Abstract
Objective
Traumatic brain injury (TBI) is a risk associated with military duty, and residual effects from TBI may adversely affect a service member's ability to complete duties. It is, therefore, important to identify factors associated with a change in job status following TBI in an active military population. On the basis of previous research, we predicted that apolipoprotein E (APOE) genotype may be 1 factor.
Design
Cohort study of military personnel who sustained a mild to moderate TBI.
Setting
Military medical clinics.
Patients or Other Participants
Fifty-two military participants were recruited through the Defense and Veterans Brain Injury Center, affiliated with Naval Medical Center San Diego and the Defense and Veterans Brain Injury Center Concussion Clinic located at the First Marine Division at Camp Pendleton.
Intervention(s)
A multivariate statistical classification approach called optimal data analysis allowed for consideration of APOE genotype alongside cognitive, emotional, psychosocial, and physical functioning.
Main Outcome Measure(s)
APOE genotype, neuropsychological, psychosocial, and clinical outcomes.
Results
We identified a model of factors that was associated with a change in job status among military personnel who experienced a mild or moderate TBI.
Conclusions
Factors associated with a change in job status are different when APOE genotype is considered. We conclude that APOE genotype may be an important genetic factor in recovery from mild to moderate head injury.
Keywords: apolipoprotein E, military, neurocognition, optimal data analysis, traumatic brain injury
Traumatic Brain Injury (TBI) represents one of the most significant health risks associated with military duty and may be associated with both combat or noncombat activities.1–3 TBI is considered to be the signature injury of the Iraq and Afghanistan wars.4 Neurocognitive impairments in memory, attention, and executive functions are commonly associated with TBI.5,6 More specifically, TBI may lead to difficulty encoding and recalling information, multitasking, abstract concept formation, and problem solving.5,7,8 In addition to the cognitive sequelae, dramatic changes in emotionality and personality following TBI have been documented,9 which may further impact quality-of-life outcomes.10 The diagnosis of TBI has also been associated with an increased risk for disciplinary problems and premature discharge from the military.11
Given the evidence that TBI has significant negative consequences on functioning, there is clear benefit to improving the identification of those patients at risk for a poor outcome. Recent work has focused on whether genetic traits may be linked to functional outcome following TBI, and 1 gene of particular interest is the apolipoprotein E (APOE) gene. The APOE gene is located on chromosome 19 and is responsible for the production of apolipoprotein. This protein is produced in response to central nervous system (CNS) injury and is involved in regulating the redistribution of cholesterol during the production of cell membranes.12 The ε4 allele of APOE (APOE-ε4) has been long associated with an increased risk for development of Alzheimer's disease13,14 and a reduced capability for CNS plastic response.15 Past work has indirectly supported the association between poor outcome following TBI and the presence of the APOE-ε4 allele. For example, Graham et al16 showed that possession of an APOE-ε4 allele was overly represented among individuals who died from TBI. Other studies have since supported this notion of an increased risk for fatal TBI in individuals who possess the APOE-ε4 allele.17 The APOE-ε4 allele has been associated with greater neurological impairment among some boxers18 and with a greater risk of prolonged coma following TBI.19
There have been relatively few studies that have investigated whether a change in functional status results from TBI in individuals with and without the APOE-ε4 allele. Teasdale and colleagues20 found that TBI patients with the APOE ε4 genotype had a poorer outcome compared with those without the gene, using the Glasgow Outcome Scale 6 months after injury. A study done by Friedman et al21 also supported this association. Taken together, these studies suggest that the presence of the APOE-ε4 genotype may represent a risk factor for poorer outcomes following TBI. However, this association has recently been contested by other researchers. Chamelian et al,22 for example, presented evidence revealing better (though not statistically significant) performances by ε4 subjects on various neurocognitive measures. There is some evidence to suggest that normal young adults with the APOE-ε4 allele may perform better than that of non-ε4 subjects on a number of neurocognitive measures, regardless of a CNS insult. Keltikangas-Jarvinen et al23 found that possession of an APOE ε4 allele was associated with increased “mental vitality, socialability, and positive emotionality” among 1577 randomly selected healthy children, adolescents, and young adults. Hubaceket al24 found that among 366 participants, those who possessed the ε4 allele achieved a mean higher level of education than those with an ε2 allele. Bloss et al25 reported better performances for children who possessed an ε4 allele versus those with an ε2 allele on a measure of visuospatial ability. Mondadori et al26 found that the APOE ε4 allele was associated with better and more efficient memory performances among 340 healthy young adults using neuropsychological and neuroimaging methods. We recently presented evidence supporting the notion that possession of an APOE ε4 allele may serve some sort of neurocognitive benefit among young active military personnel approximately 1 month following mild to moderate TBI.27
Although there is still some uncertainty as to whether the APOE ε4 allele is associated with functional outcomes following TBI, there is consensus that APOE genotype is an important factor when considering neurocognitive outcomes following TBI. Despite this general consensus, to date, few studies have investigated whether APOE genotype may be a factor in whether there is a change in job status or reduction in job responsibilities following TBI. Furthermore, no study has investigated the association between APOE genotype and other neurocognitive measures as they relate to change in job status (or fitness for duty). Using military participant groups equated on demographic variables, the present study served to address this question and assess whether APOE-ε4 genotype affects whether a change in job status or fitness for duty may occur 1 month following mild to moderate TBI.
