Abstract
Previous studies have shown that morphine-6-glucuronide (M6G), a metabolite of morphine, induces reward and psychomotor stimulation but the role of the mu opioid receptor in these actions of the drug is not fully characterized. Thus, using mice lacking exon-2 of the mu opioid receptor and their wild-type littermates/controls, we determined the role of this receptor in psychomotor stimulation, sensitization, and conditioned place preference (CPP) induced by M6G. For comparison, we also assessed the role of the mu opioid receptor in the rewarding action of morphine. For the measurement of locomotor activity and sensitization, mice were habituated to motor activity chambers for 1 h, then injected with M6G (10 mg/kg) and locomotor activity was recorded for an additional 1 h. The same treatment was given for five days and mice were tested for sensitization a week later. For the CPP experiments, mice were tested for baseline place preference on day 1, then received single or repeated alternate-day saline/drug or drug/saline conditioning and tested for CPP the following day. Mice were also tested for CPP under a drugged state. M6G induced psychomotor stimulation, a response that was enhanced upon repeated administration of the drug, showing that locomotor sensitization developed to the motor stimulatory action of M6G. However, M6G induced a weaker CPP response compared to morphine. None of these actions of M6G was detected in mice lacking the mu opioid receptor. Together, the current results suggest that M6G induces psychomotor stimulation and a weaker rewarding action via the mu opioid receptor.
Keywords: Morphine-6-glucuronide, Psychomotor sensitization, Conditioned place preference, Mu opioid receptor, Knockout mouse
1. Introduction
Morphine is used for the treatment of moderate-to-severe pain. It is metabolized in the liver to morphine-3-glucuronide (M3G) and morphine-6-glucuronide (M6G). Among the metabolites, M6G has much of morphine’s analgesic potency (Christrup, 1997; Kilpatrick and Smith, 2005; Klepstad et al., 2000; Pasternak et al., 1987). In fact, M6G is reported to be even more potent than morphine following central administration (Abbott and Palmour, 1988; Christrup, 1997; Frances et al., 1992; Kilpatrick and Smith, 2005; Klepstad et al., 2000). With M6G being currently undergoing clinical trials as an analgesic for the treatment of post-operative pain, its pharmacological actions have been thoroughly studied. These reports reveal that M6G has a similar analgesic effect but does not induce as many side effects (respiratory depression, EEG, and nausea) associated with morphine (Cann et al., 2002; Kilpatrick and Smith, 2005; Romberg et al., 2003a; Romberg et al., 2003b). However, there are limited studies determining the abuse potential of the drug.
The phenomenon of locomotor sensitization is referred to as a progressive and enduring increase in locomotor stimulation induced by repeated intermittent administration of morphine and other addictive drugs (Badiani et al., 2000; Kalivas et al., 1993; Lutfy et al., 2002; Marquez et al., 2006; Post and Rose, 1976; Shippenberg et al., 2009; Shippenberg and Heidbreder, 1995; Shuster et al., 1977; Steketee and Kalivas, 1991; Stewart and Badiani, 1993). This phenomenon is thought to mimic some aspects of addiction, e.g., compulsive drug-seeking behaviors and relapse [for review, see (Robinson and Berridge, 1993; 2000)]. M6G increases motor activity (Handal et al., 2008; Vindenes et al., 2006; Vindenes et al., 2008) and induces a robust locomotor sensitization following its single administration (Handal et al., 2008). However, the role of the mu opioid receptor in this action of M6G has not been characterized. . Accordingly, we determined the role of the mu opioid receptor in M6G-induced motor stimulation and locomotor sensitization.
The conditioned place preference (CPP) is widely used as an animal model of reward and incentive learning (Bardo and Bevins, 2000). Previous studies have shown that M6G induces CPP in rats (Abbott and Franklin, 1991) as well as in mice (Vindenes et al., 2006; Vindenes et al., 2008). However, it is not known whether its rewarding action is mediated by the mu opioid receptor. Therefore, we also examined the role of the mu opioid receptor in M6G-induced CPP under both drug-free and drugged states. For comparison, we investigated the role of the mu opioid receptor in morphine-induced CPP under the same conditions.
