Abstract
Background
The secretory protein chromogranin A (CHGA) plays a necessary role in formation of catecholamine storage vesicles and also gives rise to a catecholamine release-inhibitory fragment. Since genetic variation in the proximal human CHGA promoter predicts autonomic function and blood pressure, here we explored how a common genetic variant alters transcription of the gene.
Methods and Results
Bioinformatic analysis suggested that the common G-462A promoter variant (rs9658634) may disrupt as many as 3 transcriptional control motifs: LEF1, COUP-TF, and PPARγ-RXRα. During electrophoretic mobility shifts, chromaffin cell nuclear proteins bound specifically to the A (though not G) allele of CHGA promoter G-462A. Upon oligonucleotide affinity chromatography followed by LC-MS/MS analysis of A-allele eluates, the transcription factor LEF1 (Lymphoid Enhancer-binding Factor-1) was identified. Interaction of LEF1 with the A-allele at G-462A was confirmed by super-shift. Upon co-transfection, LEF1 discriminated between the allelic variants, especially in chromaffin cells. Allele specificity of trans-activation by LEF1 was transferable to an isolated G-462A element fused to a heterologous (SV40) promoter. Since beta-catenin (CTNNB1) can hetero-dimerize with LEF1, we tested the effect of co-transfection of this factor, and again found A-allele-specific perturbation of CHGA transcription.
Conclusions
Common genetic variation within the human CHGA promoter alters the interaction of specific factors in trans with the promoter, with LEF1 identified by proteomic analysis and confirmed by super-shift. Co-expression experiments show functional effects of LEF1 and CTNNB1 on CHGA promoter. The findings document a novel role for components of the immune and WNT pathways in control of human sympathochromaffin phenotypes.
Keywords: Chromaffin, chromogranin, catecholamine, transcription
Introduction
Chromogranin A (CHGA) is a 48 kDa acidic protein directed to the regulated secretory pathway in neuroendocrine cells, and a major constituent of regulated secretory vesicles, also designated Dense Core Granules (DCGs) 1. DCGs serve as a storage compartment for catecholamines in post-ganglionic sympathetic neurons and adrenal medullary chromaffin cells, from which all granule core contents are co-released upon stimulation by exocytosis. Studies have shown that CHGA may be both necessary and sufficient for the formation of a regulated secretory pathway 2. In addition to this important role in DCG formation, CHGA is a pro-hormone, co-released with catecholamines from the DCGs upon stimulation 3. Upon processing at dibasic sites, the pro-hormone CHGA gives rise to biologically active peptides such as the inhibitor of catecholamine release catestatin 4, the vasodilator vasostatin 5, or the dysglycemic peptide pancreastatin 6–9.
Hypertension displays substantial heritability in family studies, but the genetic contributions to control of blood pressure (BP) are still poorly understood 10–12. Based on observations in both humans and rodents, we proposed that CHGA formation and secretion may constitute “intermediate” (early or pathogenic) phenotypes for later development of hypertension 11. For instance, CHGA is over-expressed in adrenal medulla of rodent hypertension, both spontaneous 13 and acquired 14. Moreover, ablation of the CHGA gene in mice leads to substantial hypertension 15. Finally, CHGA plasma levels parallel catecholamine release in human populations 16;17, and phenotypic links between CHGA and human hypertension are reported 17–20.
A detailed study of naturally occurring genetic variations at the human CHGA locus revealed 20 common SNPs, including 8 variants in the 1.2-kbp proximal promoter 21. From the 8 promoter haplotypes inferred, two showed substantially different transcriptional activities in reporter gene experiments. These two haplotypes span 3 variants (T-1014C → G-988T → G-462A) in LD that govern CHGA expression. Further analysis of these 3 SNPs revealed a major contribution of the SNP G-462A (rs9658634) for modification of transcriptional activity of the isolated CHGA promoter 21. In addition, variations in this promoter region predict blood pressure increase after environmental stress, as well as basal blood pressure 20; initial analysis of the effect of the -462 position suggested involvement of the transcription factor COUP-TF 20.
In this study, we revisited the cellular mechanisms that may occur at the -462 position of the CHGA promoter, using an unbiased, hypothesis-free, proteomic approach. We find not only compelling new computational evidence of differing protein binding specificity between the wild-type and the variant promoter regions, but also biochemical as well as functional evidence for binding of LEF1 (Lymphoid Enhancer Factor-1) at this molecular switch region of the CHGA promoter.
Results
Bioinformatic analysis indicates five putative binding motifs in the CHGA promoter region encompassing the G-462A SNP
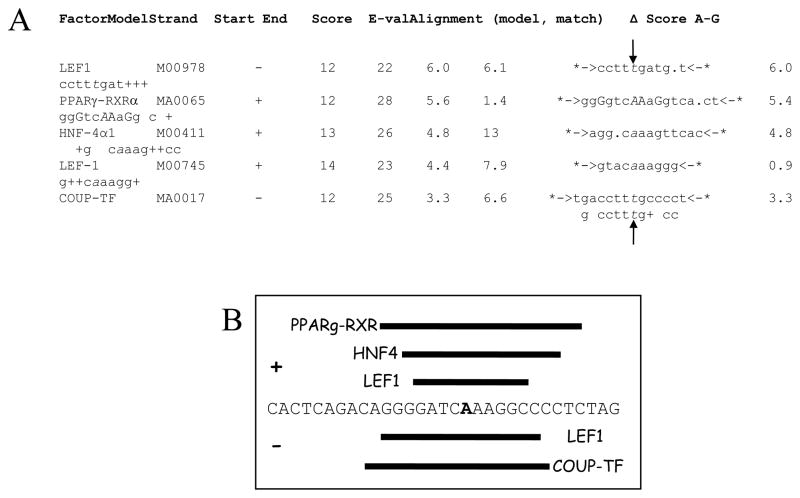
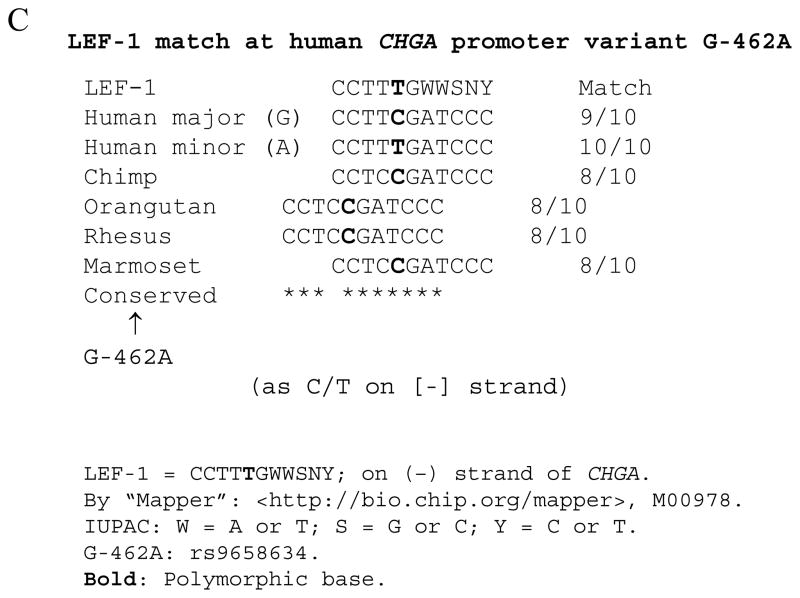
Because CHGA proximal promoter SNPs in the region T-1014C → G-988T → G-462A display peak predictions of human traits, including CHGA plasma level 21 and blood pressure response to environmental stress 20, and the SNP G-462A within this region was predominant in reporter gene experiments 20;21, we decided to search systematically for transcription binding sites around the G-462A position, using contemporary bioinformatic and proteomic approaches. Bioinformatic analysis of a 31-bp sequence surrounding the SNP (from -479 → -449; highly conserved across primates) revealed several degenerate potential binding motifs, in the JASPAR and TRANSFAC databases (Figure 1A).
