Abstract
Unremitting blood cell production throughout the lifetime of an organism is reliant on hematopoietic stem cells (HSCs). A rare and relatively quiescent cell type harbored in adult bone marrow, HSCs are, on entry into cell cycle fated to self-renew, undergo apoptosis or differentiate to progenitors (HPCs) that eventually yield specific classes of blood cells. Disruption of these HSC cell fate decisions is considered to be fundamental to the development of leukemia. Much effort has therefore been placed on understanding the molecular pathways that regulate HSC cell fate decisions and how these processes are undermined during leukemia. Transcription factors have emerged as critical regulators in this respect. Here we review the participation of zinc finger transcription factor GATA-2 in regulating normal hematopoietic stem and progenitor cell functionality, myelodysplasia and myeloid leukemia.
Keywords: GATA-2, hematopoietic stem cells, myelodysplasia, myeloid leukemia
Introduction
The GATA family of six zinc finger transcription factors modulates cell fate in multiple tissue systems through recognition of a six-nucleotide motif T/AGATAG/A consensus in target genes (Orkin, 2000). The first three members, GATA-1, 2 and 3, are predominantly expressed within the hematopoietic system and gene targeting experiments have revealed their salience to specific stages of hematopoiesis (Orkin, 2000). GATA-1 and 3 are lineage specific transcription factors, with the former affecting erythroid cells, megakaryocytes and eosinophils and the latter T-cells (Orkin, 2000). In stark contrast, GATA-2 is predominantly expressed within adult and developing hematopoietic stem cells (HSCs), myeloid progenitors and mast cells (Orlic et al., 1995; Rodrigues et al., 2008; Rodrigues et al., 2005; Tsai et al., 1994; Tsai and Orkin, 1997). Intimately linked to this expression pattern, GATA-2 is widely regarded as a pivotal regulator of HSCs and their progeny, hematopoietic progenitor cells (HPCs) (Ling et al., 2004; Rodrigues et al., 2008; Rodrigues et al., 2005; Tipping et al., 2009; Tsai et al., 1994).
Gene structure, expression, activation and turnover
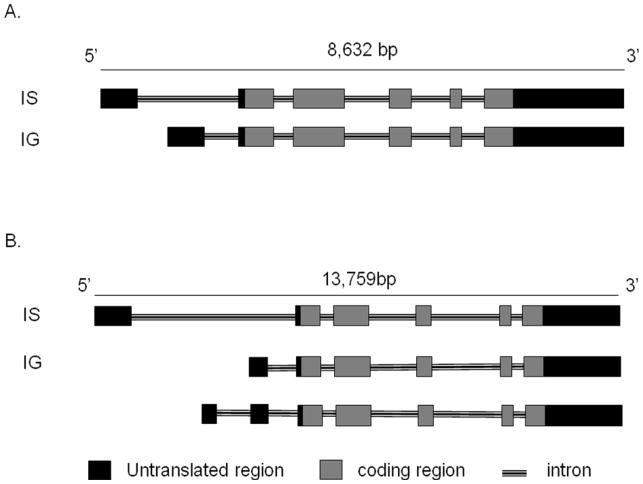
The murine GATA-2 gene spans approximately 8.5 kb, is located on chromosome 6 and produces two mRNA transcripts (Figure 1A). The human GATA-2 gene, which is approximately 14 kb, is located on chromosome 3 and produces three mRNA transcripts (Figure 1B). In both species, transcription of GATA-2 mRNAs commences from two distinct first exons, both of which encode untranslated regions, while the remaining five exons are shared by the two mRNAs transcripts (Minegishi et al., 1998; Pan et al., 2000) (Figure 1). The distal first exon of GATA-2 (IS exon), has specificity targeted to hematopoietic and neuronal lineages and the more downstream distal first exon (IG exon) is utilized by all tissues in which GATA-2 is expressed (Minegishi et al., 1998; Pan et al., 2000) (Figure 1).
Fig. 1. Mouse and human GATA-2 gene structure.