2. Materials and Methods
2.1. Subjects
Mice lacking the mu opioid receptor (Matthes et al., 1996), fully backcrossed on C57Bl/6J mouse strain, were purchased from Jackson Laboratory (Bar Harbor, Maine, USA) and used to generate heterozygous breeding pairs. Male wild-type and mu opioid receptor knockout mice were bred in house from the heterozygous breeding pairs and used for all experiments. Mice were kept 2–4 per cage with free access to food and water under a 12-h light/12-h dark cycle. All experiments were conducted according to the National Institute of Health guideline for the proper care and use of animals in research and approved by the Institutional Animal Care and Use Committee at Western University of Health Sciences (Pomona, California, USA).
2.2. Experimental Procedures
2.2.1. Locomotor Activity
Distance traveled was used as a measure of motor activity and recorded using a Videomex-V system (Columbus Instruments, Columbus, OH, USA). To determine the effect of M6G on locomotor activity, wild-type and mu opioid receptor knockout mice (n = 5 mice per genotype) were individually placed in motor activity chambers (14 cm length X 14 cm width X 22 cm height) and allowed to habituate to the activity chamber for 1 h. Mice were then injected with M6G (10 mg/kg, s.c.) and locomotor activity was measured in centimeters for 1 h (4 X 15-min bins). To determine whether sensitization develops to the motor stimulatory action of M6G, mice were treated with their respective treatment once daily for five consecutive days and tested for locomotor sensitization a week later (day 12). On this day, mice were habituated to the motor activity chambers for 1 h, injected with M6G (10 mg/kg, s.c.) and their locomotor activity was recorded, as described above.
2.2.2. Conditioned Place Preference (CPP)
Wild-type and mu opioid receptor knockout mice were tested for CPP following single and repeated (four times) alternate-day saline/M6G or M6G/saline conditioning. The description of the CPP apparatus is provided elsewhere (Marquez et al., 2006). Briefly, a 3-chambered (a smaller central gray neutral chamber and two larger conditioning chambers decorated with 2.54 cm horizontal or vertical black and white stripes) CPP apparatus was used. We used this 3-chambered apparatus because we wanted to reduce the unintentional bias due to the initial placement of animals in the apparatus. The use of this apparatus also increases the strength of the CPP response since more choices are available to the animals during the test for place preference/avoidance [(for a review, please see (Bardo and Bevins, 2000)]. The CPP protocol consisted of preconditioning, conditioning and postconditioning phases which were conducted in the absence and/or presence of each drug. On day 1 (preconditioning test day; D1), mice were placed in the neutral chamber of the CPP apparatus and allowed to freely roam the CPP chambers for 15 min. The amount of time that mice spent in each chamber was recorded. This was used as a measure of baseline preference toward the CPP chambers for each mouse. On days 2–9, mice received alternate-day saline/M6G or M6G/saline conditioning trainings in which mice were injected with saline, M6G (10 mg/kg, s.c.) or morphine and confined to the vehicle-paired or drug-paired chamber for 1 h. The CPP conditioning chambers were further made distinguishable from each other by inclusion of an olfactory cue (i.e., a 10-μL droplet of almond and orange extract was applied onto a coin-sized filter paper hanging at the right upper edge of each conditioning chamber). Mice were tested for postconditioning place preference following single (day 4) and repeated (day 10) conditioning. Four days later, mice were injected with saline and the next day with M6G or morphine and tested CPP in the presence of drug (also called drugged state or state-dependent). On these days, mice were injected with saline or the drug, placed in the neutral chamber of the CPP apparatus and allowed to free-roam the CPP chambers for 15 min. The amount of time that mice spent in each chamber was recorded.
2.3. Drugs
Morphine sulfate and M6G were generously supplied by the Drug Supply program of the National Institute on Drug Abuse Drug Supply (Bethesda, Maryland, the USA). Drugs were dissolved in normal saline (0.9% NaCl solution) and injected subcutaneously (s.c.) in the volume of 0.1ml/10g of mouse body weight. The doses of the drug was based on our previous studies, showing that a single conditioning paired with morphine (10 mg/kg, s.c.) induces a significant CPP. In order to compare the rewarding action of M6G to morphine, we also used 10 mg/kg dose of M6G.
2.4. Data Analysis
The data represent mean (±S.E.M.) distance traveled (cm) or the amount of time that mice spent in the CPP chambers (sec) and were analyzed using repeated measures analysis of variance (ANOVA). For analysis of the locomotor activity data, the factors were distance traveled over time and genotype. For analysis of the CPP data, the factors were the amount of time that mice spent in the CPP chambers and test day. The Bonferroni post-hoc test was used to reveal significant differences between various groups. P≤0.05 was considered statistically significant.
