeding was inversely associated with risk of subsequent state-reported maternal neglect (11).
Another currently funded study is examining maternal brain responses in a clinical population at high risk for maternal neglect—mothers with substance abuse disorders (88, 98, 99). We are exploring how early attachment experience may increase vulnerability to addiction and how both insecure attachment and substance abuse may impact on maternal brain and behavioral responses to infant cues.
Future directions
Thus, adaptation of the oxytocin and dopamine systems—whether through a mother’s own early childhood experience, stress during pregnancy or even breastfeeding experience—may lead to variation in infant and adult attachment, and maternal brain and endocrine responses (Figure 2). Understanding this cycle in humans may help us to better define and ultimately prevent maternal neglect. However, many unanswered questions remain. What are, for example, the human parallels of “licking and grooming”, “maternal separation” and “sensory deprivation” in rodent models? Are the neurobiological consequences just as real in humans? Further research is needed to explore whether modern obstetric and childrearing practices—such as scheduled cesarean sections, early non-maternal childcare, and lack of physical touch—may be contributing to this cycle of neglect. Additional studies are needed to explore the role of oxytocin in promoting secure mother-infant attachment.
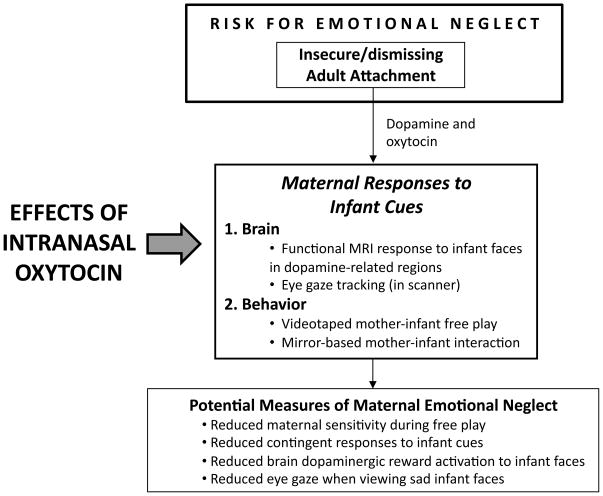
In randomized, placebo-controlled trials, intranasal oxytocin produces a broad range of social effects, including enhanced social memory, improved eye gaze when viewing faces, increased recognition and memory of facial expressions and identity, and increased manifestations of trust (100–105). We are currently conducting a study to explore the role of intranasal oxytocin in enhancing maternal brain and behavioral responses, using a double blind randomized controlled crossover design (Figure 6). This may help to elucidate brain mechanisms involved in maternal caregiving in humans, and examine how oxytocin interacts with dopaminergic brain systems, as seen in animal models of maternal behavior. We hypothesize that mothers with insecure/dismissing attachment will show increased reward activation in the VS in response to seeing their own infant’s face after receiving intranasal oxytocin. Insecure/dismissing attachment, which involves a deficit in affective information processing (25), will be used as a proxy for emotional neglect (35), and will be correlated with other neglect measures.
Figure 6.
Overview of study to examine the effects of intranasal oxytocin on maternal brain and behavioral responses to infant cues. These measures are designed to provide an insight into the neurobiology of maternal emotional neglect.
Overall, this knowledge may have practical implications for the ultimate treatment of maternal neglect, potentially providing a simple, low risk, inexpensive pharmacological intervention to support mother-infant bonding. As maternal neglect is a major public health problem with serious long-term consequences for child development and behavior, these studies may be the first step in developing novel, pharmacological treatments in support of behavioral and psychosocial interventions. Clinical populations that may benefit could include mothers with post-partum depression, addiction disorders or more general patterns of insecure attachment.
Acknowledgments
This project was supported by Award Number R01DA026437 from the National Institute on Drug Abuse, Award Number R01HD065819 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and Award Number PO DA022446 from the National Institute of Drug Abuse. The content is solely the responsibility of the authors and does not necessarily represent the official views of these institutes or the National Institutes of Health.
Footnotes
Conflict of Interests
None declared.