Methods
Participants
Eligible participants were recruited through the Defense and Veterans Brain Injury Center (DVBIC), affiliated with Naval Medical Center San Diego and the DVBIC Concussion Clinic at the 1st Marine Expeditionary Force, Camp Pendleton. After standard informed consent procedures approved by the Naval Medical Center San Diego and the VA San Diego Healthcare System, recruitment and determination of eligibility was coordinated through a comprehensive evaluation process developed by the DVBIC. Those individuals with documented mild to moderate TBI were recruited for this study. Mild TBI was defined as an initial loss of consciousness less than 15 minutes, an initial Glasgow Coma Scale score between 13 and 15, and/or a period of posttraumatic amnesia less than 24 hours. Moderate TBI was defined as an initial loss of consciousness less than 24 hours but greater than 15 minutes, an initial Glasgow Coma Scale between 8 and 12, and/or a period of post-traumatic amnesia greater than 24 hours but less than 7 days. Exclusion criteria included a history of severe or repeated head injuries, substance or alcohol abuse according to Diagnosis and Statistical Manual of Mental Disorders, Fourth Edition criteria, metabolic or other diseases known to affect the CNS, and Axis I psychiatric disorders according to Diagnosis and Statistical Manual of Mental Disorders, Fourth Edition (see Dikmen et al5 for similar criteria). Furthermore, given the nature of the statistical approach we employed, the pairwise deletion method excluded a few missing cases on any measures that entered into our statistical model. As a consequence of our enrollment and exclusion procedures, 52 subjects were enrolled in the study and 46 were acceptable for our statistical approach.
Our hypothesis was that possession of an APOE ε4 allele would be an important determinant in predicting change in job status among active military personnel along with other psychosocial and neuropsychological measures. Because of this, we were concerned with the homogeneity of means and variances in demographic information between those who possessed the APOE ε4 allele and those who did not. Levene's test for equality of variances yielded that variances in demographic characteristics were essentially equivalent. Moreover, independent-samples t test showed that there were no significant differences in means age, number of years of education, length of time since brain injury, and numerically converted rank between the 2 groups. Continuity correction of chi-square test revealed that the proportions of gender and numbers of mild versus moderate TBI were not significantly different between groups.
Materials
Within approximately 1 month after brain injury, the participants were given a series of psychosocial and neuropsychological measures described previously.27 These included sections A, D, and E of the Frontal Lobe Personality Scale; the Glasgow Assessment Schedule; the Kennedy-Johnson Post-Concussion Scale; the Beck Depression Inventory; the Beck Anxiety Inventory; and the Rand SF-36 Item Health Survey. Neuropsychological measures included the Digit Span and Digit Symbol subtests from the Wechsler Adult Intelligence Scale—Third Edition; the Paced Auditory Serial Addition Test; the Boston Naming Test; Block Design and Matrix Reasoning subtests from the Wechsler Abbreviated Scale of Intelligence (WASI); Verbal Fluency, Design Fluency, Color-Word Interference, Sorting Test, and Trail-Making subtests from the Delis-Kaplan Executive Functions System; the Logical Memory subtest from the Wechsler Memory Scale—Third Edition; the California Verbal Learning Test—Second Edition (CVLT-II); and the American National Adult Reading Test. Participants were asked to submit a buccal cheek swab to identify their APOE allele genotype according to a polymerase chain reaction– based method (see Saunders et al28). Sixteen participants possessed an APOE ε4 allele and 36 participants did not.
