Abstract
The hypothesis that schizophrenia results from a developmental, as opposed to a degenerative, process affecting the connectivity and network plasticity of the cerebral cortex is supported by findings from morphological and molecular postmortem studies. Specifically, abnormalities in the expression of protein markers of GABA neurotransmission and the lamina- and circuit-specificity of these changes in the cortex in schizophrenia, in concert with knowledge of their developmental trajectories, offer crucial insight into the vulnerability of specific cortical networks to environmental insults during different periods of development. These findings reveal potential targets for therapeutic interventions to improve cognitive function in individuals with schizophrenia, and provide guidance for future preventive strategies to preserve cortical neurotransmission in at-risk individuals.
Keywords: GABA, Glutamate, Neurotransmission, Development
1. Introduction
In the central nervous system, neuronal proliferation, cell migration, morphological and biochemical differentiation, and circuit formation rely on complex intracellular and cell–environmental interactions that control particular developmental processes. Such interactions provide different ways in which disturbances at one point in development may establish an altered trajectory for subsequent events, either as a consequence of, or compensation for, the primary alteration that eventually result in a dysfunctional state. In schizophrenia research, multiple lines of evidence support the idea that certain disturbances at specific time points during post-natal brain maturation contribute to the underlying disease process. Because the psychotic features of schizophrenia typically emerge in late adolescence or young adulthood, the substantial remodeling of cortical connections, especially in the dorsolateral prefrontal cortex (DLPFC), during this developmental period is thought to be critical (Harris et al., 2009).
Among the many clinical traits of schizophrenia, disturbances in certain cognitive processes, such as attention, context representation, and working memory represent the core features of the illness (Weinberger et al., 1986; Goldman-Rakic, 1994). Some of these cognitive deficits seem to reflect abnormal activation of the DLPFC in patients with schizophrenia, as such individuals tend to either perform poorly on working memory tasks and show reduced DLPFC activation (Weinberger et al., 1986; Perlstein et al., 2001), or perform tasks normally with increased DLPFC activation (Callicott et al., 2003). Interestingly, the functional maturation of primate DLPFC circuitry is associated with a progressive improvement of working memory performance through adolescence; indeed, the improvement in working memory performance with age depends on a progressively greater engagement of DLPFC circuitry (Alexander and Goldman, 1978; Luna et al., 2004; Crone et al., 2006). At the neuronal level, working memory performance depends on the coordinated and sustained firing of subsets of DLPFC pyramidal neurons between the temporary presentation of a stimulus cue and the later initiation of a behavioral response (Goldman-Rakic, 1995). The fine tuning and timing of excitatory synaptic activation in the DLPFC during working memory tasks have been attributed to specific inhibitory interneurons (Sawaguchi et al., 1989; Rao et al., 2000). Furthermore, several converging findings suggest that, in addition to mediating synaptic inhibition in mature circuits, GABA signaling promotes and coordinates the activity-mediated pre- and postsynaptic maturation of neuronal networks (Huang, 2009). Consequently, the working memory impairments in schizophrenia might reflect disturbances in the normal development of the DLPFC (Weinberger, 1987; Lewis and Levitt, 2002).
In the following sections we present an overview of postmortem findings of altered markers of inhibitory circuits in the DLPFC in schizophrenia and how their normal developmental trajectories provide insights into the neurodevelopmental nature of the pathology.
2. Inhibitory cortical circuits in schizophrenia
Substantial evidence suggests that dysfunction of inhibitory neurotransmission in the DLPFC appears to contribute to the cognitive deficits observed in subjects with schizophrenia (Lewis et al., 2005). Early postmortem studies suggested decreased activity for the enzyme responsible for GABA synthesis, glutamic acid decarboxylase (GAD) in schizophrenia patients (Bird et al., 1979). Despite the fact that a postmortem interval effect on GAD enzymatic activity was not excluded in this earlier study, multiple recent reports, using different techniques, consistently found lower expression levels of the 67-kDa isoform (GAD67) in the DLPFC (Akbarian et al., 1995b; Guidotti et al., 2000; Mirnics et al., 2000; Volk et al., 2000; Vawter et al., 2002; Hashimoto et al., 2003, 2005, 2008a; Straub et al., 2007; Thompson et al., 2009; Duncan et al., 2010), and other neocortical regions of subjects with schizophrenia (Woo et al., 2004; Akbarian and Huang, 2006; Hashimoto et al., 2008b). Of the two GABA synthesizing enzymes, GAD65 and GAD67, GAD67 accounts for the most GABA synthesis, influencing cellular GABA content in a gene dosage-dependent manner (Asada et al., 1997; Ji et al., 1999). However, the functional consequences of a deficit in inhibitory neurotransmission in schizophrenia are determined by whether all or a specific subpopulation(s) of GABA neurons are affected. At the cellular level, ~25–35% of GABA neurons in layers 2–5 of the DLPFC show undetectable expression of GAD67 mRNA, while the remaining GABA neurons exhibit normal levels (Akbarian et al., 1995b; Volk et al., 2000). Interestingly, the expression of GAD67 mRNA is not altered in DLPFC layer 6 of subjects with schizophrenia (Akbarian et al., 1995a; Volk et al., 2000), supporting the idea of a laminar- and circuit-specificity of altered GABA neurotransmission. Additionally, mRNA expression levels for the GABA membrane transporter (GAT1), protein responsible for presynaptic reuptake of released GABA, are also lower (Ohnuma et al., 1999) in a similar minority of GABA neurons (Volk et al., 2001). These findings suggest that both the synthesis and reuptake of GABA are altered in a subset of DLPFC inhibitory interneurons indicating a cell type/input-specific disturbance in GABA neurotransmission in schizophrenia (Fig. 1).
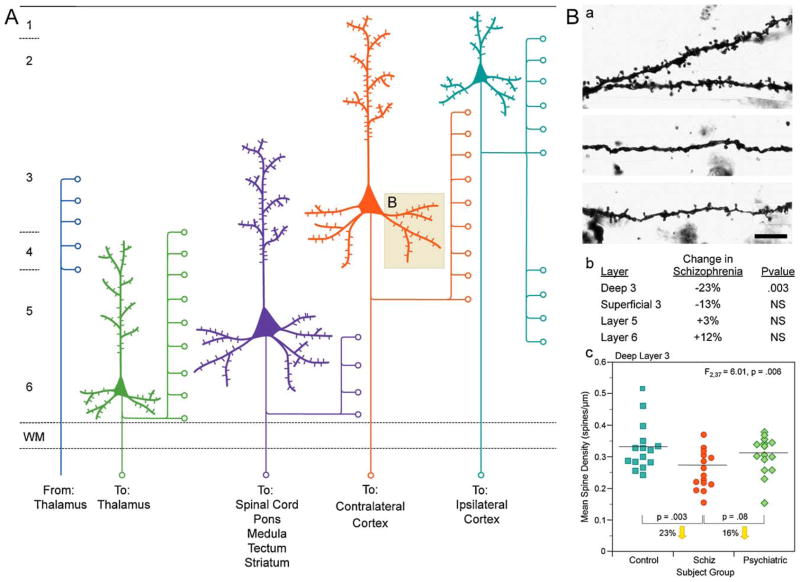
Fig. 1.
(A) Schematic representation of the main afferent and efferent pyramidal connections to and from specific cortical lamina. (B) Reduction in pyramidal neuron dendritic spines in deep layer 3 of the DLPFC in schizophrenia. (a) Golgi-impregnated basilar dendrites and spines on deep layer 3 pyramidal neurons from a normal comparison (top) and two subjects with schizophrenia (bottom). Note the reduced density of spines in the subjects with schizophrenia in these extreme examples. (b) Laminar specificity of the spine density differences in the subjects with schizophrenia relative to normal control subjects. (c) Scatter plot demonstrating the lower density of spines on the basilar dendrites of deep layer 3 pyramidal neurons in the DLPFC of subjects with schizophrenia relative to both normal and psychiatrically ill comparison subjects.
Adapted from Lewis and Gonzalez-Burgos (2008).
2.1. Lamina-specific alterations of pre- and postsynaptic markers of specific GABA circuits
The group of inhibitory GABA neurons in the primate cerebral cortex, including the DLPFC, is very heterogeneous but can be grouped into different neuronal subclasses according to a combination of specific molecular, electrophysiological, and anatomical properties. One of the molecular criteria used to distinguish cortical interneuron subpopulations is expression of the Ca2+-binding proteins, PV and calretinin (CR), and the neuropeptides somatostatin (SST) and cholecystokinin (CCK) (Conde et al., 1994; Gabbott and Bacon, 1996; DeFelipe, 1997). Furthermore, these neuronal subtypes exhibit different electrophysiological properties (Kawaguchi and Kubota, 1993; Krimer and Goldman-Rakic, 2001; Krimer et al., 2005; Zaitsev et al., 2005) and have axons of different morphological and laminar patterns and specific synaptic targets (Fig. 2) (DeFelipe, 1997).