3. Results
3.1. The role of the mu opioid receptor in the motor stimulatory action of M6G
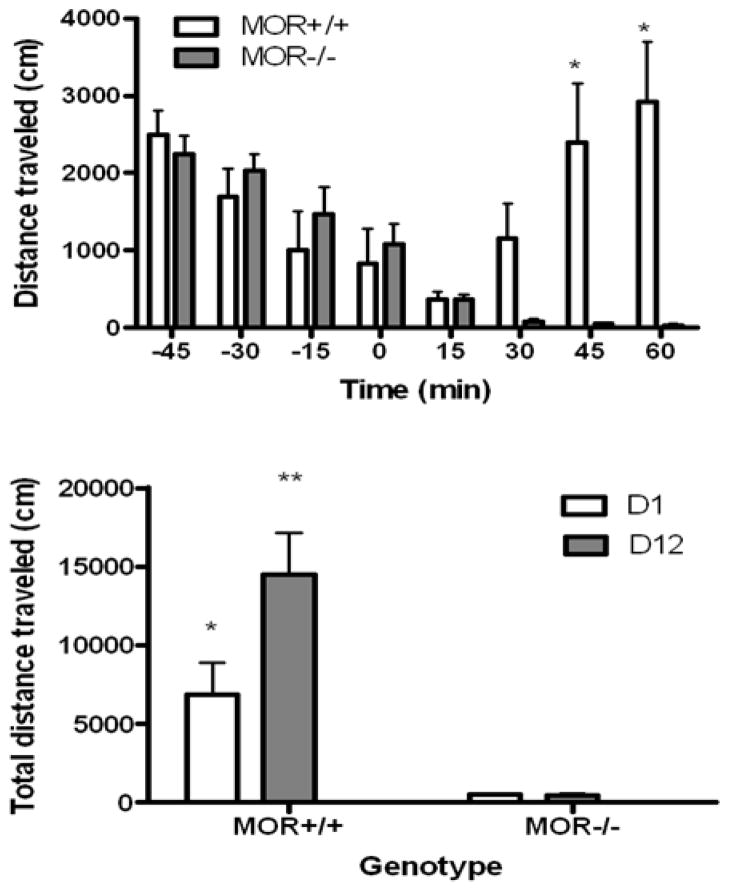
Figure 1 illustrates the increase in distance traveled (cm) following single (upper panel) and repeated (lower panel) M6G administration in mice lacking the mu opioid receptor and their wild-type littermates/controls. Analysis of the acute effect of M6G in both genotypes revealed a significant interaction between genotype and distance traveled with regards to time (F7, 56 = 11.13, p<0.001). Subsequent analysis of the data showed that M6G increased locomotor activity in wild-type but not in mu opioid receptor knockout mice (Fig. 1, upper panel). The motor stimulatory action of M6G was enhanced upon its repeated administration (Fig. 1, lower panel), as evidenced by a significant interaction between time and genotype (F1,8 = 5.08, p=0.05). Further analysis of the data showed that acute M6G increased locomotor activity in wild-type mice and the magnitude of this response was enhanced following repeated M6G administration. These results indicate that sensitization developed to the motor stimulatory action of M6G in wild-type mice. However, this phenomenon was not observed in mu opioid receptor knockout mice.
Fig. 1.
