Abstract
Background
The impact of non-HLA patient factors on the match of the selected unrelated donor (URD) for hematopoietic cell transplantation (HCT) has not been fully evaluated. National Marrow Donor Program (NMDP) data for 7486 transplants using peripheral blood stem cells (PBSC) or bone marrow from years 2000–2005 were evaluated using multivariate logistic regression to identify independent non-HLA patient factors associated with completing a more closely matched URD transplant.
Results
Advanced (intermediate and late stage) disease was significantly associated with an increased likelihood of transplant using a less-matched (partially-matched or mismatched) donor. Additionally, Black patients were 2.83 times, Asian 2.05 times, and Hispanic 1.73 times more likely to have a less-matched HCT donor than Caucasian patients. Younger patients, HCT at lower volume centers and in earlier years had significantly higher likelihood of having a less HLA matched URD transplant.
Conclusion
Our analysis provides encouraging evidence of HLA matching improvement in recent years. Initiating a patient’s URD search early in the disease process, especially for patients from non-Caucasian racial and ethnic groups, will provide the best likelihood for identifying the best available donor and making informed transplant decisions.
Keywords: Unrelated Donor, HLA match, Hematopoietic Cell Transplantation
Introduction
Allogeneic hematopoietic stem cell transplantation (HCT) is a potentially curative therapy for malignant and non-malignant hematologic disorders. Unfortunately, only 30% of patients in need of HCT will have a suitable Human Leukocyte Antigen (HLA) matched family member. For the remainder of patients, searching for an unrelated donor (URD) is an important option. The National Marrow Donor Program (NMDP) is the largest URD registry in the world and has facilitated donor searches and more than 30,000 transplants since 1987.
Previous studies have assessed the impact of HLA matching on patient outcomes such as graft failure, overall survival, disease free survival, and graft-versus-host disease. Studies have shown varying levels of how HLA mismatching impacts survival.1–4 Recent work by Lee et al. has shown important adverse effects of either allele or antigen mismatching on outcome with 9–10% lower one-year survival for each additional mismatch (7/8 and 6/8 HLA-A, -B, -C, -DRB1) compared to fully matched (8/8) transplants.5 Studies have demonstrated the need for high-resolution 4 locus typing HLA-A, -B, -C and -DRB1.3–7 The three largest studies from Morishima et al., Flomenberg et al., and Lee et al. showed no significant differences in patient outcome associated with mismatching at HLA-DQB1.3,5,6
The impact of non-HLA patient factors on the selection of an URD for proceeding to transplant has not been fully evaluated. Understanding how patient factors may impact donor selection, availability, and completion of a transplant is important given the influence of donor matching on survival. The NMDP Registry is underrepresented in available donors from non-Caucasian racial and ethnic groups. As of 2005, approximately 72% of the donor file was composed of Caucasian donors.
We analyzed the trend of patient/donor pairs undergoing transplant in the six year period of 2000–2005 as a measure reflecting both effectiveness of donor searching and availability of better matched donors. With HLA match level as an important component of favorable transplant outcome, we evaluated the factors associated with HCT using a better matched donor.
Patients and Methods
Patient Population
This observational study includes patients receiving their first transplant facilitated through the NMDP from years 2000–2005. Patient and donor pairs consented to having their data used for research and were included if baseline information was available (n=7486; 78% of domestic and 17% of international NMDP transplants). Patients were transplanted with either peripheral blood stem cells (PBSC) or bone marrow. The patient data, 94.5% in the United States and 5.5% international patients, come from 168 transplant centers. This cohort of patients is 82% Caucasian, 6% Black, 2% Asian, 8% Hispanic, and 2% other/unknown. Patient race designation was based on transplant center reporting. Hispanic patients include those specified as Hispanic race or those of Hispanic ethnicity with Caucasian or other/unknown race selected. Patient age includes 25% of patients 19 or under, 46% age 20–49, and 28% age 50 or older.
HLA Typing
HLA data used in this study were the NMDP best available HLA typings. Typing data includes 42% updated by HLA-A, -B, -C, -DRB1 high-resolution typing performed through the NMDP Donor-Recipient Pair project6,8, with the remaining 58% at various levels of resolution and number of loci tested submitted to the NMDP by the transplant center.
HLA Matching
HLA typing was evaluated for allele and antigen level mismatches across 4 loci HLA-A, -B, -C, and -DRB1. HLA-DQ was excluded due to limited effect on outcome and HLA-DP was excluded based on low rates of transplant center HLA-DP typing practices, low frequency of unrelated matching, and conflicting estimates of its impact.5,6,8,9 The cohort data were categorized into 3 HLA match levels, to evaluate HLA typing between patient and donor that included variation in resolution and number of loci typed (e.g. missing HLA-C), based on an analysis performed by NMDP/Center for International Blood and Marrow Transplant Research (CIBMTR) using survival outcomes data.10
Using this method to categorize the patient/donor matching, match outcome was categorized as “well-matched” (zero or likely no mismatches present) n=4329 (58%), “partially-matched” (one or likely one mismatch present) n=2192 (29%), and “mismatched” (≥ two or likely two or more mismatches present) n=965 (13%).