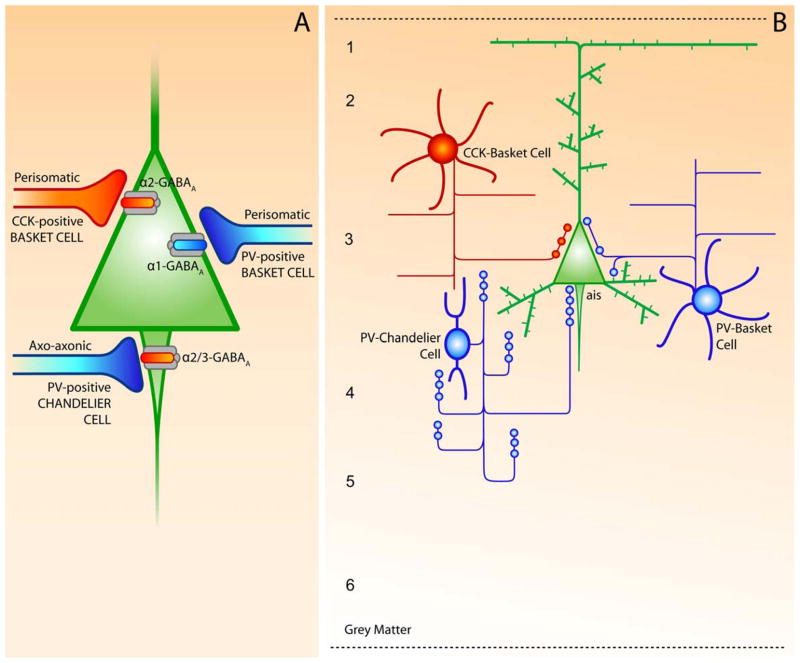
Fig. 2.
(A) Schematic summary of cortical GABA circuits describing the subcellular location and subunit-specificity of the receptors targeted by different GABA neuron subtypes. (B) Morphological and biochemical features of PV- and CCK-positive subpopulations of cortical GABA neurons in the DLPFC. The chandelier and basket neurons provide inhibitory input to the axon initial segment (ais) and the cell body and proximal dendrites, respectively, of pyramidal neurons. 1–4, layers of DLPFC.
Adapted from Lewis et al. (2005).
Both of the PV-positive interneuron subclasses, the basket cells and the chandelier neurons (Lewis and Lund, 1990; Conde et al., 1994), exhibit fast-spiking, non-adapting firing pattern (Gonzalez-Burgos et al., 2005). However, these two subpopulations of PV-positive interneurons differ in the distribution of their axons across cortical layers and in the subcellular location of their terminal contacts onto pyramidal cells. Axons from basket cells spread broadly and contact α1-containing GABAA receptors on the soma and proximal dendrites of pyramidal neurons, whereas chandelier cells project a linear array of axon terminals (termed cartridges) that synapse exclusively on the pyramidal axon initial segments enriched in either α2- or α3-subunit-containing receptors (Nusser et al., 1996; Loup et al., 1998, 2006; Nyiri et al., 2001). The proximity of these two types of perisomatic inhibitory synapses to the site of action potential generation in pyramidal neurons indicates that these GABA neurons specialize in regulating the output of cortical pyramidal neurons (Fig. 2).
At the regional level, several lamina-specific alterations of presynaptic markers of PV-positive interneurons in the DLPFC have been detected in subjects with schizophrenia: lower PV mRNA expression in layers 3 and 4, with no difference in layers 2 and 5 (Hashimoto et al., 2003), lower density of PV-positive varicosities (putative basket cell axon terminals) in layers 3 and 4, but not in layer 2 (Lewis et al., 2001; Lewis and Gonzalez-Burgos, 2008), and lower GAD67 mRNA expression in PV-positive neurons in layers 3 and 4 (Hashimoto et al., 2003). At the postsynaptic level, the laminar distribution of these changes correlates with the laminar pattern of significantly lower expression of α1 mRNA (predominantly in layers 3 and 4) reported in the DLPFC in schizophrenia (Hashimoto et al., 2008a,b; Beneyto et al., 2010) (Fig. 3). This correspondence in the distribution of expression changes in presynaptic and postsynaptic markers suggests altered inputs from the PV-positive basket cell class of GABA neurons to pyramidal neuron cell bodies in schizophrenia. Given that α1, β2, and γ2 subunits co-assemble to form ~60% of GABAA receptors in the adult cortex (Mohler, 2006) in postsynaptic receptors mediating phasic GABA neurotransmission (Farrant and Nusser, 2005), a coordinated difference in their expression levels would be expected. Consistent with this idea, transcript levels for the GABAA receptor β2 and γ2 subunits, which typically assemble with α1 subunits, have also been found reduced in the DLPFC in schizophrenia following an identical laminar pattern (Akbarian et al., 1995a; Huntsman et al., 1998; Hashimoto et al., 2008a; Beneyto et al., 2010). This constellation of findings suggests that the total number of α1β2γ2 GABAA receptors might be lower in the middle layers of the DLPFC and that a coordinated pre- and postsynaptic reduction of phasic GABA neurotransmission from PV-positive basket cells in those layers might affect subjects with schizophrenia.
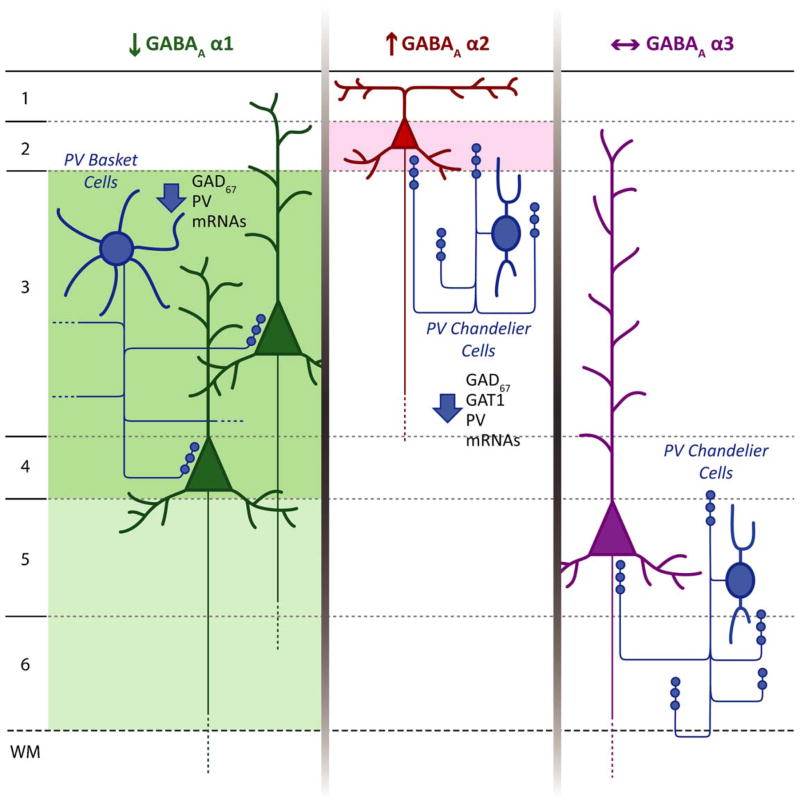
Fig. 3.
Schematic summary of hypothesized circuit-specific transcript alterations in pre- and postsynaptic markers of GABA neurotransmission in the DLPFC of subjects with schizophrenia. For each GABAA α subunit, the background shading marks the cortical layers where the indicated change in expression of that subunit was found. The laminar specificity of the decrease in α1 expression matches that of the alterations in GAD67 and PV mRNAs thought to be present in PV+ basket cells. The increase in α2 expression in layer 2 is consistent with previous findings of pre- and postsynaptic alterations in chandelier cell inputs to the axon initial segment of pyramidal cells in this location. In contrast, the absence of alterations in α3 subunit expression, which is present postsynaptic to chandelier cells in deep layer pyramidal neurons matches the failure to find significant changes in chandelier cell inputs in these layers.
Adapted from Beneyto et al. (2010).
However, α1 subunits can also co-assemble with a δ (instead of γ) subunit (Mertens et al., 1993; Saxena and Macdonald, 1994; Bianchi and Macdonald, 2003); cortical GABAA receptors containing δ subunits are extrasynaptic, have a high affinity for GABA, and mediate tonic inhibition, defined as the constant activation of extrasynaptic receptors that, by increasing input conductance, reduces the probability of generating an action potential (Farrant and Nusser, 2005). In schizophrenia, the laminar pattern of altered α1 mRNA expression changes (Beneyto et al., 2010) matches that exhibited by the δ subunit transcript in the same subjects (Maldonado-Aviles et al., 2009). These findings suggest that schizophrenia might be associated with a reduced complement of two types of α1-containing GABAA receptors in the DLPFC, possibly affecting both synaptic phasic inhibition from PV-positive basket cells (via α1β2γ2 receptors) and extrasynaptic tonic inhibition (via α1βxδ receptors) (Maldonado-Aviles et al., 2009).
