Abstract
An increased release of free fatty acids (FFA) into plasma likely contributes to the metabolic complications associated with obesity. However, the relationship between body fat and FFA metabolism is unclear because of conflicting results from different studies. The goal of our study was to determine the interrelationships between body fat, sex and plasma FFA kinetics. We determined FFA rate of appearance (Ra) in plasma, by using stable isotopically labeled tracer techniques, during basal conditions in 106 lean, overweight, and obese, non-diabetic subjects (43 men and 63 women who had 7.0–56.0 % body fat). Correlation analyses demonstrated: 1) no differences between men and women in the relationship between fat mass and total FFA Ra (µmol·min−1); 2) total FFA Ra increased linearly with increasing FM (r=0.652, P<0.001); 3) FFA Ra per kg FM decreased in a curvilinear fashion with increasing FM (r=−0.806; P<0.001); 4) FFA Ra in relationship to fat-free mass was greater in obese than lean subjects and greater in women than in men; 5) abdominal fat itself was not an important determinant of total FFA Ra. We conclude that total body fat, not regional fat distribution or sex, is an important modulator of the rate of FFA release into plasma. Although increased adiposity is associated with a decrease in fatty acid release in relationship to FM, this downregulation is unable to completely compensate for the increase in FM, so total FFA Ra and FFA Ra with respect to FFM are greater in women than in men and in obese than in lean subjects.
Keywords: fatty acid flux, lipolysis, adipose tissue, stable isotope labeled tracers
INTRODUCTION
Alterations in free fatty acid (FFA) metabolism are involved in the pathogenesis of insulin resistance, diabetes, dyslipidemia, and cardiovascular disease (CVD) (1, 2). Excessive plasma FFA availability impairs the ability of insulin to suppress hepatic glucose production and to increase skeletal muscle glucose disposal (1, 3). Plasma FFA are also the primary source of fatty acids for hepatic VLDL-triglyceride (TG) production (4–7), and plasma VLDL-TG concentration is an important risk factor for CVD (8, 9). In addition, the accumulation of intracellular fatty acid metabolites stimulates the production of inflammatory proteins (10), which can also contribute to insulin resistance and atherosclerosis (1). Therefore, understanding the factors that affect FFA metabolism has important physiological and clinical implications.
It has been hypothesized that the metabolic complications associated with obesity are due to increased body fat mass, which increases the release of FFA into plasma. However, the relationship between body fat and FFA metabolism is unclear because of conflicting results from different studies. For example, the rate of FFA release into plasma, expressed per unit of fat-free mass (FFM), in obese subjects has been reported to be both greater than (11–15) and the same as (5, 13, 16, 17) the rate of FFA release in lean subjects. The relationship between adiposity and FFA metabolism is further complicated by sex, because, at any given body mass index, women have more fat than men (18). An adequate evaluation of the influence of sex and adiposity on FFA metabolism is difficult, because it requires conducting careful metabolic studies in a large number of subjects.
The purpose of the present study was to provide a definitive evaluation of the inter-relationships between body composition, sex, and FFA kinetics by studying a large sample of men and women who had a wide range in percent body fat, but who did not have diabetes or other metabolic abnormalities that could confound the interpretation of the data. Fatty acid kinetics were determined by using intravenous stable isotopically-labeled palmitate infusion during carefully controlled metabolic conditions.
METHODS AND PROCEDURES
Subjects
A total of 106 subjects (43 men: 40 Caucasians, 3 African Americans; 63 women: 51 Caucasians, 9 African Americans, 3 Asians) who were recruited from the general public by local postings and by contacting potential participants listed in the Washington University School of Medicine volunteer database between 1999 and 2007 for measurements of FFA kinetics in our Center for Human Nutrition, had a body mass index (BMI) between 18.1 and 44.3 kg/m2, and fulfilled the inclusion criteria described below participated in this study. All subjects completed a comprehensive medical evaluation, which included a history and physical examination, standard blood and urine tests, and an oral glucose tolerance test. All subjects had normal oral glucose tolerance (19), and none had evidence of any illnesses, were taking regular medications known to affect lipid metabolism or smoked tobacco. All subjects were weight stable (<2 kg change in body weight) and untrained (regular exercise <2h per week) for at least 2 months before the study. Pregnant and lactating women were excluded. We did not collect information on menstrual cycle phase or hormonal contraceptive use by our female subjects because variations in female sex steroid availability in plasma do not affect lipolytic activity (palmitate and glycerol flux) during basal, resting postabsorptive conditions (20–26).
Written informed consent was obtained from all subjects before their participation in the study, which was approved by the Human Studies Committee and the General Clinical Research Center (GCRC) Scientific Advisory Committee at Washington University School of Medicine in St. Louis, MO.
Experimental Protocol
Body composition
Total fat mass (FM) and FFM were determined by using dual-energy x-ray absorptiometry (Hologic QDR 1000/w, Waltham, MA). Intra-abdominal and subcutaneous abdominal fat masses were assessed by using MRI (Siemens, Iselin, NJ) (27). Eight 10-mm-thick slice images were obtained beginning at the L4-L5 interspace, and analyzed for subcutaneous abdominal and intra-abdominal fat cross-sectional areas with Analyze 6.0 software (Mayo Foundation, Biomedical Imaging Resource, Rochester, MN); the areas from serial images of the abdomen were averaged.
