Abstract
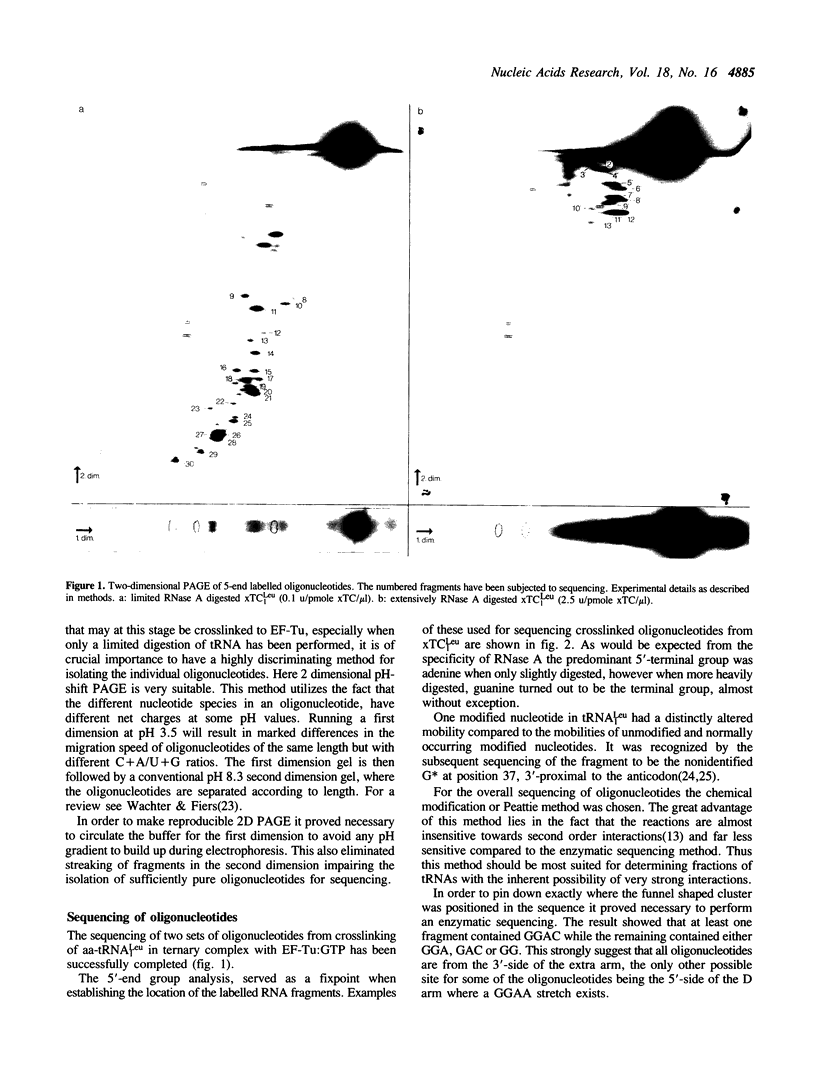
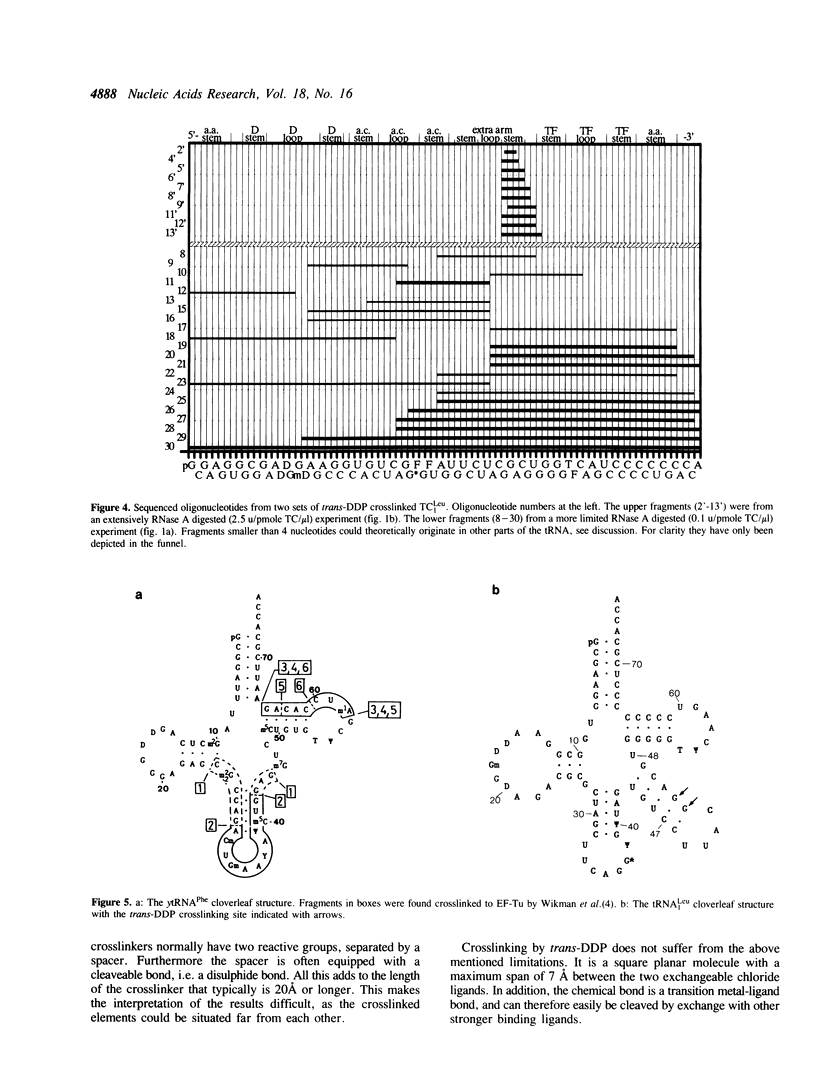
A tRNA containing a long extra arm, namely E. coli tRNA(Leu1) has been crosslinked to elongation factor Tu, with the crosslinking reagent trans-diamminedichloroplatinum(II). The nucleotide involved in the crosslinking was identified to be a guanosine in the variable region at position 47F or 47G.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamson J. K., Laue T. M., Miller D. L., Johnson A. E. Direct determination of the association constant between elongation factor Tu X GTP and aminoacyl-tRNA using fluorescence. Biochemistry. 1985 Jan 29;24(3):692–700. doi: 10.1021/bi00324a023. [DOI] [PubMed] [Google Scholar]
- Baudin F., Romby P., Romaniuk P. J., Ehresmann B., Ehresmann C. Crosslinking of transcription factor TFIIIA to ribosomal 5S RNA from X. laevis by trans-diamminedichloroplatinum (II). Nucleic Acids Res. 1989 Dec 11;17(23):10035–10046. doi: 10.1093/nar/17.23.10035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beres L., Lucas-Lenard J. Studies on the fluorescence of the Y base of yeast phenylalanine transfer ribonucleic acid. Effect of pH, aminoacylation, and interaction with elongation factor Tu. Biochemistry. 1973 Sep 25;12(20):3998–4002. doi: 10.1021/bi00744a033. [DOI] [PubMed] [Google Scholar]
- Blank H. U., Söll D. The nucleotide sequence of two leucine tRNA species from Escherichia coli K12. Biochem Biophys Res Commun. 1971 Jun 4;43(5):1192–1197. doi: 10.1016/0006-291x(71)90589-4. [DOI] [PubMed] [Google Scholar]
- Bruton C., Jakes R., Atkinson T. Gram-scale purification of methionyl-tRNA and tyrosyl-tRNA synthetases from Escherichia coli. Eur J Biochem. 1975 Nov 15;59(2):327–333. doi: 10.1111/j.1432-1033.1975.tb02459.x. [DOI] [PubMed] [Google Scholar]
- Dock-Bregeon A. C., Westhof E., Giegé R., Moras D. Solution structure of a tRNA with a large variable region: yeast tRNASer. J Mol Biol. 1989 Apr 20;206(4):707–722. doi: 10.1016/0022-2836(89)90578-0. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donis-Keller H. Phy M: an RNase activity specific for U and A residues useful in RNA sequence analysis. Nucleic Acids Res. 1980 Jul 25;8(14):3133–3142. doi: 10.1093/nar/8.14.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehresmann C., Moine H., Mougel M., Dondon J., Grunberg-Manago M., Ebel J. P., Ehresmann B. Cross-linking of initiation factor IF3 to Escherichia coli 30S ribosomal subunit by trans-diamminedichloroplatinum(II): characterization of two cross-linking sites in 16S rRNA; a possible way of functioning for IF3. Nucleic Acids Res. 1986 Jun 25;14(12):4803–4821. doi: 10.1093/nar/14.12.