A. The mouse GATA-2 gene is on chromosome 6. There are two isoforms (IS, IG) of mouse GATA-2 and exon-intron organization is shown; exons and introns are spread over 8632 nucleotides.
B. The human GATA-2 gene is located on chromosome 3. There are three isoforms (IS, IG, unnamed) of human GATA-2 and exon-intron organization is shown; exons and introns are spread over 13,579 base pair nucleotides.
Mouse GATA-2 gene expression is regulated by specific cis-acting elements that direct expression to HSCs/HPCs and repress expression during differentiation to erythroid cells, as GATA-1 expression concomitantly increases; for example, a 2.8kb site upstream of GATA-2 transcription initiation specifically confers expression in HSCs/HPCs while an upstream 1.8kb site represses expression during erythroid differentiation in vivo (Snow et al., 2010; Snow et al., 2011). These cis-regulatory components often contain GATA binding sites; thus GATA-2 gene expression is regulated by both itself and by GATA-1 (Snow et al., 2010; Snow et al., 2011).
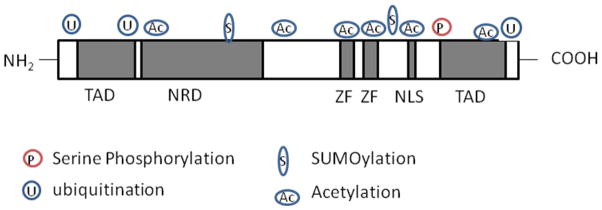
Human and mouse GATA-2 genes generate proteins of between 466–480 amino acids (49.1kDa and 50.5kDa respectively) and contain two zinc finger DNA binding domains (Figure 2). The function of the various protein domains of GATA-2 remain poorly understood. However, it is known that the function of GATA-2 in modulating hematopoietic cell fate depends critically on the two conserved zinc fingers (the N-finger and C-finger) and their flanking sequences, including two transactivation domains, a nuclear localization signal and a negative regulatory element (Minegishi et al., 2003; Tong et al., 2005) (Figure 2).
Fig. 2.
GATA-2 protein structure and post-translational modifications. The human and mouse GATA-2 genes encode proteins of between 466–480 amino acids. The GATA-2 protein contains 2 zinc fingers which serve as DNA binding domains and sites for protein-protein interaction (e.g partner protein binding). There are several sites of post-translational modifications including phosphorylation, acetylation, sumoylation and ubiquitination.
Key: TAD= Transactivation domain, NRD = negative regulatory domain, nuclear localization domain and ZF= zinc fingers.
Post-translational modifications (PTMs), including phosphorylation, acetylation, sumoylation and ubiquitination, are of considerable importance in the regulation of normal and leukemic hematopoiesis (De Felice et al., 2005; Rodriguez et al., 2011; Zhu et al., 2005). Accordingly, these PTMs are necessary for normal function, activation and turnover of GATA-2 (Chun et al., 2003; Hayakawa et al., 2004; Minegishi et al., 2005; Towatari et al., 1995) (Figure 2). Disruption of the mechanisms regulating PTMs of GATA-2 may therefore have ramifications not only for the function of GATA-2 in HSCs and HPCs, but also for leukemic disease.