Statistical Methods
Descriptive analysis was performed using frequency and univariate analysis across HLA match categories using Chi-square (categorical) or Kruskal-Wallis (continuous) tests. Ordered regression on the 3 levels of matching failed the proportional odds assumption of the cumulative logit model, so continuation ratio modeling was used to describe the odds ratios (OR) and 95% confidence intervals (CI).11,12 Binary logistic regression was performed to test well-matched pairs vs. partially-matched plus mismatched pairs (combination referred to as “less-matched”). A subsequent logistic regression comparing only the partially-matched vs. mismatched pairs (n=3138) was performed.
Logistic regression was performed using SAS statistical software (Version 9.1) with match category as the dependent variable and independent variables patient age, disease/stage at transplant, race, gender, transplant year, transplant center volume, transplant center location (international or domestic), NMDP formal search to transplant time, race matched donor status, graft source (PBSC or bone marrow), CMV serostatus of patient and donor, and conditioning intensity. Patient age was categorized into three discrete groups (≤19, 20–49, 50+) due to non-linear effects. Disease was categorized by broad disease and underlying stage if applicable. Disease categories included acute myelogenous leukemia (AML), acute lymphoblastic leukemia (ALL), chronic myelogenous leukemia (CML), myelodyplastic syndrome (MDS), lymphoma (includes Hodgkin’s and non-Hodgkin’s), other leukemias, other malignant disease, other nonmalignant disease (includes histiocytosis and inherited blood and metabolic disorders), and severe aplastic anemia (SAA).
Disease stage at transplant was categorized, as given below, for AML, ALL, CML, and MDS. For patients with AML and ALL, early stage was defined as first complete remission, intermediate stage as second complete remission or greater, and late stage as relapse or primary induction failure. Early stage in CML was defined as first chronic phase, intermediate stage included second chronic phase or greater and accelerated phase, and late stage included patients with blast crisis. Early stage disease in MDS was defined as refractory anemia and refractory anemia with ringed sideroblasts, and late stage as refractory anemia with excess blasts, refractory anemia with excess blasts in transformation, and chronic myelomonocytic leukemia.