FFA kinetics
Subjects were admitted to the inpatient unit of the GCRC at Washington University School of Medicine at 1700h. At 1900 h, they consumed a standard meal containing 15 kcal per kg body weight for lean subjects and 15 kcal per kg adjusted body weight for overweight and obese subjects. Adjusted body weight was calculated as ideal body weight (the midpoint of the medium frame of the Metropolitan Life Insurance Company Table (28)) + 0.25 × (actual body weight − ideal body weight). Of the total energy content of the meal, 57% was derived from carbohydrates, 28% from fat, and 15% from protein.
At 0530 h the following morning, after subjects fasted overnight, one catheter was inserted into a forearm vein to administer a stable isotope labeled fatty acid tracer, and a second catheter was inserted into a contralateral hand vein, which was heated to 55°C by using a thermostatically controlled box, to obtain arterialized blood samples. At 0700 h (time=0), after blood samples were obtained to determine plasma FFA, glucose, and insulin concentrations and background palmitate tracer-to-tracee ratio (TTR), a constant infusion of [2,2-2H2]palmitate (0.03 µmol·kg body wt−1·min−1), bound to human albumin, was started and maintained for 180 min. Multiple blood samples were obtained between 60 and 180 min to determine palmitate TTR in plasma.
Sample collection and analyses
Blood samples collected in heparinized tubes were analyzed immediately to determine plasma glucose concentration, by using an automated glucose analyzer (Yellow Spring Instruments Co, Yellow Springs, OH). Blood samples were collected in chilled tubes containing EDTA to determine FFA concentrations and in chilled tubes containing EDTA plus trasylol to determine insulin concentration. Samples were placed on ice, and plasma was separated by centrifugation within 30 min of collection. Plasma samples were stored at −80°C until final analyses were performed. Plasma insulin concentration was measured by using radioimmunoassay (Linco Research, St. Louis, MO). Plasma FFA concentrations were quantified by using gas chromatography (Hewlett-Packard 5890-II, Palo Alto, CA) after adding heptadecanoic acid to plasma as an internal standard (29). Plasma palmitate TTR was determined by using gas-chromatography mass-spectrometry (GC/MS; MSD 5973 system, Hewlett-Packard, Palo Alto, CA) after conversion to methyl palmitate (5, 29).
Calculations
Palmitate rate of appearance (Ra) in plasma was calculated by dividing the palmitate tracer infusion rate by the average plasma palmitate TTR value between 60 and 180 min during physiologic and isotopic steady state conditions. FFA Ra, which equals FFA rate of disappearance (Rd) from plasma during metabolic steady state (30), was derived from the proportional contribution of palmitate to total FFA concentration in plasma (31). FFA Ra was expressed in different ways: 1) FFA Ra in µmol·min−1 - the total amount of FFA released into the circulation, 2) FFA Ra in µmol·kg FM−1·min−1 - an index of FFA released into plasma with respect to endogenous TG stores in adipose tissue, and 3) FFA Ra in µmol·kg FFM−1·min−1 - an index of FFA release in relationship to tissues that consume FFA as a fuel and have high energy requirements.
An assessment of insulin resistance was made by using the homeostasis model assessment (HOMA), which is based on plasma glucose and insulin concentrations (32).
Statistical Analysis
All data were normally distributed. The Pearson product-moment correlation coefficient was determined to evaluate the relationship between anthropometric characteristics and FFA Ra. In addition, forward and backward stepwise regression analyses on the entire group of subjects (men and women) were used to identify determinants of FFA Ra, which were then used to develop a final multiple linear regression model. Models were carefully evaluated to avoid multicollinearity and ensure normal distribution of residuals. If sex was identified as a significant determinant, the same procedures were repeated for men and women separately. All data analyses were carried out by using Sigmastat vers. 2.03, SPSS Inc., Chicago, IL.
RESULTS
Subject characteristics
Body composition and metabolic characteristics of the study subjects are shown in Table 1. Both male and female groups contained lean, overweight and obese subjects. Consequently, there were large ranges in total and regional fat masses (~15–25 fold difference between the leanest and most obese subjects). FFM differed by two-fold between the leanest and most obese subjects. Although plasma glucose concentrations were similar among subjects (study population coefficient of variation [CV] = 8%), there was a large range in plasma insulin concentrations (CV = 69%), HOMA-IR values (CV = 72%), and plasma FFA concentration (CV = 32%).
Table 1.
Age, body composition and metabolic characteristics of the study subjects.
| All subjects | Men (n = 43) |
Women (n = 63) |
|
|---|---|---|---|
| Median (range) | |||
| Age (y) | 32.0 (18.0 – 59.0) | 30.7 (18.0 – 58.0) | 37.0 (21.0 – 59.0) |
| Body mass index (kg/m2) | 32.0 (18.1 – 44.3) | 26.3 (18.1 – 44.0) | 32.9 (19.8 – 44.3) |
| Fat-free mass (kg) | 56.7 (37.9 – 84.9) | 66.9 (46.8 – 84.9) | 52.2 (37.9 – 67.3) |
| Fat mass (kg) | 33.9 (4.8 – 67.5) | 16.7 (4.8 – 66.3) | 39.3 (11.5 – 67.5) |
| Fat mass (% body weight) | 36.0 (7.0 – 56.0) | 20.0 (7.0 – 51.0) | 42.0 (21.5 – 56.0) |
| Total abdominal fat mass (cm2) | 450.8 (36.0 – 927.0) | 204.9 (36.0 – 927.0) | 498.8 (73.0 – 875.0) |
| Subcutaneous abdominal fat mass (cm2) | 305.2 (30.0 – 730.7) | 107.0 (30.0 – 662.4) | 359.3 (65.0 – 730.7) |
| Intra-abdominal fat mass (cm2) | 109.2 (6.0 – 530.0) | 94.9 (6.0 – 530.0) | 117.4 (8.0 – 447.3) |
| Plasma glucose (mM) | 5.1 (4.3 – 6.1) | 5.1 (4.3 – 5.7) | 5.1 (4.3 – 6.1) |
| Plasma insulin (mU/l) | 8.8 (2.0 – 37.1) | 6.9 (2.0 – 30.4) | 10.7 (2.3 – 37.1) |
| Plasma FFA (µM) | 415 (153 – 782) | 365 (153 – 782) | 442 (161 – 689) |
| HOMA-IR | 2.08 (0.42 – 8.69) | 1.47 (0.42 – 7.06) | 2.37 (0.50 – 8.69) |
HOMA-IR: homeostasis model assessment of insulin resistance.