Figure 1.
Identification of binding motifs spanning position G-462A in the human CHGA promoter. A. Putative factors binding the CHGA promoter at the G-462A SNP position. A region of 31-bp spanning the -462A SNP in the variant CHGA promoter was analyzed using the software Mapper (http://bio.chip.org/mapper) for putative binding motifs. Shown are the hits with a score >3, the associated model and its properties, as well as the motif alignments (modelled motifs are represented between *-> <-*, with partial matches shown by +). Vertical arrows indicate the position of the G-462A variant (in italics). On the minus strand, the same variant is C/T. B. Schematic representation of the putative binding motifs and factors. Only the + strand (-479 to -449) of the region spanning the -462 SNP (bold) is represented, together with the putative motifs on the + strand (+, above the sequence) or on the reverse complement (−, below the sequence). C. LEF-1 homology match at human CHGA promoter variant G-462A across primate species.
As expected by Chen et al, one of the first five hits for the variant (minor allele, -462A) sequence was a partial match for the previously published consensus motif of COUP-TF (MA0017). However, we also found 4 new motifs spanning -462A, with even higher scores than the COUP-TF prediction (Figure 1A). The best match corresponds to a binding motif for LEF1 (M00978) with a near-perfect homology 22. The second hit is a partial match for the binding sequence of the complex PPARγ-RXRα (MA0065), whereas the third hit involves a hepatic factor, HNF4-α1 (M00411). The fourth hit corresponds to a different reported binding motif for LEF1 23 (M00745), on the reverse complement of the first hit. Of these five strongest hits, three involve the + strand of the promoter whereas the two others indicate binding on the − strand (Figure 1B).
When performed on the wild-type (major allele, G-462) sequence, the analysis failed to identify partial matches for the factors LEF1 (M00978), HNF4-α1 (M00411), or COUP-TF (MMA0017).
Therefore, our results from the bioinformatic analysis suggest a new set of proteins that putatively bind the G-462A region of the CHGA promoter, and could further explain the differences of transcriptional activity reported for the two alleles 20;21.
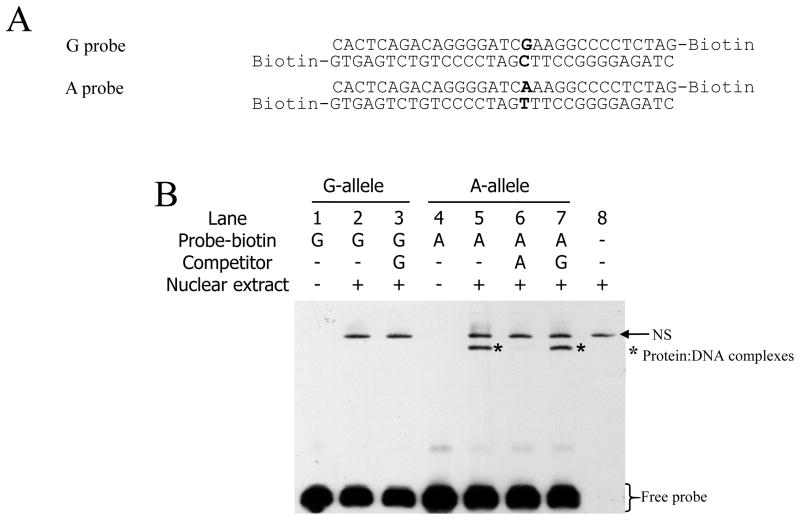
EMSA: Nuclear proteins bind to the A allele of CHGA promoter G-462A, but not to the G allele
To probe molecular mechanisms underlying the transcriptional regulation of G-462A and clearly identify the factor responsible for differences in expression, we analyzed by EMSA the binding specificity of PC12 nuclear extracts to the 31-bp sequences (alleles G-462 and -462A, Figure 2A) used in the bioinformatic analysis (Figure 1B). As shown in Figure 2B, the biotinylated double-stranded oligonucleotides incubated without PC12 nuclear extracts, are visualized at higher mobility during electrophoresis, indicating free/unbound DNA probes (Figure 2B, lanes 1 and 4). Incubation of the G allele with PC12 nuclear extracts prior to electrophoresis reveals a very low mobility band (lane 2) that is not displaced by incubation with the same allele as competitor (non biotinylated DNA, lane 3). Moreover, this very low mobility band appears as well in the nuclear extract only (i.e., no oligonucleotide probe) control (lane 8), indicating a non-specific product (NS) contained in PC12 nuclear extracts and recognized by the EMSA avidin reagents. While no additional band is seen after incubation of the G allele with PC12 nuclear extract, the A allele DNA fragment shows a second shifted band (lane 5, *), which is displaced by addition of the non-biotinylated A allele DNA fragment (lane 6), indicating specificity. On the contrary, pre-incubation with a non-biotinylated G allele DNA fragment fails to compete for the binding (lane 7), further illustrating the binding specificity of PC12 nuclear proteins for the A allele at G-462A.
Figure 2.
In vitro binding specificity of the two alleles of human CHGA G-462A region with PC12 nuclear extract. A. Sequences of the probes used for EMSA experiments. The SNP is indicated in red. B. Electro-Mobility Shift Assay (EMSA) of the two alleles G-462 and -462A with nuclear extract of PC12 cells. Biotinylated probes from (A) were incubated with nuclear extract and/or non-biotinylated competitor as indicated and the resulting complexes analyzed as described in M&M. PC12 nuclear extract alone shows a non-specific (NS) band (arrow). Protein:DNA complexes (asterisk) and free probes (brackets) are indicated.
Proteomic analysis of nuclear proteins binding the A allele of CHGA promoter G-462A: Identification of transcription factor LEF1
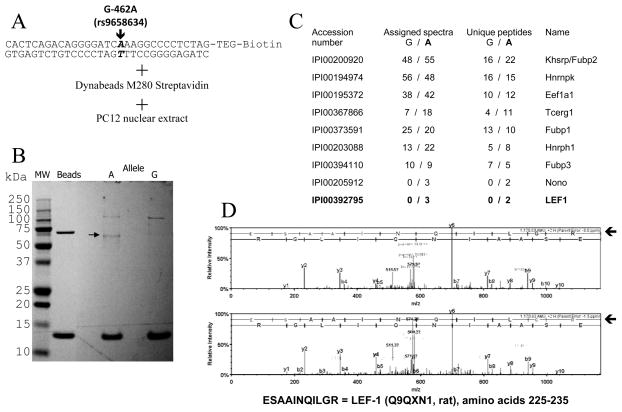
We employed oligonucleotide affinity chromatography to proceed in a hypothesis-free fashion towards identification of the trans-acting factor(s) by tandem mass spectrometry. The + strand of G or A allele DNA fragments were designed with a 3′-TEG-Biotin tag, annealed with their complementary (−) strands, and incubated with streptavidin-conjugated magnetic beads (Figure 3A). In order to minimize non-specific binding of proteins to the streptavidin or the magnetic beads, PC12 nuclear extracts were pre-cleared by incubation with streptavidin-conjugated magnetic beads alone, prior to the incubation with the magnetic beads bearing the allele-specific DNA fragments. To monitor the purification procedure, each fraction of the oligonucleotide affinity chromatography was analyzed by SDS-PAGE and Coomassie blue staining: the SDS-PAGE analysis of the elution fractions shown in Figure 3B reveals a high MW band (Mr ~120 kDa) purified with both the A and G allele DNA fragments, as well as a lower MW band (Mr ~64 kDa; arrow), purified only in presence of the A allele DNA. Eluates of the SDS-PAGE lanes were submitted for protein identification by trypsin-LC-MS/MS analysis.