Year of transplant from 2000–2005 was grouped in 2-year intervals. Transplant center volume was categorized into three levels based on the centers average NMDP transplants per year, over the 6 years represented in this study: low volume (<10 per year) n=132; medium volume (10–25) n=25; high volume (26+) n=11. NMDP formal search to transplant time, defined as the number of days between the date of first requested donor activity and date of transplant, was categorized into 4 groups (≤60, 61–120, 121–180, 181+) due to non-linear effects.
Results
Patient and Transplant Characteristics
The patient, donor, and graft characteristics stratified by HLA match category (Table 1) shows a significantly older median patient age between patients transplanted with a well-matched donor (median 41 years) compared with a mismatched donor (28 years). Search time also differs, with a median time that was 2 weeks longer for a partially-matched transplant and almost 4 weeks longer for a mismatched transplant, compared to the well-matched group. Patients with less-matched donors had a significantly greater proportion of female donors, particularly with prior pregnancies, than well-matched HCT recipients.
Table 1.
Patient, Donor and Graft Characteristics: HLA Match Category
| N Evaluated |
Well- matched |
Partially- matched |
Mismatched | P-value | |
|---|---|---|---|---|---|
| Patient | 4329 (58%) | 2192 (29%) | 965 (13%) | ||
| Median Age (range) | 7486 | 41.3 (0.13–78.1) | 36.6 (0.36 – 74.9) | 28.2 (0.38–79.3) | <0.0001 |
| % Male | 7486 | 59% | 58% | 60% | 0.78 |
| Search to Transplant Median Days (range) | 7485 | 101 (15–4110) | 116 (17–5102) | 128 (29–2744) | <0.0001 |
| Donor | |||||
| Median Age (range) | 7480 | 34.7 (18.6–61.1) | 35.7 (19.0–60.8) | 36.1 (18.6–60.4) | <0.0001 |
| Gender Male Female 0 pregnancy ≥1 pregnancy |
7343 |
67% 15% 18% |
59% 16% 25% |
56% 15% 29% |
<0.0001 |
| Graft | |||||
| Bone Marrow Graft | 7486 | 49% | 56% | 65% | <0.0001 |
| Race Matched % | 7378 | 88% | 81% | 69% | <0.0001 |
| Reduced Intensity Conditioning | 7478 | 32% | 28% | 24% | <0.0001 |
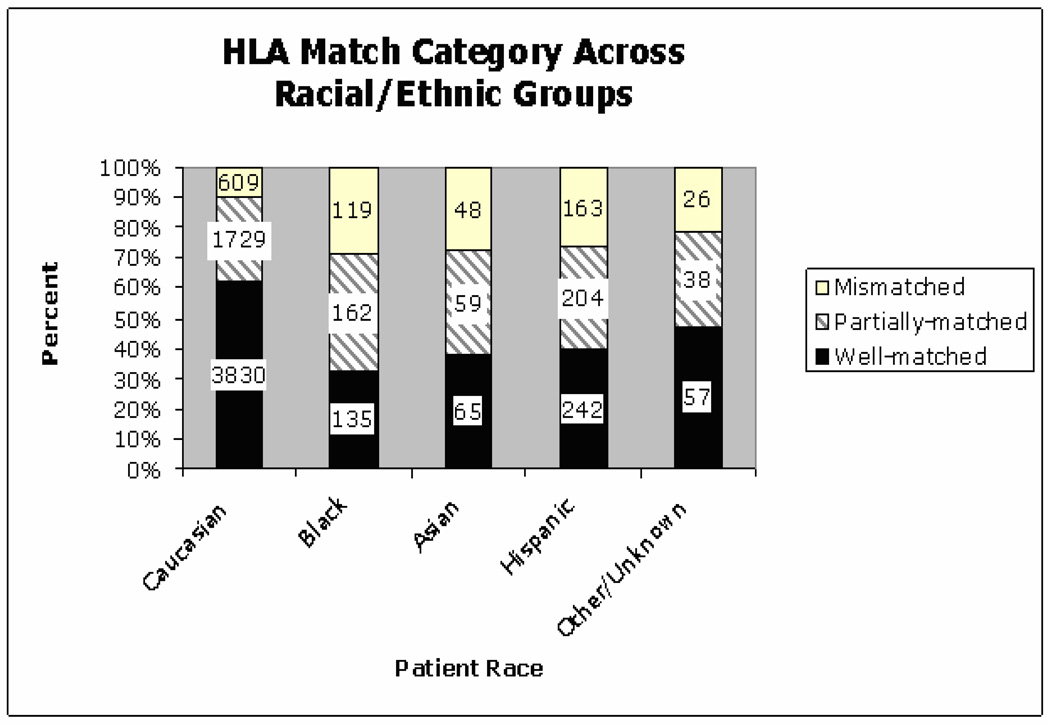
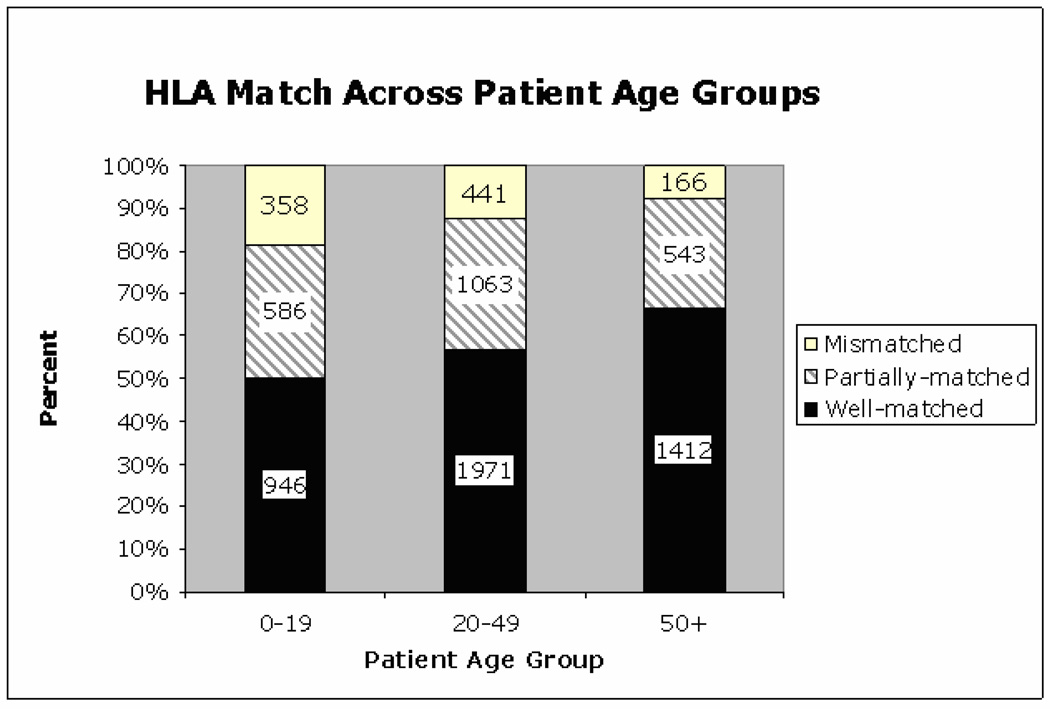
The distribution of donor HLA match across patient racial groups show Caucasian patients were transplanted mostly with well-matched donors (62%), while patients of non-Caucasian racial groups had a higher frequency of both partially and mismatched donor transplants (Figure 1). Univariate analysis of patient age and HLA match levels showed that older patients were less commonly transplanted with mismatched donors (Figure 2).