References
- 1.U.S. Department of Health and Human Services. Child Maltreatment 2009. Washington DC: U.S. Government Printing Office; 2010. [Google Scholar]
- 2.Eckenrode J, Laird M, Doris J. School Performance and Disciplinary Problems Among Abused and Neglected Children. Developmental Psychology. 1993;29(1):53–62. [Google Scholar]
- 3.Gaudin JM. Child neglect: short-term and long-term outcomes. In: Dubowitz H, editor. Neglected Children: Research, Practice and Policy. Thousand Oaks, CA: Sage Publications; 1999. pp. 89–108. [Google Scholar]
- 4.Strathearn L, Gray PH, O’Callaghan M, Wood DO. Childhood neglect and cognitive development in extremely low birth weight infants: A prospective study. Pediatrics. 2001;108(1):142–51. doi: 10.1542/peds.108.1.142. [DOI] [PubMed] [Google Scholar]
- 5.Mills R, Alati R, O’Callaghan M, Najman J, Williams G, Bor W, Strathearn L. Child abuse and neglect and cognitive function at 14: Findings from a longitudinal birth cohort. Pediatrics. 2010;127(1):4–10. doi: 10.1542/peds.2009-3479. [DOI] [PubMed] [Google Scholar]
- 6.Allen RE, Oliver JM. The effects of child maltreatment on language development. Child Abuse & Neglect. 1982;6(3):299–305. doi: 10.1016/0145-2134(82)90033-3. [DOI] [PubMed] [Google Scholar]
- 7.Fox L, Long SH, Langlois A. Patterns of Language Comprehension Deficit in Abused and Neglected Children. Journal of Speech and Hearing Disorders. 1988;53 (3):239–44. doi: 10.1044/jshd.5303.239. [DOI] [PubMed] [Google Scholar]
- 8.Egeland B, Sroufe LA, Erickson M. The developmental consequence of different patterns of maltreatment. Child Abuse & Neglect. 1983;7(4):459–69. doi: 10.1016/0145-2134(83)90053-4. [DOI] [PubMed] [Google Scholar]
- 9.Kotch JB, Lewis T, Hussey JM, English D, Thompson R, Litrownik AJ, Runyan DK, Bangdiwala SI, Margolis B, Dubowitz H. Importance of Early Neglect for Childhood Aggression. Pediatrics. 2008;121(4):725–31. doi: 10.1542/peds.2006-3622. [DOI] [PubMed] [Google Scholar]
- 10.Dubowitz H. Neglected children: research, practice, and policy. Thousand Oaks, Calif: Sage Publications; 1999. [Google Scholar]
- 11.Strathearn L, Abdullah M, Najman JM, O’Callaghan M. Does breastfeeding protect against substantiated child abuse and neglect? A 15-year cohort study. Pediatrics. 2009;123(2):483–93. doi: 10.1542/peds.2007-3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zuravin SJ. Child Neglect: A Review of Definitions and Measurement Research. In: Dubowitz H, editor. Neglected Children: Research, Practice and Policy. Thousand Oaks: Sage Publications; 1999. pp. 24–46. [Google Scholar]
- 13.Kinsley CH, Bardi M, Karelina K, Rima B, Christon L, Friedenberg J, Griffin G. Motherhood induces and maintains behavioral and neural plasticity across the lifespan in the rat. Arch Sex Behav. 2008;37(1):43–56. doi: 10.1007/s10508-007-9277-x. [DOI] [PubMed] [Google Scholar]
- 14.Kinsley CH, Trainer R, Stafisso-Sandoz G, Quadros P, Marcus LK, Hearon C, Meyer EA, Hester N, Morgan M, Kozub FJ, Lambert KG. Motherhood and the hormones of pregnancy modify concentrations of hippocampal neuronal dendritic spines. Hormones and Behavior. 2006;49(2):131–42. doi: 10.1016/j.yhbeh.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 15.Kinsley CH, Madonia L, Gifford GW, Tureski K, Griffin GR, Lowry C, Williams J, Collins J, McLearie H, Lambert KG. Motherhood improves learning and memory. Nature. 1999;402(6758):137–8. doi: 10.1038/45957. [DOI] [PubMed] [Google Scholar]
- 16.Pearson RM, Lightman SL, Evans J. Emotional sensitivity for motherhood: late pregnancy is associated with enhanced accuracy to encode emotional faces. Hormones and Behavior. 2009;56(5):557–63. doi: 10.1016/j.yhbeh.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Broad KD, Levy F, Evans G, Kimura T, Keverne EB, Kendrick KM. Previous maternal experience potentiates the effect of parturition on oxytocin receptor mRNA expression in the paraventricular nucleus. Eur J Neurosci. 1999;11(10):3725–37. doi: 10.1046/j.1460-9568.1999.00782.x. [DOI] [PubMed] [Google Scholar]
