Abstract
A new method for the construction of 2-substituted and 2,2-disubstituted chromans via Pd-catalyzed carboetherification reactions between aryl/alkeny halides and 2-(but-3-en-1-yl) phenols is described. These reactions effect formation of a C–O bond and a C–C bond to afford the chroman products in good yield, and also provide stereoselective access to tricyclic chroman derivatives.
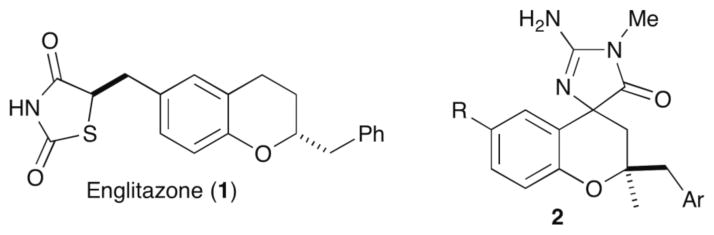
Chromans and other benzo-fused oxygen heterocycles are displayed in a number of biologically active natural products,1 including α-tocopherol2a and polyalthidin.2b In addition, 2-benzylchroman derivatives such as englitazone (1, antidiabetic activity)3a and molecules with general structure 2 (beta-secretase inhibitory activities)3b have been explored as pharmaceutical leads (Figure 1).
Figure 1.
Biologically active 2-benzylchroman derivatives
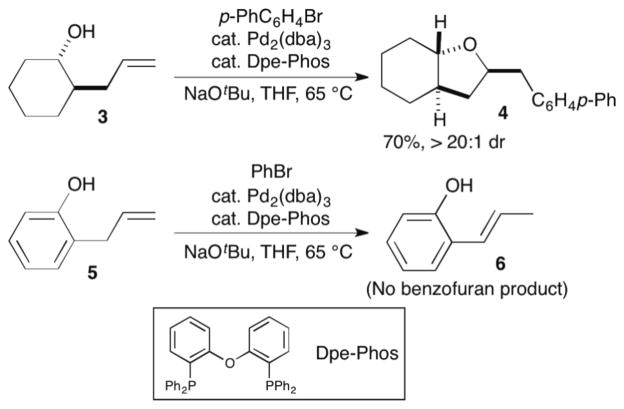
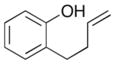
Over the past several years we have developed a new approach to the construction of substituted tetrahydrofurans via palladium-catalyzed carboetherification reactions between aryl or alkenyl halides and alcohols bearing pendant alkenes.4,5,6 For example, treatment of alcohol 3 with 4-bromobiphenyl, NaOtBu, and a catalyst composed of Pd2(dba)3/Dpe-Phos provided 4 in 70% yield with >20:1 dr (Scheme 1). We envisioned this method could provide a concise and convergent approach to benzofurans or chromans7,8 from simple starting materials (phenols bearing pendant alkenes).9 However, although the Pd-catalyzed carboetherification reactions were quite effective for the generation of tetrahydrofurans, our efforts to employ Pd-catalyzed carboetherification reactions for the synthesis of 6-membered heterocycles (e.g., tetrahydropyrans) failed to afford satisfactory results.10 In addition, the scope of our carboetherification method appeared to be limited to aliphatic alcohol substrates, as efforts to couple 2-allylphenol 5 with bromobenzene failed to generate the desired substituted benzofuran product.11 Instead, the formation of 2-(prop-1-en-1-yl)phenol 6 via double bond isomerization was observed.
Scheme 1.
Palladium-catalyzed carboetherification reactions of alcohols vs. phenols.
We have previously illustrated that the mechanism of Pd-catalyzed carboetherification reactions, such as those shown in Scheme 1, involves suprafacial insertion of the substrate alkene into the Pd–O bond of an intermediate palladium alkoxide complex.4,5a Recent mechanistic studies conducted by our group and others have shown that the rate of alkene insertion into Pd-heteroatom bonds is highly dependent on the nucleophilicity of the heteroatom, and the insertion likely occurs from an intermediate Pd-complex that bears a single phosphine ligand.12 These results suggest that two factors may be responsible for the poor reactivity of phenols such as 5 in Pd/Dpe-Phos-catalyzed carboetherifications: (a) the relatively low nucleophilicity of phenols as compared to aliphatic alcohols; and (b) use of the chelating bis-phosphine Dpe-Phos, which may disfavor generation of the reactive monophosphine intermediate. These factors apparently slow the catalytic reaction to the point that alkene isomerization of 5 to 6 occurs more rapidly than the desired transformation.