Biological function
GATA-2 function in HSCs and HPCs
The role of GATA-2 in HSCs, the common origin of all blood lineages, has been dissected extensively using both loss of function and gain of function approaches. Mice engineered to be deficient in GATA-2 (GATA-2−/−) succumb during gestation at embryonic day 10 to 11 (day e10–11) with defects in primitive hematopoiesis that culminate in severe anemia (Tsai et al., 1994). GATA-2 null/wild-type chimeric mice generated to establish the function of GATA-2 in definitive hematopoiesis show an absence of both GATA-2−/− myeloid cell output and GATA-2−/− lymphoid cells (Tsai et al., 1994). In vitro culture and analysis of GATA-2−/− embryonic stem (ES) cells and yolk-sac cells derived E9.5 null embryos reveal widespread defects in definitive hematopoiesis, including decreased responsiveness to stem-cell factor (SCF) and reduced cell survival (Tsai et al., 1994; Tsai and Orkin, 1997). Taken together, these findings point to a central function for GATA-2 in maintaining the proliferation and survival of developing HSCs. Formal demonstration of GATA-2’s function within the developing HSC compartment has been gleaned from studies with GATA-2+/− embryos. GATA-2+/− embryos exhibit profound defects in the generation, self-renewal and expansion of stem cells at the first intra-embryonic site of HSC cell generation, the aorta-gonad-mesonephros (AGM) region (Ling et al., 2004). Other key anatomical locations of HSC development in GATA-2+/− embryos, the yolk-sac and fetal liver, are also detrimentally affected in their functionality. GATA-2 regulates HSC activity during ontogeny through interaction with hematopoietic transcription factor Runx1; GATA-2+/−:Runx-1+/− compound embryos succumb at midgestation due to severe hematopoietic defects (Wilson et al., 2010). Acting as a component of a multiprotein complex with Fli-1 and Elf-1, GATA-2 also activates expression of the key hematopoietic transcription factor SCL in developing HSCs, endothelial cells and the hemangioblast (Gottgens et al., 2002). GATA-2+/− mice have also provided a tractable model by which to assess the broad impact of GATA-2 on adult hematopoiesis and adult HSCs.
GATA-2+/− adult marrow have a reduced number of functional HSCs which is mechanistically associated with increased HSC apoptosis and cellular quiescence, but not the relative ability of HSCs to form progenitors or to self-renew (Rodrigues et al., 2005). In addition, committed HPCs of the granulocyte-macrophage progenitor (GMP) lineage from GATA-2+/− mice are attenuated in number and less able to perform in functional assays while other committed HPCs, such as common myeloid progenitors (CMPs) and lymphoid progenitors, are unaffected by GATA-2 deficiency (Rodrigues et al., 2008). Furthermore, the action of GATA-2 in the GMP compartment is partly mediated through the Notch-1 target gene, HES-1. This data is broadly congruous to previous studies indicating regulation of myelopoiesis through the Notch-1-GATA-2 signaling axis (Kumano et al., 2001). Predicated on these observations, it is postulated that GATA-2 is required at differentiation stage–specific levels of the hematopoietic hierarchy, acting separately at the HSC and GMP level. The basis for this differential behavior has yet to be identified but is likely to reflect divergent GATA-2 partner protein environments and target gene programs between these two cellular compartments.
Enforced expression experiments have also been used to address the impact of GATA-2 in adult HSCs. Enforcing a 2-fold increase in GATA-2 level in murine HSCs is sufficient to reduce their number by 40-fold and cause their failure to differentiate appropriately following transplantation (Persons et al., 1999). Differentiation specific effects are also observed in mouse hematopoietic progenitor cell lines in which GATA-2 was enforced through an estrogen inducible GATA-2/ER moiety (Heyworth et al., 1999). Enforced expression of GATA-2 in human HSCs from cord blood similarly causes a level dependent block in hematopoietic reconstitution after transplantation (Tipping et al., 2009). High levels of GATA-2 in the setting of enforced expression constrains HSC proliferation and imposes cellular quiescence in cord blood HSCs via modulation of genes involved in regulating HSC proliferation, such as MEF and HES-1, and also integral components of the cell cycle machinery including CCND3, CDK4 and CDK6.
GATA-2 regulation of quiescent and apoptotic HSC fates
Thus, both loss and gain of function experiments demonstrate that GATA-2 level regulates adult HSC quiescence. However, it was largely unexpected that GATA-2+/− mice would have a larger proportion of quiescent HSC for two reasons. Firstly, the relatively high expression of GATA-2 within mammalian bone marrow has been conjectured to maintain the innate quiescence observed in HSCs (Persons et al., 1999). Secondly, as alluded to above, enforced expression experiments in HSCs show that raising GATA-2 level by 2-fold causes failure of HSC proliferation (Heyworth et al., 1999; Persons et al., 1999; Tipping et al., 2009). Using Occam’s razor, one would therefore predict that lowering GATA-2 by the same factor would in fact abate HSC quiescence. Yet the HSC compartment of GATA-2+/− marrow is less proliferative, indicating that cell cycling is exquisitely sensitive to the level of GATA-2 in either direction (Rodrigues et al., 2005; Tipping et al., 2009). It is unclear whether lower or higher levels GATA-2 regulate HSC quiescence by similar molecular mechanisms. While candidate target gene approaches have identified some of the molecular mechanisms underpinning HSC quiescence in the enforced expression setting (Tipping et al., 2009), answering this question comprehensively requires genome wide investigation into the molecular basis of HSC quiescence in the context of both enforced and reduced GATA-2 level.