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. C., Randerath K. Rapid print-readout technique for sequencing of RNA's containing modified nucleotides. Nucleic Acids Res. 1979 Aug 10;6(11):3443–3458. doi: 10.1093/nar/6.11.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack A., Ladner J. E., Klug A. Crystallographic refinement of yeast phenylalanine transfer RNA at 2-5A resolution. J Mol Biol. 1976 Dec 25;108(4):619–649. doi: 10.1016/s0022-2836(76)80109-x. [DOI] [PubMed] [Google Scholar]
- Jerez C., Sandoval A., Allende J., Henes C., Ofengand J. Specificity of the interaction of aminoacyl ribonucleic acid with a protein-guanosine triphosphate complex from wheat embryo. Biochemistry. 1969 Jul;8(7):3006–3014. doi: 10.1021/bi00835a049. [DOI] [PubMed] [Google Scholar]
- Johnson A. E., Miller D. L., Cantor C. R. Functional covalent complex between elongation factor Tu and an analog of lysyl-tRNA. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3075–3079. doi: 10.1073/pnas.75.7.3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao T., Miller D. L., Abo M., Ofengand J. Formation and properties of a covalent complex between elongation factor Tu and Phe-tRNA bearing a photoaffinity probe on its 3-(3-amino-3-carboxypropyl)uridine residue. J Mol Biol. 1983 May 25;166(3):383–405. doi: 10.1016/s0022-2836(83)80091-6. [DOI] [PubMed] [Google Scholar]
- Kisselev L. L. The role of the anticodon in recognition of tRNA by aminoacyl-tRNA synthetases. Prog Nucleic Acid Res Mol Biol. 1985;32:237–266. doi: 10.1016/s0079-6603(08)60350-5. [DOI] [PubMed] [Google Scholar]
- Labanauskas M., Connors P. G., Young J. D., Bock R. M., Anderegg J. W., Beeman W. W. Structural studies on transfer RNA: preliminary crystallographic analysis. Science. 1969 Dec 19;166(3912):1530–1532. doi: 10.1126/science.166.3912.1530. [DOI] [PubMed] [Google Scholar]
- Leberman R., Antonsson B., Giovanelli R., Guariguata R., Schumann R., Wittinghofer A. A simplified procedure for the isolation of bacterial polypeptide elongation factor EF-Tu. Anal Biochem. 1980 May 1;104(1):29–36. doi: 10.1016/0003-2697(80)90272-9. [DOI] [PubMed] [Google Scholar]
- Lockard R. E., Alzner-Deweerd B., Heckman J. E., MacGee J., Tabor M. W., RajBhandary U. L. Sequence analysis of 5'[32P] labeled mRNA and tRNA using polyacrylamide gel electrophoresis. Nucleic Acids Res. 1978 Jan;5(1):37–56. doi: 10.1093/nar/5.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie A., Ribeiro N. S., Reid B. R., Jurnak F. Relative affinities of all Escherichia coli aminoacyl-tRNAs for elongation factor Tu-GTP. J Biol Chem. 1984 Apr 25;259(8):5010–5016. [PubMed] [Google Scholar]