Figure 1.
Figure 2.
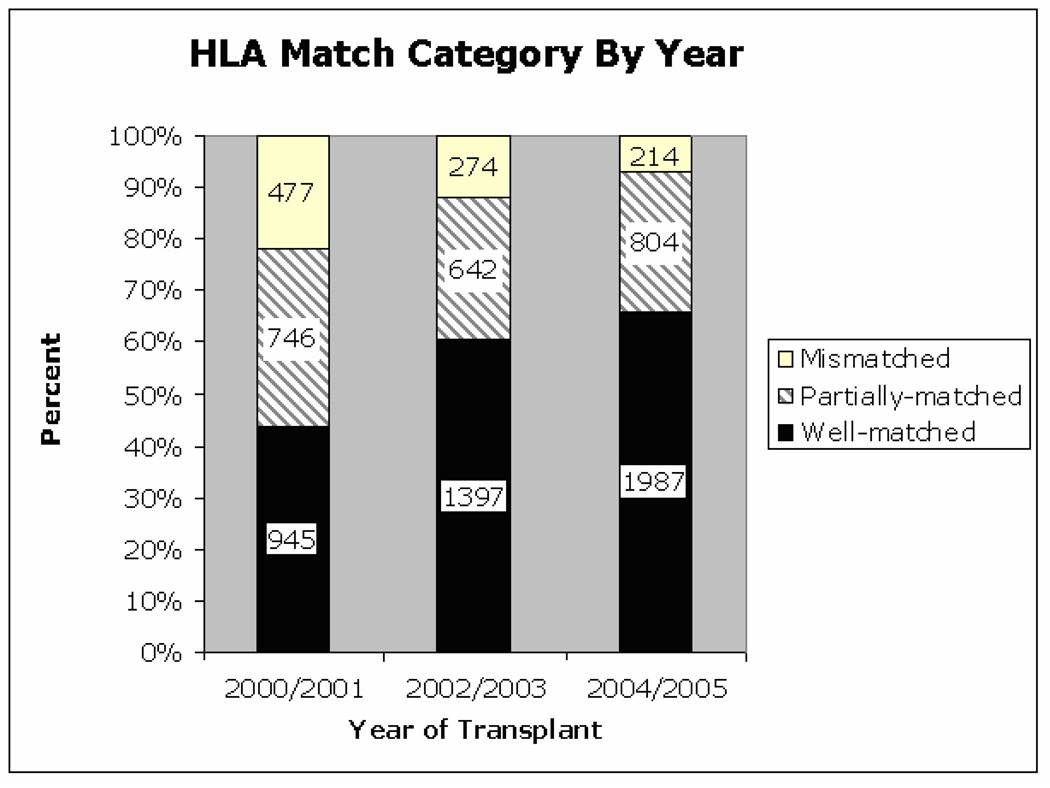
Encouragingly, over the years of study, a progressive increase in well-matched donors and decrease in partially-matched and mismatched donors was observed (Figure 3). This overall increase in transplants using well-matched donors was similar across all racial groups. The largest percentage decline in use of mismatched donors from 2000/2001 – 2004/2005 occurred in patients from non-Caucasian racial groups; Black (−22%), Asian (−19%), Hispanic (−24%) vs. Caucasian (−13%).
Figure 3.
For AML and CML, intermediate and late stage disease was associated with a somewhat higher proportion of HCT using a partially-matched or mismatched donor (Table 2). ALL and MDS showed a similar pattern, but to a lesser extent.
Table 2.
Disease/Stage: HLA Match Category
| Disease –stage | Well-matched | Partially-matched | Mismatched |
|---|---|---|---|
| Total* | 4319 (58%) | 2183 (29%) | 958 (13%) |
| AML | |||
| -Early | 553 (67%) | 199 (24%) | 75 (9%) |
| -Intermediate | 379 (53%) | 236 (33%) | 97 (14%) |
| -Late | 501 (56%) | 275 (31%) | 122 (14%) |
| ALL | |||
| -Early | 253 (55%) | 150 (33%) | 54 (12%) |
| -Intermediate | 340 (51%) | 209 (32%) | 114 (17%) |
| -Late | 164 (54%) | 86 (28%) | 55 (18%) |
| CML | |||
| -Early | 313 (59%) | 151 (29%) | 63 (12%) |
| -Intermediate | 162 (50%) | 115 (36%) | 46 (14%) |
| -Late | 31 (43%) | 27 (38%) | 14 (19%) |
| MDS | |||
| -Early | 118 (67%) | 41 (23%) | 16 (9%) |
| -Late | 263 (62%) | 123 (29%) | 40 (9%) |
| -Unknown | 91 (65%) | 37 (26%) | 12 (9%) |
| Lymphoma | 410 (64%) | 175 (27%) | 53 (8%) |
| Other Leukemia | 259 (65%) | 92 (23%) | 49 (12%) |
| Other Malignancy | 156 (63%) | 63 (26%) | 27 (11%) |
| Non-Malignancy | 154 (50%) | 91 (30%) | 60 (20%) |
| SAA | 172 (50%) | 113 (33%) | 61 (18%) |
26 patients missing disease stage: AML=14, ALL=9, CML=3
Abbreviations: AML (acute myelogenous leukemia); ALL (acute lymphoblastic leukemia); CML (chronic myelogenous leukemia); MDS (myelodysplastic syndrome); SAA (severe aplastic anemia). Non-malignant diseases include immunodeficiencies, inborn errors of metabolism, hemoglobinopathies.