Distance traveled (cm) before or after single (upper panel) or repeated (10 mg/kg once daily for four consecutive days) M6G exposure (lower panel) in mice lacking mu opioid receptor (MOR−/−) and their wild-type littermates/control (MOR+/+). The data represent mean (±S.E.M.) of 5–6 mice per genotype for each experiment. *significantly different from the knockout mice (p<0.05); **significantly different from all other group (p<0.01)
3.2. The role of the mu opioid receptor in CPP induced by single conditioning with morphine or M6G
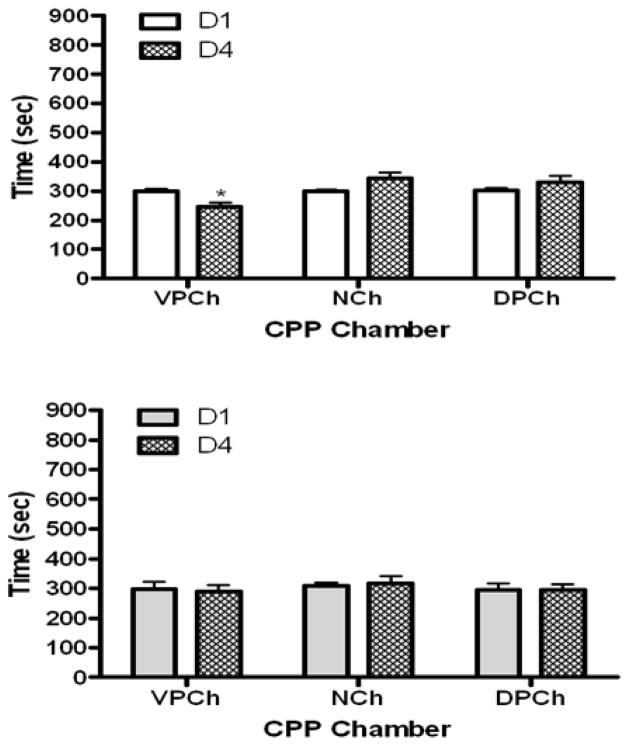
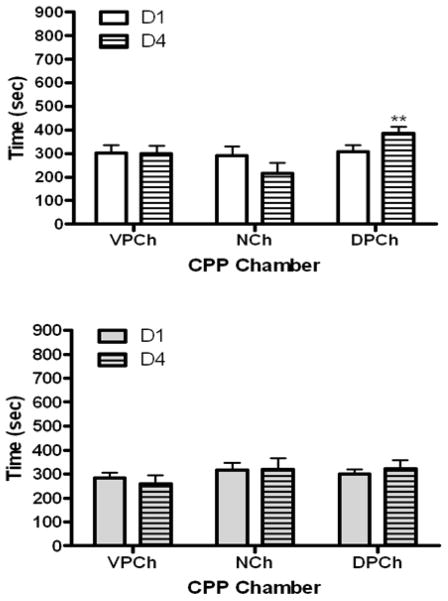
The amount of time that wild-type and mu knockout mice spent in the CPP chambers before (D1) and after (D4) single conditioning with M6G is shown in figure 2. Data analysis revealed a significant interaction between the amount of time that wild-type mice spent in the CPP chambers and test day (F2,15 = 7.17; p<0.01). Further analysis of the data demonstrated that mice did not express any preference toward the CPP chambers on the preconditioning (D1) test day (Fig. 2, upper panel). However, there was a significant decrease in the amount of time that mice spent in the vehicle-paired chamber following M6G conditioning compared to day 1 (p<0.05). On the other hand, a slight increase in the amount of time that mice spent in the drug-paired chamber was observed on postconditioning (D4) compared to preconditioning (D1) test day. Nevertheless, it was not statistically significant (p>0.05). In contrast, single morphine conditioning induced a robust CPP in wild-type mice (Fig. 3, upper panel). Analysis of the data revealed a significant interaction between the two factors (F2,15 = 12.74; p<0.001). Further analysis of the data showed that wild-type mice exhibited significantly (p<0.01) more preference toward the morphine-paired chamber on the postconditioning (D4) than preconditioning (D1) test day (Fig. 3, upper panel). Neither M6G (Fig. 2, lower panel) nor morphine (Fig. 3, lower panel) induced CPP in mu opioid receptor knockout mice. These results illustrate that single conditioning paired with morphine but not M6G induces a significant CPP that is mediated by the mu opioid receptor.
Fig. 2.
The amount of time that wild-type mice (upper panel) and mice lacking the mu receptor (lower panel) spent in the CPP chambers before (D1) and after (D4) single conditioning with M6G (10 mg/kg). The data represent mean (±S.E.M.) of 6 mice per genotype. *significantly different compared to its respective chamber on day 1 (p<0.05)
Fig. 3.