In addition to regulation of quiescence, GATA-2 also modulates HSC apoptosis (Rodrigues et al., 2005), which is a critical facet of HSC fate required to maintain the integrity of the HSC compartment. GATA-2+/− HSCs demonstrate an increased proclivity towards apoptosis in association with decreased expression of the antiapoptotic gene Bcl-xL, implying that GATA-2 and Bcl-xL act together to modulate HSC compartment size (Rodrigues et al., 2005). Relatively high levels of Bcl-xL are observed within the HSC compartment (Rodrigues et al., 2005), yet the physiological function of Bcl-xL in regulating this compartment and its relationship to GATA-2 still remains ambiguous. It is entirely possible that GATA-2 directly affects Bcl-xL expression as GATA sites are present on the promoter regions of Bcl-xL or, alternatively, Bcl-xL may function through indirect effects on other gene or signaling pathways. With other studies indicating a mechanistic link between quiescence and apoptosis pathways, including those related to the Bcl-xL family (Linette et al., 1996), it is tempting to speculate that GATA-2 and Bcl-xL jointly regulate quiescence and apoptosis in GATA-2+/− mice. By extending the notion of connectivity between quiescent and apoptotic pathways in HSCs from GATA-2+/− mice, it is also reasonable to postulate that quiescence is induced as a protective, compensatory mechanism to prevent gradual attrition of HSC numbers that would ultimately be caused by enhanced apoptosis in GATA-2+/− mice.
Medical relevance
GATA-2, myelodysplastic syndrome and leukemia
Given that transcription factors are critical regulators of gene expression and therefore HSC/HPC cell fate, it perhaps unsurprising that dysregulation of transcription factor function, in the context of mis-expression, mutations or the formation of abnormal transcription factor fusion oncogenes, is an integral, initiating and/or sustaining event in the development of leukemia.
Several lines of evidence have recently implicated mutations in GATA-2 in pre-leukemic (myelodysplastic) and leukemic disease. Two somatic mutations in the coding region of GATA-2 have been identified in the progression of chronic myeloid leukemia (CML) to blast crisis (Zhang et al., 2008). These mutations perturb the transactivation and myelomonocytic differentiation capacity of GATA-2. Mutations resulting in loss of GATA-2 function are also observed in disorders linked to an increased propensity to develop myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) including Emberger syndrome, MonoMAC syndrome and DCML deficiency, which is associated with defects in dendritic cells, monocytes, and B and NK cells (Dickinson et al., 2011; Hsu et al., 2011; Ostergaard et al., 2011). Another study shows heritable GATA-2 mutations associated with an increased tendency to develop MDS or AML without preceding hematopoietic defects (Hahn et al., 2011). All these syndromes display mutations that are broadly of two types: (i) N-terminal frameshift mutations that result in premature termination of GATA-2 protein translation, and (ii) mutations in the first and second zinc fingers of GATA-2, which are critical for both DNA binding and interactions with partner proteins. Despite common overall mechanisms leading to MDS and AML in these diseases, the specificity of the phenotype observed within each patient cohort and syndrome is presumably a reflection of the differing transcriptional activities conferred by each mutation. For example, even mutations in neighboring amino acids of a zinc finger domain of GATA-2 may confer distinct effects on interactions with partner proteins and differentially modulate target genes. Intriguingly, an identical GATA-2 mutation is observed in MonoMAC syndrome, DCML deficiency and the Hahn et al study (Dickinson et al., 2011; Hahn et al., 2011; Hsu et al., 2011); this suggests that variations in disease characteristics and progression in these settings may also be a function of the target hematopoietic cell initiating or sustaining MDS and AML, or independent co-operating mutations. Overall, the association of GATA-2 mutations with MDS or AML highlights the importance of GATA-2 to normal myeloid progenitor cell fate. Loss of GATA-2 function mutations in HSCs may also be of relevance to the diversity of lineage specific hematopoietic defects observed in Emberger, MonoMAC and DCML syndromes.