Figure 3.
Purification of sequence-specific DNA-binding proteins by affinity chromatography and identification by LC-MS/MS. A. Schematic representation of the DNA sequence used for the purification experiment. The sequence is identical to that of Figure 2, but contains a single 3′-TEG-Biotin tag. SNP G-462A allele is indicated in bold. B. Coomassie blue SDS-PAGE showing the elution fractions from both alleles, as well as beads alone, used for analysis by LC-MS/MS. The arrow indicates an Mr ~64 kDa band seen only in the “A” lane; of note, LEF1 typically migrates with a apparent Mr ~55 kDa (e.g. http://www.scbt.com/datasheet-8591-lef-1-n-17-antibody.html). C. Summary of the peptides identified by LC-MS/MS from the elution fractions. D. LC-MS/MS spectra identifying the peptide ESAAINQILGR, corresponding to amino acid residues 225–235 in rat LEF1 <http://www.uniprot.org/uniprot/Q9QXN1>. Arrows indicate the sequence reads from MS/MS.
LEF1 was identified (Figure 3C and 3D) in the A allele elution fraction (Mascot scores in Supplementary Table 1), whereas we failed to detect that factor in the G allele elution. Of note, LEF1 typically migrates with an apparent MW around 60 kDa, close to the band we noted upon elution from the A-allele (Figure 3B, arrow). Abundant cellular proteins without known DNA-binding activity (presumably non-specific contaminants; list in Supplementary Table 2) were not considered further. Several known ssDNA-binding proteins and proteins involved in transcriptional machinery were also identified (Figure 3C).
Confirmation of binding of LEF1 to the A allele of CHGA promoter: EMSA with super-shift
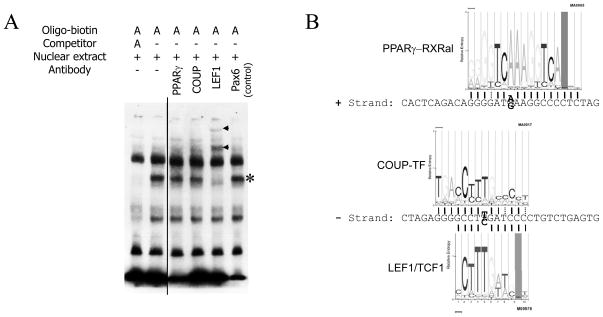
To confirm the binding of LEF1, we performed an EMSA super-shift assay. Antibodies directed against COUP-TF, PPARγ, or LEF1, as well as a negative control antibody (directed against Pax6) were added to the pre-incubated oligonucleotides and PC12 nuclear extract, prior to EMSA. While the control and the anti-COUP-TF and anti-PPARγ antibodies did not alter the migration pattern of the A-allele DNA fragment, anti-LEF1 antibody induced a super-shift of the A allele DNA fragment/protein complex (Figure 4A, lane 5). This result is consistent with the bioinformatic analysis identifying the LEF1 motif (Figure 1A and 4B). Not only is the LEF1 motif a perfect match with the variant -462A (as compared with more degenerate matches with the PPARγ and COUP-TF motifs), but the -462 SNP position is also one of the bases with the strongest specificity within the motif (Figure 4B).
Figure 4.
Identification of the binding factor of the A-allele by EMSA. A. Supershift experiment using the biotinylated A-allele probe from Figure 2. The probe was sequentially incubated with PC12 nuclear extract and antibodies directed against PPARγ, COUP-TF or LEF1 (Santa Cruz Biotechnology; see Methods). An antibody directed against Pax6 was used as a negative control (sc-32766X). The Protein:DNA complexes (asterisk, *) and the Protein:DNA:antibody complexes (arrowheads) are indicated. B. G and A allele -462 region and recognition motifs of PPARγ-RXRalpha, COUP-TF and LEF1/TCF1. Wide, thin and dotted lines indicate respectively a perfect match with the base in the motif, a second choice base, and third or fourth choice base. The variable base is presented in bold. WebLogo profiles of consensus base preference are from Chip-Mapper <http://mapper.chip.org/>.
ChIP was also undertaken (see Methods), with an antibody directed against LEF1; after immunoisolation of nucleosomes, PCR with a 152-bp amplicon spanning G-462A detected LEF1 binding to the CHGA promoter on both alleles (G and A; data not shown). However, there is a second, non-polymorphic LEF1 partial consensus match on the human CHGA promoter minus strand at position -530→-519 (ACTTTGTTGTT; Mapper score 6.1) <http://genome.ufl.edu/mapper>. Since position -530→-519 is within the inter-nucleosomal DNA fragment size range typically obtained during sonication (500–1000 bp), we cannot rely on ChIP for specific detection of LEF1 binding at the G-462A motif.
LEF1 functionally modifies CHGA promoter activity by discriminating between the two allelic variants at G-462A
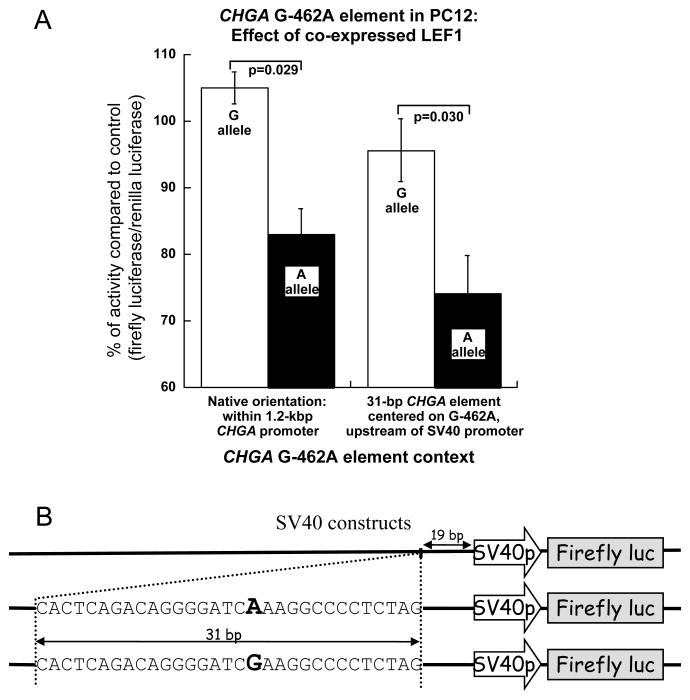
We attempted to trans-activate the two alleles of the CHGA promoter with putative binding factors identified previously, using plasmids wherein the human CHGA promoter (spanning −1142→+54 bp, or ~1.2 kbp) was cloned into pGL3-Basic (Promega) to control expression of the Firefly luciferase gene 21. The two CHGA promoters used in this study are promoter Hap-1 (containing the G-462 allele) and Hap-1/-462A (identical except for the -462A allele, created by site-directed mutagenesis). These plasmids were co-transfected into rat chromaffin cells (PC12 pheochromocytoma, Figure 5), along with a plasmid expressing human LEF1 under the control of the CMV promoter, or the plasmid pcDNA3.1 (empty CMV promoter vector) as a negative control. Co-expression of LEF1 resulted in a preferential decline in CHGA A-allele promoter expression as compared to the G-allele (p=0.029; Figure 5A, left).