- 18.Tomizawa K, Iga N, Lu YF, Moriwaki A, Matsushita M, Li ST, Miyamoto O, Itano T, Matsui H. Oxytocin improves long-lasting spatial memory during motherhood through MAP kinase cascade. Nat Neurosci. 2003;6(4):384–90. doi: 10.1038/nn1023. [DOI] [PubMed] [Google Scholar]
- 19.Numan M, Stolzenberg DS. Medial preoptic area interactions with dopamine neural systems in the control of the onset and maintenance of maternal behavior in rats. Frontiers in Neuroendocrinology. 2009;30(1):46–64. doi: 10.1016/j.yfrne.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Febo M, Shields J, Ferris CF, King JA. Oxytocin modulates unconditioned fear response in lactating dams: an fMRI study. Brain Research. 2009;1302:183–93. doi: 10.1016/j.brainres.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Febo M, Numan M, Ferris CF. Functional Magnetic Resonance Imaging Shows Oxytocin Activates Brain Regions Associated with Mother-Pup Bonding during Suckling. J Neurosci. 2005;25(50):11637–44. doi: 10.1523/JNEUROSCI.3604-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sedlak AJ, Broadhurst DD. The third national incidence study on child abuse and neglect. Washington DC: U.S. Department of Health and Human Services; 1996. [Google Scholar]
- 23.Crittenden PM. Child Neglect: Causes and Contributors. In: Dubowitz H, editor. Neglected children: Research, practice, and policy. Thousand Oaks, CA: Sage Publications; 1999. [Google Scholar]
- 24.Crittenden P. Raising Parents Attachment, parenting and child safety. Devon, U.K: Willan Publishing; 2008. [Google Scholar]
- 25.Crittenden P, Landini A. Assessing Adult Attachment. New York: W. W. Norton; 2011. [Google Scholar]
- 26.Garbarino J, Collins CC. Child Neglect. The Family With a Hole in the Middle. In: Dubowitz H, editor. Neglected children: Research, practice, and policy. Thousand Oaks, CA: Sage Publications; 1999. pp. 1–23. [Google Scholar]
- 27.Dubowitz H, Papas MA, Black MM, Starr RH., Jr Child Neglect: Outcomes in High-Risk Urban Preschoolers. Pediatrics. 2002;109(6):1100–7. doi: 10.1542/peds.109.6.1100. [DOI] [PubMed] [Google Scholar]
- 28.Sturge-Apple ML, Davies PT, Cummings EM. Impact of hostility and withdrawal in interparental conflict on parental emotional unavailability and children’s adjustment difficulties. Child Development. 2006;77(6):1623–41. doi: 10.1111/j.1467-8624.2006.00963.x. [DOI] [PubMed] [Google Scholar]
- 29.Strathearn L. Exploring the Neurobiology of Attachment. In: Mayes LC, Fonagy P, Target M, editors. Developmental Science and Psychoanalysis: Integration and Innovation. London: Karnac Press; 2006. pp. 117–30. [Google Scholar]
- 30.Bowlby J. Attachment and loss. London: Hogarth Press; 1969. [Google Scholar]
- 31.Ainsworth MD, Bell SM. Attachment, exploration, and separation: illustrated by the behavior of one-year-olds in a strange situation. Child Dev. 1970;41(1):49–67. [PubMed] [Google Scholar]
- 32.George C, Kaplin N, Main M. Unpublished manuscript. 3. 1996. Adult Attachment Interview. [Google Scholar]
- 33.van IJzendoorn MH, Bakermans-Kranenburg MJ. The first 10,000 Adult Attachment Interviews: Distributions of adult attachment representations in clinical and non-clinical groups. Attachment & Human Development. 2009;11(3):223–63. doi: 10.1080/14616730902814762. [DOI] [PubMed] [Google Scholar]
- 34.Crittenden PM. An Information-Processing Perspective on the Behavior of Neglectful Parents. Criminal Justice and Behavior. 1993;20(1):27–48. [Google Scholar]
- 35.Adam EK, Gunnar MR, Tanaka A. Adult attachment, parent emotion, and observed parenting behavior: mediator and moderator models. Child Dev. 2004;75 (1):110–22. doi: 10.1111/j.1467-8624.2004.00657.x. [DOI] [PubMed] [Google Scholar]
- 36.Crowell JA, Feldman SS. Mothers’ internal models of relationships and children’s behavioral and developmental status: a study of mother-child interaction. Child Dev. 1988;59(5):1273–85. doi: 10.1111/j.1467-8624.1988.tb01496.x. [DOI] [PubMed] [Google Scholar]