The dependent variable (DV) was dichotomous: “job change” (JC) or “no job change” (NJC). Job change was defined as any reduction in duties following TBI for any reason and included a medical hold, rehabilitation or assignment to light/limited duties (n = 24), reduction of duties because of the brain injury problems (n = 3), a referral to Medical Board (n = 3), or administrative separation (n = 1). All other participants who had no change in job status (n = 18) were categorized as “NJC.”
Analysis strategy
The data were analyzed with Optimal Data Analysis (ODA) with the use of the APA copyrighted Windows-based computer program.29 ODA is a nonlinear statistical method used to solve multivariate classification problems. Independent of assumptions such as multivariate normality, additivity, equality of group sizes, the number of variables, and multicollinearity, ODA can accurately provide a hierarchical classification tree model whereby observed data are categorized into each level of the dependent categorical variable according to pathways delineated by “nodes” in the tree model (see references 29–31 for details). It should be noted that ODA is not limited in any way by size of participant groups.29,30
In ODA, an independent variable (IV) is referred to as an attribute, and a DV is referred to as the class variable.29,30 Attributes can be both continuous and categorical; however, the class variable must be dichotomous or multicategorical. ODA first selects the best categorical borderline for each attribute (cutpoint or decision rule), which achieves the maximum percentage of observations correctly classified (percentage accuracy in classification or PAC) in each group of the class variable. Instead of coefficient values, ODA reports an effect strength for sensitivity (ESS), which indicates the percentage of how many observations actually belonging to a group are correctly classified. The formula for ESS is the following:
where C = 100/(the number of categories in the dependent categorical variable). For instance, this study examined a dichotomous DV (ie, “JC” and “NJC”), hence C in our study was 100/2 = 50. ESS is directly correlated with PAC. In other words, the higher the PAC in each group of the class variable, the higher the ESS. ODA further analyzes leave-one-out (LOO) validity to evaluate the stability of classification performance. Every time 1 observation is removed, LOO analysis runs a classification performance again to check whether the classification performance (eg, the value of ESS) is constant across the data. Finally, to evaluate the significance level of the classification performance, the Fisher exact probability test is run by ODA.
In an ODA tree model, the strongest attribute that shows the highest ESS, LOO stability, and significant P-value enters the top node of the hierarchical tree model.29,30 Alternatively, ODA is also able to allow a certain attribute to enter into the top node manually. Once the top attribute is selected, then observed data of the attribute are further classified into several groups, and ODA can detect the next strongest attribute within each group. That attribute enters the second node of the tree model. These procedures—finding the strongest attribute, classifying the data of the attribute into groups, and then finding the strongest attribute within each group again—continues until no significant attribute is detected. In the current study, we defined the variable possession of an APOE ε4 allele as the top node so that we could test whether a change in job status was significantly predicted by different sets of psychosocial or neuropsychological measures when initially considering the possession of the ε4 allele. One hundred fifty-four IVs were entered into the model. In ODA, the number of variables does not affect the results or inferences.29,30 This is because ODA analyzes the whole effect of each IV on the DV individually, rather than the partial effect of each IV on DV within a whole regression equation (the latter approach is used by logistic regression and discriminant analysis). To finalize the classification tree model, a sequentially rejective Sidak Bonferroni-type multiple comparisons procedure was used to control type I error rate per comparison and maximize statistical power. If the significance levels of any attributes are beyond P value per comparison, these attributes are pruned from the model.