Molecular markers of contacts from PV-positive chandelier cells onto pyramidal neurons in the DLPFC are also altered in schizophrenia. Ankyrin-G immunoreactivity in pyramidal neuron axon initial segments (Cruz et al., 2009b), and GAT1 immunoreactivity in chandelier neuron axon cartridges have both been reported to be lower in schizophrenia (Woo et al., 1998; Pierri et al., 1999). Interestingly, these alterations in the DLPFC are correlated with significantly increased protein immunoreactivity (Fig. 4A) (Volk et al., 2002) and transcript expression (Beneyto et al., 2010) of GABAA α2 subunit in schizophrenia, suggesting a chandelier-pyramidal cell connectivity dysfunction. However, in contrast to the laminar distribution of the alterations described for PV-basket cell markers, chandelier findings all preferentially or selectively affect DLPFC layers 2 and superficial 3 of subjects with schizophrenia. Presynaptic alterations of chandelier neuron axon cartridges are less prominent in layers 5–6 (Woo et al., 1998; Pierri et al., 1999), consistent with the un altered expression in schizophrenia of GABAA α3, which is the predominant subunit in the GABAA receptors on the axon initial segment in layers 5–6 (Figs. 2 and 3) (Nusser et al., 1996; Loup et al., 1998, 2006). Together, these findings suggest that an interaction between GABA cell type and laminar location might confer vulnerability in the disease.
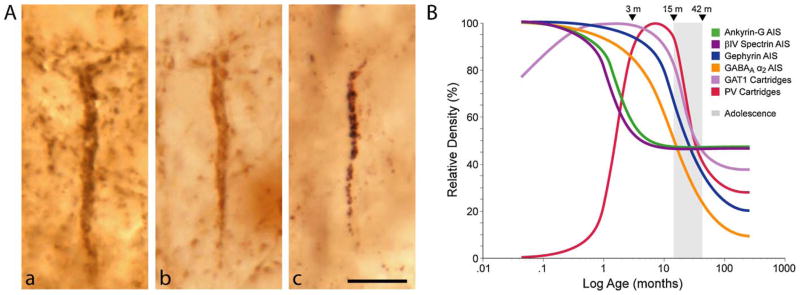
Fig. 4.
(A) Pre- and postsynaptic markers of chandelier neuron inputs to the axon initial segment of pyramidal neurons. (a and b) Immunoreactivity for GAT1 (a) and parvalbumin (b) clearly identifies vertical arrays of chandelier neuron axon terminals (cartridges) that are located below the cell bodies of unlabelled pyramidal neurons. (c) Immunoreactivity for the GABAA α2 subunit is localized postsynaptically, in the axon initial segment of pyramidal neurons. From Lewis et al. (2005). (B) Schematic summary of the trajectories of pyramidal neuron axon initial segment and chandelier neuron axon cartridges labeled with different markers across postnatal development in area 46 of monkey DLPFC. Lines for each marker represent the percent maximal value achieved plotted against age in months after birth on a log scale. Arrowheads demarcate the indicated ages in months, and the shaded area indicates the approximate age range corresponding to adolescence in this species.
From Cruz et al. (2009a,b).
As mentioned above, an approximately 25–35% reduction in the expression of GAD67 mRNA occurs across layers 2 through 5 (Akbarian et al., 1995b; Guidotti et al., 2000; Volk et al., 2000; Straub et al., 2007) of the DLPFC in subjects with schizophrenia. The alterations in PV-positive cells can account for the decreased GAD67 transcript levels in layers deep 3 and 4 (Hashimoto et al., 2003), but recent studies suggest that other GABA neurons are affected in other cortical layers. For example, the subpopulation of CCK-containing GABA cells might contribute to the GABA neurotransmission deficit in layer 2 (Eggan et al., 2008). CCK-positive cells belong to two classes of local circuit neurons, narrow arbor basket cells connecting layers 2 and 4, and medium arbor neurons with axons confined to layers 2 and superficial 3 (Lund and Lewis, 1993). The synaptic targets of these CCK-basket neurons are GABAA receptors enriched in α2 subunits. The idea that the CCK-positive cells contribute to the GAD67 deficiency in the superficial layers is supported by findings in schizophrenia of reduced cannabinoid receptor 1 (CB1R) mRNA and protein, which is heavily localized to the axon terminals of CCK neurons (Marsicano and Lutz, 1999; Galarreta et al., 2004; Bodor et al., 2005). Importantly, lower CB1R mRNA levels in layers 2 through superficial 3 correlate with CCK and GAD67 mRNA deficits in the DLPFC in schizophrenia (Eggan et al., 2008). In the primate DLPFC, the highest densities of both CB1R- and CCK-positive neurons are found in these layers, and both CB1R- and CCK-positive axon terminals densely innervate layer 4 (Oeth and Lewis, 1990; Eggan and Lewis, 2007). The convergence of all these findings suggests that, in schizophrenia, GABA neurotransmission might also be altered in the subset of CB1R- and CCK-containing GABA neurons that provide inhibitory projections from the superficial to middle cortical layers in the DLPFC.
3. Development of prefrontal cortical circuitry
Given the extensive excitatory intra- and interareal cortical connections that layer 3 pyramidal neurons provide (Jones, 1984; Barbas, 1992; Levitt et al., 1993), developmental-related structural and neurochemical alterations in excitatory and inhibitory inputs to these neurons may be important determinants of the functional maturation of prefrontal cortical circuitry in schizophrenia. In the neocortex of both monkeys and humans, the extended period of adolescence is characterized by a massive synaptic pruning which eliminates exuberant excitatory synapses previously produced by rapid perinatal synaptogenesis (Rakic et al., 1986; Huttenlocher and Dabholkar, 1997). During postnatal development of the primate cerebral cortex, the period of synaptic proliferation, with the subsequent retraction of many of these contacts until a steady adult number of synapses level is achieved in individual pyramidal neurons (Anderson et al., 1995). Synaptic pruning during adolescence has been linked to neurodevelopmental models of schizophrenia because the clinical symptoms typically arise in early adulthood (Lewis and Levitt, 2002). Altered expression of certain synaptic proteins in schizophrenia suggested the possibility that the exuberant synapses present before adolescence somehow compensated for a dysfunction in excitatory transmission in individuals with schizophrenia (Mirnics et al., 2001). Alternatively, such alterations in synaptic protein expression might disturb the mechanisms of adolescence-related synapse elimination leading, for instance, to excessive synapse pruning in the illness (Feinberg, 1990; Keshavan et al., 1994).
In the monkey DLPFC, the developmental trajectory of excitatory synaptic density is paralleled by similar changes in the density of dendritic spines in pyramidal neurons (Anderson et al., 1995). Although the overall pattern of postnatal changes in synaptic density has been found to be similar in all cortical regions studied, differences in the precise time course of these changes have been observed at the regional, laminar, and cellular levels. For example, in layer 3 of human cerebral cortex, total synaptic density peaks at 6 months of age in primary visual cortex (Huttenlocher and de Courten, 1987) but at 2 years of age in prefrontal regions (Huttenlocher, 1979). Laminar differences in the temporal pattern of postnatal synaptic production and elimination have been described in several regions of monkey neocortex, including the DLPFC. In this region, peripubertal synaptic density reduction was found in the supragranular layers but not infragranular layers (Bourgeois et al., 1994). Within a cortical layer, there may also be important differences among classes of neurons in the timing of synaptic production and elimination (Gonzalez-Burgos et al., 2008). These comparisons suggest that the elimination of prefrontal cortical axospinous synapses and dendritic spines during the peripubertal age may follow different time courses for specific subpopulations of pyramidal neurons.
Overall, the time course of synaptic development strongly suggests that the connectivity of the primate DLPFC undergoes substantial changes during adolescence. However, due to the apparent regional, laminar, and cellular specificity in the timing and magnitude of maturational changes in cortical synaptic density, it is important to understand how specific and interrelated components of DLPFC circuitry change during postnatal development as such knowledge may inform the developmental origin of the circuit alterations observed in the DLPFC in schizophrenia.