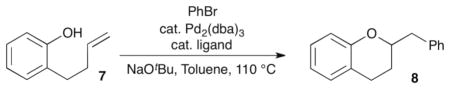
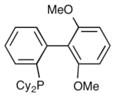
The hypothesis outlined above suggests that use of catalysts bearing monodentate phosphine ligands may provide improved results in carboetherification reactions of phenol derivatives, as these ligands could potentially facilitate the key alkene insertion step. In addition, we also anticipated that the conversion of 2-(but-3-en-1-yl)phenol (7) to chroman 8 (Table 1) may be more straightforward than the analogous transformation of 2-allylphenol (5) to a benzofuran derivative, as 7 should be much less prone to base-mediated alkene isomerization than 5. Thus, we elected to initially examine the coupling of 7 with bromobenzene using the biaryl monophosphine ligand S-Phos. We had previously found this ligand provided good results in other challenging carboetherification reactions,5e and we were gratified to discover these conditions afforded the desired product 8 in 86% isolated yield. A quick survey of related biaryl phosphines did not lead to any further improvement in yield (entries 2–5), and Dpe-Phos produced 8 in only 30% yield (entry 6).
Table 1.
Optimization of ligand for the coupling of 7 with bromobenzenea
 | |||
|---|---|---|---|
| Entry | Ligand | Yieldb | |
| 1 |
|
S-Phos | 84% (75%) |
| 2 |
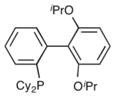
|
RuPhos | 76% |
| 3 |
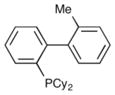
|
Me-Phos | 31% |
| 4 |
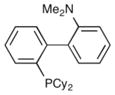
|
Dave-Phos | 12% |
| 5 |
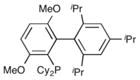
|
Brett-Phos | 47% |
| 6 |
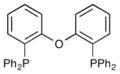
|
Dpe-Phos | 30% |
Conditions: 1.0 equiv 7, 2.0 equiv PhBr, 2.0 equiv NaOtBu, 2 mol % Pd2(dba)3, 4 mol % ligand, toluene (0.25 M), 110 °C.
Yields were determined by 1H NMR analysis of crude reaction mixtures using phenanthrene as an internal standard. The yield in parentheses is an isolated yield of pure product.
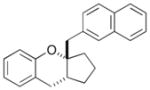
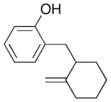
In order to examine the scope of the phenol carboetherification reactions, a number of substrates with different substitution patterns were synthesized and subjected to the optimized reaction conditions. As shown in Table 2, substrates 7, 9, and 10 were converted to 2-substituted- or 2,2-disubstituted chromans in moderate to good yield (entries 1–9). In addition, cyclopentane- and cyclohexane-derived substrates 11–12 were transformed to tricyclic products 22–24 with high diastereoselectivity (entries 10–12). However, stereocontrol was poor in the reaction of 13 with 4-bromo- tert-butylbenzene (2:1 dr), and a significant amount (ca. 25%) of an inseparable unidentified low molecular weight side product was also formed (entry 13). In addition, substrates bearing internal alkenes failed to undergo the desired transformation; no reaction was observed at temperatures up to 140 °C. Although these new reaction conditions were generally effective for the preparation of chromans, efforts to transform 5 to substituted benzofuran 26 were still only modestly successful; the desired product was generated in 37% yield (entry 14).
Table 2.