The amount of time that wild-type mice (upper panel) and mice lacking the mu receptor (lower panel) spent in the CPP chambers before (D1) and after (D4) single conditioning with morphine (10 mg/kg). The data represent mean (±S.E.M.) of 6–7 mice per genotype. **significantly different compared to its respective chamber on day 1 (p<0.01)
3.3. The role of the mu opioid receptor in state-dependent CPP induced by M6G
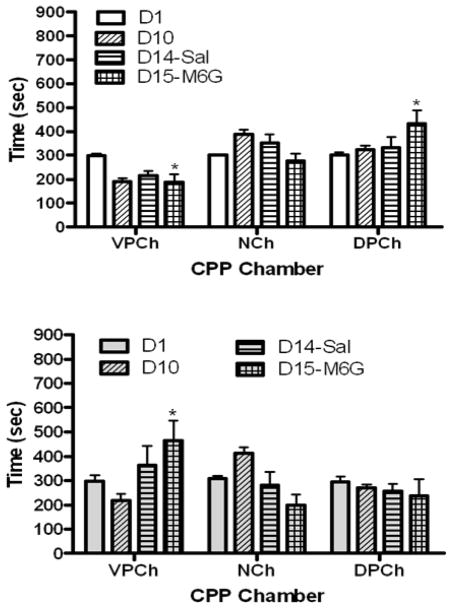
Figure 4 depicts the amount of time that mice lacking the mu opioid receptor and their wild-type littermates/controls spent in the CPP chambers on each test day. Analysis of the data in wild-type mice (Fig. 4, upper panel) revealed a significant interaction between the amount of time that mice spent in the CPP chambers and test day (F6,45 = 6.72, p<0.0001). Further analysis of the data showed that the amount of time that wild-type mice spent in the vehicle-paired chamber and drug-paired chamber was comparable on day 1 (Fig. 4, upper panel). When mice were tested for preference toward the CPP chambers following repeated conditioning (D10), there was a significant (p<0.05) decrease in the amount of time that mice spent in the vehicle-paired chamber on this day compared to day 1 (D1). There was a slight increase in the amount of time that mice spent in the M6G-paired chamber on this day compared to day 1 but the difference was not statistically significant (p>0.05). A similar pattern was observed when mice were challenged with saline four days later. However, mice showed a significant CPP following a challenge dose of M6G, as evidenced by a significant (p<0.05) increase in the amount of time that mice spent in the M6G-paired chamber on this day compared to other test days (p<0.05). However, these changes were absent in mice lacking the mu opioid receptor (Fig. 4, lower panel). Data analysis revealed a significant interaction between the two factors (F6,45 = 5.24, p<0.005). Further analysis of the data showed that knockout mice spent significantly greater amount of time in the saline-paired chamber following M6G challenge compared to day 1 and day 10 (p<0.05 or better; Fig. 4, lower panel). These results suggest that M6G induces a weak CPP via the mu opioid receptor.
Fig. 4.
The amount of time that wild-type (upper panel) and knockout (lower panel) mice spent in CPP chambers before (D1) and after (D10) repeated conditioning with M6G (10 mg/kg) and following a saline (D14) or M6G (5 mg/kg, D15) challenge. The data represent the mean (±S.E.M.) of 6 mice per genotype. *significantly different compared to their respective chamber on day 1 (p<0.05)
3.4. The role of the mu opioid receptor in state-dependent CPP induced by morphine
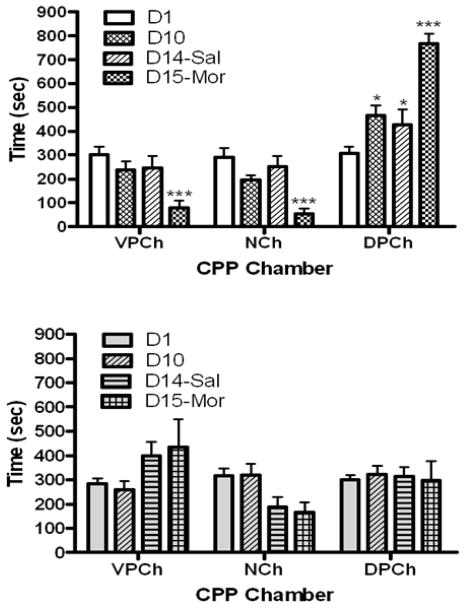
The amount of time that mice lacking the mu opioid receptor and their wild-type littermates/controls spent in the CPP chambers before (D1) and after (D10) repeated conditioning with morphine is shown in figure 5. Analysis of the data revealed a significant interaction between the amount of time that wild-type mice spent in the CPP chamber and test day (F6,45 = 26.43, p<0.0001). Further analysis of the data suggested that repeated morphine conditioning induced a robust CPP in wild-type mice, as evidenced by a significant increase in the amount of time that mice spent in the morphine-paired chamber on day 10 compared to day 1. This CPP response was still evident when mice were challenged with saline on day 14 (D14-Sal). Furthermore, this response was significantly increased following morphine challenge (Fig. 5, upper panel; compare the amount of time that mice spent in the drug-paired chamber on this day (D15-MOR) versus other days). In contrast, morphine failed to induce CPP in mice lacking the mu opioid receptor (Fig. 5, lower panel). Data analysis revealed a significant interaction between the two factors ((F6,54 = 3.40; p<0.01). Similar to M6G, when mice lacking the mu opioid receptor were tested in the presence of morphine, there was an increase in the amount of time that mice spent in the vehicle-paired chamber although it was not statistically significant (Fig. 5, lower panel).