3.1. Postnatal developmental of presynaptic GABA markers
In monkey DLPFC, the overall density of symmetric synapses, which identify all GABAergic terminals, does not change markedly throughout postnatal development (Bourgeois et al., 1994). This observation is also supported by the consistent density of GAT1-labeled puncta during this period, but is in contrast with the fact that the density of PV-positive terminals exhibits marked laminar and region-specific changes over a protracted period of postnatal development (Erickson and Lewis, 2002). In monkey DLPFC, these changes in PV expression correspond to a 10 fold increase of PV-immunoreactive puncta density in the superficial and middle layers during the postnatal period from extremely low levels in the newborn stage (Erickson and Lewis, 2002). Given that, among the PV-positive terminals in the superficial and middle cortical layers, those from thalamocortical axons and chandelier cells contribute little, if at all, to the observed developmental trends in the density of PV-positive puncta (Lund and Lewis, 1993), the observed changes are likely to reflect alterations within GABAergic terminals arising from PV-containing basket cells (Erickson and Lewis, 2002). However, in contrast with this idea, qualitative studies of basket cells axon terminals by Golgi impregnation reveal no obvious changes in their axonal morphology between infant and adult prefrontal cortex (Lund and Lewis, 1993). This suggests that the apparent discrepancy in developmental trajectories between total GABAergic and PV-positive terminals reflects changes in the immunodetectability of the latter, corresponding to altered protein concentration levels, rather than an increase in the number of such terminals. Interestingly, changes in PV content have been reported to be associated with corresponding increases or decreases in neuronal activity (Heizmann, 1984), which indicates that these changes in PV protein expression levels between newborn and adult stages may correspond to alterations in the activity of PV-positive basket cells during postnatal development.
The PV-positive chandelier cells also show developmental changes in the expression of several of their molecular markers. Presynaptically, PV and GAT1 immunoreactivity levels in chandelier axon cartridges are not detectable or extremely low at birth in monkey DLPFC, rise following different developmental trajectories and reach peak levels early in postnatal development that are sustained until approximately 15 months of age. After this, the expression of these two markers rapidly declines during adolescence until lower stable adult levels are achieved (Fig. 4B) (Anderson et al., 1995; Conde et al., 1996; Cruz et al., 2003). Together, these findings suggest that the maturation of inhibitory inputs to the axon initial segment of prefrontal pyramidal neurons is a complex process that may differentially affect the firing patterns of subpopulations of pyramidal neurons at specific postnatal time points (Cruz et al., 2003). In fact, the developmental trajectories of proteins that regulate synapse formation (ankyrin-G and βIV spectrin) and receptor clustering (gephyrin) at the axon initial segment reflect specific types of changes during the perinatal period and adolescence (Fig. 4B). In particular, these trajectories reveal a two-phase developmental process of GABAergic synaptic stability and GABA neurotransmission at chandelier cell inputs to pyramidal neurons that parallels the protracted functional maturation of primate DLPFC circuitry (Cruz et al., 2009a).
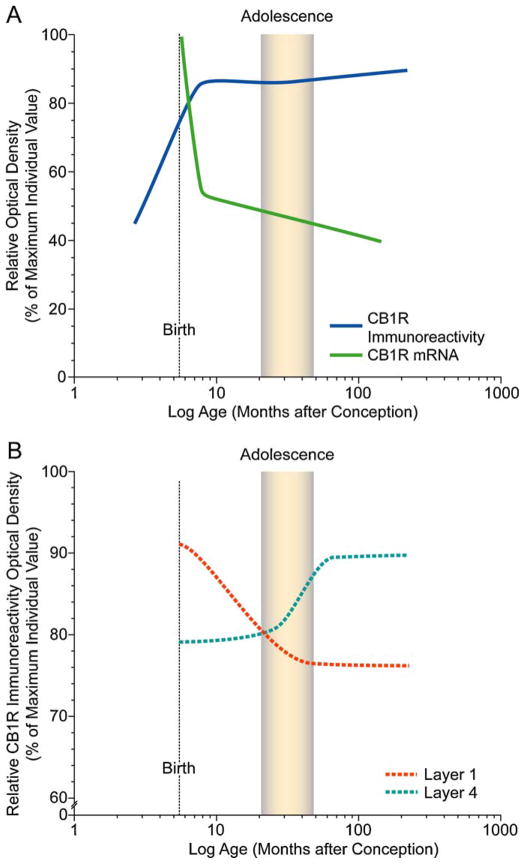
In contrast to the postnatal developmental trajectory of PV-positive GABA interneurons, the density of CCK-positive basket cells in supragranular layers is most prominent at birth and falls to a constant adult-like level by 1 year of age (Oeth and Lewis, 1993). This pattern is opposite to the developmental trajectories of the density of dendritic spines and PV-immunoreactive cartridges, which both increase while CCK immunoreactivity declines. In the rodent neocortex and hippocampus, CB1R-immunoreactive cells and axons undergo distinct developmental refinements in relative density and laminar distribution, and in the hippocampus, these refinements parallel those of CCK-immunoreactive cells and axons (Morozov and Freund, 2003; Deshmukh et al., 2007). Analysis of changes in the levels of CB1R immunoreactivity and mRNA expression during pre- and postnatal development in the primate DLPFC show that the overall level of CB1R immunoreactivity robustly increases during the pre- and perinatal periods and then remains stable throughout postnatal development (Eggan et al., 2010). However, laminar-specific developmental changes are present in innervation density, with a decrease in layers 1–2 that is most marked during the first postnatal year and an increase in layer 4 that is prominent during adolescence. In contrast, CB1R mRNA expression is highest at birth, markedly decreases during the first 3 postnatal months, and then remains stable through development and into adulthood with a distinct peak in CB1R mRNA expression in layer 2 (Fig. 5) (Eggan et al., 2010). Thus, the relative levels and laminar distribution of both CB1R immunoreactivity and mRNA exhibit distinctive patterns and different rates of change, eventually achieving peaks of mRNA expression in layer 2 and of CB1R-IR axons in layer 4. These findings suggest a shifting role of CB1Rs in cortical circuitry that may contribute to the functional maturation of the DLPFC and, given its role in endocannabinoid signaling (Freund et al., 2003), to age-specific vulnerabilities to cannabis exposure during both the perinatal and adolescent periods of development (Fig. 5).
Fig. 5.
Schematic summary figures illustrating trajectories of overall CB1R immunoreactivity and mRNA levels (A) and density of CB1R-IR axons in layers 1 and 4 (B) across development of monkey DLPFC. Lines were generated by plotting the density as a percent of the maximum density value for individual animals for each marker as a function of age in months after conception on a log scale, fitted by Loess regression analysis, and smoothed by hand. The shaded area indicates the approximate age range corresponding to adolescence (15–42 months; Plant 1988) in macaque monkey. Note the different developmental time courses in overall CB1R immunoreactivity and mRNA levels (A) and in the laminar distribution of CB1R immunoreactivity (B).
From Eggan et al. (2010).
Together, these data are consistent with the hypothesis of a high degree of cellular specificity in the postnatal refinement of monkey prefrontal cortical circuitry. However, the functional consequences of these developmental changes in basket and chandelier cell outputs are largely determined by the laminar location of their targeted pyramidal cells and by the developmental trajectories of their specific postsynaptic molecular counterparts, such as α1- and α2 subunit enriched GABAA receptors.
3.2. Protracted developmental trajectories of postsynaptic GABAA receptor subunits
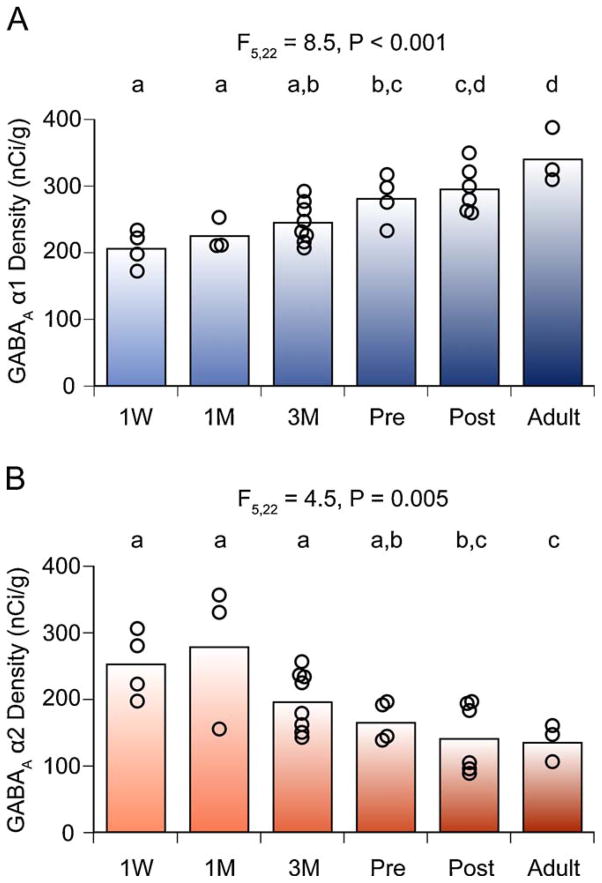
At the postsynaptic level, α1, β2, and δ subunits in primate DLPFC GABAA receptors have similar laminar patterns of expression and undergo similar developmental trajectories, which are distinct from those followed by GABAA α2 and α4 subunits (Fillman et al., 2010; Hashimoto et al., 2009; Maldonado-Aviles et al., 2009). For example, expression of mRNAs encoding GABAA receptor α1 and α2 subunits in monkey prefrontal cortex exhibit opposite trajectories during an extended period of postnatal development characterized by a progressive change with age, including significant differences between prepubertal and adult animals (Fig. 6) (Hashimoto et al., 2009). This divergent trajectory is shaped by a gradual postnatal increase of α1 subunit mRNA expression toward peak levels in the adult, and by a progressive decline of α2 subunit mRNA from its highest levels in neonates to the lowest levels of expression in adult animals (Hashimoto et al., 2009). Although, in general, protein levels of these two subunits follow the same developmental trajectory, the magnitude of the age-related changes in α1 subunit expression appears to be greater for protein than for mRNA levels (Cruz et al., 2003; Hashimoto et al., 2009). This difference might reflect developmental changes in translational or post-translational mechanisms that regulate the turnover of the subunit proteins.
