Abstract
Aim of this study was to determine whether an increase in adiposity, without a concomitant increase in intrahepatic triglyceride (IHTG) content, is associated with a deterioration in metabolic function. To this end, multi-organ insulin sensitivity, assessed by using a two-stage hyperinsulinemic-euglycemic clamp procedure in conjunction with stable isotopically labeled tracer infusion, and very low density lipoprotein (VLDL) kinetics, assessed by stable isotopically labeled tracer infusion and mathematical modeling, were determined in 10 subjects with class I obesity (body mass index [BMI]: 31.6±0.3 kg/m2; 37±2% body fat; visceral adipose tissue [VAT]: 1225±144 cm3) and 10 subjects with class III obesity (BMI: 41.5±0.5 kg/m2; 43±2% body fat; VAT: 2121±378 cm3), matched on age, sex and IHTG content (14±4% and 14±3%, respectively). No differences between class I and class III obese groups were detected in insulin-mediated suppression of palmitate (67±3% and 65±3, respectively; P=0.635) and glucose (67±3% and 73±5%, respectively; P=0.348) rates of appearance in plasma, and the insulin-mediated increase in glucose disposal (218±18% and 193±30%, respectively; P=0.489). In 15 addition, no differences between class I and class III obese groups were detected in secretion rates of VLDL-triglyceride (6.5±1.0 and 6.0±1.4 µmol/l·min, respectively; P=0.787) and VLDL-apolipoprotein B-100 (0.40±0.05 and 0.41±0.04 nmol/l·min, respectively; P=0.866), and plasma clearance rates of VLDL-triglyceride (31 [16–59] and 29 [18–46] ml/min, respectively; P=0.888) and VLDL-apolipoprotein B-100 (15 [11–19] and 17 [11–25] ml/min, respectively; P=0.608). We conclude that increased adiposity without a concomitant increase in IHTG content does not cause additional abnormalities in adipose tissue, skeletal muscle, and hepatic insulin sensitivity, or VLDL metabolism.
Keywords: Liver, Adipose Tissue, Very-Low-Density Lipoproteins, VLDL, Visceral Fat, Insulin Resistance
INTRODUCTION
Obesity is associated with multiple medical co-morbidities and reduced survival (1). In general, the risk of obesity-related complications and mortality increases with increasing body mass index (BMI), which has led to a classification scheme that separates obesity into distinct BMI categories used to identify patients who have “high” (class I obesity: BMI 5 30.0–34.9 kg/m2), “very high” (class II obesity: BMI 35.0–39.9 kg/m2) and “extremely high” (class III obesity: BMI ≥40.0 kg/m2) health risk (2). These guidelines imply that body fat mass is an important determinant of obesity-related disease, because BMI correlates directly with percent body fat (3,4).
Although the major metabolic complications of obesity, namely insulin resistance, type 2 diabetes and hypertriglyceridemia, increase linearly with increasing BMI (5–8), the relationship between metabolic risk and BMI is affected by several factors, such as sex, ethnic background and body fat distribution (9). Recently, data from several studies have found that increased intrahepatic triglyceride (IHTG) content is an important marker of multi-organ insulin resistance and increased hepatic very low-density lipoprotein (VLDL)-triglyceride (TG) secretion rate (10–12). In fact, insulin sensitivity in the liver, skeletal muscle and adipose tissue is inversely correlated with IHTG content in obese subjects who have the same BMI and percent body fat (12), and normal IHTG content is an important predictor of “metabolically-benign” obesity (13). Therefore, it is possible that the increase in metabolic complications associated with increasing BMI reflects an increase in liver fat, because IHTG content, degree of steatosis and the prevalence of non-alcoholic fatty liver disease (NAFLD) generally increase with increasing BMI values (14–18).
The purpose of the present study was to determine whether an increase in whole-body adiposity and BMI without a concomitant increase in IHTG content is associated with augmented metabolic dysfunction. Accordingly, we evaluated multi-organ insulin sensitivity by using a two-stage hyperinsulinemic clamp procedure in conjunction with stable isotope tracer infusion, and VLDL kinetics by using stable isotope tracers in conjunction with mathematical modeling, in men and women with class I (BMI 30.0–34.9 kg/m2) and class III (BMI ≥40.0 kg/m2) obesity who were matched on IHTG content.
METHODS AND PROCEDURES
Subjects
Among a group of 42 obese men and women studied previously (11,12,19), we identified 10 subjects with class I obesity (3 men and 7 women; age 44.9 ± 3.4 yr) and 10 subjects with class III obesity (3 men and 7 women; age 43.0 ± 4.0 yr), who could be matched on age, sex, and IHTG. In each group, 4 subjects had normal IHTG content (i.e., <5.6% of liver volume based on data obtained from a normal population, who had normal fasting serum glucose and liver enzyme concentrations and did not have diabetes (16)) and 6 subjects had increased IHTG content (≥10% of liver volume). We did not have knowledge of any outcome measures when the matching was performed. All subjects completed a comprehensive medical evaluation, which included a detailed history and physical examination, routine blood tests, a 12-lead electrocardiogram, and a 2-h oral glucose tolerance test. Exclusion criteria included smoking, alcohol consumption ≥20 g/d, use of medications known to affect carbohydrate and lipid metabolism, history or evidence of liver disease (other than fat accumulation), severe hypertriglyceridemia (≥300 mg/dl), and diabetes. All subjects had been weight-stable (≤2% change in body weight) and sedentary (exercise <1 h/wk) for at least 2 months before enrollment. Subjects provided their written informed consent before participating in the study, which was approved by the Human Research Protection Office and the Center for Applied Research Sciences Advisory Committee of Washington University School of Medicine in St. Louis, MO.