Figure 5.
Effect of LEF1 on the regulation of CHGA promoter transcriptional activity in chromaffin (rat PC12) cells. A. Luciferase constructs containing the 1.2-kbp CHGA proximal promoter (left panel) with the -462 SNP A allele (Hap-1/-462A) or G allele (Hap-1/-G462) or the SV40 constructs illustrated in panel B were co-transfected with a plasmid expressing the LEF1 transcription factor or an empty plasmid control (No TF; pcDNA3.1) in PC12 cells. 24h post transfection, the regulatory effects of LEF1 were analyzed by gene reporter assay in as described in Methods. Each transfection experiment included a transfection efficiency control plasmid, pRL-TK (Promega). CHGA promoter transcriptional activity is expressed as the ratio of Firefly luciferase/Renilla luciferase activity with co-transfection of LEF1 plasmid over the negative control pcDNA3.1 (in %). The bars represent standard errors. B. Schematic representation of the constructs used in the assays. The 31-bp fragments spanning the position -462 (red or blue) of the CHGA promoter were inserted 19 bp upstream of the SV40p, in the plasmid pGL3-Promoter.
Allele specificity of trans-activation by LEF1 is transferable to an isolated G-462A element in a new promoter (SV40) context
The reporter gene assays described above were performed on two haplotypes of the CHGA promoter (~1.2 kb). To probe context-dependence of the LEF1 response, we designed a new reporter construct in which the 31-bp sequence flanking the -462 polymorphic site (G-462 versus -462A, Figure 1B) was inserted just upstream of the SV40 promoter in the pGL3-Promoter/luciferase reporter plasmid (Invitrogen) (Figure 5B). These modified pGL3-Promoter plasmids (SV40, +G or +A), as well as the control pGL3-Promoter plasmid, were co-transfected with the pCMV plasmid expressing human LEF1. This experiment allows us to understand whether the differential G/A response to LEF1 can occur independent of the usual position of the G-462A region, upstream of its cognate core promoter and TATA box. Once again, co-transfection with LEF1 significantly decreased expression from the A allele as compared to the G allele (p=0.030; Figure 5A, right) in this new context.
Taken together, our results from reporter gene assays show that LEF1, identified by bioinformatics and proteomic, differentially affects CHGA promoter depending on the nucleotide at position G-462A.
Role of the WNT/beta-catenin (CTNNB1) pathway in cooperation with LEF1
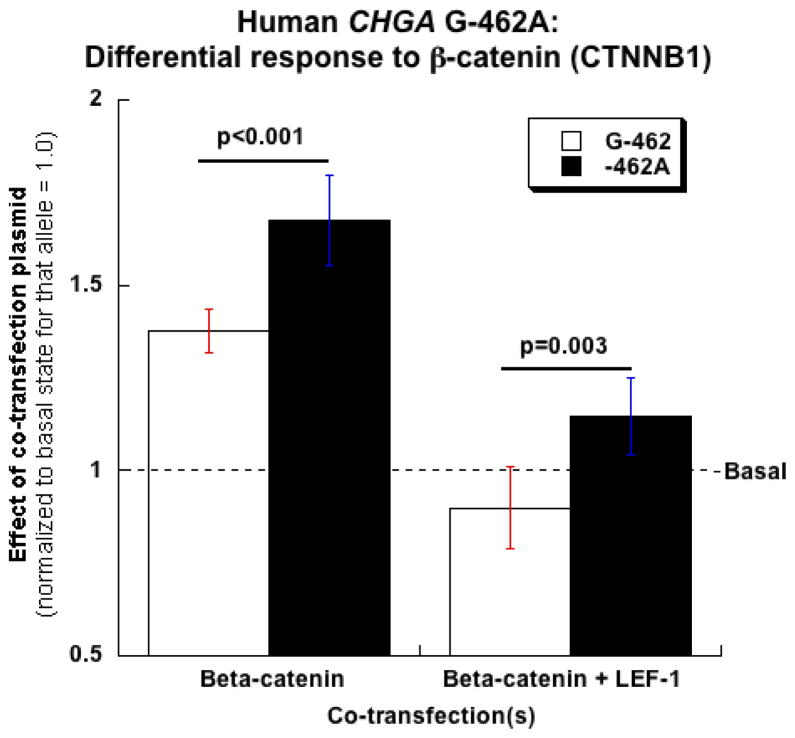
Since LEF1 characteristically hetero-dimerizes with the WNT signal transduction pathway component CTNNB1 24, and thus constitutes an efferent limb of WNT pathway signaling, we tested whether CTNNB1 perturbed CHGA expression. Transfected CTNNB1 trans-activated the co-transfected human CHGA promoter in chromaffin cells (Figure 6), and the effect was greater for the A than the G allele at G-462A (A>G, p<0.001). When CTNNB1 and LEF1 were co-transfected, the A>G difference persisted (p=0.003).
Figure 6.
Effect of beta-catenin (CTNNB1) on CHGA promoter transcriptional activity in chromaffin (rat PC12) cells. Luciferase constructs containing the 1.2-kbp CHGA proximal promoter (left panel) with the -462 SNP A-allele (Hap-1/-462A) or G-allele (Hap-1/-G462) were co-transfected with plasmids expressing either CTNNB1 or LEF1 in PC12 cells. 24h post transfection, the regulatory effects of LEF1 were analyzed by gene reporter assay in as described in M&M. Each transfection experiment included a transfection efficiency control plasmid, pRL-TK (Promega). CHGA promoter transcriptional activity is expressed as the ratio of Firefly luciferase/Renilla luciferase activity with co-transfection of CTNNB1 or CTNNB1 and LEF1 normalized to the basal state of the promoter (co-transfection with pcDNA3.1). The bars represent standard errors.
Discussion
Overview
Sympathetic signaling and outflow are key components in the regulation of blood pressure. Associations of the sympathetic pathway with hypertension and “intermediate” phenotypes contributing to blood pressure control have been documented 11. Chromogranin A plays crucial roles in the sympathoadrenal system; because of its involvement in DCG formation, regulation of catecholamine release 1, and production of bioactive peptides, CHGA may represent a key candidate gene for the regulation of the autonomic contributions to blood pressure 25. Accumulating evidence gathered in different models now document direct links between CHGA and blood pressure regulation 13–15;20. The plasma catestatin (CHGA catecholamine release-inhibitory peptide) levels were reduced in subjects with established hypertension and their at-risk siblings 26–28. In addition, variations in the CHGA promoter were associated to plasma CHGA concentration and blood pressure 20;21. Although the SNP rs9658634 at position G-462A appears to be the most important functional variant in the promoter 21, the molecular mechanisms underlying its effects are incompletely understood.
In this study, we combined an un-biased, hypothesis-free, proteomic screening approach, along with bioinformatic analyses and gene reporter assays, in order to identify the trans-acting factor(s) affecting the -462 region of the human CHGA promoter.