Fig. 6.
Expression levels of GABAA receptor α1 and α2 subunit mRNAs in the monkey DLPFC during development. The optical densities for α1 (A) and α2 (B) subunit mRNAs within area 46 are individually plotted for each age group. The mean values for each age group are indicated as bars. During postnatal development, α1 mRNA levels increased, whereas α2 mRNA levels declined. Age groups that do not share the same letter are statistically different at α ≤ 05. Note that the shift in α subunit expression is a progressive and protracted process that lasts through adolescence. M, months; Pre, prepuberty; Post, postpuberty; W, week.
From Hashimoto et al. (2009).
These opposite trajectories might reflect a developmental replacement of α2 subunits with α1 subunits at synapses made by PV-containing basket cells on pyramidal neurons, whereas α2 subunits remain predominant in synapses made by CCK-containing basket cells into adulthood. The idea that the observed changes in the ratio of α1 to α2 subunit protein levels during adolescence occur at the single-synapse level, at least in inputs onto pyramidal neurons, is consistent with the developmental changes of the electrophysiological properties of mIPSPs observed in pyramidal neurons (Hashimoto et al., 2009). For example, α1-subunit-containing GABAA receptors have faster deactivation kinetics than those containing α2 subunits (Farrant and Nusser, 2005), and the duration of mIPSPs in pyramidal neurons are shorter in postpubertal animals than prepubertal animals (Hashimoto et al., 2009). As mentioned earlier, in mature circuits, GABAA α1 subunit predominates at synapses made by PV-positive basket cells (Klausberger et al., 2002), whereas the α2 subunit is post-synaptic to the axon terminals of PV-positive chandelier neurons (Nusser et al., 1996; Loup et al., 1998; Nyiri et al., 2001; Loup et al., 2006) and to PV-negative, putative CCK-containing basket cells, in adult rat hippocampus (Nyiri et al., 2001). Given that the α1 subunit is ubiquitously and abundantly expressed in the adult cortex (Fritschy and Mohler, 1995; Mohler, 2006), the post-natal increase in α1 subunit mRNA levels across cortical layers (Hashimoto et al., 2009) suggests that this increase is likely to occur at a large proportion of cortical GABA synapses. In contrast, the marked decrease in cortical α2 mRNA expression with age (Hashimoto et al., 2009) is likely to reflect a postnatal downregulation of the α2 subunit in GABA synapses furbished by these two cell types. Consistent with this interpretation, the density of α2-subunit-immunoreactive puncta (presumably at axosomatic and axodendritic synapses) is markedly lower in post-adolescent animals compared with animals of younger age (Cruz et al., 2003). Together, these developmental changes suggest that during the postnatal period, the composition of GABAA receptors shifts from α2 to α1 subunits in diverse populations of GABA synapses on both pyramidal and GABA neurons in a cell input-specific manner.
3.3. Functional implications of the protracted development of GABAA receptor subunits
At the functional level, experience dependent plasticity relies on the recruitment of α1-subunit-containing GABAA receptors to inhibitory synapses made by PV-containing basket cells on pyramidal neuron soma (Katagiri et al., 2007). Therefore, the increase in α1 subunits at GABA synapses might also be crucial for engaging neuronal plasticity in the developing primate DLPFC. In the neocortex, PV-containing basket cells are extensively and mutually interconnected into networks (Kisvarday et al., 1993) that by virtue of synchronizing pyramidal cell firing, are believed to play a central role in generating gamma band (30–80 Hz) oscillations (Bartos et al., 2007). Gamma oscillations are thought to provide a temporal structure for cortical information processing, including those dependent on DLPFC circuitry such as working memory (Jensen et al., 2007). The generation of gamma oscillations requires strong and fast inhibitory connections among PV-containing neurons and between PV-containing basket neurons and pyramidal cells (Bartos et al., 2007), which appear to be provided by the fast deactivation kinetics of α1-subunit-containing GABAA receptors in these synapses (Klausberger et al., 2002). Consistent with this idea, an increase in α1 subunit expression during development might be important for establishing the network properties required to efficiently generate the gamma oscillations associated with cognitive functions. The faster kinetics of inhibitory inputs to pyramidal neurons during postnatal development (Hashimoto et al., 2009) are consistent with increased α1 subunit expression at inhibitory synapses on pyramidal neurons, such as those made by PV-containing basket neurons (Klausberger et al., 2002). Given that each PV-containing neuron innervates a large number of pyramidal neurons (Lewis et al., 2005), faster inhibition by PV-containing neurons across postnatal development might contribute to an improved ability to synchronize populations of pyramidal neurons at high frequencies. Therefore, it has been suggested that increased α1 subunit levels at the synapses between both PV-containing neurons and PV-containing and pyramidal neurons during postnatal development, might have synergistic roles in the generation of cortical gamma band oscillations (Hashimoto et al., 2009). Consistent with this interpretation, in humans both working memory performance (Luna et al., 2004; Crone et al., 2006) and gamma band power (Uhlhaas et al., 2006) increase across postnatal development, including adolescence, and into early adulthood.
Given the protracted developmental profiles of α1 and α2 subunit expression, the elevated α2 subunit expression and the decreased α1 mRNA levels in schizophrenia might reflect a developmental dysregulation of GABAA receptor α subunit expression, in which the changes in subunit expression with age fail to undergo their full course. This disruption might contribute to the cognitive deficits in patients with schizophrenia by compromising neuronal plasticity and gamma band oscillations, both of which seem to be critical for the cognitive processes mediated by DLPFC circuitry (Gonzalez-Burgos and Lewis, 2008).
On the other hand, this complex and protracted postnatal maturation of the inputs from PV-containing GABA neurons in the primate DLPFC provides a number of opportunities for any disturbances, even subtle ones, to have their effects amplified as they alter the trajectories of the developmental events that follow. In particular, the marked developmental changes in the axon terminals of PV-containing basket and chandelier neurons, and their postsynaptic receptors, during the perinatal period and adolescence raise the possibility that the alterations of these markers in schizophrenia reflect a disturbance in these patterns of development. These temporal correlations may explain how a range of environmental factors (e.g. labor-delivery complications, urban place of rearing, and marijuana use during adolescence) are all associated with increased risk for the appearance of schizophrenia later in life. Although it seems unlikely that the GABA-related disturbances in schizophrenia represent an arrest of development, they may reflect an alteration of DLPFC circuitry that makes it unable to support higher levels of working memory load, rendering the impaired performance in schizophrenia analogous to the immature levels of working memory function seen in children (Diamond et al., 2002; Luna et al., 2004; Crone et al., 2006).
4. Conclusions
Alterations in the expression of genes (those that mediate inherited susceptibility and perhaps other genes that are downstream of their control) (Choi et al., 2009) generally results from combination of several factors. These altered patterns of gene expression may be influenced, or even triggered, by environmental phenomena during a particular period of development. Thus, the resultant altered course of developmental trajectories would be due to a combination of genetic changes, which when combined with particular stressors during development, drive the system towards a critical threshold for an altered phenotype. These altered patterns of gene expression have a cumulative effect that leads to additional disturbances in subsequent developmental processes. The impact of these alterations in gene expression and their sequelae becomes relatively stabilized once developmental processes approach completion, being only modestly affected by adult levels of plasticity but more subject than the normal brain to further adverse functional consequences from other distressing factors (e.g. drug abuse, stress, etc.).