Body composition analyses
Body composition was assessed approximately 1–2 weeks before the hyperinsulinemiceuglycemic clamp procedure was performed. Total body fat and fat-free mass (FFM) were determined by using dual-energy X-ray absorptiometry (Delphi-W densitometer, Hologic, Waltham, MA) (20). Intra-abdominal and subcutaneous abdominal adipose tissue volumes were quantified by using magnetic resonance imaging (Siemens, Iselin, NJ; ANALYZE 7.0 software, Mayo Foundation, Minnesota, USA) (21); eight 10-mm-thick slice images were obtained proximally at the L4-L5 interspace and were analyzed for intra-abdominal and subcutaneous fat volume. IHTG content was measured by using proton magnetic resonance spectroscopy (1.5T Siemens Magneton Vision scanner; Siemens, Erlanger, Germany) (22). Three 2×2×2 cm3 voxels were examined in each subject, and the values were averaged to determine intrahepatic TG content; the coefficient of variation was 1.5%.
Hyperinsulinemic-euglycemic clamp procedure
Subjects were admitted to the Clinical Research Unit at Washington University School of Medicine on the evening before the study. At 1900 h, they consumed a standard meal containing ~12 kcal/kg FFM (55% of total energy from carbohydrate, 30% from fat, and 15% from protein). Subjects then fasted (except for water) and rested in bed until completion of the clamp procedure the next day. At 0500 h the following morning, one catheter was inserted into a forearm vein to infuse stable isotope labeled tracers (Cambridge Isotope Laboratories, Andover, MA), dextrose and insulin, and a second catheter was inserted into a radial artery in the contralateral hand to obtain blood samples. At 0600 h (time = 0), a primed, continuous infusion of [6,6-2H2]glucose (0.25 µmol/kg·min; priming dose: 22.5 µmol/kg), dissolved in 0.9% NaCl solution, was started and maintained for 5.5 h (until the end of clamp stage 1). At 0800 h, continuous infusions of [2,2-2H2]palmitate (infusion rate: 0.035 µmol/kg·min), dissolved in 25% human albumin solution, and [1,1,2,3,3-2H5]glycerol (0.08 µmol/kg·min; priming dose: 1.2 µmol/kg), dissolved in 0.9% NaCl solution, were started and maintained for 3.5 h (until the end of clamp stage 1). At 0930 h (3.5 h after starting the glucose tracer infusion), a two-stage hyperinsulinemic-euglycemic clamp procedure was started and continued for 6 h. During stage 1 of the clamp procedure (from 3.5 to 5.5 h), insulin was infused at a rate of 20 mU/m2 body surface area (BSA)·min (initiated with a priming dose of 80 mU/m2 BSA·min for 5 min and then 40 mU/m2 15 BSA·min for 5 min). During stage 2 of the clamp procedure (from 5.5 to 9.5 h), insulin was infused at a rate of 50 mU/m2 BSA·min (initiated with a priming dose of 200 mU/m2 BSA·min for 5 min and then 100 mU/m2 BSA·min for 5 min). The low-dose insulin infusion rate was used to evaluate adipose tissue insulin sensitivity (suppression of lipolysis) and hepatic insulin sensitivity (suppression of glucose production) and the high-dose insulin infusion rate was used to evaluate skeletal muscle insulin sensitivity (stimulation of skeletal muscle glucose uptake) (12,23). Euglycemia was maintained at a blood glucose concentration of ~100 mg/dl throughout stages 1 and 2, by infusing 20% dextrose solution enriched to 2.5% with [6,6-2H2]glucose. The infusion rates of [6,6-2H2]glucose, [1,1,2,3,3-2H5]glycerol, and [2,2-2H2]palmitate were reduced by 50% during clamp stage 1, and [6,6-2H2]glucose infusion rate was reduced by 75% during clamp stage 2, to account for changes in hepatic glucose production and adipose tissue lipolytic rates.
Blood samples were obtained immediately before starting the tracer infusion and every 10 min during the final 30 min of the basal period and stages 1 and 2 of the clamp procedure 5 to determine plasma insulin and substrate concentrations and plasma glucose, glycerol, and palmitate tracer-to-tracee ratios (TTRs). Blood samples were collected in chilled tubes containing sodium EDTA. Samples were placed on ice, and plasma was separated by centrifugation within 30 min of collection and stored at −80°C until final analyses were performed. A small amount of blood (~1 ml) was collected into heparinized tubes every 10 min during insulin infusion to monitor plasma glucose concentrations.
VLDL kinetics study
Approximately 1 week after the hyperinsulinemic-euglycemic clamp procedure, subjects were readmitted to the Clinical Research Unit in the evening. At 1800 h, they consumed a meal containing ~12 kcal/kg FFM (55% of total energy as carbohydrates, 30% as fat, and 15% as protein). Subjects then fasted (except for water) and rested in bed until completion of the VLDL kinetics study the next day. At 0500 h the following morning, one catheter was inserted into a forearm vein to administer stable isotope labeled tracers, and a second catheter was inserted into a vein in the contralateral hand, which was heated to 55°C by using a thermostatically controlled box, to obtain arterialized blood samples. At 0600 h (time = 0), a bolus of [1,1,2,3,3-2H5]glycerol (75 µmol/kg), dissolved in 0.9% NaCl solution, was administered through the catheter in the forearm vein, and constant infusions of [2,2-2H2]palmitate (0.024 µmol/kg·min), dissolved in 25% human albumin solution, and [5,5,5-2H3]leucine (0.12 µmol/kg·min; priming dose: 7.2 µmol/kg), dissolved in 0.9% NaCl solution, were started and maintained for 12 h.
Blood samples were collected immediately before starting the tracer infusion and at 5, 15, 30, 60, 90, and 120 min and then every hour for 10 h after starting the tracer infusion to determine glycerol and palmitate TTR in plasma and VLDL-TG, and leucine TTR in plasma and VLDL-apolipoprotein B-100 (apoB-100). Blood samples were collected in chilled tubes containing sodium EDTA. Samples were placed on ice, and plasma was separated by centrifugation within 30 min of collection. Aliquots of plasma (~3 ml) were kept in the refrigerator for immediate isolation of VLDL, as previously described (24,25). The remaining plasma samples were stored at −80°C until final analyses were performed.