Multivariate Analysis
Multivariate logistic regression analysis was performed to analyze the factors associated with having a transplant using a donor from the 3 HLA match levels (Table 3). The effect of race on HLA matching was pronounced. Black patients were 2.83 times, Asian 2.05 times, and Hispanic 1.73 times more likely to proceed using a less-matched (partial or mismatched) donor than Caucasian patients. This effect was also shown when comparing the less-matched donors between the partially-matched and mismatched groups. Older patient age was associated with significantly greater likelihood of having a well-matched donor selected. Patients age 50 and over were significantly less likely to have a less-matched donor (OR=0.73, 95% CI 0.62–0.85, P<0.0001), compared to patients under age 20. Patient age was also analyzed by decade (data not shown) and the findings were concordant with the 3 discrete age groups shown.
Table 3.
Factors Associated with a Less-Matched Transplant: Multivariate Analysis*
| Partially/Mismatched vs. Well-matched (ref) (N=7449) |
Mismatched vs. Partially-matched (ref) (N=3138) |
|||||
|---|---|---|---|---|---|---|
| Variable | OR | 95% CI | P-Value | OR | 95% CI | P-Value |
| Age | ||||||
| 0–19 (ref) | 1.00 | - | 0.0004 | 1.00 | - | 0.0087 |
| 20–49 | 0.86 | 0.75–0.98 | 0.75 | 0.61–0.92 | ||
| 50+ | 0.73 | 0.62–0.85 | 0.70 | 0.54–0.91 | ||
| Gender | ||||||
| Male (ref) | 1.00 | - | 0.94 | 1.00 | - | 0.42 |
| Female | 1.00 | 0.90–1.10 | 0.94 | 0.80–1.10 | ||
| Race | ||||||
| Caucasian (ref) | 1.00 | - | <0.0001 | 1.00 | - | <0.0001 |
| Black | 2.83 | 2.26–3.54 | 1.85 | 1.41–2.44 | ||
| Asian | 2.05 | 1.47–2.85 | 2.14 | 1.42–3.22 | ||
| Hispanic | 1.73 | 1.41–2.12 | 1.87 | 1.42–2.45 | ||
| Other/Unknown | 1.56 | 0.94–2.56 | 2.17 | 1.10–4.27 | ||
| Race Matched with donor | ||||||
| Yes (ref) | 1.00 | - | <0.0001 | 1.00 | - | 0.0004 |
| No | 1.60 | 1.37–1.86 | 1.45 | 1.17–1.79 | ||
| Unknown | 1.12 | 0.66–1.91 | 0.52 | 0.20–1.34 | ||
| Disease | ||||||
| AML -Early** | 1.00 | - | <0.0001 | 1.00 | - | 0.15 |
| -Inter. | 1.55 | 1.25–1.93 | 1.00 | 0.69–1.45 | ||
| -Late | 1.50 | 1.23–1.85 | 1.17 | 0.82–1.67 | ||
| ALL -Early** | 1.00 | - | 1.00 | - | ||
| -Inter. | 0.95 | 0.74–1.23 | 1.26 | 0.84–1.89 | ||
| -Late | 0.90 | 0.66–1.22 | 1.74 | 1.08–2.81 | ||
| CML -Early** | 1.00 | - | 1.00 | - | ||
| -Inter. | 1.84 | 1.36–2.48 | 1.18 | 0.73–1.89 | ||
| -Late | 2.35 | 1.38–4.02 | 1.44 | 0.68–3.04 | ||
| MDS -Early** | 1.00 | - | 1.00 | - | ||
| -Late | 1.48 | 1.00–2.19 | 0.96 | 0.47–1.95 | ||
| -Unkn. | 1.08 | 0.66–1.78 | 0.82 | 0.33–2.02 | ||
| Lymphoma † | 1.02 | 0.81–1.28 | 0.74 | 0.48–1.12 | ||
| Other Leukemia † | 0.93 | 0.72–1.22 | 1.30 | 0.82–2.05 | ||
| Other Malignancy † | 1.00 | 0.73–1.37 | 1.07 | 0.62–1.84 | ||
| Non-Malignancy † | 1.13 | 0.84–1.53 | 0.97 | 0.61–1.54 | ||
| SAA † | 1.14 | 0.86–1.51 | 0.89 | 0.57–1.39 | ||
| Year of Transplant | ||||||
| 2004/2005 (ref) | 1.00 | - | <0.0001 | 1.00 | - | <0.0001 |
| 2002/2003 | 1.28 | 1.14–1.44 | 1.73 | 1.40–2.15 | ||
| 2000/2001 | 2.47 | 2.18–2.79 | 2.69 | 2.20–3.30 | ||
| Search time to HCT (days) | ||||||
| 0–60 (ref) | 1.00 | - | <0.0001 | 1.00 | - | <0.0001 |
| 61–120 | 1.29 | 1.06–1.57 | 1.83 | 1.23–2.73 | ||
| 121–180 | 1.87 | 1.51–2.31 | 2.07 | 1.36–3.14 | ||
| 181+ | 2.41 | 1.94–2.99 | 2.61 | 1.72–3.96 | ||
| Transplant Center volume (per year) | ||||||
| High (26+) (ref) | 1.00 | - | 0.0085 | 1.00 | - | 0.06 |
| Medium (10–25) | 1.14 | 1.01–1.29 | 1.11 | 0.90–1.37 | ||
| Low (<10) | 1.20 | 1.07–1.36 | 1.28 | 1.04–1.57 | ||
| Transplant Center location | ||||||
| Domestic (ref) | 1.00 | - | <0.0001 | 1.00 | - | 0.61 |
| International | 2.06 | 1.63–2.62 | 0.92 | 0.68–1.26 | ||
Separate adjusted regressions comparing well-matched vs. partial or mismatched and comparing partial vs. mismatched transplants.