Relationship among plasma FFA concentration, FFA Ra, and body composition
Total FFA Ra (µmol·min−1) accounted for ~30% of the variation in plasma FFA concentration (r = 0.538; P < 0.001). There was no relationship between BMI or percent body fat or FM and plasma FFA concentration.
The relationships between FFA Ra and body composition in men and women are summarized in Table 2. There was no difference between men and women in the relationship between all measures of adiposity (BMI, FM, percent body fat, total abdominal fat, subcutaneous abdominal fat, and intra-abdominal fat) and total FFA Ra and FFA Ra expressed per kg FM. However, the intercepts of the regression lines describing the relationship between all measures of adiposity and FFA Ra per kg FFM were significantly greater (P ≤ 0.05) in women than in men, so the data for men and women are presented separately.
Table 2.
Pearson product-moment correlation coefficients for the relationships between FFA rate of appearance (Ra) into plasma and variables of body composition in men and women.
| FFA Ra (µmol·min−1) |
FFA Raa (µmol·kg FM−1·min−1) |
FFA Rab (µmol·kg FFM−1·min−1) |
||
|---|---|---|---|---|
| Men | Women | |||
| Body mass index (kg/m2) | 0.661† | −0.650† | 0.661† | 0.324** |
| Fat mass (kg) | 0.652† | −0.806† | 0.714† | 0.333** |
| Fat mass (% body weight) | 0.557† | −0.817† | 0.719† | 0.294* |
| Total abdominal fat mass (cm2) | 0.677† | −0.649† | 0.632† | 0.466† |
| Subcutaneous-abdominal fat mass (cm2) | 0.573† | −0.678† | 0.626† | 0.318* |
| Intra-abdominal fat mass (cm2) | 0.546† | −0.471** | 0.509† | 0.524† |
| Fat-free mass (kg) | N.D. | N.S. | N.S. | N.S. |
curvilinear fit.
Data for men (n = 43) and women (n = 63) are presented separately because the intercept of the regression lines for men and women, respectively was significantly different (P ≤ 0.05).
P < 0.05;
P ≤ 0.001;
P < 0.001;
N.S. = not significant.
N.D. = not determined because of marked sex differences (see Figure 1, bottom panel).
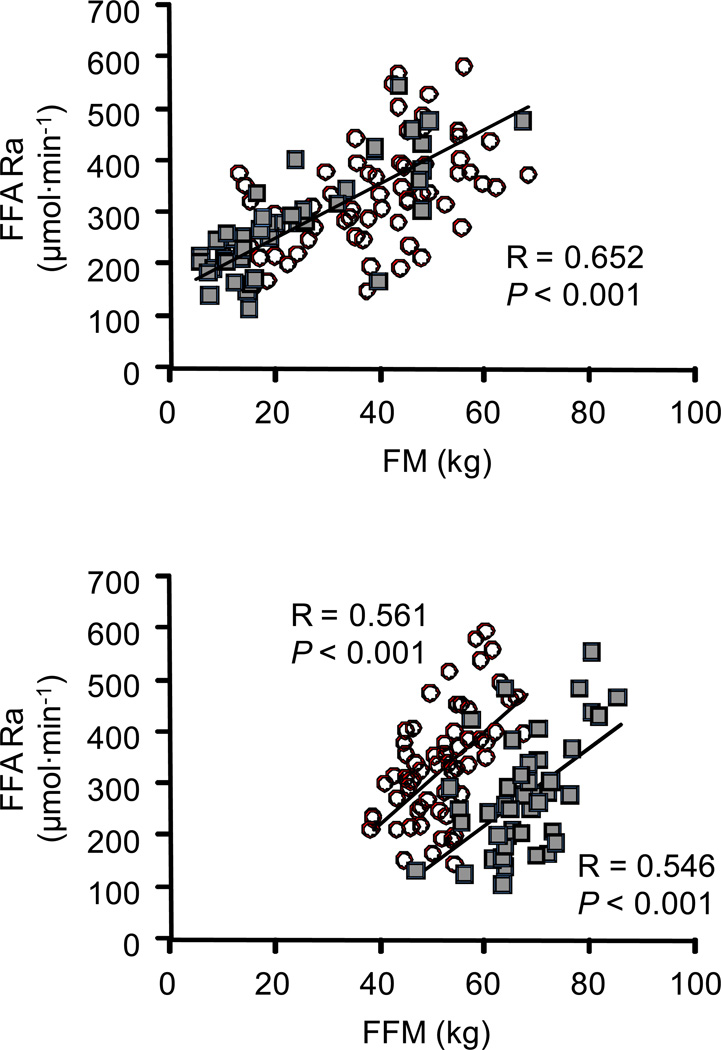
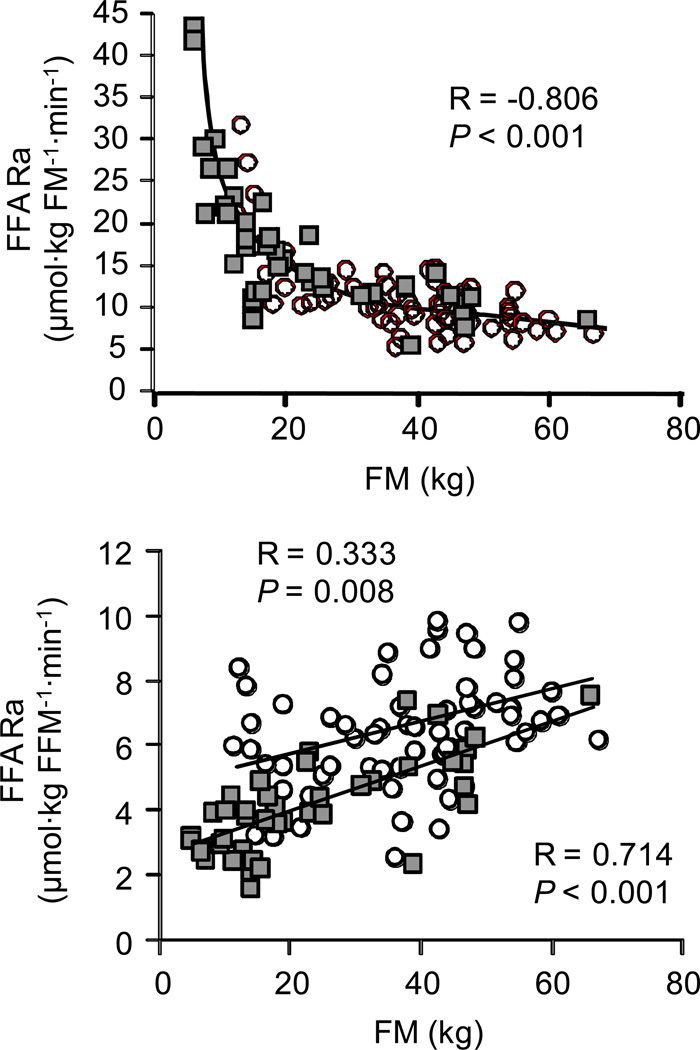
Total FFA Ra (µmol·min−1) correlated directly with all measures of adiposity because of the close relationship among the different assessments of adiposity; FM was correlated with BMI (r = 0.923; P < 0.001), percent body fat (r = 0.940; P < 0.001), total abdominal fat mass (r = 0.861; P < 0.001), subcutaneous abdominal fat mass (r = 0.863; P < 0.001) and intra-abdominal fat mass (r = 0.510; P < 0.001). The