Overexpression of GATA-2 is also implicated in leukemic disease and is an indicator of poor prognosis in AML (Vicente et al., 2011). That GATA-2 overexpression confers enhanced cellular quiescence provides a plausible mechanism by which its overexpression could cause weak responses to chemotherapy and contribute to poor prognosis in AML (Tipping et al., 2009); elevated GATA-2 level may similarly induce quiescence in leukemic cells, allowing them to evade chemotherapeutic killing. Enforced expression of GATA-2 forestalling proliferation of MLL-ENL oncogene driven leukemic cells lends credence to this idea (Bonadies et al., 2011). Set against the backdrop of loss and gain of function experiments in normal HSCs/HPCs, these data underscore the pathological importance to maintain appropriate threshold levels of GATA-2 in the hematopoietic system.
In poor prognosis AML, GATA-2 overexpression also mirrors that of Evi-1 overexpression; Evi-1 is also a well established marker of poor prognosis AML and a critical regulator of both HSC and leukemic stem cell (LSC) function and GATA-2 is a target gene of Evi-1 in both HSCs and LSCs (Goyama et al., 2008; Valk et al., 2004). However, the function of GATA-2 in LSCs - which are considered primary drivers of leukemic disease and the origin of leukemic relapse (Hope et al., 2004) – is unclear and merits investigation. Furtherance of developing therapies to combat poor disease prognosis associated with high GATA-2 expression in leukemia will be dependent on selective identification of GATA-2 partner proteins and druggable target genes in LSCs and should advertently shed light on the feasibility of specific targeting of LSCs and leukemic tumor burden rather than normal hematopoietic cells.
Concluding remarks
GATA-2 functions as a crucial level-dependent regulator of hematopoietic stem and committed progenitor cells. Consequently, dysregulated GATA-2 expression and function is emerging as a key regulator of myelodysplasia and myeloid leukemia. Evaluating GATA-2 function in LSCs will be necessary to understand disease progression in GATA-2 related leukemia and in developing specific therapeutics to eradicate leukemic disease in this context. Continued investigation into GATA-2 function in HSCs/HPCs is of interest in and of itself as it may offer insights into pathways amenable to manipulation of normal HSC/HPC function in the resurgent field of gene therapy and in cord blood transplantation for adults.