Sample analyses
Plasma glucose concentration was determined by using an automated glucose analyzer (YSI 2300 STAT plus, Yellow Spring Instrument Co., Yellow Springs, OH). Plasma insulin was measured by using a chemiluminescent immunoassay method (Immulite 1000, Diagnostic Products Corporation, Los Angeles, CA). Plasma high-density lipoprotein (HDL) cholesterol was determined by using commercially available assays. Low-density lipoprotein (LDL) cholesterol was calculated by the equation of Friedewald and colleagues (26). Plasma free fatty acid (FFA) concentrations were quantified by gas chromatography (HP 5890 Series II GC, Hewlett-Packard, Palo Alto, CA) (27). Plasma VLDL-TG concentration was determined by using a colorimetric enzymatic kit (SIGMA Chemicals, St. Louis, MO), and VLDL-apoB-100 concentration by using a turbidimetric immunoassay (Wako Pure Chemical Industries, Osaka, Japan). Plasma free glycerol, glucose, palmitate and leucine TTRs, glycerol and palmitate TTRs in VLDL-TG, and leucine TTR in VLDL-apoB-100 were determined by gas chromatography - mass spectrometry (Agilent Technologies/HP 6890 Series GC System – 5973 Mass Selective Detector, Hewlett-Packard, Palo Alto, CA), as described previously (24,25,27–29).
Calculations
Glucose, glycerol, and palmitate kinetics
Isotopic steady-state conditions were achieved during the final 30 min of the basal period and stages 1 and 2 of the clamp procedure; Steele’s equation for steady-state conditions (30) was therefore used to calculate substrate kinetics. Endogenous rate of appearance (Ra) of glucose, palmitate and glycerol in plasma was calculated by dividing the respective tracer infusion rate by the average plasma substrate TTR during the last 30 min of the basal period and stages 1 and 2 of the clamp procedure. Glucose rate of disappearance (Rd) was calculated as the sum of endogenous glucose Ra and the infusion rate of exogenous glucose. The hepatic insulin sensitivity index was calculated as the inverse of the product of basal hepatic glucose Ra and fasting plasma insulin concentration (31).
VLDL-TG and VLDL-apoB-100 kinetics
The fractional turnover rate (FTR) of VLDL-TG was determined by fitting the TTR time-courses of free glycerol in plasma and glycerol in VLDL-TG to a compartmental model (28). The total rate of VLDL-TG secretion (in µmol/l·min), which represents the amount of VLDL-TG secreted by the liver per unit of plasma, was calculated by multiplying the FTR of VLDL-TG (in pools/min) by the steady-state plasma VLDL-TG concentration (in µmol/l). The plasma clearance rate of VLDL-TG (in ml/min) was calculated as the production rate (in µmol/min) divided by the plasma concentration (in µmol/ml).
The relative contributions of systemic plasma FFA and non-systemic fatty acids to total VLDL-TG production were calculated by fitting palmitate TTR in plasma and VLDL-TG to a compartmental model (24,28,32,33). This model provides an estimate of the extent of dilution of systemic plasma FFA by unlabeled non-systemic sources of palmitate before being incorporated into VLDL-TG. These non-systemic fatty acids are derived from pools of fatty acids that are not labeled with tracer during the palmitate tracer infusion study, and include: 1) fatty acids released from pre-existing, slowly turning over TG stores in the liver and visceral adipose tissue which releases fatty acids directly into the portal vein, 2) fatty acids derived from local lipolysis of plasma lipoproteins that are taken up by the liver without mixing with the systemic plasma pool, and 3) fatty acids derived from hepatic de novo lipogenesis (34).
The FTR of VLDL-apoB-100 was calculated by fitting the TTR time-courses of free leucine in plasma and leucine in VLDL-apoB-100 to a compartmental model (24,25). The rate of VLDL-apoB-100 secretion and the plasma clearance rate of VLDL-apoB-100 were calculated based on plasma VLDL-apoB-100 concentration and VLDL-apoB-100 FTR as described above for VLDL-TG. A molecular mass of 512723 g/mol for apoB-100 was used for unit conversions (35). The kinetic parameters of VLDL-apoB-100 are indices of the secretion rate and plasma clearance rate of VLDL particles, because each VLDL particle contains a single molecule of apoB-100 (36).
Statistical analysis
All data sets were tested for normality according to the Kolmogorov-Smirnov procedure. Not-normally distributed variables were log-transformed for analysis and back-transformed for presentation as means and 95% confidence intervals. Results for the remaining parameters are presented as means ± SEM. Differences between groups were examined by using Student’s independent t test, preceded by Levene’s test to assess the equality of group variances on each dependent variable. Relationships between variables of interest were evaluated with correlation analysis. A P-value < 0.05 was considered statistically significant. Analyses were performed by using SPSS version 17 (SPSS, Chicago, IL)
Based on an in-house assessment of intra-individual variability in basal VLDL-TG and VLDL-apoB-100 kinetics and in glucose kinetics during both basal conditions and insulin infusion, determined by studying obese persons on two separate occasions under identical conditions, a sample size of 10 subjects per group would allow us to detect between-group differences of 12% in plasma VLDL-TG concentration, 19–31% in VLDL-TG kinetics, 15–20% in plasma VLDL-apoB-100 concentration and kinetics, 10–17% in basal glucose and FFA kinetics, and 17–26% in hepatic, adipose tissue and skeletal muscle insulin sensitivity, with an alpha value of 0.05 and power of 80% (beta = 0.2) for two-sided tests.
RESULTS
Body composition and plasma insulin and substrate concentrations
All measures of adiposity (total body fat, percent body fat, total abdominal fat, abdominal subcutaneous fat, and intra-abdominal fat) were greater in subjects with class III than those with class I obesity (Table 1). By design, IHTG same was the same in both groups (P = 0.879). There were no significant differences between groups in plasma insulin and substrate concentrations (Table 2).