relationship between FM and total FFA Ra is shown in Figure 1, top panel. Total FFA Ra correlated directly with FFM in both men and women; however, at any given amount of FFM, total FFA Ra was greater in women than in men (Figure 1, bottom panel). There was a curvilinear, inverse relationship between FFA Ra expressed per kg FM and all measures of adiposity; the relationship between FM and FFA Ra per kg FM is shown in Figure 2, top panel. FFA Ra expressed per kg FFM was directly correlated with all measures of adiposity both in men and women but FFA Ra per kg FFM was greater in women than in men over the entire range of adiposity (Figure 2, bottom panel).
Figure 1.
Relationship between FM and total FFA Ra (top panel) and FFM and total FFA Ra (bottom panel) in men (n = 43; filled squares) and women (n = 63; open circles). The strength of the relationship was evaluated by using the Pearson product-moment correlation coefficient (R-value) and corresponding P-values. FFA: free fatty acid; FFM: fat-free mass; FM: fat mass; Ra: rate of appearance in plasma.
Figure 2.
Relationship between FM and FFA Ra per kg FM (top panel) and FM and FFA Ra per kg FFM (bottom panel) in men (n = 43; filled squares) and women (n = 63; open circles). The strength of the relationship was evaluated by using the Pearson product-moment correlation coefficient (R-value) and corresponding P-values. The intercepts of the regression lines describing the relationship between FM and FFA Ra per kg FFM for men and women were different (P <0.002). FFA: free fatty acid; FFM: fat-free mass; FM: fat mass; Ra: rate of appearance in plasma.
Multiple linear regression analysis to identify predictors of FFA flux
Multiple linear regression analyses (including sex, age, BMI, FM, FFM, percent body fat, intra-abdominal fat mass, subcutaneous abdominal fat mass, total abdominal fat mass, insulin concentration and HOMA-IR as independent variables) identified total body FM as the single best determinant of both total FFA Ra and FFA Ra per kg FFM in men (r = 0.791 and 0.714, respectively, P < 0.001) and intra-abdominal fat mass as the single best predictor of both total FFA Ra and FFA Ra per kg FFM in women (r = 0.648 and r = 0.524, respectively; P <0.001). Percent body fat was identified as the single best determinant of FFA Ra in relationship to FM with no difference between men and women (r=−0.817, P <0.001). None of the other model variables significantly improved the determination of FFA flux.