Fig. 5.
The amount of time that wild-type (upper panel) and knockout (lower panel) mice spent in the CPP chambers prior to (D1) and after (D10) repeated conditioning with morphine (10 mg/kg) and following a saline (D14) or morphine (5 mg/kg, D15) challenge. The data represent the mean (±S.E.M.) of 6–7 mice per genotype. *significantly different compared to the amount of time that the mice spent in this chamber on day 1 (p<0.05), ***significantly different compared to the amount of time that mice spent in this chamber on day 1 (p<0.001)
4. Discussion
The main findings of the present study are that morphine induced a robust CPP under both drug-free and drugged states; whereas, M6G exerted a strong CPP under a drugged state only. M6G stimulated locomotor activity and induced locomotor sensitization. M6G-mediated state-dependent CPP and hyperlocomotion were abolished in mice lacking the mu opioid receptor, suggesting the involvement of the mu opioid receptor in these actions of M6G. Overall, the present findings illustrate that M6G may have a weaker rewarding action than morphine.
M6G has been shown to possess a potent analgesic effect which is, at least, equivalent to morphine (Abbott and Palmour, 1988; Christrup, 1997; Frances et al., 1992; Kilpatrick and Smith, 2005; Klepstad et al., 2000). Surprisingly, M6G elicits antinociception in mice lacking exon-1, but not exon-2, of the mu opioid receptor (Schuller et al., 1999). In contrast, morphine fails to reduce nociception in either knockout line (Matthes et al., 1996; Schuller et al., 1999). These findings raise the possibility that the action of morphine and M6G may be mediated via different types of mu opioid receptors. Indeed, M6G and morphine have been proposed to bind to different mu opioid receptor (Pan et al., 2005). Thus, the current study was designed to shed some light on the role of the mu opioid receptor in M6G-induced motor stimulation and CPP.
M6G has been shown to induce psychomotor stimulation (Handal et al., 2008; Vindenes et al., 2006; Vindenes et al., 2008) and locomotor sensitization following single M6G administration in mice (Handal et al., 2008). In the present study, we also observed that M6G significantly increased locomotor activity in wild-type mice. Additionally, we found that this response was enhanced upon its repeated administration. On the other hand, M6G did not increase locomotion and failed to elicit locomotor sensitization in mice lacking the mu opioid receptor. Overall, these results suggest that the psychomotor stimulatory action of M6G and its ability to induce behavioral sensitization are mediated by the mu opioid receptor.
Morphine and other addictive drugs are widely abused, in part, due to their positive reinforcing and rewarding actions. We observed a robust CPP response following single or repeated morphine conditioning, as evidenced by a significant increase in the amount of time that mice spent in the morphine-paired chamber on the postconditioning as compared to preconditioning test day or compared to the saline-paired side on the postconditioning test day (p<0.01). We also found that the CPP response was significantly greater in magnitude when mice were tested in the presence of morphine, illustrating that morphine induced a greater CPP response under a drugged state when compared to drug-free state. However, these actions of morphine were abolished in knockout mice. Overall, these results suggest that the rewarding action of morphine, under both drug-free and drugged states, is mediated by the mu opioid receptor. This is consistent with a previous report showing that morphine failed to induce CPP under a drug-free state (Matthes et al., 1996). A number of studies have shown that M6G induces CPP in mice, and this response is comparable to morphine (Vindenes et al., 2006; Vindenes et al., 2008). However, we only observed a significant CPP when we compared the amount of time that mice spent in the M6G-paired versus saline-paired chamber on the postconditioning test day. In fact, we found a small increase in the amount of time that mice spent in the drug-paired chamber after compared to before conditioning. Nonetheless, a significant reduction in the vehicle-paired chamber was observed between the two test days, raising the possibility that M6G may have weaker rewarding action than morphine. Consistent with this notion, we discovered that wild-type mice exhibited a robust CPP under a drugged state, i.e., in the presence of M6G. Interestingly, these changes were not observed in mice lacking the mu opioid receptor, suggesting that M6G exerts its rewarding action via the mu opioid receptor.