Cis- and trans- mechanisms at human CHGA G-462A: Bioinformatic and experimental approaches
Bioinformatic analysis of a putative protein binding site in the promoter region spanning the -462 SNP revealed partial binding motifs for the transcription factors LEF1, HNF4-α1, COUP-TF and the heterodimeric complex PPARγ-RXRα. Whereas the putative binding of COUP-TF was already suggested 20, the four other binding motifs were novel. The partial motif for PPARγ-RXRα in the CHGA promoter was of importance since CHGA expression responded to retinoic acid 20, and also because PPARγ has been linked to multiple complex diseases and disorders, including not only metabolic disease 29;30 (reminiscent of the phenotypes associated to CHGA KO-mice, 8;15), but also hypertension 31–33. As previously reported, COUP-TF is a putative binding protein of this promoter region that increases expression in PC12 cells; this effect appears linked to the -462 region and showed selectivity between the two alleles 20. However, this transcription factor was not identified in the proteomic approach and an antibody directed against COUP-TF failed to super-shift DNA-protein complexes during EMSA. Our unbiased proteomic approach identified LEF1 as binding the SNP region.
Co-transfection of LEF1 acts differentially on the two alleles in gene reporter experiments in PC12 cells, and this effect is transferable to a heterologous (SV40) core promoter by the -462 region. Finally, an anti-LEF1 antibody demonstrated super-shifting of DNA-protein complexes in EMSA, identifying LEF1 as a factor binding the promoter region at the minor allele (A). Taken together, our results suggest that the factor LEF1 binds specifically the minor (A) allele at G-462A, inducing selective repression of its activity.
Role of transcription factor LEF1
LEF1 was first identified as a T-cell specific transcription factor (also named TCF1α) regulating of expression of the TCRα gene 23;34. The LEF1 transcription factor is developmentally regulated and expressed in pre-B and T lymphocytes 35 and is a component of the Wnt signaling pathway 36; indeed, LEF1 bears a β-catenin (CTNNB1) binding domain, whereby hetero-dimerization potentiates the transcriptional activity of LEF1 37. However, LEF1 is also expressed in sympathochromaffin cell types, such as PC12 chromaffin cells 36 (our data), and human pheochromocytoma 38, as well as the normal adrenal gland 39. In the mouse adrenal gland, LEF1 expression follows a circadian rhythm 40, and LEF1 expression in rat adrenal medulla increases after multiple rounds of immobilization stress 41. Targeted ablation of the LEF1 locus in the mouse results in a pleiotropic spectrum of consequences, including alterations in some populations of neural crest-derived cells 42. Here we showed that CTNNB1 expression augmented human CHGA promoter activity (Figure 6), with a A>G preference at G-462A, either alone (p<0.001) or in combination with LEF1 co-expression (p=0.003); thus, CTNNB1 may reverse the usual suppression of CHGA transcription by LEF1 (Figure 5), in CHGA allele-specific fashion. The results open the way to understanding a new effect of WNT/CTNNB1 signaling: control of the sympathochromaffin phenotype.
Conclusions and perspectives
Here, we identified LEF1 as a polymorphism-dependent transcriptional repressor of the CHGA promoter, centering on the common functional variant G-462A. Given our results and the observations that the BP circadian rhythm synchronizes with changes in LEF1 expression 40, and experimental stress induces elevations in BP and LEF1 expression 40, it is plausible to postulate that LEF1 might have a role in catecholamine synthesis and/or secretion as well as DCG formation, perhaps in concert with the WNT/CTNNB1 pathway. Future experimentation is likely to reveal new roles of this transcription factor and its implications for human autonomic biochemistry, physiology and disease.
Materials and Methods
Cell culture
The rat adrenomedullary chromaffin cell line PC12 43 was grown in high-glucose Dulbecco’s modification of Eagle’s medium with penicillin G (100 U/ml) and streptomycin sulfate (100 mg/ml), supplemented with 10% horse serum and 5% fetal bovine serum.
Promoter/luciferase reporter plasmids
Human CHGA promoter/reporter plasmids were constructed essentially as previously described 21. Haplotype-1 promoter fragment corresponding to CHGA −1142/+54 bp (with respect to the cap-site) was amplified from genomic DNA of known homozygotes, and cloned between the site KpnI and XhoI in the pGL3-Basic vector (Promega Inc., Madison, WI, USA). The rs9658634 SNP was reintroduced by site-directed mutagenesis (QuikChange; Stratagene, La Jolla, CA, USA). For modification of the pGL3-Promoter plasmid, the oligonucleotides CACTCAGACAGGGGATCGAAGGCCCCTCTAG, CACTCAGACAGGGGATCAAAGGCCCCTCTAG were annealed with their complements, and cloned into SmaI of pGL3-Promoter. The constructs were sequence-veried, and purified on columns (Qiagen, Valencia, CA, USA).
Transfection and reporter assay
PC12 cell line was transfected at 50–60% confluence by the liposome method (TransfectIN, Bio-Rad) according to the manufacturer; with 500 ng reporter plasmid and 2.75 μl of TransfectIN per well of a 24-well plate. For Renilla luciferase control, 50 ng of plasmid pRL-TK (Promega Inc.) was co-transfected. For trans-activation experiments, 50 ng of plasmid in pCMV vector/transcription factor or pcDNA3.1 (control) was co-transfected. Co-transfected pCMV-driven trans-activator plasmid cDNAs were: human LEF1 (Open Biosystems, Huntsville, AL), or Xenopus CTNNB1 (Open Biosystems). Cells were lysed 18–24 hr post transfection in 100 mM KHPO4 pH 7.8, 1 mM DTT, 0.1% Triton X-100. For dual-luciferase measurements, Stop & Glo (Promega Inc.) as well as homemade buffers were used 44. Each experimental condition was repeated a minimum of four times (replicates).
EMSA: Electrophoretic Mobility Shift Assay and supershift
PC12 nuclear extracts were obtained using nuclear extraction kit (Cayman). The oligonucleotide probes (see above promoter/luciferase reporter plasmids) were biotin-labeled using the Biotin 3′ End DNA Labeling Kit (Pierce) and annealed. Nuclear-extract-binding reactions were performed at room temperature for 20 min using 7 μg of nuclear extract and 20 fmoles of biotin-labeled oligonucleotide in 15 μl final volume, using a LightShift chemiluminescent EMSA kit (Pierce). Protein–oligonucleotide probe complexes were resolved using native 5% polyacrylamide gels, and transferred onto nitrocellulose membranes. The biotin-tagged oligonucleotides were detected using a Chemiluminescent Nucleic Acid Detection Module (Pierce), in which a streptavidin-horseradish peroxidase conjugate was hybridized to the membrane, washed, and visualized by luminol chemiluminescence. For competition experiments, unlabelled competitor oligonucleotides were pre-incubated at 100-fold excess with the nuclear extract for 5 minutes. In super-shift assays, 1 μl of antibody was added before migration and incubated 20 minutes. The antibodies were from Santa Cruz Biotechnology: PPARγ, sc-7273X; COUP-TF, sc-30180X; and LEF1, sc-8591X. Details of the LEF1 motif sequence specificity, by position weight matrix derived from experimental data45, are available at the Chip-Mapper46 interface for the Transfac database, at <http://snpper.chip.org/mapper-pages/factors.html>.