DISCUSSION
It has been hypothesized that many of the metabolic abnormalities associated with obesity are caused by excessive FFA release from adipose tissue into plasma. However, the relationship between body fat and FFA metabolism has been confusing because of conflicting data from different studies and potential sex-related differences in FFA metabolism. By studying more than 100 non-diabetic men and women, the results from the present study have clarified several issues regarding the influence of adiposity and sex on FFA kinetics. First, our data demonstrate that sexual dimorphism in FFA kinetics is primarily due to differences in body composition between men and women. We found that the rate of FFA release into plasma per unit of FM is the same in men and women, but total FFA Ra in relationship to FFM is greater in women than men, because women have more body fat than men. Second, total FFA Ra and FFA Ra expressed per unit of FFM increase with increasing adiposity whereas FFA release per unit of FM decreases in a curvilinear fashion with increasing body fat. Therefore, obesity is associated with a decrease in the rate of FFA release from adipose tissue. However, this downregulation in the rate of FFA release per unit of body fat does not completely compensate for the increase in total body fat, so total FFA Ra and FFA availability in relation to FFM are increased. Third, abdominal fat distribution does not have an important independent influence on FFA kinetics. These data underscore the importance of total body fat mass in regulating the basal FFA flux.
Total body adiposity was an important predictor of the rate of FFA release into plasma, independent of sex and body fat distribution. The progressive increase in FFA Ra with increasing FM helps explain the reason for the conflicting results from previous studies, which reported that the rate of FFA release into plasma, expressed per unit of FFM, is increased (11–15) or the same (5, 13, 16, 17) in obese compared with lean subjects. The apparent discrepancy between these studies could be due to differences in the relationship between body FM and FFM and the inherent variability in FFA kinetics at any given amount of body FM. In addition, our data suggest the reason for sexual dimorphism in FFA kinetics, i.e., greater basal FFA Ra relative to either FFM (33) or resting energy expenditure (34) in women than in men, is because women generally have more body fat and less FFM than men (18). In a previous study, Nielson et al. (34) found there was a linear relationship between FFA release and resting energy expenditure (REE), and that REE predicted most of the inter-individual variation in the rate of FFA release. We also observed a direct liner relationship between FFM, which is closely related to REE (34); however, the relationship between FM and FFA Ra was stronger and FM was a better predictor of FFA Ra in our study.
Our data contradict the current dogma that adipose tissue lipolytic activity, and the rate of FFA release into the circulation, are abnormal in obesity (35–40). This notion is based, in large part, on data from studies that found FFA Ra expressed per unit of FFM was increased in obese subjects (11–14). However, the use of ratios to determine whether FFA Ra is “normal” can be misleading when used to compare groups of subjects who have marked differences in body composition, because the relationship between FFA kinetics and all components of body composition have y and x intercepts that are significantly different from zero (e.g. Figure 1, top panel). Therefore, FFA kinetics normalized by body composition (e.g., FFA Ra per kg FM or FFA Ra per kg FFM) will be different between groups who differ in body composition because of mathematical bias rather than true differences in FFA metabolism (41). The most appropriate approach for evaluating whether FFA kinetics are “abnormal” in obesity is by using regression curves among subjects who have a large range in percent body fat mass. The data from the present study demonstrate that basal FFA Ra correlated closely with body FM in lean, overweight and obese subjects, and data from obese subjects followed the same regression curve as data from overweight and lean subjects. Therefore, FFA Ra is “normal” (i.e., as expected) in overweight and obese men and women, even though FFA Ra in relationship to FM decreases and FFA Ra in relationship to FFM increases with increasing amounts of body fat.
The rate of FFA release into plasma per unit of FM progressively decreased with increasing FM. This downregulation has beneficial effects because it helps prevent excessive FFA release into plasma, which could have adverse metabolic consequences (1). However, the decrease in FFA release is not able to completely compensate for the increase in total FM, so total FFA Ra increased with increasing amounts of body fat. The increase in FFA delivery to the liver and skeletal muscle can contribute to insulin resistance in these organs because excessive FFA uptake impairs insulin mediated suppression of glucose production by the liver (1, 3) and insulin-mediated glucose uptake by skeletal muscle (1, 3).
The relative suppression of FFA Ra with increasing adiposity seems surprising, because insulin is the major regulator of basal adipose tissue lipolytic rates and FFA Ra (42, 43) and obesity is associated with adipose tissue insulin resistance (13, 44, 45). Therefore, other regulatory mechanisms must exist that inhibit FFA release from adipose tissue TG, which more than offset the increase in lipolysis induced by insulin resistance. It is unlikely that the differences in FFA Ra we observed between our lean and obese subjects were due to differences in intracellular adipocyte fatty acid re-esterification which would prevent FFA release into plasma, because data from arteriovenous balance studies across adipose tissue in human subjects found re-esterification does not occur during basal, postabsorptive conditions (46). The data obtained in vivo from the present study are consistent with data obtained from ex vivo studies conducted in adipocytes prepared from adipose tissue biopsies obtained from lean and obese subjects. Although lipolytic rate per cell was greater in large adipocytes from obese subjects than in small adipocytes from lean subjects, lipolytic rate in relationship to cell volume (i.e., total TG content) was lower in large than small adipocytes (47, 48).