Table 1.
Body composition of study subjects
| Class I obese | Class III obese | |
|---|---|---|
| Body mass index (kg/m2) | 31.6 ± 0.3 | 41.5 ± 0.5* |
| Weight (kg) | 91.3 ± 3.1 | 115.8 ± 1.5* |
| Body fat (%) | 37.2 ± 1.9 | 42.5 ± 2.0* |
| Fat mass (kg) | 33.0 ± 2.2 | 48.6 ± 2.4* |
| Fat-free mass (kg) | 55.6 ± 2.6 | 65.8 ± 2.5* |
| Total abdominal fat (cm3) | 4123 ± 227 | 6442 ± 153* |
| Intra-abdominal fat (cm3) | 1225 ± 144 | 2121 ± 378* |
| Subcutaneous abdominal fat (cm3) | 2898 ± 280 | 4428 ± 317* |
| Intrahepatic triglyceride content (%) | 13.7 ± 3.8 | 14.4 ± 3.4 |
Values are means ± SEM.
Value is significantly different from class I obese subjects, P < 0.05.
Table 2.
Plasma insulin and substrate concentrations
| Class I obese | Class III obese | |
|---|---|---|
| Insulin (µU/ml) | 14 (11, 18) | 16 (9, 28) |
| Glucose (mg/dl) | 98 ± 2 | 96 ± 3 |
| Free fatty acids (mmol/l) | 0.42 ± 0.03 | 0.50 ± 0.04 |
| VLDL-TG (mg/dl) | 59 ± 12 | 66 ± 16 |
| VLDL-apoB-100 (mg/dl) | 4.7 ± 0.9 | 5.1 ± 1.0 |
| HDL-cholesterol (mg/dl) | 45 ± 4 | 44 ± 5 |
| LDL-cholesterol (mg/dl) | 93 ± 8 | 93 ± 11 |
Values are means ± SEM, except for plasma insulin concentrations which are means and 95% confidence intervals. There are no significant differences between groups. VLDL, very low-density lipoprotein; TG, triglyceride; apoB-100, apolipoprotein B-100; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Basal substrate kinetics and insulin sensitivity
Basal whole-body glycerol Ra and palmitate Ra were ~55% greater in class III obese (332 ± 33 and 158 ± 13 µmol/min, respectively) than in class I obese (206 ± 21 and 106 ± 11 µmol/min, respectively) subjects (P < 0.01 for both). However expressing glycerol Ra and palmitate Ra per kg of fat mass eliminated the differences between groups (class III obese: 5 6.9 ± 0.7 and 3.3 ± 0.3 µmol/kg fat mass·min for palmitate Ra and glycerol Ra, respectively; class I obese: 6.4 ± 0.7 and 3.3 ± 0.4 µmol/kg fat mass·min, respectively; P > 0.58 for both). Basal endogenous glucose Ra in subjects with class III obesity was not different from that obtained in subjects with class I obesity (846 ± 32 and 824 ± 47 µmol/min, respectively; P = 0.692).
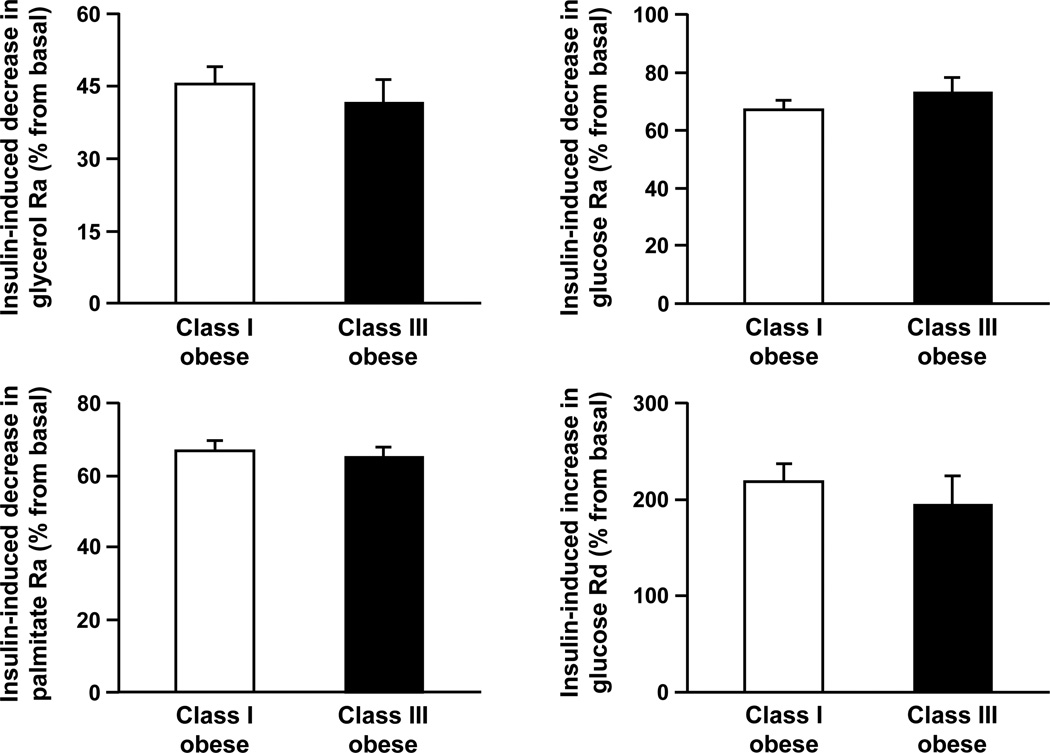
Plasma insulin concentrations during the clamp procedure were similar in subjects with class I and III obesity (stage 1: 48 ± 2 and 49 ± 5 µU/ml, respectively, P = 0.753; stage 2: 113 ± 5 and 112 ± 8 µU/ml, respectively, P = 0.951). There were no significant differences between groups in insulin-mediated suppression of glycerol Ra (P = 0.516), palmitate Ra (P = 0.635), and glucose Ra (P = 0.348) during stage 1, and insulin-mediated stimulation of glucose Rd (P = 0.489) during stage 2 of the clamp procedure (Figure 1). The hepatic insulin sensitivity index was 0.49 (0.38, 0.62) and 0.48 (0.26, 0.90) in subjects with class I and class III obesity, respectively (P = 0.995).