Results
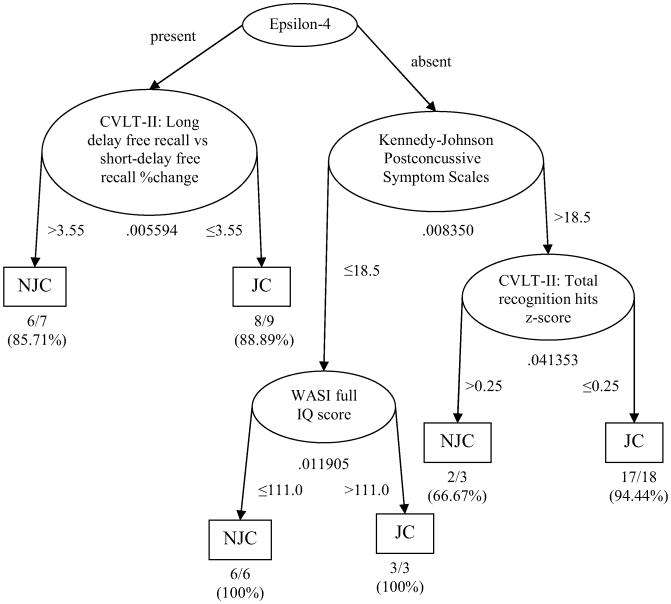
Because of the pairwise deletion method, 46 participants entered in ODA. Figure 1 shows a hierarchical classification tree model generated to predict whether or not the military personnel experienced a change in job status following the TBI. When the participant possessed the APOE ε4 allele, the analyses revealed that job status was determined by a measure of cognitive functioning, specifically the change in percentage of words recalled following a 20-minute delay (long-delay free recall) versus the number of words recalled immediately after presentation (short-delay free recall) on the CVLT-II, a well-established measure of verbal learning and memory. If the percentage change between long-delay free recall and short-delay free recall (defined as ([long-delay free recall raw score]–[short-delay free recall raw score])/[short-delay free recall raw score]) was greater than 3.55%, the participants were correctly predicted as having no change in work status with 85.71% accuracy. On the other hand, if the percentage change was at 3.55% or below, the participants were correctly predicted to have a change in their job status with 88.89% accuracy. On the basis of these findings, it appears that the change in percentage of the long-delay free recall versus the short-delay free recall from the CVLT-II is a useful index to predict job status if the military personnel possess the APOE ε4 allele.
Figure 1.
ODA Hierarchical Tree Model for predicting job status when APOE ε4 was entered as the first node (N = 46). Ellipses represent nodes, arrow lines represent branches, and rectangles represent prediction endpoints. Numbers under each ellipse (node) indicate the Fisher exact P value for each node. Numbers next to arrows indicate the cutpoint for classifying observations into the categories (“no job change” or “job change”) for each node. Finally, fractions and percentages below each prediction endpoint indicate the absolute number or percentage of the observations correctly classified. NJC, No job change; JC, Job change.
However, when participants did not possess the APOE ε4 allele, the Kennedy-Johnson Post-Concussive Symptom Scale became the primary determinant. The Kennedy-Johnson Post-Concussive Symptom Scale is a self-report measure of symptoms commonly associated with postconcussive syndrome (higher score indicates worse postconcussive symptoms). Performances on the WASI (a scale of intelligence) and CVLT-II total recognition hits (a measure of recognition memory) were secondary determinants. If the military personnel scored 18.5 or lower on the Kennedy-Johnson Post-Concussive Symptom Scale, their job status was predicted by WASI Full IQ score; those who scored 110.0 or lower on WASI Full IQ score remained on their duties with 100% accuracy; on the other hand, those who scored higher than 111.0 on WASI Full IQ score were more likely to change their job with 100% accuracy. For the military personnel who did not possess the APOE ε4 allele and scored higher than 18.5 on the Kennedy-Johnson Post-Concussive Symptom Scale, the standard score for total recognition hits from the CVLT-II (higher z score indicates better recognition) predicted job status. If they scored greater than 0.25 of z score on total recognition hits, their job status was predicted as NJC with 66.67% accuracy. However, if the z score was 0.25 or lower, it was predicted that the military personnel changed their job with 94.44% accuracy.
The totality of the above scheme for overall classification accuracy was 91.30% (see Table 1), which indicated that 91.30% of the cases were correctly classified into either JC or NJC categories. The effect strength (or “effect size”) of this overall classification tree model was strong (see the criteria provided by Yarnold and Soltysik29) and the model was significant (P = .531330 × 10−7). Thus, job status was predicted by the change in percentage of the long-delay free recall versus the short-delay free recall from the CVLT-II, the number of symptoms endorsed on the Kennedy-Johnson Post-Concussive Symptom Scale, WASI Full IQ, and/or total recognition hits z score from the CVLT-II, depending on whether the military personnel possessed the APOE ε4 allele genotype.