Purification of sequence-specific DNA-binding proteins by affinity chromatography
Nuclear extracts were prepared from PC12 cells as described for EMSA. Affinity purification was performed as described 47;48 with modifications. 3′-TEG-Biotinylated versions of the forward oligonucleotide probes used for EMSA were annealed to their unlabelled complements. 25 pmoles of the ds-oligonucleotides were captured on 150 μg of M280 streptavidin magnetic beads for 30 min (Dynal Biotech, Oslo, Norway; Invitrogen). All purification procedures were carried out at 4oC. Crude nuclear extracts (~115 μg) were mixed with 150 μg of salmon sperm DNA in a 10 mM Tris pH 7.5, 50 mM KCl, 1 mM DTT buffer, and incubated 30 min with streptavidin beads to clear extracts from proteins binding the streptavidin or the beads. Pre-cleared extract was then incubated with beads coated with G- or A-allele oligonucleotides. Beads were washed three times with 100 mM Tris pH 7.5, 100 mM KCl buffer. The beads were eluted in 50 μl of Laemmli buffer. Elutions were separated on SDS-PAGE and Coomassie blue stained (SimplyBlue SafeStain, Invitrogen). Coomassie blue-stained protein bands were excised and in-gel digested with trypsin 49 and analyzed by LC electrospray ionization MS as described 50;51. Briefly, samples were loaded onto a capillary column with integrated spray tip (75 um I.D., 10 um tip, New Objective, Woburn, MA), which was packed in-house with C18 reversed phase material (Zorbax SB-C18, 5 um particle size, Agilent, Santa Clara, CA) to a length of 10 cm. The reversed phase elution was achieved by of a linear gradient of 0–60% acetonitrile in 0.1% formic acid within 60 minutes at a flow rate of 300 nl/min. The eluate was introduced into a Thermo LTQ-Orbitrap mass spectrometer (ThermoFisher, Waltham, MA) via a nano-spray source. Mass spectrometric analysis was conducted by recording precursor ion scans at a resolution of 60,000 in the Orbitrap Fourier-transform analyzer followed by collision induced dissociation MS/MS scans of the top 5 ions in the linear ion trap (cycle time approx. 1 s). An active exclusion window of 90 s was employed. Data were analyzed on a Sorcerer Solo system running Sorcerer-Sequest (rev11) against the IPI rat database (v3.61, RAT, 39876 entries) and by using the Mascot algorithm (V. 27 rev.11, Matrix Science, London, UK). Scaffold (version Scaffold_2_06_02, Proteome Software Inc., Portland, OR) was used to validate MS/MS based peptide and protein identifications. Peptide identifications were accepted if they could be established at greater than 95,0% probability as specified by the Peptide Prophet algorithm 52. Protein identifications were accepted if they could be established at greater than 99,0% probability and contained at least 2 identified peptides. Protein probabilities were assigned by Protein Prophet 53. Proteins that contained similar peptides and could not be differentiated based on MS/MS analysis alone were grouped to satisfy the principle of parsimony.
Chromatin ImmunoPrecipitation (ChIP)
ChIP was accomplished by modification of procedures previously described by us54. PC12 chromaffin cells were transfected with particular CHGA promoter haplotype/reporters to obtain G versus A alleles for the G-462A variant. ChIP assays were carried out using the Imprint® ChIP kit (CHP1; Sigma, St. Louis, MO). Cells (~3×106 in transfected 10-cm plates) were cross-linked in 1% formaldehyde for 10 min at room temp and washed x3 with ice-cold PBS, then resuspended in nuclear preparation buffer. Chromatin was sonicated to achieve inter-nucleosomal cleavage (Branson Sonifier) until DNA was fragmented to ~500–1000 bp size. After 10-min centrifugation, samples were incubated with specific or control antibodies pre-adsorbed to polystyrene wells at room temp for 1.5 hours with rotation. Characteristics of the specific antibody were: goat polyclonal anti-LEF1, SCBT sc-8591X. Control antibodies were from the Sigma ChIP kit: pre-immune normal mouse IgG (as a negative control), and anti-RNA polymerase II (as a positive control). The adsorbed immune complex washed 6–7 times and eluted by “DNA release buffer” including proteinase K digestion at 65°C for 15 min, then cross-links were reversed with “reversing solution” with heating in at 65°C for 1.5 hours. The DNA was subsequently extracted and purified with GenElute Binding Column G (Sigma). Immunoprecipitated nucleosomal DNA samples were analyzed by PCR using primers forming a 152-bp amplicon that bracketed the G-462A (sense: 5′-AGAGAGAAGCCTCACTCAGACAG-3′, antisense: 5′-CACCCCGTGCTATTTTTCCTA-3′) site in the human CHGA promoter. Extracted DNA from the chromatin fractions before antibody adsorption/elution was used as a positive control (“input DNA”). To ensure that the PCR amplification was in the linear range, reactions with different amounts of input DNA samples were carried out for various (typically 15–30) cycle numbers; a linear range of amplification typically occurred at ~25 cycles. After amplification, PCR products were separated on 1.5% agarose TBE gels. In this reporter system, the transfected plasmid is incorporated into the chromatin fraction of the cell54.
Statistics
Analyses were performed in SPSS. Results are expressed as the mean value +/− one SEM. Cell culture experiments were typically done with 4 replicates per condition. Parametric statistics (T-test) were used to evaluate differences between experimental conditions, since descriptive statistics were consistent with approximately normal distributions of the data points (skewness from −0.8 to +0.8).
Supplementary Material
Acknowledgments
Funding sources: National Institutes of Health, Department of Veterans Affairs. Mass spectrometry studies were performed at Salk Institute under the La Jolla Interdisciplinary Neuroscience Center grant <http://www.lajollaneuroscience.org/>.
Glossary of abbreviations
- CHGA
Chromogranin A
- CTNNB1
Beta-catenin
- DCG
Dense Core Granule (of the regulated neuroendocrine secretory pathway)
- EMSA
Electrophoretic Mobility Shift Assay
- ESI-MS/MS
ElectroSpray Ionization, followed by 2-dimensional (tandem) Mass Spectrometry
- LD
Linkage Disequilibrium
- PC12
Rat pheochromocytoma (chromaffin) cell line
Footnotes
Conflict of interest disclosures: None.