Reference group for each diagnostic category
Reference group early stage AML
Bold type indicates OR and 95% CI statistically different than reference group
Advanced disease was associated with increased likelihood of transplant with a donor mismatch. AML-intermediate stage was 1.55 times more likely (95% CI 1.25–1.93, P<0.0001) and AML-late stage 1.50 times more likely (95% CI 1.23–1.85, P<0.0001) to have a less-matched HCT than AML-early stage. Similarly, intermediate and late stage CML was associated with less-matched donors, OR=1.84 (95% CI 1.36–2.48, P<0.0001) and OR=2.35 (95% CI 1.38–4.02, P=0.0017) compared to early stage CML, respectively. Similar associations were noted for late stage MDS OR=1.48 (95% CI 1.00–2.19, P=0.052), but not for the other malignant or the non-malignant diseases.
Other factors beyond patient demographics including transplant center, year of transplant, and search time to transplant also showed an association with the HLA match outcome. Transplant centers with the lowest volume of transplants were more likely than high volume centers to select a less-matched donor, OR=1.20 (95% CI 1.07–1.36, P=0.003). There were also increased odds of less-matched transplants from international transplant centers.
Encouragingly, mismatches were less frequent in more recent years. HCT in 2000/2001 were 2.47 times (95% CI 2.18–2.79, P<0.0001) more likely to be less-matched compared to years 2004/2005. Transplants between pairs not racially matched were 1.6 times more likely to be less-matched. Neither graft source, conditioning intensity, nor patient and donor CMV serostatus were significantly associated with matching category (data not shown) and therefore, were not included in the final model.
Because international transplants might be associated with greater ethnic and thus HLA disparity, we performed a subset analysis (n=7049) excluding the 5.5% of patients from international transplant centers. These results were similar to the full cohort analysis (data not shown).
Discussion
This analysis demonstrates that both donor file HLA diversity as well as search and donor selection practices have greatly improved from 2000–2005 with 44% of transplants being well-matched in 2000/2001 compared to 66% in 2004/2005. The NMDP required transplant centers to type patient/donor pairs at high-resolution HLA-A, -B, -C and -DRB1 starting in June 2005 (near the study end). The practice of HLA typing at the allele level including the HLA-C locus was already increasing at many centers. This new NMDP requirement may have assisted other centers to identify which donor options might be the best donor match. Some of the early year transplant pairs may have been considered a match based on standard HLA typing technology prior to transplant, then upon subsequent high-resolution testing were found to contain mismatches. This trend to better HLA matching is a success reflecting advances in the donor file diversity, improvement in typing technology, implementation of evidence-based recommendations, and donor selection strategy.13
Other factors have influenced the availability and selection of better matched donors. Greater transplant center experience and volume may improve a patient’s chances of transplanting with a well-matched donor. Small volume transplant centers have 20% greater likelihood of proceeding to transplant with a less-matched donor compared to high volume centers and are 28% more likely to choose a mismatched than a partially-matched donor. Improvements in HLA search strategy, early initiation of a search, and other factors may increase the chances of HCT with a better matched donor, even in less-experienced centers. Immunogenetic consultation may be of particular importance in these less-experienced transplant centers.