- 37.Sroufe LA, Egeland B, Carlson E, Collin WA. The development of the person: The Minnesota study of risk and adaptation from birth to adulthood. New York: Guilford; 2005. [Google Scholar]
- 38.van IJzendoorn MH. Adult attachment representations, parental responsiveness, and infant attachment: a meta-analysis on the predictive validity of the Adult Attachment Interview. Psychol Bull. 1995;117(3):387–403. doi: 10.1037/0033-2909.117.3.387. [DOI] [PubMed] [Google Scholar]
- 39.Shah PE, Fonagy P, Strathearn L. Is Attachment Transmitted Across Generations? The Plot Thickens. Clinical Child Psychology and Psychiatry. 2010;15 (3):329–46. doi: 10.1177/1359104510365449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strathearn L, Fonagy P, Amico JA, Montague PR. Adult attachment predicts mother’s brain and oxytocin response to infant cues. Neuropsychopharmacology. 2009;34(13):2655–66. doi: 10.1038/npp.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Insel TR, Young LJ. The neurobiology of attachment. Nat Rev Neurosci. 2001;2(2):129–36. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- 42.Lim MM, Young LJ. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Hormones and Behavior. 2006;50(4):506–17. doi: 10.1016/j.yhbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 43.Insel TR. Is social attachment an addictive disorder? Physiology & Behavior. 2003;79(3):351–7. doi: 10.1016/s0031-9384(03)00148-3. [DOI] [PubMed] [Google Scholar]
- 44.Champagne FA, Meaney MJ. Stress During Gestation Alters Postpartum Maternal Care and the Development of the Offspring in a Rodent Model. Biological Psychiatry. 2006;59(12):1227–35. doi: 10.1016/j.biopsych.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 45.Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286(5442):1155–8. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- 46.Pruessner JC, Champagne F, Meaney MJ, Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C]raclopride. Journal of Neuroscience. 2004;24(11):2825–31. doi: 10.1523/JNEUROSCI.3422-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weaver ICG, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7(8):847–54. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 48.Champagne FA, Weaver ICG, Diorio J, Dymov S, Szyf M, Meaney MJ. Maternal Care Associated with Methylation of the Estrogen Receptor-α1b Promoter and Estrogen Receptor-α Expression in the Medial Preoptic Area of Female Offspring. Endocrinology. 2006;147(6):2909–15. doi: 10.1210/en.2005-1119. [DOI] [PubMed] [Google Scholar]
- 49.Pedersen CA. Oxytocin control of maternal behavior. Regulation by sex steroids and offspring stimuli. Ann NY Acad Sci. 1997;807:126–45. doi: 10.1111/j.1749-6632.1997.tb51916.x. [DOI] [PubMed] [Google Scholar]
- 50.Hillerer KM, Reber SO, Neumann ID, Slattery DA. Exposure to Chronic Pregnancy Stress Reverses Peripartum-Associated Adaptations: Implications for Postpartum Anxiety and Mood Disorders. Endocrinology. 2011;152(10) doi: 10.1210/en.2011-1091. in press. [DOI] [PubMed] [Google Scholar]
- 51.Champagne FA, Meaney MJ. Transgenerational effects of social environment on variations in maternal care and behavioral response to novelty. Behav Neurosci. 2007;121(6):1353–63. doi: 10.1037/0735-7044.121.6.1353. [DOI] [PubMed] [Google Scholar]
- 52.Francis DD, Diorio J, Plotsky PM, Meaney MJ. Environmental Enrichment Reverses the Effects of Maternal Separation on Stress Reactivity. Journal of Neuroscience. 2002;22(18):7840–3. doi: 10.1523/JNEUROSCI.22-18-07840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fonagy P, Steele H, Steele M. Maternal Representations of Attachment during Pregnancy Predict the Organization of Infant-Mother Attachment at One Year of Age. Child Development. 1991;62(5):891–905. doi: 10.1111/j.1467-8624.1991.tb01578.x. [DOI] [PubMed] [Google Scholar]