The findings reviewed above converge on the idea that a deficiency in GABA signaling is a pathogenetic mechanism including reduced GAD67 mRNA expression and GABA synthesis in the parvalbumin-expressing subpopulation of GABA neurons in the DLPFC of individuals with schizophrenia. Despite apparent compensatory responses including the decrease in the levels of presynaptic GAT1 and the upregulation of α2-containing postsynaptic GABAA receptors, the resulting pathophysiological process – changes in the perisomatic inhibitory regulation of pyramidal neurons that are required for gamma frequency oscillations – contributes to the impairments in working memory function that represent a core feature of the clinical syndrome of schizophrenia. The observed abnormalities in the expression of these protein markers and the lamina- and cellular-specificity of these changes in schizophrenia suggest that these disturbances depend both upon the timing of normal maturational events and circuitry-specific susceptibility. However, these abnormalities in GABA neurons are unlikely to be the only contributors to working memory dysfunction in this disorder; in fact, changes in dopamine and glutamate neurotransmission in the DLPFC also seem to be involved (Weickert et al., 2007; Lewis and Gonzalez-Burgos, 2008). The real value of determining the molecular and morphological abnormalities that advance the understanding of the pathophysiology of schizophrenia rests in the prediction of novel targets for pharmacological intervention. The observations reviewed above may reveal targets for new drug development that are selective for the neural networks that are specifically altered in the disease; furthermore, understanding their developmental trajectories may provide insight into when preemptive interventions might be most successful.
References
- Akbarian S, Huang HS. Molecular and cellular mechanisms of altered GAD1/GAD67 expression in schizophrenia and related disorders. Brain Res Rev. 2006;52:293–304. doi: 10.1016/j.brainresrev.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Huntsman MM, Kim JJ, Tafazzoli A, Potkin SG, Bunney WE, Jr, Jones EG. GABAA receptor subunit gene expression in human prefrontal cortex: comparison of schizophrenics and controls. Cereb Cortex. 1995a;5:550–560. doi: 10.1093/cercor/5.6.550. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, Jr, Jones EG. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995b;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Goldman PS. Functional development of the dorsolateral prefrontal cortex: an analysis utlizing reversible cryogenic depression. Brain Res. 1978;143:233–249. doi: 10.1016/0006-8993(78)90566-8. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Classey JD, Conde F, Lund JS, Lewis DA. Synchronous development of pyramidal neuron dendritic spines and parvalbumin-immunoreactive chandelier neuron axon terminals in layer III of monkey prefrontal cortex. Neuroscience. 1995;67:7–22. doi: 10.1016/0306-4522(95)00051-j. [DOI] [PubMed] [Google Scholar]
- Asada H, Kawamura Y, Maruyama K, Kume H, Ding RG, Kanbara N, Kuzume H, Sanbo M, Yagi T, Obata K. Cleft palate and decreased brain gamma-aminobutyric acid in mice lacking the 67-kDa isoform of glutamic acid decarboxylase. Proc Natl Acad Sci USA. 1997;94:6496–6499. doi: 10.1073/pnas.94.12.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H. Architecture and cortical connections of the prefrontal cortex in the rhesus monkey. Adv Neurol. 1992;57:91–115. [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Beneyto M, Abbott A, Hashimoto T, Lewis DA. Lamina-specific alterations in cortical GABAA receptor subunit expression in schizophrenia. Cerebral Cortex. 2010 doi: 10.1093/cercor/bhq169. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi MT, Macdonald RL. Neurosteroids shift partial agonist activation of GABA(A) receptor channels from low- to high-efficacy gating patterns. J Neurosci. 2003;23:10934–10943. doi: 10.1523/JNEUROSCI.23-34-10934.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird ED, Spokes EG, Iversen LL. Increased dopamine concentration in limbic areas of brain from patients dying with schizophrenia. Brain. 1979;102:347–360. doi: 10.1093/brain/102.2.347. [DOI] [PubMed] [Google Scholar]
- Bodor AL, Katona I, Nyiri G, Mackie K, Ledent C, Hajos N, Freund TF. Endocannabinoid signaling in rat somatosensory cortex: laminar differences and involvement of specific interneuron types. J Neurosci. 2005;25:6845–6856. doi: 10.1523/JNEUROSCI.0442-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois JP, Goldman-Rakic PS, Rakic P. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb Cortex. 1994;4:78–96. doi: 10.1093/cercor/4.1.78. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Verchinski BA, Marenco S, Egan MF, Weinberger DR. Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. Am J Psychiatry. 2003;160:2209–2215. doi: 10.1176/appi.ajp.160.12.2209. [DOI] [PubMed] [Google Scholar]
- Choi KH, Zepp ME, Higgs BW, Weickert CS, Webster MJ. Expression profiles of schizophrenia susceptibility genes during human prefrontal cortical development. J Psychiatry Neurosci. 2009;34:450–458. [PMC free article] [PubMed] [Google Scholar]
- Conde F, Lund JS, Jacobowitz DM, Baimbridge KG, Lewis DA. Local circuit neurons immunoreactive for calretinin, calbindin D-28k or parvalbumin in monkey prefrontal cortex: distribution and morphology. J Comp Neurol. 1994;341:95–116. doi: 10.1002/cne.903410109. [DOI] [PubMed] [Google Scholar]
- Conde F, Lund JS, Lewis DA. The hierarchical development of monkey visual cortical regions as revealed by the maturation of parvalbumin-immunoreactive neurons. Brain Res Dev Brain Res. 1996;96:261–276. doi: 10.1016/0165-3806(96)00126-5. [DOI] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, Donohue S, van Leijenhorst L, Bunge SA. Neurocognitive development of the ability to manipulate information in working memory. Proc Natl Acad Sci USA. 2006;103:9315–9320. doi: 10.1073/pnas.0510088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz DA, Eggan SM, Lewis DA. Postnatal development of pre- and post-synaptic GABA markers at chandelier cell connections with pyramidal neurons in monkey prefrontal cortex. J Comp Neurol. 2003;465:385–400. doi: 10.1002/cne.10833. [DOI] [PubMed] [Google Scholar]
- Cruz DA, Lovallo EM, Stockton S, Rasband M, Lewis DA. Postnatal development of synaptic structure proteins in pyramidal neuron axon initial segments in monkey prefrontal cortex. J Comp Neurol. 2009a;514:353–367. doi: 10.1002/cne.22006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz DA, Weaver CL, Lovallo EM, Melchitzky DS, Lewis DA. Selective alterations in postsynaptic markers of chandelier cell inputs to cortical pyramidal neurons in subjects with schizophrenia. Neuropsychopharmacology. 2009b;34:2112–2124. doi: 10.1038/npp.2009.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelipe J. Types of neurons, synaptic connections and chemical characteristics of cells immunoreactive for calbindin-D28K, parvalbumin and calretinin in the neocortex. J Chem Neuroanat. 1997;14:1–19. doi: 10.1016/s0891-0618(97)10013-8. [DOI] [PubMed] [Google Scholar]
- Deshmukh S, Onozuka K, Bender KJ, Bender VA, Lutz B, Mackie K, Feldman DE. Postnatal development of cannabinoid receptor type 1 expression in rodent somatosensory cortex. Neuroscience. 2007;145:279–287. doi: 10.1016/j.neuroscience.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A, Kirkham N, Amso D. Conditions under which young children can hold two rules in mind and inhibit a prepotent response. Dev Psychol. 2002;38:352–362. [PubMed] [Google Scholar]
- Duncan CE, Webster MJ, Rothmond DA, Bahn S, Elashoff M, Shannon Weickert C. Prefrontal GABA(A) receptor alpha-subunit expression in normal postnatal human development and schizophrenia. J Psychiatr Res. 2010;44:673–681. doi: 10.1016/j.jpsychires.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Eggan SM, Hashimoto T, Lewis DA. Reduced cortical cannabinoid 1 receptor messenger RNA and protein expression in schizophrenia. Arch Gen Psychiatry. 2008;65:772–784. doi: 10.1001/archpsyc.65.7.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggan SM, Lewis DA. Immunocytochemical distribution of the cannabinoid CB1 receptor in the primate neocortex: a regional and laminar analysis. Cereb Cortex. 2007;17:175–191. doi: 10.1093/cercor/bhj136. [DOI] [PubMed] [Google Scholar]