Table 1. Demographic information for the participant sample.
| APOE genotype | ||||
|---|---|---|---|---|
| Demographic information | ε4 | Non-ε4 | t or (χ2) | P |
| N | 16 | 30 | ||
| Age | 22.56 ± 3.76 | 25.23 ± 6.101 | −1.769 | .081 |
| Gender (M/F) | 13/3 | 29/1 | (1.484) | .223 |
| Education | 12.50 ± 1.09 | 13.00 ± 2.11 | −0.830 | .411 |
| Rank | 3.53 ± 1.13 | 4.07 ± 1.46 | −1.239 | .223 |
| Days from injury | 38.73 ± 13.80 | 39.97 ± 12.40 | −0.301 | .765 |
| Mild/moderate | 8/8 | 13/17 | (0.015) | .903 |
Discussion
In the present study, we present evidence suggesting that possession of an APOE ε4 allele may be one of many differential factors when considering whether a mild or moderate TBI leads to a change or reduction in job duties among active military personnel. Using a nonlinear statistical approach to this multivariate classification problem, we showed that APOE ε4 carriers had a different set of factors that were significant to the issue of change in job status when compared with non-ε4 carriers. More specifically, performance on CVLT-II long-delay free recall versus short-delay free recall was the only variable significantly associated with job status outcomes among ε4 carriers. The Kennedy-Johnson Post-Concussive Symptom Scales, CVLT-II recognition hits standard score, and the WASI Full Scale IQ score were significantly associated with job status outcomes for non-ε4 carriers. Our overall model was found to be predictive and accurate according to previously published conventions.29
A significant observation from our model is that a change in job status was not associated with a measure of postconcussive symptoms among ε4 carriers, only performance on a measure of immediate versus delayed memory. In contrast, postconcussive symptom severity was the first significant factor for change in job status among non-ε4 carriers. If non-ε4 participants were above the cutpoint for postconcussive symptom severity, then recognition memory was the determining factor. If non-ε4 participants were below the cutpoint, then IQ score was the determining factor. This difference in factor structure may suggest that TBI postconcussive symptom severity has less of an effect upon outcome variables for ε4 carriers versus non-ε4 carriers. We recently suggested27,32 that young ε4 participants may experience some neurocognitive benefit following mild to moderate head injury. These current findings may therefore be viewed as tentative support for this notion.
Our results are relatively novel with respect to the existing literature. Few, if any, investigators have considered genetics when attempting to determine factors that may lead to a change in job status following TBI. Most, however, have considered neurocognitive outcomes as pivotal in predicting potential changes in employment following TBI. Ownsworth and McKenna33 reviewed 85 studies and determined that employment outcome depended on “preinjury occupational status, functional status at discharge, global cognitive functioning, perceptual ability, executive functioning, involvement in vocational rehabilitation services, and emotional status.”(p765) Boake et al34 reported that neuropsychological testing done before rehabilitation discharge was predictive of “long-term productivity” among nonpenetrating TBI survivors. Sherer et al35 reviewed 23 studies and supported the relationship between neuropsychological test results and employment outcomes. Interestingly, Simpson and Schmitter-Edgecombe36 reported that a questionnaire of frontal lobe functioning differentiated employed survivors from unemployed survivors of TBI. In our study, executive function measures from the Delis-Kaplan Executive Functions System were entered into the model but were not found to be significantly predictive of change in job status. Multiple differences between our study and that of Simpson and Schmitter-Edgecombe may account for this discrepancy. One explanation may be that our study considered performances on a range of measures sensitive to many different neurocognitive domains, while Simpson and Schmitter-Edgecombe assessed performances on only 1 measure of 1 neurocognitive domain. Another explanation may be found in the difference in severity of TBI between participant groups. The average coma duration of the Simpson and Schmitter-Edgecombe study population was 520 hours (SD = 549), suggestive of a more severe TBI than our mild to moderate TBI population. Another difference is in follow-up time between the 2 studies. Simpson and Schmitter-Edgecombe's population was assessed at 10.42 years (SD = 9.19) after injury, while we assessed our participants approximately 1 month after their injury (APOE ε4 group: 38.73 ± 13.80 days; APOE non-ε4 group: 39.97 ± 12.40 days). We contribute to the growing body of literature concerned with the relationship between neuropsychological ability post-TBI and employment outcomes by providing one of the first considerations of the interaction between genetics and neuropsychological performance.