Acknowledgments
Work in the authors’ laboratories is supported by NIH grant P20RR018757 (NPR), BD Biosciences (NPR), the UK Medical Research Council (TE) and UK Leukemia and Lymphoma Research (TE). Additional support to NPR laboratory is provided by the administrative and flow cytometry cores within the NIH COBRE at Roger Williams Medical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bonadies N, Foster SD, Chan WI, Kvinlaug BT, Spensberger D, Dawson MA, Spooncer E, Whetton AD, Bannister AJ, Huntly BJ, et al. Genome-wide analysis of transcriptional reprogramming in mouse models of acute myeloid leukaemia. PloS one. 2011;6:e16330. doi: 10.1371/journal.pone.0016330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun TH, Itoh H, Subramanian L, Iniguez-Lluhi JA, Nakao K. Modification of GATA-2 transcriptional activity in endothelial cells by the SUMO E3 ligase PIASy. Circ Res. 2003;92:1201–1208. doi: 10.1161/01.RES.0000076893.70898.36. [DOI] [PubMed] [Google Scholar]
- De Felice L, Tatarelli C, Mascolo MG, Gregorj C, Agostini F, Fiorini R, Gelmetti V, Pascale S, Padula F, Petrucci MT, et al. Histone deacetylase inhibitor valproic acid enhances the cytokine-induced expansion of human hematopoietic stem cells. Cancer Res. 2005;65:1505–1513. doi: 10.1158/0008-5472.CAN-04-3063. [DOI] [PubMed] [Google Scholar]
- Dickinson RE, Griffin H, Bigley V, Reynard LN, Hussain R, Haniffa M, Lakey JH, Rahman T, Wang XN, McGovern N, et al. Exome sequencing identifies GATA-2 mutation as the cause of dendritic cell, monocyte, B and NK lymphoid deficiency. Blood. 2011;118:2656–2658. doi: 10.1182/blood-2011-06-360313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottgens B, Nastos A, Kinston S, Piltz S, Delabesse EC, Stanley M, Sanchez MJ, Ciau-Uitz A, Patient R, Green AR. Establishing the transcriptional programme for blood: the SCL stem cell enhancer is regulated by a multiprotein complex containing Ets and GATA factors. The EMBO journal. 2002;21:3039–3050. doi: 10.1093/emboj/cdf286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyama S, Yamamoto G, Shimabe M, Sato T, Ichikawa M, Ogawa S, Chiba S, Kurokawa M. Evi-1 is a critical regulator for hematopoietic stem cells and transformed leukemic cells. Cell Stem Cell. 2008;3:207–220. doi: 10.1016/j.stem.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Hahn CN, Chong CE, Carmichael CL, Wilkins EJ, Brautigan PJ, Li XC, Babic M, Lin M, Carmagnac A, Lee YK, et al. Heritable GATA2 mutations associated with familial myelodysplastic syndrome and acute myeloid leukemia. Nat Genet. 2011;43:1012–1017. doi: 10.1038/ng.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa F, Towatari M, Ozawa Y, Tomita A, Privalsky ML, Saito H. Functional regulation of GATA-2 by acetylation. Journal of leukocyte biology. 2004;75:529–540. doi: 10.1189/jlb.0603389. [DOI] [PubMed] [Google Scholar]
- Heyworth C, Gale K, Dexter M, May G, Enver T. A GATA-2/estrogen receptor chimera functions as a ligand-dependent negative regulator of self-renewal. Genes & development. 1999;13:1847–1860. doi: 10.1101/gad.13.14.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope KJ, Jin L, Dick JE. Acute myeloid leukemia originates from a hierarchy of leukemic stem cell classes that differ in self-renewal capacity. Nat Immunol. 2004;5:738–743. doi: 10.1038/ni1080. [DOI] [PubMed] [Google Scholar]
- Hsu AP, Sampaio EP, Khan J, Calvo KR, Lemieux JE, Patel SY, Frucht DM, Vinh DC, Auth RD, Freeman AF, et al. Mutations in GATA2 are associated with the autosomal dominant and sporadic monocytopenia and mycobacterial infection (MonoMAC) syndrome. Blood. 2011;118:2653–2655. doi: 10.1182/blood-2011-05-356352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumano K, Chiba S, Shimizu K, Yamagata T, Hosoya N, Saito T, Takahashi T, Hamada Y, Hirai H. Notch1 inhibits differentiation of hematopoietic cells by sustaining GATA-2 expression. Blood. 2001;98:3283–3289. doi: 10.1182/blood.v98.12.3283. [DOI] [PubMed] [Google Scholar]