Pd-catalyzed carboetherification reactions of phenols bearing pendant alkenesa
| Entry | Substrate | RBr | Product | Yieldb |
|---|---|---|---|---|
| 1 |
 7 |
|
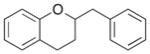 8 |
75% |
| 2 | 7 |
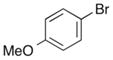
|
14 |
57% |
| 3 | 7 |
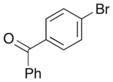
|
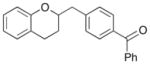 15 |
51% |
| 4 |
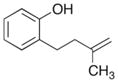 9 |
|
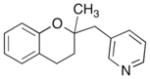 16 |
87% |
| 5 | 9 |
|
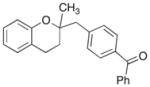 17 |
71% |
| 6 | 9 |
|
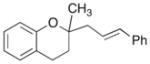 18 |
83% |
| 7 | 9 |
|
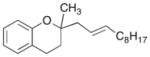 19 |
68% |
| 8 |
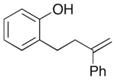 10 |
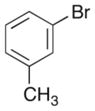
|
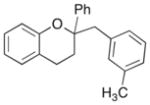 20 |
54% |
| 9 | 10 |
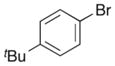
|
 21 |
56% |
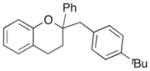
| 10 |
 11 |
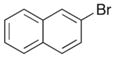
|
 22 |
59% >20:1 dr |
| 11 |
 12 |
|
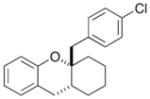 23 |
71% >20:1 dr |
| 12 | 12 |
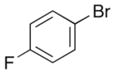
|
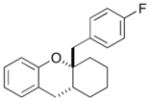 24 |
63% >20:1 dr |
| 13 |
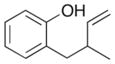 13 |
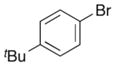
|
25 |
ca. 47%c 2:1 dr |
| 14 |
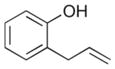 5 |
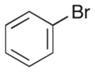
|
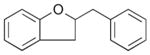 26 |
37% |
Conditions: 1.0 equiv phenol substrate, 2.0 equiv R–Br, 2.0 equiv NaOtBu, 2 mol % Pd2(dba)3, 4 mol % S-Phos, toluene (0.25 M), 110 °C.
Isolated yield (average of two experiments).
The two inseparable stereoisomeric products were isolated in 63% yield and ca. 75% purity. The remaining 25% of the mixture was primarily composed of an unidentifed low molecular weight side product that appears to be derived from the phenol substrate.
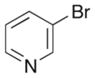
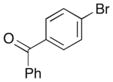
A range of different electrophiles were examined in the carboetherification reactions of 7, 9, and 10. As shown in Table 2, aryl halides bearing chloride, fluoride, methoxy and diaryl ketone functionality were successfully converted to the desired products. Alkenyl halides were also effective coupling partners in these reactions (entries 6–7), and the coupling of 9 with the heteroaryl halide 3-bromopyridine also proceeded smoothly (entry 4). However, the scope of carboetherification reactions involving 11 and 12 was not as broad, and use of aryl halides that were relatively electron rich or electron deficient led to poor reactivity or low yields.
In conclusion, we have developed a new method for the construction of 2-substituted chroman derivatives via Pd-catalyzed carboetherification reactions. These transformations employ simple substrates, and provide access to a number of different derivatives in a straightforward manner. In addition these are the first examples of Pd-catalyzed alkene carboetherification reactions between aryl bromides and alkenyl phenols, and are also rare cases in which six-membered oxygen heterocycles are generated via 1,2-alkene carboheterofunctionalization processes. Future studies will be directed towards the development of catalysts for enantioselective variants of these transformations.
Supplementary Material
Footnotes
Electronic Supplementary Information (ESI) available: [experimental procedures and spectral data for all new compounds reported in the text].
Notes and references
- 1.Shen HC. Tetrahedron. 2009;65:3931. [Google Scholar]
- 2.Zingg J-M, Azzi A. Curr Med Chem. 2004;11:1113. doi: 10.2174/0929867043365332.and references cited therein; Zafra-Polo MC, Gonzalez MC, Tormo JR, Estornell E, Cortes D. J Nat Prod. 1996;59:913. doi: 10.1021/np960492m.
- 3.Urban FJ, Moore BS. J Heterocycl Chem. 1992;29:431.and references cited therein; Hunt KW, Rizzi JP, Cook A. 2011072064A1. PCT Int Appl WO. 2011
- 4.For reviews, see: Wolfe JP. Eur J Org Chem. 2007:571.Wolfe JP. Synlett. 2008:2913.