Figure 1.
Insulin-induced suppression of glycerol, palmitate and glucose rates of appearance (Ra) and insulin-induced stimulation of glucose rate of disappearance (Rd) in subjects with class I and III obesity, matched on intrahepatic triglyceride content. Values are means ± SEM. There are no significant differences between groups.
VLDL kinetics
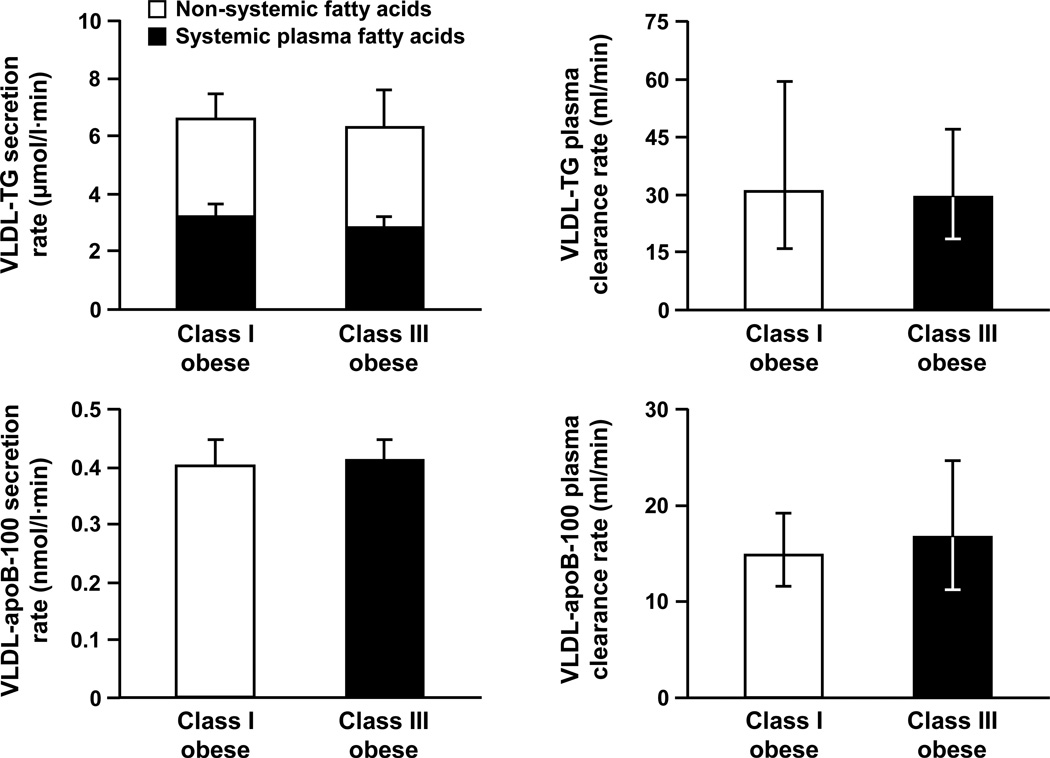
There were no significant differences between groups in hepatic VLDL-TG (P = 0.787) and VLDL-apoB-100 (P = 0.866) secretion rates (Figure 2). The relative contribution of systemic and non-systemic sources of fatty acids to total VLDL-TG production did not differ between groups (P = 0.628): systemic plasma FFA accounted for 57 ± 8% of all fatty acids in VLDL-TG in class I obese subjects and for 52 ± 7% in class III obese subjects; non-systemic fatty acids contributed 43 ± 8% and 48 ± 7%, respectively. Therefore, there were no significant differences between groups in the absolute secretion rates of VLDL-TG derived from either systemic plasma FFA (P = 0.461) or non-systemic fatty acids (P = 0.918) (Figure 2). The plasma clearance rates of VLDL-TG (P = 0.888) and VLDL-apoB-100 (P = 0.608) were not significantly different between groups (Figure 2).
Figure 2.
Basal very low density lipoprotein (VLDL) triglyceride (TG) and apolipoprotein B-100 (apoB-100) kinetics in subjects with class I and III obesity, matched on intrahepatic triglyceride content. Values are means ± SEM, except for plasma clearance rates which are means and 95% confidence intervals. There are no significant differences between groups.
Relationship between IHTG content and metabolic function
For the whole group of subjects (n = 20), and consistent with our previous observations 10 (11,12,19), IHTG content was directly correlated with VLDL-TG secretion rate (r = 0.462, P = 0.040) and basal plasma FFA Ra (r = 0.513, P = 0.021), inversely correlated with the hepatic insulin sensitivity index (r = −0.576, P = 0.008), inversely correlated with insulin-mediated stimulation of glucose Rd (skeletal muscle insulin sensitivity) (r = −0.627, P = 0.003), and tended to correlate inversely with insulin-mediated suppression of FFA Ra (adipose tissue insulin sensitivity) (r = −0.406, P = 0.076).
DISCUSSION
An increase in BMI is often associated with an increase in IHTG content (14–18) and obesity-related metabolic complications (5–8). In the present study we evaluated the importance of an increase in BMI and adiposity on insulin action and VLDL kinetics independent of any changes in IHTG. The novel finding from our study is that the increase in metabolic dysfunction associated with an increase in whole-body adiposity and BMI (5–8) does not occur without a concomitant increase in liver fat. Despite considerable differences in BMI values, body fat mass, and visceral adipose tissue (VAT) mass in our subjects with class I and class III obesity, there were no significant differences in liver, skeletal muscle, and adipose tissue insulin sensitivity or VLDL kinetics between groups who were matched on IHTG content. These results support the notion that IHTG is an important marker of obese metabolic function (10–13) and demonstrate that an increase in BMI and body fat mass alone do not necessarily cause an increase in metabolic abnormalities.