- Linette GP, Li Y, Roth K, Korsmeyer SJ. Cross talk between cell death and cell cycle progression: BCL-2 regulates NFAT-mediated activation. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:9545–9552. doi: 10.1073/pnas.93.18.9545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling KW, Ottersbach K, van Hamburg JP, Oziemlak A, Tsai FY, Orkin SH, Ploemacher R, Hendriks RW, Dzierzak E. GATA-2 plays two functionally distinct roles during the ontogeny of hematopoietic stem cells. The Journal of experimental medicine. 2004;200:871–882. doi: 10.1084/jem.20031556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minegishi N, Ohta J, Suwabe N, Nakauchi H, Ishihara H, Hayashi N, Yamamoto M. Alternative promoters regulate transcription of the mouse GATA-2 gene. J Biol Chem. 1998;273:3625–3634. doi: 10.1074/jbc.273.6.3625. [DOI] [PubMed] [Google Scholar]
- Minegishi N, Suzuki N, Kawatani Y, Shimizu R, Yamamoto M. Rapid turnover of GATA-2 via ubiquitin-proteasome protein degradation pathway. Genes Cells. 2005;10:693–704. doi: 10.1111/j.1365-2443.2005.00864.x. [DOI] [PubMed] [Google Scholar]
- Minegishi N, Suzuki N, Yokomizo T, Pan X, Fujimoto T, Takahashi S, Hara T, Miyajima A, Nishikawa S, Yamamoto M. Expression and domain-specific function of GATA-2 during differentiation of the hematopoietic precursor cells in midgestation mouse embryos. Blood. 2003;102:896–905. doi: 10.1182/blood-2002-12-3809. [DOI] [PubMed] [Google Scholar]
- Orkin SH. Diversification of haematopoietic stem cells to specific lineages. Nature reviews Genetics. 2000;1:57–64. doi: 10.1038/35049577. [DOI] [PubMed] [Google Scholar]
- Orlic D, Anderson S, Biesecker LG, Sorrentino BP, Bodine DM. Pluripotent hematopoietic stem cells contain high levels of mRNA for c-kit, GATA-2, p45 NF-E2, and c-myb and low levels or no mRNA for c-fms and the receptors for granulocyte colony-stimulating factor and interleukins 5 and 7. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:4601–4605. doi: 10.1073/pnas.92.10.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostergaard P, Simpson MA, Connell FC, Steward CG, Brice G, Woollard WJ, Dafou D, Kilo T, Smithson S, Lunt P, et al. Mutations in GATA2 cause primary lymphedema associated with a predisposition to acute myeloid leukemia (Emberger syndrome) Nat Genet. 2011;43:929–931. doi: 10.1038/ng.923. [DOI] [PubMed] [Google Scholar]
- Pan X, Minegishi N, Harigae H, Yamagiwa H, Minegishi M, Akine Y, Yamamoto M. Identification of human GATA-2 gene distal IS exon and its expression in hematopoietic stem cell fractions. Journal of biochemistry. 2000;127:105–112. doi: 10.1093/oxfordjournals.jbchem.a022570. [DOI] [PubMed] [Google Scholar]
- Persons DA, Allay JA, Allay ER, Ashmun RA, Orlic D, Jane SM, Cunningham JM, Nienhuis AW. Enforced expression of the GATA-2 transcription factor blocks normal hematopoiesis. Blood. 1999;93:488–499. [PubMed] [Google Scholar]
- Rodrigues NP, Boyd AS, Fugazza C, May GE, Guo Y, Tipping AJ, Scadden DT, Vyas P, Enver T. GATA-2 regulates granulocyte-macrophage progenitor cell function. Blood. 2008;112:4862–4873. doi: 10.1182/blood-2008-01-136564. [DOI] [PubMed] [Google Scholar]
- Rodrigues NP, Janzen V, Forkert R, Dombkowski DM, Boyd AS, Orkin SH, Enver T, Vyas P, Scadden DT. Haploinsufficiency of GATA-2 perturbs adult hematopoietic stem-cell homeostasis. Blood. 2005;106:477–484. doi: 10.1182/blood-2004-08-2989. [DOI] [PubMed] [Google Scholar]