- Metz-Boutigue M. H., Reinbolt J., Ebel J. P., Ehresmann C., Ehresmann B. Crosslinking of elongation factor Tu to tRNA(Phe) by trans-diamminedichloroplatinum (II). Characterization of two crosslinking sites on EF-Tu. FEBS Lett. 1989 Mar 13;245(1-2):194–200. doi: 10.1016/0014-5793(89)80220-0. [DOI] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petsko G. A., Phillips D. C., Williams R. J., Wilson I. A. On the protein crystal chemistry of chloroplatinite ions: general principles and interactions with triose phosphate isomerase. J Mol Biol. 1978 Apr 15;120(3):345–359. doi: 10.1016/0022-2836(78)90423-0. [DOI] [PubMed] [Google Scholar]
- Petsko G. A. Preparation of isomorphous heavy-atom derivatives. Methods Enzymol. 1985;114:147–156. doi: 10.1016/0076-6879(85)14015-2. [DOI] [PubMed] [Google Scholar]
- Roberts J. J., Pascoe J. M. Cross-linking of complementary strands of DNA in mammalian cells by antitumour platinum compounds. Nature. 1972 Feb 4;235(5336):282–284. doi: 10.1038/235282a0. [DOI] [PubMed] [Google Scholar]
- Silberklang M., Gillum A. M., RajBhandary U. L. The use of nuclease P1 in sequence analysis of end group labeled RNA. Nucleic Acids Res. 1977 Dec;4(12):4091–4108. doi: 10.1093/nar/4.12.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukalo M. A., Kubler M. D., Kern D., Mougel M., Ehresmann C., Ebel J. P., Ehresmann B., Giegé R. trans-Diamminedichloroplatinum(II), a reversible RNA-protein cross-linking agent. Application to the ribosome and to an aminoacyl-tRNA synthetase/tRNA complex. Biochemistry. 1987 Aug 11;26(16):5200–5208. doi: 10.1021/bi00390a045. [DOI] [PubMed] [Google Scholar]
- Wikman F. P., Romby P., Metz M. H., Reinbolt J., Clark B. F., Ebel J. P., Ehresmann C., Ehresmann B. Crosslinking of elongation factor Tu to tRNA(Phe) by trans-diamminedichloroplatinum (II). Characterization of two crosslinking sites in the tRNA. Nucleic Acids Res. 1987 Jul 24;15(14):5787–5801. doi: 10.1093/nar/15.14.5787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikman F. P., Siboska G. E., Petersen H. U., Clark B. F. The site of interaction of aminoacyl-tRNA with elongation factor Tu. EMBO J. 1982;1(9):1095–1100. doi: 10.1002/j.1460-2075.1982.tb01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. D., Bock R. M., Nishimura S., Ishikura H., Yamada Y., RajBhandary U. L., Labanauska M., Connors P. G. Structural studies on transfer RNA: crystallization of formylmethionine and leucine transfer RNA's. Science. 1969 Dec 19;166(3912):1527–1528. doi: 10.1126/science.166.3912.1527. [DOI] [PubMed] [Google Scholar]
- de Bruijn M. H., Klug A. A model for the tertiary structure of mammalian mitochondrial transfer RNAs lacking the entire 'dihydrouridine' loop and stem. EMBO J. 1983;2(8):1309–1321. doi: 10.1002/j.1460-2075.1983.tb01586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]