Abdominal (upper body) obesity is associated with a greater risk of metabolic diseases than femoral/gluteal (lower body) obesity (49). It has been hypothesized that the relationship observed between visceral fat and peripheral insulin resistance is due to increased rates of FFA release into plasma (50). However, in our subjects, intra-abdominal fat mass itself was not an important determinant of FFA flux. Although multivariate regression analysis identified intra-abdominal fat mass as the best predictor of both total FFA Ra and FFA Ra per kg FFM in women, this was most likely a reflection of the inherent instability in multivariate analyses when there are correlated predictors, such as intra-abdominal fat and total body fat mass. Nielson and colleagues (51) found the contribution of FFA derived from lipolysis of visceral adipose tissue to systemic FFA flux was only ~6% in lean and ~14% in obese subjects. Therefore, increased visceral fat mass, itself, is not an important contributor to whole-body FFA flux, and FFA release from visceral fat is unlikely to be responsible for insulin resistance in skeletal muscle.
The results from the present study provide a comprehensive evaluation of the relationship between body composition and FFA kinetics in human subjects. Our data demonstrate that total body fat mass is an important determinant of the rate of FFA release into plasma in both men and women during basal conditions. In addition, the rate of FFA release from adipose tissue decreases with increasing body FM. However, the downregulation of FFA release is unable to completely compensate for the increase in FM, so total FFA Ra is greater in women than in men and in obese than in lean subjects. These findings provide additional insights into the mechanisms responsible for metabolic complications associated with obesity, and provide a framework for interpreting data from other studies evaluating the pathophysiology of FFA kinetics in human subjects.
ACKNOWLEDGEMENTS
The authors wish to thank Danielle Hindman, Jennifer C. McCrea and Megan E. Steward for assistance in subject recruitment, the nursing staff of the Center for Applied Research Sciences for their help in performing the studies, Freida Custodio, Junyoung Kwon, Adewole Okunade, and Gary Skolnick for their technical assistance, and the study subjects for their participation.
This publication was made possible by Grant Number UL1 RR024992 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), NIH grants DK 37948, AT 0110, AR 49869, HD 057796, DK 56341 (Clinical Nutrition Research Unit), and RR 00954 (Biomedical Mass Spectrometry Resource), and grants from the International Life Sciences Foundation, and the American Heart Association (0365436Z and 0510015Z).
Footnotes
DISCLOSURES
The authors have no financial or personal interest in any company or organization sponsoring the research, including advisory board affiliations.
REFERENCES
- 1.Boden G. Fatty acid-induced inflammation and insulin resistance in skeletal muscle and liver. Curr Diab Rep. 2006;6:177–181. doi: 10.1007/s11892-006-0031-x. [DOI] [PubMed] [Google Scholar]
- 2.Petersen KF, Shulman GI. Pathogenesis of skeletal muscle insulin resistance in type 2 diabetes mellitus. Am J Cardiol. 2002;90:11G–18G. doi: 10.1016/s0002-9149(02)02554-7. [DOI] [PubMed] [Google Scholar]
- 3.Boden G, Chen X, Ruiz J, White JV, Rossetti L. Mechanisms of fatty acid-induced inhibition of glucose uptake. J Clin Invest. 1994;93:2438–2446. doi: 10.1172/JCI117252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwarz JM, Neese RA, Turner S, Dare D, Hellerstein MK. Short-term alterations in carbohydrate energy intake in humans. Striking effects on hepatic glucose production, de novo lipogenesis, lipolysis, and whole-body fuel selection. J Clin Invest. 1995;96:2735–2743. doi: 10.1172/JCI118342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mittendorfer B, Patterson BW, Klein S. Effect of weight loss on VLDL-triglyceride and apoB-100 kinetics in women with abdominal obesity. Am J Physiol Endocrinol Metab. 2003;284:E549–E556. doi: 10.1152/ajpendo.00379.2002. [DOI] [PubMed] [Google Scholar]
- 6.Magkos F, Wright DC, Patterson BW, Mohammed BS, Mittendorfer B. Lipid metabolism response to a single, prolonged bout of endurance exercise in healthy young men. Am J Physiol Endocrinol Metab. 2006;290:E355–E362. doi: 10.1152/ajpendo.00259.2005. [DOI] [PubMed] [Google Scholar]
- 7.Barrows BR, Parks EJ. Contributions of different fatty acid sources to very low-density lipoprotein-triacylglycerol in the fasted and fed states. J Clin Endocrinol Metab. 2006;91:1446–1452. doi: 10.1210/jc.2005-1709. [DOI] [PubMed] [Google Scholar]
- 8.Cullen P. Evidence that triglycerides are an independent coronary heart disease risk factor. Am J Cardiol. 2000;86:943–949. doi: 10.1016/s0002-9149(00)01127-9. [DOI] [PubMed] [Google Scholar]