- 5.(a) Hay MB, Wolfe JP. J Am Chem Soc. 2005;127:16468. doi: 10.1021/ja054754v. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wolfe JP, Rossi MA. J Am Chem Soc. 2004;126:1620. doi: 10.1021/ja0394838. [DOI] [PubMed] [Google Scholar]; (c) Hay MB, Wolfe JP. Tetrahedron Lett. 2006;47:2793. doi: 10.1016/j.tetlet.2006.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Hay MB, Hardin AR, Wolfe JP. J Org Chem. 2005;70:3099. doi: 10.1021/jo050022+. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Ward AF, Wolfe JP. Org Lett. 2009;11:2209. doi: 10.1021/ol900594h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.For recent, complementary alkene difunctionalization reactions of unsaturated alcohols that generate disubstituted tetrahydrofurans with formation of both a C–C and a C–O bond, see: Fries P, Halter D, Kleinschek A, Hartung J. J Am Chem Soc. 2011;133:3906. doi: 10.1021/ja108403s.Chen Z, Falck JR. Angew Chem, Int Ed. 2011;50:6626. doi: 10.1002/anie.201101857.Zhang G, Cui L, Wang Y, Zhang L. J Am Chem Soc. 2010;132:1474. doi: 10.1021/ja909555d.Protti S, Dondi D, Fagnoni M, Albini A. Eur J Org Chem. 2008:2240.Yang X, Fang X, Yang X, Zhao M, Han Y, Shen Y, Wu F. Tetrahedron. 2008;64:2259.
- 7.For recent reviews on the synthesis of chromans, see (a) Reference 1; Tang Y, Oppenheimer J, Song Z, You L, Zhang X, Hsung RP. Tetrahedron. 2006;62:10785.Ferreira SB, de F, da Silva C, Pinto AC, Gonzaga DTG, Ferreira VF. J Heterocycl Chem. 2009;46:1080.Shi Y-L, Shi M. Org Biomol Chem. 2007;5:1499. doi: 10.1039/b618984a.
- 8.For selected recent syntheses of chroman derivatives, see: Enders D, Yang X, Wang C, Raabe G, Runsik J. Chem Asian J. 2011;6:2255. doi: 10.1002/asia.201100320.Xin Z, Zhang Y, Tao H, Xue J, Li Y. Synlett. 2011:1579.Hernandez-Torres G, Carreno MC, Urbano A, Colobert F. Eur J Org Chem. 2011:3864.Wang X-F, Hua Q-L, Cheng Y, An X-L, Yang Q-Q, Chen J-R, Xiao W-J. Angew Chem, Int Ed. 2010;49:8379. doi: 10.1002/anie.201004534.Leibeling M, Koester DC, Pawliczek M, Schild SC, Werz DB. Nature Chem Biol. 2010;6:199. doi: 10.1038/nchembio.302.
- 9.Two other Pd-catalyzed alkene carboetherification methods have successfully been employed for synthesis of benzofurans or chromans from phenols bearing pendant alkenes. However, these transformations appear to be limited to alkenes bearing a substituent on the internal carbon atom. For Pd-catalyzed oxyalkynylation reactions between unsaturated phenols and hypervalent iodoalkyne reagents, see: Nicolai S, Erard S, Gonzalez DF, Waser J. Org Lett. 2010;12:384. doi: 10.1021/ol9027286.For Pd-catalyzed domino Wacker/Heck reactions between unsaturated phenols and α,β-unsaturated esters or ketones, see: Tietze LF, Spiegel DA, Stecker F, Major J, Raith C, Große C. Chem Eur J. 2008;14:8956. doi: 10.1002/chem.200800967.Tietze LF, Zinngrebe J, Spiegel DA, Stecker F. Heterocycles. 2007;74:473.Tietze LF, Stecker F, Zinngrebe J, Sommer KM. Chem Eur J. 2006;12:8770. doi: 10.1002/chem.200600849.Tietze LF, Sommer KM, Zinngrebe J, Stecker F. Angew Chem, Int Ed. 2005;44:257. doi: 10.1002/anie.200461629.
- 10.Only a single example of a Pd-catalyzed alkene difunctionalization reaction that generates a tetrahydropyran product has been reported. See reference 6c.
- 11.Hay MB, Ward AF, Wolfe JP. unpublished results
- 12.(a) Neukom JD, Perch NS, Wolfe JP. Organometallics. 2011;30:1269. [Google Scholar]; (b) Neukom JD, Perch NS, Wolfe JP. J Am Chem Soc. 2010;132:6276. doi: 10.1021/ja9102259. [DOI] [PubMed] [Google Scholar]; (c) Hanley PS, Markovic D, Hartwig JF. J Am Chem Soc. 2010;132:6302. doi: 10.1021/ja102172m. [DOI] [PubMed] [Google Scholar]; (d) Hanley PS, Hartwig JF. J Am Chem Soc. 2011;133:15661. doi: 10.1021/ja205722f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.