References
- 1.Taupenot L, Harper KL, O’Connor DT. The chromogranin-secretogranin family. N Engl J Med. 2003;348:1134–1149. doi: 10.1056/NEJMra021405. [DOI] [PubMed] [Google Scholar]
- 2.Kim T, Tao-Cheng JH, Eiden LE, Loh YP. Chromogranin A, an “on/off” switch controlling dense-core secretory granule biogenesis. Cell. 2001;106:499–509. doi: 10.1016/s0092-8674(01)00459-7. [DOI] [PubMed] [Google Scholar]
- 3.Takiyyuddin MA, Cervenka JH, Hsiao RJ, Barbosa JA, Parmer RJ, O’Connor DT. Chromogranin A. Storage and release in hypertension. Hypertension. 1990;15:237–246. doi: 10.1161/01.hyp.15.3.237. [DOI] [PubMed] [Google Scholar]
- 4.Mahata SK, O’Connor DT, Mahata M, Yoo SH, Taupenot L, Wu H, Gill BM, Parmer RJ. Novel autocrine feedback control of catecholamine release. A discrete chromogranin a fragment is a noncompetitive nicotinic cholinergic antagonist. J Clin Invest. 1997;100:1623–1633. doi: 10.1172/JCI119686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aardal S, Helle KB, Elsayed S, Reed RK, Serck-Hanssen G. Vasostatins, comprising the N-terminal domain of chromogranin A, suppress tension in isolated human blood vessel segments. J Neuroendocrinol. 1993;5:405–412. doi: 10.1111/j.1365-2826.1993.tb00501.x. [DOI] [PubMed] [Google Scholar]
- 6.Cadman PE, Rao F, Mahata SK, O’Connor DT. Studies of the dysglycemic peptide, pancreastatin, using a human forearm model. Ann N Y Acad Sci. 2002;971:528–529. doi: 10.1111/j.1749-6632.2002.tb04518.x. [DOI] [PubMed] [Google Scholar]
- 7.O’Connor DT, Cadman PE, Smiley C, Salem RM, Rao F, Smith J, Funk SD, Mahata SK, Mahata M, Wen G, Taupenot L, Gonzalez-Yanes C, Harper KL, Henry RR, Sanchez-Margalet V. Pancreastatin: multiple actions on human intermediary metabolism in vivo, variation in disease, and naturally occurring functional genetic polymorphism. J Clin Endocrinol Metab. 2005;90:5414–5425. doi: 10.1210/jc.2005-0408. [DOI] [PubMed] [Google Scholar]
- 8.Gayen JR, Saberi M, Schenk S, Biswas N, Vaingankar SM, Cheung WW, Najjar SM, O’Connor DT, Bandyopadhyay G, Mahata SK. A novel pathway of insulin sensitivity in chromogranin A null mice: a crucial role for pancreastatin in glucose homeostasis. J Biol Chem. 2009;284:28498–28509. doi: 10.1074/jbc.M109.020636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friese RS, Gayen JR, Mahapatra NR, Schmid-Schonbein GW, O’Connor DT, Mahata SK. Global metabolic consequences of the chromogranin A-null model of hypertension: transcriptomic detection, pathway identification, and experimental verification. Physiol Genomics. 2010;40:195–207. doi: 10.1152/physiolgenomics.00164.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MIALL WE, OLDHAM PD. The hereditary factor in arterial blood-pressure. Br Med J. 1963;1:75–80. doi: 10.1136/bmj.1.5323.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Connor DT, Insel PA, Ziegler MG, Hook VY, Smith DW, Hamilton BA, Taylor PW, Parmer RJ. Heredity and the autonomic nervous system in human hypertension. Curr Hypertens Rep. 2000;2:16–22. doi: 10.1007/s11906-000-0053-8. [DOI] [PubMed] [Google Scholar]
- 12.Timberlake DS, O’Connor DT, Parmer RJ. Molecular genetics of essential hypertension: recent results and emerging strategies. Curr Opin Nephrol Hypertens. 2001;10:71–79. doi: 10.1097/00041552-200101000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Schober M, Howe PR, Sperk G, Fischer-Colbrie R, Winkler H. An increased pool of secretory hormones and peptides in adrenal medulla of stroke-prone spontaneously hypertensive rats. Hypertension. 1989;13:469–474. doi: 10.1161/01.hyp.13.5.469. [DOI] [PubMed] [Google Scholar]
- 14.Takiyyuddin MA, De Nicola L, Gabbai FB, Dinh TQ, Kennedy B, Ziegler MG, Sabban EL, Parmer RJ, O’Connor DT. Catecholamine secretory vesicles. Augmented chromogranins and amines in secondary hypertension. Hypertension. 1993;21:674–679. doi: 10.1161/01.hyp.21.5.674. [DOI] [PubMed] [Google Scholar]
- 15.Mahapatra NR, O’Connor DT, Vaingankar SM, Hikim AP, Mahata M, Ray S, Staite E, Wu H, Gu Y, Dalton N, Kennedy BP, Ziegler MG, Ross J, Mahata SK. Hypertension from targeted ablation of chromogranin A can be rescued by the human ortholog. J Clin Invest. 2005;115:1942–1952. doi: 10.1172/JCI24354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dimsdale JE, O’Connor DT, Ziegler M, Mills P. Chromogranin A correlates with norepinephrine release rate. Life Sci. 1992;51:519–525. doi: 10.1016/0024-3205(92)90029-o. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y, Rao F, Rodriguez-Flores JL, Mahata M, Fung MM, Stridsberg M, Vaingankar SM, Wen G, Salem RM, Das M, Cockburn MG, Schork NJ, Ziegler MG, Hamilton BA, Mahata SK, Taupenot L, O’Connor DT. Naturally occurring human genetic variation in the 3′-untranslated region of the secretory protein chromogranin A is associated with autonomic blood pressure regulation and hypertension in a sex-dependent fashion. J Am Coll Cardiol. 2008;52:1468–1481. doi: 10.1016/j.jacc.2008.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Connor DT. Plasma chromogranin A. Initial studies in human hypertension. Hypertension. 1985;7:I76–I79. doi: 10.1161/01.hyp.7.3_pt_2.i76. [DOI] [PubMed] [Google Scholar]
- 19.Takiyyuddin MA, Parmer RJ, Kailasam MT, Cervenka JH, Kennedy B, Ziegler MG, Lin MC, Li J, Grim CE, Wright FA. Chromogranin A in human hypertension. Influence of heredity. Hypertension. 1995;26:213–220. doi: 10.1161/01.hyp.26.1.213. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y, Rao F, Rodriguez-Flores JL, Mahapatra NR, Mahata M, Wen G, Salem RM, Shih PA, Das M, Schork NJ, Ziegler MG, Hamilton BA, Mahata SK, O’Connor DT. Common genetic variants in the chromogranin A promoter alter autonomic activity and blood pressure. Kidney Int. 2008;74:115–125. doi: 10.1038/ki.2008.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wen G, Mahata SK, Cadman P, Mahata M, Ghosh S, Mahapatra NR, Rao F, Stridsberg M, Smith DW, Mahboubi P, Schork NJ, O’Connor DT, Hamilton BA. Both rare and common polymorphisms contribute functional variation at CHGA, a regulator of catecholamine physiology. Am J Hum Genet. 2004;74:197–207. doi: 10.1086/381399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giese K, Amsterdam A, Grosschedl R. DNA-binding properties of the HMG domain of the lymphoid-specific transcriptional regulator LEF-1. Genes Dev. 1991;5:2567–2578. doi: 10.1101/gad.5.12b.2567. [DOI] [PubMed] [Google Scholar]
- 23.Waterman ML, Fischer WH, Jones KA. A thymus-specific member of the HMG protein family regulates the human T cell receptor C alpha enhancer. Genes Dev. 1991;5:656–669. doi: 10.1101/gad.5.4.656. [DOI] [PubMed] [Google Scholar]
- 24.Barker N, Morin PJ, Clevers H. The Yin-Yang of TCF/beta-catenin signaling. Adv Cancer Res. 2000;77:1–24. doi: 10.1016/s0065-230x(08)60783-6. [DOI] [PubMed] [Google Scholar]
- 25.Gayen JR, Gu Y, O’Connor DT, Mahata SK. Global disturbances in autonomic function yield cardiovascular instability and hypertension in the chromogranin a null mouse. Endocrinology. 2009;150:5027–5035. doi: 10.1210/en.2009-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kennedy BP, Mahata SK, O’Connor DT, Ziegler MG. Mechanism of cardiovascular actions of the chromogranin A fragment catestatin in vivo. Peptides. 1998;19:1241–1248. doi: 10.1016/s0196-9781(98)00086-2. [DOI] [PubMed] [Google Scholar]