- 9.Castelli WP. Cholesterol and lipids in the risk of coronary artery disease--the Framingham Heart Study. Can J Cardiol. 1988;4 Suppl A:5A–10A. [PubMed] [Google Scholar]
- 10.Shoelson SE, Lee J, Yuan M. Inflammation and the IKK beta/I kappa B/NF-kappa B axis in obesity- and diet-induced insulin resistance. Int J Obes Relat Metab Disord. 2003;27 Suppl 3:S49–S52. doi: 10.1038/sj.ijo.0802501. [DOI] [PubMed] [Google Scholar]
- 11.Wolfe RR, Peters EJ, Klein S, Holland OB, Rosenblatt J, Gary H. Effect of short-term fasting on lipolytic responsiveness in normal and obese human subjects. Am J Physiol. 1987;252:E189–E196. doi: 10.1152/ajpendo.1987.252.2.E189. [DOI] [PubMed] [Google Scholar]
- 12.Horowitz JF, Coppack SW, Paramore D, Cryer PE, Zhao G, Klein S. Effect of short-term fasting on lipid kinetics in lean and obese women. Am J Physiol. 1999;276:E278–E284. doi: 10.1152/ajpendo.1999.276.2.E278. [DOI] [PubMed] [Google Scholar]
- 13.Jensen MD, Haymond MW, Rizza RA, Cryer PE, Miles JM. Influence of body fat distribution on free fatty acid metabolism in obesity. J Clin Invest. 1989;83:1168–1173. doi: 10.1172/JCI113997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanaley JA, Cryer PE, Jensen MD. Fatty acid kinetic responses to exercise. Effects of obesity, body fat distribution, and energy-restricted diet. J Clin Invest. 1993;92:255–261. doi: 10.1172/JCI116559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bjorntorp P, Bergman H, Varnauskas E. Plasma free fatty acid turnover rate in obesity. Acta Med Scand. 1969;185:351–356. doi: 10.1111/j.0954-6820.1969.tb07347.x. [DOI] [PubMed] [Google Scholar]
- 16.Horowitz JF, Klein S. Whole body and abdominal lipolytic sensitivity to epinephrine is suppressed in upper body obese women. Am J Physiol Endocrinol Metab. 2000;278:E1144–E1152. doi: 10.1152/ajpendo.2000.278.6.E1144. [DOI] [PubMed] [Google Scholar]
- 17.Mittendorfer B, Fields DA, Klein S. Excess body fat in men decreases plasma fatty acid availability and oxidation during endurance exercise. Am J Physiol Endocrinol Metab. 2004;286:E354–E362. doi: 10.1152/ajpendo.00301.2003. [DOI] [PubMed] [Google Scholar]
- 18.Gallagher D, Heymsfield SB, Heo M, Jebb SA, Murgatroyd PR, Sakamoto Y. Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. Am J Clin Nutr. 2000;72:694–701. doi: 10.1093/ajcn/72.3.694. [DOI] [PubMed] [Google Scholar]
- 19.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 20.Magkos F, Patterson BW, Mittendorfer B. No effect of menstrual cycle phase on VLDL-triglyceride and VLDL-apolipoprotein B-100 metabolism. Am J Physiol Endocrinol Metab. 2006;291:E1243–E1249. doi: 10.1152/ajpendo.00246.2006. [DOI] [PubMed] [Google Scholar]
- 21.Heiling VJ, Jensen MD. Free fatty acid metabolism in the follicular and luteal phases of the menstrual cycle. J Clin Endocrinol Metab. 1992;74:806–810. doi: 10.1210/jcem.74.4.1548345. [DOI] [PubMed] [Google Scholar]
- 22.Horton TJ, Miller EK, Bourret K. No effect of menstrual cycle phase on glycerol or palmitate kinetics during 90 min of moderate exercise. J Appl Physiol. 2006;100:917–925. doi: 10.1152/japplphysiol.00491.2005. [DOI] [PubMed] [Google Scholar]
- 23.Casazza GA, Jacobs KA, Suh SH, Miller BF, Horning MA, Brooks GA. Menstrual cycle phase and oral contraceptive effects on triglyceride mobilization during exercise. J Appl Physiol. 2004;97:302–309. doi: 10.1152/japplphysiol.00050.2004. [DOI] [PubMed] [Google Scholar]
- 24.Van Pelt RE, Gozansky WS, Hickner RC, Schwartz RS, Kohrt WM. Acute modulation of adipose tissue lipolysis by intravenous estrogens. Obesity (Silver Spring) 2006;14:2163–2172. doi: 10.1038/oby.2006.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tchernof A, Desmeules A, Richard C, et al. Ovarian hormone status and abdominal visceral adipose tissue metabolism. J Clin Endocrinol Metab. 2004;89:3425–3430. doi: 10.1210/jc.2003-031561. [DOI] [PubMed] [Google Scholar]
- 26.Mauriege P, Imbeault P, Prud'Homme D, Tremblay A, Nadeau A, Despres JP. Subcutaneous adipose tissue metabolism at menopause: importance of body fatness and regional fat distribution. J Clin Endocrinol Metab. 2000;85:2446–2454. doi: 10.1210/jcem.85.7.6687. [DOI] [PubMed] [Google Scholar]
- 27.Racette SB, Horowitz JF, Mittendorfer B, Klein S. Racial differences in lipid metabolism in women with abdominal obesity. Am J Physiol Regul Integr Comp Physiol. 2000;279:R944–R950. doi: 10.1152/ajpregu.2000.279.3.R944. [DOI] [PubMed] [Google Scholar]
- 28.Metropolitan Life Insurance Company. 1983 Metropolitan height and weight tables. Stat Bull Metrop Life Found. 1983;64:3–9. [PubMed] [Google Scholar]