Our findings extend the observations reported from a series of previous studies that evaluated the inverse paradigm of our study design. In those studies, the importance of an increase in IHTG content on insulin action and VLDL kinetics was assessed when BMI and percent body fat were kept constant (19,37–41). The results were consistent across studies, and demonstrated that increased IHTG content is associated with multi-organ insulin resistance and increased VLDL-TG secretion rate among subjects with the same BMI and percent body fat. Moreover, there was a direct relationship between the amount of IHTG and the degree of insulin resistance in liver, skeletal muscle and adipose tissue (12,37,38) and hepatic VLDL-TG and VLDL-apoB-100 secretion rate (10,19). We also found that IHTG content was directly associated with metabolic dysregulation in our group of subjects, which supports our conclusion that increasing whole-body adiposity does not cause additional metabolic abnormalities without a concomitant increase in IHTG.
Increased VAT mass is also associated with metabolic dysfunction, particularly insulin resistance and dyslipidemia (42–44). However, VAT often correlates with IHTG content (10,12,38,41,45), so it is possible that VAT is associated with metabolic dysfunction because of its relationship with IHTG. The selection of our subjects resulted in a dissociation between VAT and IHTG content; those with class III obesity had nearly twice the volume of VAT as those with class I obesity despite having the same amount of IHTG. Therefore, our data demonstrate that increased VAT mass is not associated with increased metabolic dysfunction when IHTG does not also increase. These findings are consistent with data from previous studies that found IHTG content is more closely associated with insulin resistance (12,13,38) and VLDL-TG and VLDL-apoB-100 production (10) than VAT. Moreover, we recently found no differences in multi-organ insulin sensitivity and VLDL-TG secretion rate in obese subjects matched on IHTG content but who differed markedly in VAT, whereas both insulin resistance and VLDL-TG secretion rate were greater in obese subjects who had high rather than normal IHTG content, but matched on VAT (11). These data suggest that the association between VAT and metabolic dysfunction reported previously (42–44) is not likely a causal relationship. In fact, the hypothesis that excessive release of fatty acids or adipokines from VAT is responsible for the insulin resistance associated with visceral adiposity has not been supported by data from studies conducted in obese people that evaluated the contribution of VAT to portal vein and systemic fatty acid flux (46,47) and portal vein adipokine concentrations (48). Therefore, the summation of these data underscores the importance of IHTG content as a marker of abnormal insulin action and VLDL metabolism, and helps explain why patients with NAFLD have such a high prevalence rate of diabetes and dyslipidemia (49). However, it is not known whether the relationship between excessive IHTG content and metabolic dysfunction is causal or a simple association.
The absence of an increase in metabolic dysfunction despite an increase in adiposity in our study subjects does not mean that adipose tissue, itself, does not contribute to the metabolic abnormalities associated with obesity. Our data are consistent with the idea that the specific characteristics of adipose tissue are more important than the amount of body fat in determining the risk of obesity-related metabolic disease. Accordingly, increased fat cell size (50), increased adipose tissue lipolytic activity (51), adipose tissue inflammatory cell infiltration (52), adipose tissue hypoxia (53), and adipose tissue endoplasmic reticulum stress (54) are associated with insulin resistance. In addition, an accumulation of femoral and gluteal fat is cardioprotective and lower body obesity is associated with decreased risk of metabolic disease (55). Our data suggest that the accumulation of ectopic fat in other organs, particularly the liver, might be a marker of adipose tissue pathology.
In summary, we found that a marked increase in BMI, total body fat and VAT is not associated with increased insulin resistance or alterations in VLDL-TG and VLDL-apoB-100 metabolism in obese subjects when there is no concomitant increase in IHTG content. These results have important physiological and clinical implications because they indicate that liver fat modifies the metabolic risk associated with progressively increasing BMI values. Additional studies are needed to determine whether excessive IHTG, itself, causes metabolic dysfunction and should therefore be a distinct therapeutic target in obese patients.
ACKNOWLEDGEMENTS
The authors thank Jennifer McCrea, Adewole Okunade, Freida Custodio and Jennifer Shew for technical assistance, the staff of the Clinical Research Unit for their help in performing the studies, and the study subjects for their participation.
This study was supported by National Institutes of Health grants DK 37948, DK 56341 (Clinical Nutrition Research Unit), RR024992 (Clinical and Translational Science Award), and RR-00954 (Biomedical Mass Spectrometry Resource), and grant 0510015Z from the American Heart Association.
Footnotes
Disclosure: There are no financial conflicts with the subject matter or materials discussed in this manuscript with any of the authors.