Limitations of the present study include a lack of longitudinal data to build a more predictive model of JC. All data used were collected at one time point approximately 1 month following TBI. Although this allowed for mapping of multivariate associations between outcome variables, neurocognitive and psychological data immediately following TBI would have allowed for a better predictive multivariate categorical model for change in job status 1 month later. Another limitation of the present study was our relatively low participant numbers. However, it should be noted that, since ODA utilizes a pairwise deletion method, ODA is arguably more powerful than other statistical classification approaches that employ a listwise deletion method and are thus significantly limited by any incomplete data (eg, logistic regression analysis, predictive discriminant analysis; see references 29 and 30 for discussion). A third limitation of the present study was our use of an active military population, which may reduce the generalizability of our results to the civilian sector. A fourth limitation is the lack of inclusion of other factors, which may have influenced the present results, such as pre-injury job history. Since we did not have data on these factors, we could not include them in the present model. A fifth limitation was our grouping of multiple different changes in job status post-TBI together in 1 general “JC” category. It could be argued that there are different sets of factors that lead to different types of changes in job status. In addition, changes in job status may have been caused by factors other than TBI.
In summary, we provide support for the notion that APOE genotype, in association with other neurocognitive and clinical symptom measures, may be an important factor when considering change in job status approximately 1 month following mild to moderate TBI in a military population. We also provide tentative support for the notion that young APOE ε4 carriers may experience a neurocognitive benefit following mild to moderate TBI. Future longitudinal research would help clarify the predictive value of our present multivariate classification tree model.
Table 2. Optimal data analysis classification performance summary (N = 46).
| Performance index | Performance parameter | |
|
| ||
| Overall classification accuracy | 42/46 (91.30%) | |
| Sensitivity (No Job Change) | 14/16 (87.50%) | |
| Sensitivity (Job Change) | 28/30 (93.33%) | |
| Effect strength for sensitivity | 80.83% | |
| Predictive value (No Job Change) | 14/16 (87.50%) | |
| Predictive value (Job Change) | 28/30 (93.33%) | |
| Effect strength for predictive value | 80.83% | |
| Effect strength overall | 80.83% | |
| Cross-classification table (P= .531330 × 10−7) | ||
|
| ||
| Respondents' predicted status | ||
|
|
||
| Respondents' actual status | No Job Change | Job Change |
|
|
|
|
| No Job Change | 14 | 2 |
| Job Change | 2 | 28 |
Overall classification accuracy is the percentage of the observations classified correctly. Sensitivity is the percentage of how many observations were correctly classified among observations that actually belong to a given category. Predictive value is the percentage of how many observations were correctly classified among observations that were predicted as a given category. Higher percentage indicates greater classification performance. Effect strength overall is the mean of effect strength for sensitivity and effect strength for predictive value. According to Yarnold and Soltysik,29 the effect strength is strong for the present model (75% < ES < 90%).
Acknowledgments
The views expressed in this article do not necessarily reflect those of the funding agency, the US Navy, the US Marine Corps, the Department of Defense, or the US Government.
This work was supported by a merit review research program from the Medical Research Service of the Department of Veterans Affairs and by the Defense and Veterans Brain Injury Center; resources and use of facilities were also provided by the Naval Medical Center, San Diego. The authors gratefully acknowledge the staff, patients, and volunteers at Naval Medical Center San Diego and US Marine Corps Base Camp Pendleton for their assistance with this study.
Footnotes
There were no actual or potential conflicts of interest for the authors that could have inappropriately influenced the present work. Subjects were recruited in accordance with Internal Review Board–approved policies and procedures. Standard professional and ethical guidelines were upheld during the research study and the manuscript preparation.