- 29.Patterson BW, Zhao G, Elias N, Hachey DL, Klein S. Validation of a new procedure to determine plasma fatty acid concentration and isotopic enrichment. J Lipid Res. 1999;40:2118–2124. [PubMed] [Google Scholar]
- 30.Wolfe RR. Radioactive and Stable Isotope Tracers in Biomedicine: Principles and Practice of Kinetic Analysis. New York: Wiley-Liss; 1992. [Google Scholar]
- 31.Mittendorfer B, Liem O, Patterson BW, Miles JM, Klein S. What does the measurement of whole-body fatty acid rate of appearance in plasma by using a fatty acid tracer really mean? Diabetes. 2003;52:1641–1648. doi: 10.2337/diabetes.52.7.1641. [DOI] [PubMed] [Google Scholar]
- 32.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 33.Mittendorfer B, Horowitz JF, Klein S. Effect of gender on lipid kinetics during endurance exercise of moderate intensity in untrained subjects. Am J Physiol Endocrinol Metab. 2002;283:E58–E65. doi: 10.1152/ajpendo.00504.2001. [DOI] [PubMed] [Google Scholar]
- 34.Nielsen S, Guo Z, Albu JB, Klein S, O'Brien PC, Jensen MD. Energy expenditure, sex, and endogenous fuel availability in humans. J Clin Invest. 2003;111:981–988. doi: 10.1172/JCI16253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bays H. Adiposopathy: role of adipocyte factors in a new paradigm. Expert Rev Cardiovasc Ther. 2005;3:187–189. doi: 10.1586/14779072.3.2.187. [DOI] [PubMed] [Google Scholar]
- 36.Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest. 2002;32 Suppl 3:14–23. doi: 10.1046/j.1365-2362.32.s3.3.x. [DOI] [PubMed] [Google Scholar]
- 37.Boden G. Free fatty acids (FFA), a link between obesity and insulin resistance. Front Biosci. 1998;3:d169–d175. doi: 10.2741/a272. [DOI] [PubMed] [Google Scholar]
- 38.Bairras C, Mauriege P, Bukowiecki L, Atgie C. Regulation of lypolysis in white adipose tissues of lean and obese Zucker rats. J Physiol Biochem. 2007;63:287–296. doi: 10.1007/BF03165760. [DOI] [PubMed] [Google Scholar]
- 39.Langin D, Dicker A, Tavernier G, et al. Adipocyte lipases and defect of lipolysis in human obesity. Diabetes. 2005;54:3190–3197. doi: 10.2337/diabetes.54.11.3190. [DOI] [PubMed] [Google Scholar]
- 40.Hoffstedt J, Arner P, Hellers G, Lonnqvist F. Variation in adrenergic regulation of lipolysis between omental and subcutaneous adipocytes from obese and non-obese men. J Lipid Res. 1997;38:795–804. [PubMed] [Google Scholar]
- 41.Tanner JM. Fallacy of per-weight and per-surface area standards, and their relation to spurious correlation. J Appl Physiol. 1949;2:1–15. doi: 10.1152/jappl.1949.2.1.1. [DOI] [PubMed] [Google Scholar]
- 42.Horowitz JF, Mora-Rodriguez R, Byerley LO, Coyle EF. Lipolytic suppression following carbohydrate ingestion limits fat oxidation during exercise. Am J Physiol. 1997;273:E768–E775. doi: 10.1152/ajpendo.1997.273.4.E768. [DOI] [PubMed] [Google Scholar]
- 43.Jensen MD, Caruso M, Heiling V, Miles JM. Insulin regulation of lipolysis in nondiabetic and IDDM subjects. Diabetes. 1989;38:1595–1601. doi: 10.2337/diab.38.12.1595. [DOI] [PubMed] [Google Scholar]
- 44.Albu JB, Curi M, Shur M, Murphy L, Matthews DE, Pi-Sunyer FX. Systemic resistance to the antilipolytic effect of insulin in black and white women with visceral obesity. Am J Physiol. 1999;277:E551–E560. doi: 10.1152/ajpendo.1999.277.3.E551. [DOI] [PubMed] [Google Scholar]
- 45.Coppack SW, Evans RD, Fisher RM, et al. Adipose tissue metabolism in obesity: lipase action in vivo before and after a mixed meal. Metabolism. 1992;41:264–272. doi: 10.1016/0026-0495(92)90269-g. [DOI] [PubMed] [Google Scholar]
- 46.Coppack SW, Persson M, Judd RL, Miles JM. Glycerol and nonesterified fatty acid metabolism in human muscle and adipose tissue in vivo. Am J Physiol. 1999;276:E233–E240. doi: 10.1152/ajpendo.1999.276.2.E233. [DOI] [PubMed] [Google Scholar]
- 47.Goldrick RB, McLoughlin GM. Lipolysis and lipogenesis from glucose in human fat cells of different sizes. Effects of insulin, epinephrine, and theophylline. J Clin Invest. 1970;49:1213–1223. doi: 10.1172/JCI106335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arner P, Engfeldt P, Ostman J. Relationship between lipolysis, cyclic, AMP fat-cell size in human adipose tissue during fasting and in diabetes mellitus. Metabolism. 1979;28:198–209. doi: 10.1016/0026-0495(79)90065-9. [DOI] [PubMed] [Google Scholar]
- 49.Krotkiewski M, Bjorntorp P, Sjostrom L, Smith U. Impact of obesity on metabolism in men and women. Importance of regional adipose tissue distribution. J Clin Invest. 1983;72:1150–1162. doi: 10.1172/JCI111040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Despres JP. Is visceral obesity the cause of the metabolic syndrome? Ann Med. 2006;38:52–63. doi: 10.1080/07853890500383895. [DOI] [PubMed] [Google Scholar]
- 51.Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. J Clin Invest. 2004;113:1582–1588. doi: 10.1172/JCI21047. [DOI] [PMC free article] [PubMed] [Google Scholar]