Patients with early stage leukemia had transplants with a mismatched donor less often than those with intermediate and late stage disease. This association could reflect either more urgent, late stage transplants with insufficient time to identify better matched donors or alternatively, no better donor identified during early stage and disease progression resulting in the transplant performed with two adverse factors – advanced stage and a lesser-matched donor.5 Since disease stage at transplant is a potentially modifiable factor based on clinical decisions, earlier searching with selection of the best matched donor and HCT at an earlier disease stage might be a preferred decision-making strategy, if no effective alternatives are available.
Extended duration searches have not been, and were not in this study, associated with transplantation using a better matched donor.14 This emphasizes the need to balance the patient’s disease status and urgency with donor matching. Longer search times may not yield better HLA matched donors. Regression analysis showed that patients transplanted more than 6 months from the initiation of donor searching were significantly (2.4 times) more likely to use a less-matched donor than those transplanted promptly (within 60 days of searching).
Transplant using a mismatch donor was less common for older patients. This may reflect deliberate physician decisions to avoid the added mismatch-associated morbidity and mortality in already higher risk, older recipients. The recent increase in transplants for older patients using reduced intensity treatment regimens are designed for better treatment tolerance. These reduced intensity transplants were more often performed using more stringent HLA-matching, perhaps defined by pre-specified protocol.
Patients from non-Caucasian racial groups were more often transplanted with lesser-matched donors. In this study, we evaluated only patients proceeding to transplant and did not address the recognized racial disparity in the likelihood of finding a suitable donor. Increased recruitment of donors from non-Caucasian racial and ethnic groups as well as specific decision-making about URD vs. alternate therapies, including umbilical cord blood HCT, is needed to further increase the likelihood of good outcome for patients potentially eligible for HCT.
International transplant centers were twice as likely to select a less-matched donor. For patients with uncommon HLA typing, accessing the NMDP’s diverse donor pool may have been the best option for the patient, possibly explaining, at least in part, this observation.
We have studied a unique aspect of donor searching, the association of patient non-HLA factors with selection of HLA matched stem cell donors. Based upon an assumption that the donor selected by the transplant center was the best available donor, we are encouraged that both availability and use of well-matched donors increased over the study interval, particularly for non-Caucasian racial and ethnic subgroups. Use of new advances, including the recent NMDP HapLogicSM match calculation algorithm, could further improve identification of better HLA matched donors for searching patients leading to more successful URD HCT.
These results identify statistically significant evidence of the impact of race, disease/stage, age and transplant center experience on the selection of HLA well-matched donors. We suggest that all centers should utilize current HLA strategy and worldwide resources available to aid in donor selection.14 Initiating a patient’s URD search early in the disease process, especially for patients from non-Caucasian racial and ethnic groups, will provide the best scenario for making informed transplant decisions. Additional focus on donor recruitment efforts and strategies, perhaps including umbilical cord blood, can help narrow the gap for non-Caucasian patient groups at increased risk of transplants with HLA mismatching.
Acknowledgements
This work was supported by Public Health Service Grant U24-CA76518 from the National Cancer Institute, the National Institute of Allergy and Infectious Diseases, and the National Heart, Lung and Blood Institute; Office of Naval Research (grant to the NMDP N00014-06-1-0704); Health Resources and Services Administration (DHHS); and grants from AABB; Aetna; American Society for Blood and Marrow Transplantation; Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Association of Medical Microbiology and Infectious Disease Canada; Astellas Pharma US, Inc.; Baxter International, Inc.; Bayer HealthCare Pharmaceuticals; BloodCenter of Wisconsin; Blue Cross and Blue Shield Association; Bone Marrow Foundation; Canadian Blood and Marrow Transplant Group; Celgene Corporation; CellGenix, GmbH; Centers for Disease Control and Prevention; ClinImmune Labs; CTI Clinical Trial and Consulting Services; Cubist