- Eggan SM, Mizoguchi Y, Stoyak SR, Lewis DA. Development of cannabinoid 1 receptor protein and messenger RNA in monkey dorsolateral prefrontal cortex. Cereb Cortex. 2010;20:1164–1174. doi: 10.1093/cercor/bhp179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson SL, Lewis DA. Postnatal development of parvalbumin- and GABA transporter-immunoreactive axon terminals in monkey prefrontal cortex. J Comp Neurol. 2002;448:186–202. doi: 10.1002/cne.10249. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Feinberg I. Cortical pruning and the development of schizophrenia. Schizophr Bull. 1990;16:567–570. doi: 10.1093/schbul/16.4.567. [DOI] [PubMed] [Google Scholar]
- Fillman SG, Duncan CE, Webster MJ, Elashoff M, Weickert CS. Developmental co-regulation of the [beta] and [gamma] GABAA receptor subunits with distinct [alpha] subunits in the human dorsolateral prefrontal cortex. Int J Neurosci. 2010;28:513–519. doi: 10.1016/j.ijdevneu.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Mohler H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Bacon SJ. Local circuit neurons in the medial prefrontal cortex (areas 24a,b,c, 25 and 32) in the monkey: II. Quantitative areal and laminar distributions. J Comp Neurol. 1996;364:609–636. doi: 10.1002/(SICI)1096-9861(19960122)364:4<609::AID-CNE2>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Galarreta M, Erdelyi F, Szabo G, Hestrin S. Electrical coupling among irregular-spiking GABAergic interneurons expressing cannabinoid receptors. J Neurosci. 2004;24:9770–9778. doi: 10.1523/JNEUROSCI.3027-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci. 1994;6:348–357. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Krimer LS, Povysheva NV, Barrionuevo G, Lewis DA. Functional properties of fast spiking interneurons and their synaptic connections with pyramidal cells in primate dorsolateral prefrontal cortex. J Neurophysiol. 2005;93:942–953. doi: 10.1152/jn.00787.2004. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Kroener S, Zaitsev AV, Povysheva NV, Krimer LS, Barrionuevo G, Lewis DA. Functional maturation of excitatory synapses in layer 3 pyramidal neurons during postnatal development of the primate prefrontal cortex. Cereb Cortex. 2008;18:626–637. doi: 10.1093/cercor/bhm095. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Lewis DA. GABA neurons and the mechanisms of network oscillations: implications for understanding cortical dysfunction in schizophrenia. Schizophr Bull. 2008;34:944–961. doi: 10.1093/schbul/sbn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR, Impagnatiello F, Pandey G, Pesold C, Sharma R, Uzunov D, Costa E, DiGiorgi Gerevini V. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- Harris LW, Lockstone HE, Khaitovich P, Weickert CS, Webster MJ, Bahn S. Gene expression in the prefrontal cortex during adolescence: implications for the onset of schizophrenia. BMC Med Genom. 2009;2:28. doi: 10.1186/1755-8794-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Arion D, Unger T, Maldonado-Aviles JG, Morris HM, Volk DW, Mirnics K, Lewis DA. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2008a;13:147–161. doi: 10.1038/sj.mp.4002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Bazmi HH, Mirnics K, Wu Q, Sampson AR, Lewis DA. Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. Am J Psychiatry. 2008b;165:479–489. doi: 10.1176/appi.ajp.2007.07081223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Bergen SE, Nguyen QL, Xu B, Monteggia LM, Pierri JN, Sun Z, Sampson AR, Lewis DA. Relationship of brain-derived neurotrophic factor and its receptor TrkB to altered inhibitory prefrontal circuitry in schizophrenia. J Neurosci. 2005;25:372–383. doi: 10.1523/JNEUROSCI.4035-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Nguyen QL, Rotaru D, Keenan T, Arion D, Beneyto M, Gonzalez-Burgos G, Lewis DA. Protracted developmental trajectories of GABAA receptor alpha1 and alpha2 subunit expression in primate prefrontal cortex. Biol Psychiatry. 2009;65:1015–1023. doi: 10.1016/j.biopsych.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, Sampson AR, Lewis DA. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heizmann CW. Parvalbumin, an intracellular calcium-binding protein; distribution, properties and possible roles in mammalian cells. Experientia. 1984;40:910–921. doi: 10.1007/BF01946439. [DOI] [PubMed] [Google Scholar]
- Huang ZJ. Activity-dependent development of inhibitory synapses and innervation pattern: role of GABA signalling and beyond. J Physiol. 2009;587:1881–1888. doi: 10.1113/jphysiol.2008.168211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntsman MM, Tran BV, Potkin SG, Bunney WE, Jr, Jones EG. Altered ratios of alternatively spliced long and short gamma2 subunit mRNAs of the gamma-amino butyrate type A receptor in prefrontal cortex of schizophrenics. Proc Natl Acad Sci USA. 1998;95:15066–15071. doi: 10.1073/pnas.95.25.15066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex—developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, de Courten C. The development of synapses in striate cortex of man. Hum Neurobiol. 1987;6:1–9. [PubMed] [Google Scholar]
- Jensen O, Kaiser J, Lachaux JP. Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci. 2007;30:317–324. doi: 10.1016/j.tins.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Ji F, Kanbara N, Obata K. GABA and histogenesis in fetal and neonatal mouse brain lacking both the isoforms of glutamic acid decarboxylase. Neurosci Res. 1999;33:187–194. doi: 10.1016/s0168-0102(99)00011-5. [DOI] [PubMed] [Google Scholar]
- Jones E, editor. Laminar Distribution of Cortical Efferent Cells. Plenum Press; New York: 1984. pp. 521–553. [Google Scholar]
- Katagiri H, Fagiolini M, Hensch TK. Optimization of somatic inhibition at critical period onset in mouse visual cortex. Neuron. 2007;53:805–812. doi: 10.1016/j.neuron.2007.02.026. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. Correlation of physiological subgroupings of nonpyramidal cells with parvalbumin- and calbindinD28k-immunoreactive neurons in layer V of rat frontal cortex. J Neurophysiol. 1993;70:387–396. doi: 10.1152/jn.1993.70.1.387. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Anderson S, Pettegrew JW. Is schizophrenia due to excessive synaptic pruning in the prefrontal cortex? The Feinberg hypothesis revisited. J Psychiatr Res. 1994;28:239–265. doi: 10.1016/0022-3956(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Kisvarday ZF, Beaulieu C, Eysel UT. Network of GABAergic large basket cells in cat visual cortex (area 18): implication for lateral disinhibition. J Comp Neurol. 1993;327:398–415. doi: 10.1002/cne.903270307. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Roberts JD, Somogyi P. Cell type- and input-specific differences in the number and subtypes of synaptic GABA(A) receptors in the hippocampus. J Neurosci. 2002;22:2513–2521. doi: 10.1523/JNEUROSCI.22-07-02513.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krimer LS, Goldman-Rakic PS. Prefrontal microcircuits: membrane properties and excitatory input of local, medium, and wide arbor interneurons. J Neurosci. 2001;21:3788–3796. doi: 10.1523/JNEUROSCI.21-11-03788.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krimer LS, Zaitsev AV, Czanner G, Kroner S, Gonzalez-Burgos G, Povysheva NV, Iyengar S, Barrionuevo G, Lewis DA. Cluster analysis-based physiological classification and morphological properties of inhibitory neurons in layers 2–3 of monkey dorsolateral prefrontal cortex. J Neurophysiol. 2005;94:3009–3022. doi: 10.1152/jn.00156.2005. [DOI] [PubMed] [Google Scholar]
- Levitt JB, Lewis DA, Yoshioka T, Lund JS. Topography of pyramidal neuron intrinsic connections in macaque monkey prefrontal cortex (areas 9 and 46) J Comp Neurol. 1993;338:360–376. doi: 10.1002/cne.903380304. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Cruz DA, Melchitzky DS, Pierri JN. Lamina-specific deficits in parvalbumin-immunoreactive varicosities in the prefrontal cortex of subjects with schizophrenia: evidence for fewer projections from the thalamus. Am J Psychiatry. 2001;158:1411–1422. doi: 10.1176/appi.ajp.158.9.1411. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Gonzalez-Burgos G. Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacology. 2008;33:141–165. doi: 10.1038/sj.npp.1301563. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci. 2002;25:409–432. doi: 10.1146/annurev.neuro.25.112701.142754. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Lund JS. Heterogeneity of chandelier neurons in monkey neocortex: corticotropin-releasing factor- and parvalbumin-immunoreactive populations. J Comp Neurol. 1990;293:599–615. doi: 10.1002/cne.902930406. [DOI] [PubMed] [Google Scholar]