- 54.Baskerville TA, Douglas AJ. Dopamine and Oxytocin Interactions Underlying Behaviors: Potential Contributions to Behavioral Disorders. CNS Neuroscience & Therapeutics. 2010;16(3):e92–e123. doi: 10.1111/j.1755-5949.2010.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ferguson JN, Young LJ, Insel TR. The neuroendocrine basis of social recognition. Frontiers in Neuroendocrinology. 2002;23(2):200–24. doi: 10.1006/frne.2002.0229. [DOI] [PubMed] [Google Scholar]
- 56.McClure SM, Daw ND, Montague PR. A computational substrate for incentive salience. Trends Neurosci. 2003;26(8):423–8. doi: 10.1016/s0166-2236(03)00177-2. [DOI] [PubMed] [Google Scholar]
- 57.Numan M. Hypothalamic neural circuits regulating maternal responsiveness toward infants. Behav Cogn Neurosci Rev. 2006;5(4):163–90. doi: 10.1177/1534582306288790. [DOI] [PubMed] [Google Scholar]
- 58.Francis DD, Champagne FC, Meaney MJ. Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. J Neuroendocrinol. 2000;12(12):1145–8. doi: 10.1046/j.1365-2826.2000.00599.x. [DOI] [PubMed] [Google Scholar]
- 59.Pedersen CA, Prange AJ., Jr Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proc Natl Acad Sci USA. 1979;76 (12):6661–5. doi: 10.1073/pnas.76.12.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pedersen CA, Caldwell JD, Walker C, Ayers G, Mason GA. Oxytocin activates the postpartum onset of rat maternal behavior in the ventral tegmental and medial preoptic areas. Behav Neurosci. 1994;108(6):1163–71. doi: 10.1037//0735-7044.108.6.1163. [DOI] [PubMed] [Google Scholar]
- 61.Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci. 2001;21(20):8278–85. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nat Genet. 2000;25(3):284–8. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- 63.Keverne EB, Kendrick KM. Oxytocin facilitation of maternal behavior in sheep. Ann NY Acad Sci. 1992;652:83–101. doi: 10.1111/j.1749-6632.1992.tb34348.x. [DOI] [PubMed] [Google Scholar]
- 64.Champagne F, Meaney MJ. Like mother, like daughter: evidence for non-genomic transmission of parental behavior and stress responsivity. Prog Brain Res. 2001;133:287–302. doi: 10.1016/s0079-6123(01)33022-4. [DOI] [PubMed] [Google Scholar]
- 65.Champagne FA. Maternal imprints and the origins of variation. Hormones and Behavior. 2011;60(1):4–11. doi: 10.1016/j.yhbeh.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc Natl Acad Sci USA. 2001;98(22):12736–41. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lovic V, Gonzalez A, Fleming AS. Maternally separated rats show deficits in maternal care in adulthood. Dev Psychobiol. 2001;39(1):19–33. doi: 10.1002/dev.1024. [DOI] [PubMed] [Google Scholar]
- 68.Winslow JT, Noble PL, Lyons CK, Sterk SM, Insel TR. Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacology. 2003;28(5):910–8. doi: 10.1038/sj.npp.1300128. [DOI] [PubMed] [Google Scholar]
- 69.Heim C, Young LJ, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. Lower CSF oxytocin concentrationsin women with a history of childhood abuse. Molecular Psychiatry. 2009;14(10):954–8. doi: 10.1038/mp.2008.112. [DOI] [PubMed] [Google Scholar]
- 70.Feldman R, Gordon I, Zagoory-Sharon O. The cross-generation transmission of oxytocin in humans. Horm Behav. 2010;58(4):669–76. doi: 10.1016/j.yhbeh.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 71.Champagne FA, Chretien P, Stevenson CW, Zhang TY, Gratton A, Meaney MJ. Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat. Journal of Neuroscience. 2004;24(17):4113–23. doi: 10.1523/JNEUROSCI.5322-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Numan M, Numan MJ, Pliakou N, Stolzenberg DS, Mullins OJ, Murphy JM, Smith CD. The effects of D1 or D2 dopamine receptor antagonism in the medial preoptic area, ventral pallidum, or nucleus accumbens on the maternal retrieval response and other aspects of maternal behavior in rats. Behav Neurosci. 2005;119(6):1588–604. doi: 10.1037/0735-7044.119.6.1588. [DOI] [PubMed] [Google Scholar]
- 73.Keer SE, Stern JM. Dopamine Receptor Blockade in the Nucleus Accumbens Inhibits Maternal Retrieval and Licking, but Enhances Nursing Behavior in Lactating Rats. Physiology & Behavior. 1999;67(5):659–69. doi: 10.1016/s0031-9384(99)00116-x. [DOI] [PubMed] [Google Scholar]