- Rodriguez S, Wang L, Mumaw C, Srour EF, Lo Celso C, Nakayama K, Carlesso N. The SKP2 E3 ligase regulates basal homeostasis and stress-induced regeneration of HSCs. Blood. 2011;117:6509–6519. doi: 10.1182/blood-2010-11-321521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow JW, Trowbridge JJ, Fujiwara T, Emambokus NE, Grass JA, Orkin SH, Bresnick EH. A single cis element maintains repression of the key developmental regulator Gata2. PLoS genetics. 2010:6. doi: 10.1371/journal.pgen.1001103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow JW, Trowbridge JJ, Johnson KD, Fujiwara T, Emambokus NE, Grass JA, Orkin SH, Bresnick EH. Context-dependent function of “GATA switch” sites in vivo. Blood. 2011;117:4769–4772. doi: 10.1182/blood-2010-10-313031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipping AJ, Pina C, Castor A, Hong D, Rodrigues NP, Lazzari L, May GE, Jacobsen SE, Enver T. High GATA-2 expression inhibits human hematopoietic stem and progenitor cell function by effects on cell cycle. Blood. 2009;113:2661–2672. doi: 10.1182/blood-2008-06-161117. [DOI] [PubMed] [Google Scholar]
- Tong Q, Tsai J, Tan G, Dalgin G, Hotamisligil GS. Interaction between GATA and the C/EBP family of transcription factors is critical in GATA-mediated suppression of adipocyte differentiation. Molecular and cellular biology. 2005;25:706–715. doi: 10.1128/MCB.25.2.706-715.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towatari M, May GE, Marais R, Perkins GR, Marshall CJ, Cowley S, Enver T. Regulation of GATA-2 phosphorylation by mitogen-activated protein kinase and interleukin-3. J Biol Chem. 1995;270:4101–4107. doi: 10.1074/jbc.270.8.4101. [DOI] [PubMed] [Google Scholar]
- Tsai FY, Keller G, Kuo FC, Weiss M, Chen J, Rosenblatt M, Alt FW, Orkin SH. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature. 1994;371:221–226. doi: 10.1038/371221a0. [DOI] [PubMed] [Google Scholar]
- Tsai FY, Orkin SH. Transcription factor GATA-2 is required for proliferation/survival of early hematopoietic cells and mast cell formation, but not for erythroid and myeloid terminal differentiation. Blood. 1997;89:3636–3643. [PubMed] [Google Scholar]
- Valk PJ, Verhaak RG, Beijen MA, Erpelinck CA, Barjesteh van Waalwijk van Doorn-Khosrovani S, Boer JM, Beverloo HB, Moorhouse MJ, van der Spek PJ, Lowenberg B, et al. Prognostically useful gene-expression profiles in acute myeloid leukemia. The New England journal of medicine. 2004;350:1617–1628. doi: 10.1056/NEJMoa040465. [DOI] [PubMed] [Google Scholar]
- Vicente C, Vazquez I, Conchillo A, Garcia-Sanchez MA, Marcotegui N, Fuster O, Gonzalez M, Calasanz MJ, Lahortiga I, Odero MD. Overexpression of GATA2 predicts an adverse prognosis for patients with acute myeloid leukemia and it is associated with distinct molecular abnormalities. Leukemia. 2011 doi: 10.1038/leu.2011.235. [DOI] [PubMed] [Google Scholar]
- Wilson NK, Foster SD, Wang X, Knezevic K, Schutte J, Kaimakis P, Chilarska PM, Kinston S, Ouwehand WH, Dzierzak E, et al. Combinatorial transcriptional control in blood stem/progenitor cells: genome-wide analysis of ten major transcriptional regulators. Cell Stem Cell. 2010;7:532–544. doi: 10.1016/j.stem.2010.07.016. [DOI] [PubMed] [Google Scholar]
- Zhang SJ, Ma LY, Huang QH, Li G, Gu BW, Gao XD, Shi JY, Wang YY, Gao L, Cai X, et al. Gain-of-function mutation of GATA-2 in acute myeloid transformation of chronic myeloid leukemia. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:2076–2081. doi: 10.1073/pnas.0711824105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Zhou J, Peres L, Riaucoux F, Honore N, Kogan S, de The H. A sumoylation site in PML/RARA is essential for leukemic transformation. Cancer cell. 2005;7:143–153. doi: 10.1016/j.ccr.2005.01.005. [DOI] [PubMed] [Google Scholar]