- Loup F, Picard F, Andre VM, Kehrli P, Yonekawa Y, Wieser HG, Fritschy JM. Altered expression of alpha3-containing GABAA receptors in the neocortex of patients with focal epilepsy. Brain. 2006;129:3277–3289. doi: 10.1093/brain/awl287. [DOI] [PubMed] [Google Scholar]
- Loup F, Weinmann O, Yonekawa Y, Aguzzi A, Wieser HG, Fritschy JM. A highly sensitive immunofluorescence procedure for analyzing the subcellular distribution of GABAA receptor subunits in the human brain. J Histochem Cytochem. 1998;46:1129–1139. doi: 10.1177/002215549804601005. [DOI] [PubMed] [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Dev. 2004;75:1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Lund JS, Lewis DA. Local circuit neurons of developing and mature macaque prefrontal cortex: Golgi and immunocytochemical characteristics. J Comp Neurol. 1993;328:282–312. doi: 10.1002/cne.903280209. [DOI] [PubMed] [Google Scholar]
- Maldonado-Aviles JG, Curley AA, Hashimoto T, Morrow AL, Ramsey AJ, O’Donnell P, Volk DW, Lewis DA. Altered markers of tonic inhibition in the dorsolateral prefrontal cortex of subjects with schizophrenia. Am J Psychiatry. 2009;166:450–459. doi: 10.1176/appi.ajp.2008.08101484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Lutz B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur J Neurosci. 1999;11:4213–4225. doi: 10.1046/j.1460-9568.1999.00847.x. [DOI] [PubMed] [Google Scholar]
- Mertens S, Benke D, Mohler H. GABAA receptor populations with novel subunit combinations and drug binding profiles identified in brain by alpha 5-and delta-subunit-specific immunopurification. J Biol Chem. 1993;268:5965–5973. [PubMed] [Google Scholar]
- Mirnics K, Middleton FA, Lewis DA, Levitt P. Analysis of complex brain disorders with gene expression microarrays: schizophrenia as a disease of the synapse. Trends Neurosci. 2001;24:479–486. doi: 10.1016/s0166-2236(00)01862-2. [DOI] [PubMed] [Google Scholar]
- Mirnics K, Middleton FA, Marquez A, Lewis DA, Levitt P. Molecular characterization of schizophrenia viewed by microarray analysis of gene expression in prefrontal cortex. Neuron. 2000;28:53–67. doi: 10.1016/s0896-6273(00)00085-4. [DOI] [PubMed] [Google Scholar]
- Mohler H. GABA(A) receptor diversity and pharmacology. Cell Tissue Res. 2006;326:505–516. doi: 10.1007/s00441-006-0284-3. [DOI] [PubMed] [Google Scholar]
- Morozov YM, Freund TF. Post-natal development of type 1 cannabinoid receptor immunoreactivity in the rat hippocampus. Eur J Neurosci. 2003;18:1213–1222. doi: 10.1046/j.1460-9568.2003.02852.x. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Benke D, Fritschy JM, Somogyi P. Differential synaptic localization of two major gamma-aminobutyric acid type A receptor alpha subunits on hippocampal pyramidal cells. Proc Natl Acad Sci USA. 1996;93:11939–11944. doi: 10.1073/pnas.93.21.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyiri G, Freund TF, Somogyi P. Input-dependent synaptic targeting of alpha(2)-subunit-containing GABA(A) receptors in synapses of hippocampal pyramidal cells of the rat. Eur J Neurosci. 2001;13:428–442. doi: 10.1046/j.1460-9568.2001.01407.x. [DOI] [PubMed] [Google Scholar]
- Oeth KM, Lewis DA. Cholecystokinin innervation of monkey prefrontal cortex: an immunohistochemical study. J Comp Neurol. 1990;301:123–137. doi: 10.1002/cne.903010112. [DOI] [PubMed] [Google Scholar]
- Oeth KM, Lewis DA. Postnatal development of the cholecystokinin innervation of monkey prefrontal cortex. J Comp Neurol. 1993;336:400–418. doi: 10.1002/cne.903360307. [DOI] [PubMed] [Google Scholar]
- Ohnuma T, Augood SJ, Arai H, McKenna PJ, Emson PC. Measurement of GABAergic parameters in the prefrontal cortex in schizophrenia: focus on GABA content, GABA(A) receptor alpha-1 subunit messenger RNA and human GABA transporter-1 (HGAT-1) messenger RNA expression. Neuroscience. 1999;93:441–448. doi: 10.1016/s0306-4522(99)00189-x. [DOI] [PubMed] [Google Scholar]
- Perlstein WM, Carter CS, Noll DC, Cohen JD. Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. Am J Psychiatry. 2001;158:1105–1113. doi: 10.1176/appi.ajp.158.7.1105. [DOI] [PubMed] [Google Scholar]
- Pierri JN, Chaudry AS, Woo TU, Lewis DA. Alterations in chandelier neuron axon terminals in the prefrontal cortex of schizophrenic subjects. Am J Psychiatry. 1999;156:1709–1719. doi: 10.1176/ajp.156.11.1709. [DOI] [PubMed] [Google Scholar]
- Rakic P, Bourgeois JP, Eckenhoff MF, Zecevic N, Goldman-Rakic PS. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science. 1986;232:232–235. doi: 10.1126/science.3952506. [DOI] [PubMed] [Google Scholar]
- Rao SG, Williams GV, Goldman-Rakic PS. Destruction and creation of spatial tuning by disinhibition: GABA(A) blockade of prefrontal cortical neurons engaged by working memory. J Neurosci. 2000;20:485–494. doi: 10.1523/JNEUROSCI.20-01-00485.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaguchi T, Matsumura M, Kubota K. Delayed response deficits produced by local injection of bicuculline into the dorsolateral prefrontal cortex in Japanese macaque monkeys. Exp Brain Res. 1989;75:457–469. doi: 10.1007/BF00249897. [DOI] [PubMed] [Google Scholar]
- Saxena NC, Macdonald RL. Assembly of GABAA receptor subunits: role of the delta subunit. J Neurosci. 1994;14:7077–7086. doi: 10.1523/JNEUROSCI.14-11-07077.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub RE, Lipska BK, Egan MF, Goldberg TE, Callicott JH, Mayhew MB, Vakkalanka RK, Kolachana BS, Kleinman JE, Weinberger DR. Allelic variation in GAD1 (GAD67) is associated with schizophrenia and influences cortical function and gene expression. Mol Psychiatry. 2007;12:854–869. doi: 10.1038/sj.mp.4001988. [DOI] [PubMed] [Google Scholar]
- Thompson M, Weickert CS, Wyatt E, Webster MJ. Decreased glutamic acid decarboxylase(67) mRNA expression in multiple brain areas of patients with schizophrenia and mood disorders. J Psychiatr Res. 2009;43:970–977. doi: 10.1016/j.jpsychires.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Linden DE, Singer W, Haenschel C, Lindner M, Maurer K, Rodriguez E. Dysfunctional long-range coordination of neural activity during Gestalt perception in schizophrenia. J Neurosci. 2006;26:8168–8175. doi: 10.1523/JNEUROSCI.2002-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vawter MP, Crook JM, Hyde TM, Kleinman JE, Weinberger DR, Becker KG, Freed WJ. Microarray analysis of gene expression in the prefrontal cortex in schizophrenia: a preliminary study. Schizophr Res. 2002;58:11–20. doi: 10.1016/s0920-9964(01)00377-2. [DOI] [PubMed] [Google Scholar]
- Volk D, Austin M, Pierri J, Sampson A, Lewis D. GABA transporter-1 mRNA in the prefrontal cortex in schizophrenia: decreased expression in a subset of neurons. Am J Psychiatry. 2001;158:256–265. doi: 10.1176/appi.ajp.158.2.256. [DOI] [PubMed] [Google Scholar]
- Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry. 2000;57:237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- Volk DW, Pierri JN, Fritschy JM, Auh S, Sampson AR, Lewis DA. Reciprocal alterations in pre- and postsynaptic inhibitory markers at chandelier cell inputs to pyramidal neurons in schizophrenia. Cereb Cortex. 2002;12:1063–1070. doi: 10.1093/cercor/12.10.1063. [DOI] [PubMed] [Google Scholar]
- Weickert CS, Webster MJ, Gondipalli P, Rothmond D, Fatula RJ, Herman MM, Kleinman JE, Akil M. Postnatal alterations in dopaminergic markers in the human prefrontal cortex. Neuroscience. 2007;144:1109–1119. doi: 10.1016/j.neuroscience.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I Regional cerebral blood flow evidence. Arch Gen Psychiatry. 1986;43:114–124. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- Woo TU, Walsh JP, Benes FM. Density of glutamic acid decarboxylase 67 messenger RNA-containing neurons that express the N-methyl-D-aspartate receptor subunit NR2A in the anterior cingulate cortex in schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2004;61:649–657. doi: 10.1001/archpsyc.61.7.649. [DOI] [PubMed] [Google Scholar]
- Woo TU, Whitehead RE, Melchitzky DS, Lewis DA. A subclass of prefrontal gamma-aminobutyric acid axon terminals are selectively altered in schizophrenia. Proc Natl Acad Sci USA. 1998;95:5341–5346. doi: 10.1073/pnas.95.9.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaitsev AV, Gonzalez-Burgos G, Povysheva NV, Kroner S, Lewis DA, Krimer LS. Localization of calcium-binding proteins in physiologically and morphologically characterized interneurons of monkey dorsolateral prefrontal cortex. Cereb Cortex. 2005;15:1178–1186. doi: 10.1093/cercor/bhh218. [DOI] [PubMed] [Google Scholar]