- 74.Spielewoy C, Roubert C, Hamon M, Nosten-Bertrand M, Betancur C, Giros B. Behavioural disturbances associated with hyperdopaminergia in dopamine-transporter knockout mice. Behav Pharmacol. 2000;11(3–4):279–90. doi: 10.1097/00008877-200006000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Montague PR, Hyman SE, Cohen JD. Computational roles for dopamine in behavioural control. Nature. 2004;431(7010):760–7. doi: 10.1038/nature03015. [DOI] [PubMed] [Google Scholar]
- 76.Berns GS, McClure SM, Pagnoni G, Montague PR. Predictability modulates human brain response to reward. J Neurosci. 2001;21(8):2793–8. doi: 10.1523/JNEUROSCI.21-08-02793.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McClure SM, Berns GS, Montague PR. Temporal prediction errors in a passive learning task activate human striatum. Neuron. 2003;38(2):339–46. doi: 10.1016/s0896-6273(03)00154-5. [DOI] [PubMed] [Google Scholar]
- 78.Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal Pathways in Primates Form an Ascending Spiral from the Shell to the Dorsolateral Striatum. Journal of Neuroscience. 2000;20(6):2369–82. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hall FS, Wilkinson LS, Humby T, Inglis W, Kendall DA, Marsden CA, Robbins TW. Isolation Rearing in Rats: Pre-and Postsynaptic Changes in Striatal Dopaminergic Systems. Pharmacology Biochemistry and Behavior. 1998;59(4):859–72. doi: 10.1016/s0091-3057(97)00510-8. [DOI] [PubMed] [Google Scholar]
- 80.Meaney MJ, Brake W, Gratton A. Environmental regulation of the development of mesolimbic dopamine systems: a neurobiological mechanism for vulnerability to drug abuse? Psychoneuroendocrinology. 2002;27(1–2):127–38. doi: 10.1016/s0306-4530(01)00040-3. [DOI] [PubMed] [Google Scholar]
- 81.Shahrokh DK, Zhang TY, Diorio J, Gratton A, Meaney MJ. Oxytocin-Dopamine Interactions Mediate Variations in Maternal Behavior in the Rat. Endocrinology. 2010;151(5):2276–86. doi: 10.1210/en.2009-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ross HE, Cole CD, Smith Y, Neumann ID, Landgraf R, Murphy AZ, Young LJ. Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience. 2009;162(4):892–903. doi: 10.1016/j.neuroscience.2009.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Olazabal DE, Young LJ. Oxytocin receptors in the nucleus accumbens facilitate “spontaneous” maternal behavior in adult female prairie voles. Neuroscience. 2006;141(2):559–68. doi: 10.1016/j.neuroscience.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 84.Broad KD, Kendrick KM, Sirinathsinghji DJ, Keverne EB. Changes in oxytocin immunoreactivity and mRNA expression in the sheep brain during pregnancy, parturition and lactation and in response to oestrogen and progesterone. Journal of Neuroendocrinology. 1993;5(4):435–44. doi: 10.1111/j.1365-2826.1993.tb00505.x. [DOI] [PubMed] [Google Scholar]
- 85.Kendrick KM, Keverne EB, Chapman C, Baldwin BA. Intracranial dialysis measurement of oxytocin, monoamine and uric acid release from the olfactory bulb and substantia nigra of sheep during parturition, suckling, separation from lambs and eating. Brain Res. 1988;439(1–2):1–10. doi: 10.1016/0006-8993(88)91455-2. [DOI] [PubMed] [Google Scholar]
- 86.Sarnyai Z, Vecsernyes M, Laczi F, Biro E, Szabo G, Kovacs GL. Effects of cocaine on the contents of neurohypophyseal hormones in the plasma and in different brain structures in rats. Neuropeptides. 1992;23(1):27–31. doi: 10.1016/0143-4179(92)90006-i. [DOI] [PubMed] [Google Scholar]