M6G has been shown to bind to the kappa opioid receptor (Kilpatrick and Smith, 2005), and activation of this receptor is associated with aversion in rodents and dysphoria in humans [for review, see (Herz, 1998)]. It is tempting to suggest that this may have altered the rewarding action of M6G, thereby leading to a weaker reward following conditioning with M6G. However, morphine also has some kappa opioid receptor agonistic action. Nevertheless, our data suggest that the activity of M6G and morphine at the mu and kappa opioid receptors may alter the rewarding actions of these drugs. In fact, mice lacking the mu opioid receptor spent significantly more time in the vehicle-paired chamber following M6G challenge on day 15 compared to other days. Notably, morphine induced a similar response in mu knockout mice. Considering that the increase in the amount of time that mice lacking the mu opioid receptor spent in the vehicle-paired chamber was not associated with a significant reduction in the amount of time that mice spent in the drug-paired chamber, this may not indicate an aversive response. However, the increase in the amount of time that mu opioid receptor knockout mice spent in the vehicle-paired chamber may be due to an increase in the familiarity of the drug-paired context, thereby leading to exploration of the unfamiliar more than familiar CPP chamber [for review, see (Bardo and Bevins, 2000)] or a decrease in exploration between the conditioning chambers. Given that mice would require more time to move to and from the vehicle-paired chamber to the drug-paired chamber in the three-chambered CPP apparatus, the decrease in exploration may have been exacerbated by the use of the three chambered CPP apparatus used in the current study. In view of that our CPP chambers did not allow for the simultaneous measurement of locomotor activity, further studies are needed to explore these possibilities.
5. Conclusion
The current results suggest that the motor stimulatory and rewarding actions of M6G are mediated via the mu opioid receptors. However, the rewarding action of M6G is weaker than morphine. This is consistent with a growing body of literature claiming that M6G is a potent analgesic with fewer side effects than its parent compound, morphine.
Acknowledgments
The current studies were supported in part by the Department of Pharmaceutical Sciences, College of Pharmacy, Western University of Health Sciences (Pomona, CA) and in part by a NIDA funded Grant DA # R01-016682 to KL and DA # R24 DA017298.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott FV, Franklin KB. Morphine-6-glucuronide contributes to rewarding effects of opiates. Life Sci. 1991;48:1157–1163. doi: 10.1016/0024-3205(91)90453-i. [DOI] [PubMed] [Google Scholar]
- Abbott FV, Palmour RM. Morphine-6-glucuronide: analgesic effects and receptor binding profile in rats. Life Sci. 1988;43:1685–1695. doi: 10.1016/0024-3205(88)90479-1. [DOI] [PubMed] [Google Scholar]
- Badiani A, Oates MM, Robinson TE. Modulation of morphine sensitization in the rat by contextual stimuli. Psychopharmacology (Berl) 2000;151:273–282. doi: 10.1007/s002130000447. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Cann C, Curran J, Milner T, Ho B. Unwanted effects of morphine-6-glucoronide and morphine. Anaesthesia. 2002;57:1200–1203. doi: 10.1046/j.1365-2044.2002.02624_2.x. [DOI] [PubMed] [Google Scholar]
- Christrup LL. Morphine metabolites. Acta anaesthesiologica Scandinavica. 1997;41:116–122. doi: 10.1111/j.1399-6576.1997.tb04625.x. [DOI] [PubMed] [Google Scholar]
- Frances B, Gout R, Monsarrat B, Cros J, Zajac JM. Further evidence that morphine-6 beta-glucuronide is a more potent opioid agonist than morphine. J Pharmacol Exp Ther. 1992;262:25–31. [PubMed] [Google Scholar]
- Handal M, Ripel A, Skurtveit S, Morland J. Behavioural sensitization in mice induced by morphine-glucuronide metabolites. Pharmacol Biochem Behav. 2008;90:578–585. doi: 10.1016/j.pbb.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Herz A. Opioid reward mechanisms: a key role in drug abuse? Canadian journal of physiology and pharmacology. 1998;76:252–258. doi: 10.1139/cjpp-76-3-252. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Sorg BA, Hooks MS. The pharmacology and neural circuitry of sensitization to psychostimulants. Behav Pharmacol. 1993;4:315–334. [PubMed] [Google Scholar]