Pharmaceuticals; Cylex Inc.; CytoTherm; DOR BioPharma, Inc.; Dynal Biotech, an Invitrogen Company; Enzon Pharmaceuticals, Inc.; European Group for Blood and Marrow Transplantation; Gambro BCT, Inc.; Gamida Cell, Ltd.; Genzyme Corporation; Histogenetics, Inc.; HKS Medical Information Systems; Hospira, Inc.; Infectious Diseases Society of America; Kiadis Pharma; Kirin Brewery Co., Ltd.; Merck & Company; The Medical College of Wisconsin; MGI Pharma, Inc.; Michigan Community Blood Centers; Millennium Pharmaceuticals, Inc.; Miller Pharmacal Group; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Nature Publishing Group; New York Blood Center; Novartis Oncology; Oncology Nursing Society; Osiris Therapeutics, Inc.; Otsuka Pharmaceutical Development & Commercialization, Inc.; Pall Life Sciences; PDL BioPharma, Inc; Pfizer Inc; Pharmion Corporation; Saladax Biomedical, Inc.; Schering Plough Corporation; Society for Healthcare Epidemiology of America; StemCyte, Inc.; StemSoft Software, Inc.; Sysmex; Teva Pharmaceutical Industries; The Marrow Foundation; THERAKOS, Inc.; Vidacare Corporation; Vion Pharmaceuticals, Inc.; ViraCor Laboratories; ViroPharma, Inc.; and Wellpoint, Inc. Any opinions, findings and conclusions or recommendations expressed in this article are those of the author(s) and do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, National Marrow Donor Program, or any other agency of the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Petersdorf EW, Gooley TA, Anasetti C, et al. Optimizing outcome after unrelated marrow transplantation by comprehensive matching of HLA class I and II alleles in the donor and recipient. Blood. 1998;92:3515–3520. [PubMed] [Google Scholar]
- 2.Petersdorf EW, Hansen JA, Martin PJ, et al. Major-histocompatibility-complex class I alleles and antigens in hematopoietic-cell transplantation. N Engl J Med. 2001;345:1794–1800. doi: 10.1056/NEJMoa011826. [DOI] [PubMed] [Google Scholar]
- 3.Morishima Y, Sasazuki T, Inoko H, et al. The clinical significance of human leukocyte antigen (HLA) allele compatibility in patients receiving a marrow transplant from serologically HLA-A, HLA-B, and HLA-DR matched unrelated donors. Blood. 2002;99:4200–4206. doi: 10.1182/blood.v99.11.4200. [DOI] [PubMed] [Google Scholar]
- 4.Sasazuki T, Juji T, Morishima Y, et al. Effect of matching of class I HLA alleles on clinical outcome after transplantation of hematopoietic stem cells from an unrelated donor. Japan Marrow Donor Program. N Engl J Med. 1998;339:1177–1185. doi: 10.1056/NEJM199810223391701. [DOI] [PubMed] [Google Scholar]
- 5.Lee SJ, Klein J, Haagenson M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110:4576–4583. doi: 10.1182/blood-2007-06-097386. [DOI] [PubMed] [Google Scholar]
- 6.Flomenberg N, Baxter-Lowe LA, Confer D, et al. Impact of HLA class I and class II high-resolution matching on outcomes of unrelated donor bone marrow transplantation: HLA-C mismatching is associated with a strong adverse effect on transplantation outcome. Blood. 2004;104:1923–1930. doi: 10.1182/blood-2004-03-0803. [DOI] [PubMed] [Google Scholar]
- 7.Petersdorf EW, Anasetti C, Martin PJ, et al. Limits of HLA mismatching in unrelated hematopoietic cell transplantation. Blood. 2004;104:2976–2980. doi: 10.1182/blood-2004-04-1674. [DOI] [PubMed] [Google Scholar]
- 8.Hurley CK, Fernandez-Vina M, Hildebrand WH, et al. A high degree of HLA disparity arises from limited allelic diversity: analysis of 1775 unrelated bone marrow transplant donor-recipient pairs. Hum Immunol. 2007;68:30–40. doi: 10.1016/j.humimm.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Shaw BE, Marsh SG, Mayor NP, Russell NH, Madrigal JA. HLA-DPB1 matching status has significant implications for recipients of unrelated donor stem cell transplants. Blood. 2006;107:1220–1226. doi: 10.1182/blood-2005-08-3121. [DOI] [PubMed] [Google Scholar]
- 10.Weisdorf D, Spellman S, Haagenson M, et al. Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: revised definitions to predict survival. Biol Blood Marrow Transplant. 2008;14:748–758. doi: 10.1016/j.bbmt.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armstrong BG, Sloan M. Ordinal regression models for epidemiologic data. Am J Epidemiol. 1989;129:191–204. doi: 10.1093/oxfordjournals.aje.a115109. [DOI] [PubMed] [Google Scholar]
- 12.Bender R, Benner A. Calculating ordinal regression models in SAS and S-Plus. Biometrical Journal. 2000;42:677–699. [Google Scholar]
- 13.Davis JW, Buck K, Dorr L, et al. Evaluation of unrelated donor search strategy proficiencies. Biol Blood Marrow Transplant. 2006;12(2):31. [abstract 79]. [Google Scholar]
- 14.Bray RA, Hurley CK, Kamani NR, et al. National Marrow Donor Program HLA matching guidelines for unrelated adult hematopoietic cell transplants. Biol Blood Marrow Transplant. 2008;14(9):45–53. doi: 10.1016/j.bbmt.2008.06.014. [DOI] [PubMed] [Google Scholar]