- 87.McGregor IS, Callaghan PD, Hunt GE. From ultrasocial to antisocial: a role for oxytocin in the acute reinforcing effects and long-term adverse consequences of drug use? Br J Pharmacol. 2008;154(2):358–68. doi: 10.1038/bjp.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Strathearn L, Mayes LC. Cocaine addiction in mothers: potential effects on maternal care and infant development. Annals of the New York Academy of Sciences. 2010;1187(1):1–183. doi: 10.1111/j.1749-6632.2009.05142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Swain JE. The human parental brain: In vivo neuroimaging. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2011;35:1242–54. doi: 10.1016/j.pnpbp.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lorberbaum JP, Newman JD, Horwitz AR, Dubno JR, Lydiard RB, Hamner MB, Bohning DE, George MS. A potential role for thalamocingulate circuitry in human maternal behavior. Biol Psychiatry. 2002;51(6):431–45. doi: 10.1016/s0006-3223(01)01284-7. [DOI] [PubMed] [Google Scholar]
- 91.Swain JE, Tasgin E, Mayes L, Feldman R, Constable RT, Leckman JF. Maternal brain response to own baby cry is affected by cesarean section delivery. Journal of Child Psychology and Psychiatry. 2008;49(10):1042–52. doi: 10.1111/j.1469-7610.2008.01963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nissen E, Gustavsson P, Widstrom AM, Uvnas-Moberg K. Oxytocin, prolactin, milk production and their relationship with personality traits in women after vaginal delivery or Cesarean section. J Psychosom Obstet Gynaecol. 1998;19(1):49–58. doi: 10.3109/01674829809044221. [DOI] [PubMed] [Google Scholar]
- 93.Strathearn L, Li J, Fonagy P, Montague PR. What’s in a smile? Maternal brain responses to infant facial cues. Pediatrics. 2008;122(1):40–51. doi: 10.1542/peds.2007-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Glocker ML, Langleben DD, Ruparel K, Loughead JW, Valdez JN, Griffin MD, Sachser N, Gur RC. Baby schema modulates the brain reward system in nulliparous women. Proc Natl Acad Sci USA. 2009;106(22):9115–9. doi: 10.1073/pnas.0811620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage. 2004;21(3):1155–66. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 96.Noriuchi M, Kikuchi Y, Senoo A. The Functional Neuroanatomy of Maternal Love: Mother’s Response to Infant’s Attachment Behaviors. Biological Psychiatry. 2008;63(4):415–23. doi: 10.1016/j.biopsych.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 97.Kim P, Feldman R, Mayes LC, Eicher V, Thompson N, Leckman JF, Swain JE. Breastfeeding, brain activation to own infant cry, and maternal sensitivity. Journal of child psychology and psychiatry. 2011;52(8):907–15. doi: 10.1111/j.1469-7610.2011.02406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Strathearn L, Kosten TR. Does chronic cocaine use affect a mother’s brain response to baby face cues? A pilot fMRI study. The College on Problems of Drug Dependence 70th Annual Scientific Meeting; San Juan, Puerto Rico. 2008. [Google Scholar]
- 99.Rutherford H, Williams S, Moy S, Mayes L, Johns J. Disruption of maternal parenting circuitry by addictive process: rewiring of reward and stress systems. Frontiers in Psychiatry. 2011;2:37. doi: 10.3389/fpsyt.2011.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves “mind-reading” in humans. Biol Psychiatry. 2007;61(6):731–3. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 101.Savaskan E, Ehrhardt R, Schulz A, Walter M, Schachinger H. Post-learning intranasal oxytocin modulates human memory for facial identity. Psychoneuroendocrinology. 2008;33(3):368–74. doi: 10.1016/j.psyneuen.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 102.Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E. Oxytocin Shapes the Neural Circuitry of Trust and Trust Adaptation in Humans. Neuron. 2008;58 (4):639–50. doi: 10.1016/j.neuron.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 103.Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435(7042):673–6. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- 104.Guastella AJ, Mitchell PB, Mathews F. Oxytocin Enhances the Encoding of Positive Social Memories in Humans. Biological Psychiatry. 2008;64(3):256–8. doi: 10.1016/j.biopsych.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 105.Guastella AJ, Mitchell PB, Dadds MR. Oxytocin Increases Gaze to the Eye Region of Human Faces. Biological Psychiatry. 2008;63(1):3–5. doi: 10.1016/j.biopsych.2007.06.026. [DOI] [PubMed] [Google Scholar]