References
- 1.Galarneau MR, Woodruff SI, Dye JL, Mohrle CR, Wade AL. Traumatic brain injury during Operation Iraqi Freedom: findings from the United States Navy-Marine Corps Combat Trauma Registry. J Neurosurg. 2008;108:950–957. doi: 10.3171/JNS/2008/108/5/0950. [DOI] [PubMed] [Google Scholar]
- 2.Sayer NA, Chiros CE, Sigford B, et al. Characteristics and rehabilitation outcomes among patients with blast and other injuries sustained during the global war on terror. Arch Phys Med Rehabil. 2008;89:163–170. doi: 10.1016/j.apmr.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 3.Belanger HG, Kretzmer T, Yoash-Gantz R, Pickett T, Tupler LA. Cognitive sequelae of blast-related versus other mechanisms of brain trauma. J Int Neuropsycholo Soc. doi: 10.1017/S1355617708090036. In press. [DOI] [PubMed] [Google Scholar]
- 4.Warden D. Military TBI during the Iraq and Afghanistan wars. J Head Trauma Rehabil. 2006;21:298–402. doi: 10.1097/00001199-200609000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Dikmen SS, Machamer JE, Winn HR, Temkin NR. Neuropsychological outcome at 1-year post head injury. Neuropsychology. 1995;9:80–90. [Google Scholar]
- 6.Levin HS, Benton AL, Grossman RG. Neurobehavioral Consequences of Closed Head Injury. New York, NY: Oxford Press; 1982. [Google Scholar]
- 7.Brooks DN. Long and short term memory in head injured patients. Cortex. 1975;11:329–340. doi: 10.1016/s0010-9452(75)80025-6. [DOI] [PubMed] [Google Scholar]
- 8.Gronwall D, Wrightson P. Delayed recovery of intellectual function after minor head injury. Lancet. 1974;2:995–997. doi: 10.1016/s0140-6736(74)91939-4. [DOI] [PubMed] [Google Scholar]
- 9.McAllister TW. Neuropsychiatric sequelae of head injuries. Psychiatr Clin N Am. 1992;15:395–412. [PubMed] [Google Scholar]
- 10.Levine B, Dawson D, Boutet I, Schwartz ML, Stuss DT. Assessment of strategic self-regulation in traumatic brain injury: its relationship to injury severity and psychosocial outcome. Neuropsychology. 2000;14:491–500. doi: 10.1037//0894-4105.14.4.491. [DOI] [PubMed] [Google Scholar]
- 11.Ommaya AK, Salazar AM, Dannenberg AL, Ommaya AK, Chervinsky AB, Schwab K. Outcome after traumatic brain injury in the U.S. military medical system. J Trauma. 1996;41:972–975. doi: 10.1097/00005373-199612000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- 13.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 14.Schmechel DE, Saunders AM, Strittmatter WJ, et al. Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci. 1993;90:9649–9653. doi: 10.1073/pnas.90.20.9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arendt T, Schindler C, Bruckner MK, et al. Plastic neuronal remodeling is impaired in patients with Alzheimer's disease carrying apolipoprotein epsilon 4 allele. J Neurosci. 1997;17(2):516–529. doi: 10.1523/JNEUROSCI.17-02-00516.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graham DI, Gentleman SM, Nicoll JAR, et al. Is there a genetic basis for the deposition of β-amyloid after fatal head injury? Cell Mol Neurobiol. 1999;19:19–30. doi: 10.1023/a:1006956306099. [DOI] [PubMed] [Google Scholar]
- 17.Nicoll JA, Roberts GW, Graham DI. Amyloid beta-protein, APOE genotype and head injury. Ann N Y Acad Sci. 1996;777:271–275. doi: 10.1111/j.1749-6632.1996.tb34431.x. [DOI] [PubMed] [Google Scholar]
- 18.Jordan CD, Relkin NR, Ravdin LD, et al. Apolipoprotein E epsilon-4 associated with chronic traumatic brain injury in boxing. JAMA. 1997;278(2):136–140. [PubMed] [Google Scholar]
- 19.Sorbi S, Nacmias B, Piacentini S, et al. ApoE as a prognostic factor for post traumatic coma. Nat Med. 1995;9:852. doi: 10.1038/nm0995-852. [DOI] [PubMed] [Google Scholar]
- 20.Teasdale G, Nicoll J, Murray G, Fiddes M. Association of apoE polymorphism with outcome after head injury. Lancet. 1997;350:1069–1071. doi: 10.1016/S0140-6736(97)04318-3. [DOI] [PubMed] [Google Scholar]