- Kilpatrick GJ, Smith TW. Morphine-6-glucuronide: actions and mechanisms. Medicinal research reviews. 2005;25:521–544. doi: 10.1002/med.20035. [DOI] [PubMed] [Google Scholar]
- Klepstad P, Kaasa S, Borchgrevink PC. Start of oral morphine to cancer patients: effective serum morphine concentrations and contribution from morphine-6-glucuronide to the analgesia produced by morphine. Eur J Clin Pharmacol. 2000;55:713–719. doi: 10.1007/s002280050003. [DOI] [PubMed] [Google Scholar]
- Lutfy K, Khaliq I, Carroll FI, Maidment NT. Orphanin FQ/nociceptin blocks cocaine-induced behavioral sensitization in rats. Psychopharmacology (Berl) 2002;164:168–176. doi: 10.1007/s00213-002-1192-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez P, Baliram R, Gajawada N, Friedman TC, Lutfy K. Differential involvement of enkephalins in analgesic tolerance, locomotor sensitization, and conditioned place preference induced by morphine. Behav Neurosci. 2006;120:10–15. doi: 10.1037/0735-7044.120.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dolle P, Tzavara E, Hanoune J, Roques BP, Kieffer BL. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- Pan L, Xu J, Yu R, Xu MM, Pan YX, Pasternak GW. Identification and characterization of six new alternatively spliced variants of the human mu opioid receptor gene, Oprm. Neuroscience. 2005;133:209–220. doi: 10.1016/j.neuroscience.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Pasternak GW, Bodnar RJ, Clark JA, Inturrisi CE. Morphine-6-glucuronide, a potent mu agonist. Life Sci. 1987;41:2845–2849. doi: 10.1016/0024-3205(87)90431-0. [DOI] [PubMed] [Google Scholar]
- Post RM, Rose H. Increasing effects of repetitive cocaine administration in the rat. Nature. 1976;260:731–732. doi: 10.1038/260731a0. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95(Suppl 2):S91–117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Romberg R, Olofsen E, Sarton E, Teppema L, Dahan A. Pharmacodynamic effect of morphine-6-glucuronide versus morphine on hypoxic and hypercapnic breathing in healthy volunteers. Anesthesiology. 2003a;99:788–798. doi: 10.1097/00000542-200310000-00008. [DOI] [PubMed] [Google Scholar]
- Romberg R, Sarton E, Teppema L, Matthes HW, Kieffer BL, Dahan A. Comparison of morphine-6-glucuronide and morphine on respiratory depressant and antinociceptive responses in wild type and mu-opioid receptor deficient mice. British journal of anaesthesia. 2003b;91:862–870. doi: 10.1093/bja/aeg279. [DOI] [PubMed] [Google Scholar]
- Schuller AG, King MA, Zhang J, Bolan E, Pan YX, Morgan DJ, Chang A, Czick ME, Unterwald EM, Pasternak GW, Pintar JE. Retention of heroin and morphine-6 beta-glucuronide analgesia in a new line of mice lacking exon 1 of MOR-1. Nat Neurosci. 1999;2:151–156. doi: 10.1038/5706. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Chefer VI, Thompson AC. Delta-opioid receptor antagonists prevent sensitization to the conditioned rewarding effects of morphine. Biol Psychiatry. 2009;65:169–174. doi: 10.1016/j.biopsych.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippenberg TS, Heidbreder C. Sensitization to the conditioned rewarding effects of cocaine: pharmacological and temporal characteristics. J Pharmacol Exp Ther. 1995;273:808–815. [PubMed] [Google Scholar]
- Shuster L, Yu G, Bates A. Sensitization to cocaine stimulation in mice. Psychopharmacology (Berl) 1977;52:185–190. doi: 10.1007/BF00439108. [DOI] [PubMed] [Google Scholar]
- Steketee JD, Kalivas PW. Sensitization to psychostimulants and stress after injection of pertussis toxin into the A10 dopamine region. J Pharmacol Exp Ther. 1991;259:916–924. [PubMed] [Google Scholar]
- Stewart J, Badiani A. Tolerance and sensitization to the behavioral effects of drugs. Behav Pharmacol. 1993;4:289–312. [PubMed] [Google Scholar]
- Vindenes V, Handal M, Ripel A, Boix F, Morland J. Conditioned place preference induced by morphine and morphine-6-glucuronide in mice. Pharmacol Biochem Behav. 2006;85:292–297. doi: 10.1016/j.pbb.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Vindenes V, Handal M, Ripel A, Thaulow CH, Vindenes HB, Boix F, Morland J. Different time schedules affect conditioned place preference after morphine and morphine-6-glucuronide administration. Pharmacol Biochem Behav. 2008;89:374–383. doi: 10.1016/j.pbb.2008.01.012. [DOI] [PubMed] [Google Scholar]