REFERENCES
- 1.Klein S, Wadden T, Sugerman HJ. AGA technical review on obesity. Gastroenterology. 2002;123:882–932. doi: 10.1053/gast.2002.35514. [DOI] [PubMed] [Google Scholar]
- 2.National Institutes of Health. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults - The Evidence Report. Obes Res. 1998;6 Suppl 2:51S–209S. [PubMed] [Google Scholar]
- 3.Gallagher D, Heymsfield SB, Heo M, Jebb SA, Murgatroyd PR, Sakamoto Y. Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. Am J Clin Nutr. 2000;72:694–701. doi: 10.1093/ajcn/72.3.694. [DOI] [PubMed] [Google Scholar]
- 4.Gallagher D, Visser M, Sepulveda D, Pierson RN, Harris T, Heymsfield SB. How useful is body mass index for comparison of body fatness across, age, sex, and ethnic groups? Am J Epidemiol. 1996;143:228–239. doi: 10.1093/oxfordjournals.aje.a008733. [DOI] [PubMed] [Google Scholar]
- 5.Colditz GA, Willett WC, Stampfer MJ, Manson JE, Hennekens CH, Arky RA, et al. Weight as a risk factor for clinical diabetes in women. Am J Epidemiol. 1990;132:501–513. doi: 10.1093/oxfordjournals.aje.a115686. [DOI] [PubMed] [Google Scholar]
- 6.Esteghamati A, Khalilzadeh O, Anvari M, Ahadi MS, Abbasi M, Rashidi A. Metabolic syndrome and insulin resistance significantly correlate with body mass index. Arch Med Res. 2008;39:803–808. doi: 10.1016/j.arcmed.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Ferrannini E, Natali A, Bell P, Cavallo-Perin P, Lalic N, Mingrone G. Insulin resistance and hypersecretion in obesity. European Group for the Study of Insulin Resistance (EGIR) J Clin Invest. 1997;100:1166–1173. doi: 10.1172/JCI119628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris MI, Flegal KM, Cowie CC, Eberhardt MS, Goldstein DE, Little RR, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988–1994. Diabetes Care. 1998;21:518–524. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- 9.Sumner AE. The relationship of body fat to metabolic disease: influence of sex and ethnicity. Gend Med. 2008;5:361–371. doi: 10.1016/j.genm.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adiels M, Taskinen MR, Packard C, Caslake MJ, Soro-Paavonen A, Westerbacka J, et al. Overproduction of large VLDL particles is driven by increased liver fat content in man. Diabetologia. 2006;49:755–765. doi: 10.1007/s00125-005-0125-z. [DOI] [PubMed] [Google Scholar]
- 11.Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW, et al. Intrahepatic, fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci U S A. 2009;106:15430–15435. doi: 10.1073/pnas.0904944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korenblat KM, Fabbrini E, Mohammed BS, Klein S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology. 2008;134:1369–1375. doi: 10.1053/j.gastro.2008.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stefan N, Kantartzis K, Machann J, Schick F, Thamer C, Rittig K, et al. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med. 2008;168:1609–1616. doi: 10.1001/archinte.168.15.1609. [DOI] [PubMed] [Google Scholar]
- 14.Perseghin G, Lattuada G, De Cobelli F, Ragogna F, Ntali G, Esposito A, et al. Habitual physical activity is associated with intrahepatic fat content in humans. Diabetes Care. 2007;30:683–688. doi: 10.2337/dc06-2032. [DOI] [PubMed] [Google Scholar]
- 15.Sabir N, Sermez Y, Kazil S, Zencir M. Correlation of abdominal fat accumulation and liver steatosis: importance of ultrasonographic and anthropometric measurements. Eur J Ultrasound. 2001;14:121–128. doi: 10.1016/s0929-8266(01)00153-7. [DOI] [PubMed] [Google Scholar]
- 16.Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–E468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 17.Church TS, Kuk JL, Ross R, Priest EL, Biltoft E, Blair SN. Association of cardiorespiratory fitness, body mass index, and waist circumference to nonalcoholic fatty liver disease. Gastroenterology. 2006;130:2023–2030. doi: 10.1053/j.gastro.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 18.Thomas EL, Hamilton G, Patel N, O'Dwyer R, Dore CJ, Goldin RD, et al. Hepatic triglyceride content and its relation to body adiposity: a magnetic resonance imaging and proton magnetic resonance spectroscopy study. Gut. 2005;54:122–127. doi: 10.1136/gut.2003.036566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fabbrini E, Mohammed BS, Magkos F, Korenblat KM, Patterson BW, Klein S. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology. 2008;134:424–431. doi: 10.1053/j.gastro.2007.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Genton L, Hans D, Kyle UG, Pichard C. Dual-energy X-ray absorptiometry and body composition: differences between devices and comparison with reference methods. Nutrition. 2002;18:66–70. doi: 10.1016/s0899-9007(01)00700-6. [DOI] [PubMed] [Google Scholar]
- 21.Abate N, Burns D, Peshock RM, Garg A, Grundy SM. Estimation of adipose tissue mass by magnetic resonance imaging: validation against dissection in human cadavers. J Lipid Res. 1994;35:1490–1496. [PubMed] [Google Scholar]
- 22.Frimel TN, Deivanayagam S, Bashir A, O'Connor R, Klein S. Assessment of intrahepatic triglyceride content using magnetic resonance spectroscopy. J Cardiometab Syndr. 2007;2:136–138. doi: 10.1111/j.1559-4564.2007.07168.x. [DOI] [PubMed] [Google Scholar]
- 23.Groop LC, Bonadonna RC, DelPrato S, Ratheiser K, Zyck K, Ferrannini E, et al. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J Clin Invest. 1989;84:205–213. doi: 10.1172/JCI114142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Magkos F, Patterson BW, Mittendorfer B. Reproducibility of stable isotope-labeled tracer measures of VLDL-triglyceride and VLDL-apolipoprotein B-100 kinetics. J Lipid Res. 2007;48:1204–1211. doi: 10.1194/jlr.D600048-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Mittendorfer B, Patterson BW, Klein S. Effect of weight loss on VLDL-triglyceride and apoB-100 kinetics in women with abdominal obesity. Am J Physiol Endocrinol Metab. 2003;284:E549–E556. doi: 10.1152/ajpendo.00379.2002. [DOI] [PubMed] [Google Scholar]