- 21.Friedman G, Froom P, Sazbon L, et al. ApoE-ε4 genotype predicts a poor outcome in survivors of traumatic brain injury. Neurology. 1999;52:244–248. doi: 10.1212/wnl.52.2.244. [DOI] [PubMed] [Google Scholar]
- 22.Chamelian L, Reis M, Feinstein A. Six-month recovery from mild to moderate traumatic brain injury: the role of APOE-e4 allele. Brain. 2004;127:2621–2628. doi: 10.1093/brain/awh296. [DOI] [PubMed] [Google Scholar]
- 23.Keltikangas-Jarvinen L, Raikkonen K, Lehtimaki T. Dependence between apolipoprotein E phenotypes and temperament in children, adolescents, and young adults. Psychosom Med. 1993;55:155–163. doi: 10.1097/00006842-199303000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Hubacek JA, Pitha J, Skodova Z, Adamkova V, Lanska V, Poledne R. A possible role of apolipoprotein E polymorphism in predisposition to higher education. Neuropsychobiology. 2001;43:200–203. doi: 10.1159/000054890. [DOI] [PubMed] [Google Scholar]
- 25.Bloss CS, Delis DC, Salmon DP, Bondi MW. APOE genotype is associated with left-handedness and visuospatial skills in children. Neurobiol Aging. doi: 10.1016/j.neurobiolaging.2008.05.021. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mondadori CRA, de Quervain DJF, Buchmann A, et al. Better memory and neural efficiency in young apolipoprotein E epsilon4 carriers. Cereb Cortex. 2007;17:1934–1947. doi: 10.1093/cercor/bhl103. [DOI] [PubMed] [Google Scholar]
- 27.Han SD, Drake AI, Cessante LM, et al. APOE and recovery from TBI in a U.S. military population: evidence for a compensatory mechanism? J Neurol Neurosurg Psychiatr. 2007;78:1103–1108. doi: 10.1136/jnnp.2006.108183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saunders AM, Strittmatter WJ, Schmechel DE. Association of apolipoprotein E allele e4 with late-onset familial and sporadic Alzheimer's disease. Neurology. 1993;43:1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- 29.Yarnold PR, Soltysik RC. Optimal Data Analysis: A Guide book With Software for Windows. Washington, DC: American Psychological Association; 2005. [Google Scholar]
- 30.Soltysik RC, Yarnold PR. ODA 1.0: Optimal Data Analysis for DOS. Chicago: Optimal Data Analysis; 1993. [Google Scholar]
- 31.Yarnold PR, Soltysik RC, Bennett CL. Predicting in-hospital mortality of patients with AIDS-related pneumocystis carinii pneumonia: an example of hierarchically optimal classification tree analysis. Stat Med. 1997;16:1451–1463. doi: 10.1002/(sici)1097-0258(19970715)16:13<1451::aid-sim571>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 32.Han SD, Bondi MW. Revision of the APOE compensatory mechanism recruitment hypothesis. Alzheimer's Demen. 2008;4(4):251–254. doi: 10.1016/j.jalz.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 33.Ownsworth T, McKenna K. Investigation of factors related to employment outcome following traumatic brain injury: a critical review and conceptual model. Disabil Rehabil. 2004;26(13):765–784. doi: 10.1080/09638280410001696700. [DOI] [PubMed] [Google Scholar]
- 34.Boake C, Millis SR, High WM, et al. Using early neuropsychologic testing to predict long-term productivity outcome from traumatic brain injury. Arch Phys Med Rehabil. 2001;82:761–768. doi: 10.1053/apmr.2001.23753. [DOI] [PubMed] [Google Scholar]
- 35.Sherer M, Novack TA, Sander AM, Struchen MA, Alderson A, Thompson RN. Neuropsychological assessment and employment outcome after traumatic brain injury: a review. Clin Neuropsychol. 2002;16(2):157–178. doi: 10.1076/clin.16.2.157.13238. [DOI] [PubMed] [Google Scholar]
- 36.Simpson A, Schmitter-Edgecombe M. Prediction of employment status following traumatic brain injury using a behavioural measure of frontal lobe functioning. Brain Inj. 2002;16(12):1075–1091. doi: 10.1080/02699050210155249. [DOI] [PubMed] [Google Scholar]