- 27.O’Connor DT, Kailasam MT, Kennedy BP, Ziegler MG, Yanaihara N, Parmer RJ. Early decline in the catecholamine release-inhibitory peptide catestatin in humans at genetic risk of hypertension. J Hypertens. 2002;20:1335–1345. doi: 10.1097/00004872-200207000-00020. [DOI] [PubMed] [Google Scholar]
- 28.O’Connor DT, Zhu G, Rao F, Taupenot L, Fung MM, Das M, Mahata SK, Mahata M, Wang L, Zhang K, Greenwood TA, Shih PA, Cockburn MG, Ziegler MG, Stridsberg M, Martin NG, Whitfield JB. Heritability and genome-wide linkage in US and australian twins identify novel genomic regions controlling chromogranin a: implications for secretion and blood pressure. Circulation. 2008;118:247–257. doi: 10.1161/CIRCULATIONAHA.107.709105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meirhaeghe A, Cottel D, Amouyel P, Dallongeville J. Association between peroxisome proliferator-activated receptor gamma haplotypes and the metabolic syndrome in French men and women. Diabetes. 2005;54:3043–3048. doi: 10.2337/diabetes.54.10.3043. [DOI] [PubMed] [Google Scholar]
- 30.Semple RK, Chatterjee VK, O’Rahilly S. PPAR gamma and human metabolic disease. J Clin Invest. 2006;116:581–589. doi: 10.1172/JCI28003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim K, Lee S, Valentine RJ. Association of pro12Ala polymorphism in the peroxisome proliferative-activated receptor gamma2 gene with obesity and hypertension in Korean women. J Nutr Sci Vitaminol (Tokyo) 2007;53:239–246. doi: 10.3177/jnsv.53.239. [DOI] [PubMed] [Google Scholar]
- 32.Leibovitz E, Schiffrin EL. PPAR activation: a new target for the treatment of hypertension. J Cardiovasc Pharmacol. 2007;50:120–125. doi: 10.1097/FJC.0b013e318062153b. [DOI] [PubMed] [Google Scholar]
- 33.Lu Z, Dong B, Mo X, Chen T, Wu H, Zhang Y, Xiao H. Pro12Ala polymorphism in PPAR gamma 2 associated with essential hypertension in Chinese nonagenarians/centenarians. Exp Gerontol. 2008;43:1108–1113. doi: 10.1016/j.exger.2008.08.046. [DOI] [PubMed] [Google Scholar]
- 34.Waterman ML, Jones KA. Purification of TCF-1 alpha, a T-cell-specific transcription factor that activates the T-cell receptor C alpha gene enhancer in a context-dependent manner. New Biol. 1990;2:621–636. [PubMed] [Google Scholar]
- 35.Travis A, Amsterdam A, Belanger C, Grosschedl R. LEF-1, a gene encoding a lymphoid-specific protein with an HMG domain, regulates T-cell receptor alpha enhancer function [corrected] Genes Dev. 1991;5:880–894. doi: 10.1101/gad.5.5.880. [DOI] [PubMed] [Google Scholar]
- 36.Porfiri E, Rubinfeld B, Albert I, Hovanes K, Waterman M, Polakis P. Induction of a beta-catenin-LEF-1 complex by wnt-1 and transforming mutants of beta-catenin. Oncogene. 1997;15:2833–2839. doi: 10.1038/sj.onc.1201462. [DOI] [PubMed] [Google Scholar]
- 37.Novak A, Dedhar S. Signaling through beta-catenin and Lef/Tcf. Cell Mol Life Sci. 1999;56:523–537. doi: 10.1007/s000180050449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dahia PL, Ross KN, Wright ME, Hayashida CY, Santagata S, Barontini M, Kung AL, Sanso G, Powers JF, Tischler AS, Hodin R, Heitritter S, Moore F, Dluhy R, Sosa JA, Ocal IT, Benn DE, Marsh DJ, Robinson BG, Schneider K, Garber J, Arum SM, Korbonits M, Grossman A, Pigny P, Toledo SP, Nose V, Li C, Stiles CD. A HIF1alpha regulatory loop links hypoxia and mitochondrial signals in pheochromocytomas. PLoS Genet. 2005;1:72–80. doi: 10.1371/journal.pgen.0010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ge X, Yamamoto S, Tsutsumi S, Midorikawa Y, Ihara S, Wang SM, Aburatani H. Interpreting expression profiles of cancers by genome-wide survey of breadth of expression in normal tissues. Genomics. 2005;86:127–141. doi: 10.1016/j.ygeno.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 40.Oster H, Damerow S, Hut RA, Eichele G. Transcriptional profiling in the adrenal gland reveals circadian regulation of hormone biosynthesis genes and nucleosome assembly genes. J Biol Rhythms. 2006;21:350–361. doi: 10.1177/0748730406293053. [DOI] [PubMed] [Google Scholar]
- 41.Liu X, Serova L, Kvetnansky R, Sabban EL. Identifying the stress transcriptome in the adrenal medulla following acute and repeated immobilization. Ann N Y Acad Sci. 2008;1148:1–28. doi: 10.1196/annals.1410.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Genderen C, Okamura RM, Farinas I, Quo RG, Parslow TG, Bruhn L, Grosschedl R. Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev. 1994;8:2691–2703. doi: 10.1101/gad.8.22.2691. [DOI] [PubMed] [Google Scholar]
- 43.Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dyer BW, Ferrer FA, Klinedinst DK, Rodriguez R. A noncommercial dual luciferase enzyme assay system for reporter gene analysis. Anal Biochem. 2000;282:158–161. doi: 10.1006/abio.2000.4605. [DOI] [PubMed] [Google Scholar]
- 45.Leung T, Soll I, Arnold SJ, Kemler R, Driever W. Direct binding of Lef1 to sites in the boz promoter may mediate pre-midblastula-transition activation of boz expression. Dev Dyn. 2003;228:424–432. doi: 10.1002/dvdy.10408. [DOI] [PubMed] [Google Scholar]
- 46.Marinescu VD, Kohane IS, Riva A. The MAPPER database: a multi-genome catalog of putative transcription factor binding sites. Nucleic Acids Res. 2005;33:D91–D97. doi: 10.1093/nar/gki103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Masternak K, Barras E, Zufferey M, Conrad B, Corthals G, Aebersold R, Sanchez JC, Hochstrasser DF, Mach B, Reith W. A gene encoding a novel RFX-associated transactivator is mutated in the majority of MHC class II deficiency patients. Nat Genet. 1998;20:273–277. doi: 10.1038/3081. [DOI] [PubMed] [Google Scholar]
- 48.Yaneva M, Tempst P. Affinity capture of specific DNA-binding proteins for mass spectrometric identification. Anal Chem. 2003;75:6437–6448. doi: 10.1021/ac034698l. [DOI] [PubMed] [Google Scholar]
- 49.Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 50.Herrera F, Chen Q, Fischer WH, Maher P, Schubert DR. Synaptojanin-1 plays a key role in astrogliogenesis: possible relevance for Down’s syndrome. Cell Death Differ. 2009;16:910–920. doi: 10.1038/cdd.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schubert D, Herrera F, Cumming R, Read J, Low W, Maher P, Fischer WH. Neural cells secrete a unique repertoire of proteins. J Neurochem. 2009;109:427–435. doi: 10.1111/j.1471-4159.2009.05968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem. 2002;74:5383–5392. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- 53.Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem. 2003;75:4646–4658. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- 54.Zhang K, Zhang L, Rao F, Brar B, Rodriguez-Flores JL, Taupenot L, O’Connor DT. Human tyrosine hydroxylase natural genetic variation: delineation of functional transcriptional control motifs disrupted in the proximal promoter. Circ Cardiovasc Genet. 2010;3:187–198. doi: 10.1161/CIRCGENETICS.109.904813. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.