- 26.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 27.Patterson BW, Zhao G, Elias N, Hachey DL, Klein S. Validation of a new procedure to determine plasma fatty acid concentration and isotopic enrichment. J Lipid Res. 1999;40:2118–2124. [PubMed] [Google Scholar]
- 28.Patterson BW, Mittendorfer B, Elias N, Satyanarayana R, Klein S. Use of stable isotopically labeled tracers to measure very low density lipoprotein-triglyceride turnover. J Lipid Res. 2002;43:223–233. [PubMed] [Google Scholar]
- 29.Klein S, Mittendorfer B, Eagon JC, Patterson B, Grant L, Feirt N, et al. Gastric bypass surgery improves metabolic and hepatic abnormalities associated with nonalcoholic fatty liver disease. Gastroenterology. 2006;130:1564–1572. doi: 10.1053/j.gastro.2006.01.042. [DOI] [PubMed] [Google Scholar]
- 30.Steele R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci. 1959;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- 31.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 32.Magkos F, Mittendorfer B. Stable isotope-labeled tracers for the investigation of fatty acid and triglyceride metabolism in humans in vivo. Clin Lipidol. 2009;4:215–230. doi: 10.2217/clp.09.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Magkos F, Patterson BW, Mohammed BS, Klein S, Mittendorfer B. Women produce fewer but triglyceride-richer very low-density lipoproteins than men. J Clin Endocrinol Metab. 2007;92:1311–1318. doi: 10.1210/jc.2006-2215. [DOI] [PubMed] [Google Scholar]
- 34.Lewis GF. Fatty acid regulation of very low density lipoprotein production. Curr Opin Lipidol. 1997;8:146–153. doi: 10.1097/00041433-199706000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Law SW, Grant SM, Higuchi K, Hospattankar A, Lackner K, Lee N, et al. Human liver apolipoprotein B-100 cDNA: complete nucleic acid and derived amino acid sequence. Proc Natl Acad Sci U S A. 1986;83:8142–8146. doi: 10.1073/pnas.83.21.8142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elovson J, Chatterton JE, Bell GT, Schumaker VN, Reuben MA, Puppione DL, et al. Plasma very low density lipoproteins contain a single molecule of apolipoprotein B. J Lipid Res. 1988;29:1461–1473. [PubMed] [Google Scholar]
- 37.Seppala-Lindroos A, Vehkavaara S, Hakkinen AM, Goto T, Westerbacka J, Sovijarvi A, et al. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab. 2002;87:3023–3028. doi: 10.1210/jcem.87.7.8638. [DOI] [PubMed] [Google Scholar]
- 38.Hwang JH, Stein DT, Barzilai N, Cui MH, Tonelli J, Kishore P, et al. Increased intrahepatic triglyceride is associated with peripheral insulin resistance: in vivo MR imaging and spectroscopy studies. Am J Physiol Endocrinol Metab. 2007;293:E1663–E1669. doi: 10.1152/ajpendo.00590.2006. [DOI] [PubMed] [Google Scholar]
- 39.Bugianesi E, Gastaldelli A, Vanni E, Gambino R, Cassader M, Baldi S, et al. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms. Diabetologia. 2005;48:634–642. doi: 10.1007/s00125-005-1682-x. [DOI] [PubMed] [Google Scholar]
- 40.Kotronen A, Juurinen L, Tiikkainen M, Vehkavaara S, Yki-Jarvinen H. Increased liver fat, impaired insulin clearance, and hepatic and adipose tissue insulin resistance in type 2 diabetes. Gastroenterology. 2008;135:122–130. doi: 10.1053/j.gastro.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 41.Deivanayagam S, Mohammed BS, Vitola BE, Naguib GH, Keshen TH, Kirk EP, et al. Nonalcoholic fatty liver disease is associated with hepatic and skeletal muscle insulin resistance in overweight adolescents. Am J Clin Nutr. 2008;88:257–262. doi: 10.1093/ajcn/88.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ross R, Aru J, Freeman J, Hudson R, Janssen I. Abdominal adiposity and insulin resistance in obese men. Am J Physiol Endocrinol Metab. 2002;282:E657–E663. doi: 10.1152/ajpendo.00469.2001. [DOI] [PubMed] [Google Scholar]
- 43.Ross R, Freeman J, Hudson R, Janssen I. Abdominal obesity, muscle composition, and insulin resistance in premenopausal women. J Clin Endocrinol Metab. 2002;87:5044–5051. doi: 10.1210/jc.2002-020570. [DOI] [PubMed] [Google Scholar]
- 44.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 45.Jakobsen MU, Berentzen T, Sorensen TI, Overvad K. Abdominal obesity and fatty liver. Epidemiol Rev. 2007;29:77–87. doi: 10.1093/epirev/mxm002. [DOI] [PubMed] [Google Scholar]
- 46.Klein S. The case of visceral fat: argument for the defense. J Clin Invest. 2004;113:1530–1532. doi: 10.1172/JCI22028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. J Clin Invest. 2004;113:1582–1588. doi: 10.1172/JCI21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56:1010–1013. doi: 10.2337/db06-1656. [DOI] [PubMed] [Google Scholar]
- 49.Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 50.Weyer C, Foley JE, Bogardus C, Tataranni PA, Pratley RE. Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts type II diabetes independent of insulin resistance. Diabetologia. 2000;43:1498–1506. doi: 10.1007/s001250051560. [DOI] [PubMed] [Google Scholar]
- 51.Martin ML, Jensen MD. Effects of body fat distribution on regional lipolysis in obesity. J Clin Invest. 1991;88:609–613. doi: 10.1172/JCI115345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 53.Stuart Wood I, de Heredia FP, Wang B, Trayhurn P. Cellular hypoxia and adipose tissue dysfunction in obesity. Proc Nutr Soc. 2009;68:370–377. doi: 10.1017/S0029665109990206. [DOI] [PubMed] [Google Scholar]
- 54.Gregor MF, Yang L, Fabbrini E, Mohammed BS, Eagon JC, Hotamisligil GS, et al. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes. 2009;58:693–700. doi: 10.2337/db08-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lemieux I. Energy partitioning in gluteal-femoral fat: does the metabolic fate of triglycerides affect coronary heart disease risk? Arterioscler Thromb Vasc Biol. 2004;24:795–797. doi: 10.1161/01.ATV.0000126485.80373.33. [DOI] [PubMed] [Google Scholar]