Abstract
Medicaid programs use preferred drug lists to limit high-cost prescribing, but their effect on promoting more effective and safer prescribing is unknown. We examined the impact of the May 2007 rosiglitazone safety warnings on rosiglitazone availability on state Medicaid preferred drug list programs and antihyperglycemic medication prescribing among Medicaid beneficiaries. Nearly all state Medicaid programs provided rosiglitazone coverage, with minimal change after the safety warnings. Monthly rosiglitazone use was consistently higher among states that provided coverage, although the safety warnings were associated with prescribing changes among all antihyperglycemic medications. Medicaid programs could better utilize incentive formularies to promote high-quality prescribing.
Keywords: Medicaid, Vulnerable populations, Pharmaceuticals
Medicaid is the government-sponsored health insurance program for individuals and families with low incomes and minimal resources in the United States (1). Jointly funded by state and federal governments, each state manages its Medicaid program, allowing for differences in eligibility, design and benefits. More than 40 state Medicaid programs have adopted varying incentive formulary designs, such as preferred drug lists and prior authorization programs, in an effort to manage pharmaceutical costs (2). In essence, a preferred drug list represents a list of medications for which the state Medicaid program provides full coverage without prior notification, authorization, or review. Often, medications included on a preferred drug list are the least costly among any class of therapeutics. Any medication not included on a preferred drug list generally requires prior authorization for coverage, whereby the prescribing physician must obtain approval from the state Medicaid program prior to prescribing.
While Medicaid preferred drug lists and prior authorization programs have consistently been effective at limiting high-cost prescribing (3–5), their role in promoting higher-quality prescribing, prescribing that is both effective and safe, has rarely been studied. The effect of these programs on promoting more-effective prescribing is inconsistent (6). Furthermore, few studies have examined how these programs incorporate safety concerns into their formulary design. In one study examining Medicaid prior authorization policies, there were no coverage changes in response to the Food and Drug Administration’s (FDA) safety warning on atypical antipsychotic use among elderly adults with dementia (7).
Thiazolidinediones (TZDs) are a class of medications widely used to lower blood glucose in patients with type 2 diabetes mellitus (8) and their safety has been increasingly questioned (9, 10), particularly since safer and cheaper alternatives are widely available: metformin and sulfonylureas. Two TZDs are currently available, pioglizazone and rosiglitazone. Pioglitazone carries a black box warning of increased risk of heart failure (11). Rosiglitazone carries the same warning but was also subject to greater scrutiny after a May 2007 meta-analysis suggested it increased risk of myocardial infarction (12). This meta-analysis triggered a safety alert by the FDA that same month (13) and Congressional hearings on the topic shortly thereafter in June 2007. In September 2010 the FDA updated rosiglitazone’s product label to include information on cardiovascular risks and in May 2011, the FDA implemented a risk evaluation and mitigation strategy, which restricts the drug’s availability and applies specified criteria for use (14, 15).
Several studies have demonstrated that rosiglitazone prescribing decreased precipitously after this combination of events that included the published meta-analysis, the FDA’s 2007 safety alert, and the surrounding media attention (8, 16–19). However, none of these studies focused on prescribing for Medicaid beneficiaries to understand the role incentive formulary designs, such as preferred drug lists and prior authorization programs, could play in the promotion of safer, higher-quality prescribing, a critical issue because of the prevalence of type 2 diabetes among beneficiaries (20) and Medicaid programs role as payor for disadvantaged, vulnerable populations.
Examining the response of Medicaid programs to the 2007 rosiglitazone safety warnings, which includes the published meta-analysis, FDA safety alert, and the surrounding media attention (12, 13), provides a useful natural experiment to inform expectations for the potential impact that preferred drug list programs may have both in limiting medications when safety concerns are raised and in promoting safer, alternative medications. Our objective was to examine changes in rosiglitazone coverage by state Medicaid programs and the impact the May 2007 rosiglitazone safety warnings had within the Medicaid population on prescribing of rosiglitazone, pioglitazone, metformin, and sulfonylureas, all medications which have been shown to have similar effectiveness for managing glucose levels in diabetes care, albeit with varying safety profiles and impact on clinical outcomes (21).
METHODS
Data Sources
We conducted a retrospective cohort study using interrupted time-series methods. We used data from the Xponent™ database, published by IMS HEALTH (Collegeville, PA), a provider of market intelligence to the pharmaceutical and health care industries, for the period January 2006 through June 2009. The Xponent™ database captures more than 70% of all prescriptions dispensed in the United States and uses a patented projection methodology to represent 100% of the prescriptions dispensed in the United States. Using Monte Carlo simulation studies, the Xponent™ database has been validated and sampling errors have been estimated to be approximately 3% (22).
The Xponent™ database includes state-level monthly data on thetotal number of prescriptions dispensed to patients by payor for all antihyperglycemic agents and forindividual brands (excluding insulin). We limited our sample to medication prescriptions paid for by state Medicaid programs; prescriptions covered by private insurance plans via Medicaid managed care programs were not identifiable for analysis.
Preferred Drug Lists
We surveyed the Medicaid programs from all fifty states and the District of Columbia to determine whether rosiglitazone or pioglitazone were covered as preferred drug list medications without prior authorization for each year between 2006 and 2009. Again, preferred drug lists are lists of medications for which state Medicaid programs provide full coverage without prior authorization; any medication not included on the list generally requires prior authorization for coverage. We first searched state Medicaid websites to identify preferred drug lists. If no preferred drug list was found, we contacted state Medicaid program officials by email or by telephone to make determinations. Covered includes coverage under the preferred drug list or use of an open formulary; not covered implies prior authorization requirements were in place.
Main Outcome Measures
We categorized sixteen distinct antihyperglycemic drug entities into four classes of medications: metformin (i.e., biguanides, but metformin is the only one available in this class in the U.S.), sulfonylureas, TZDs, and all others. State-level proportions of total prescriptions for each drug class and individual drug were calculated for each month of study. For combination drugs and drug classes, the prescription was attributed to each drug and drug class; however, the denominator counted combination prescriptions as one prescription. Our main outcome of interest was the proportion of rosiglitazone prescriptions among all non-insulin, antihyperglycemic medication prescriptions.
Statistical Analysis
We first used descriptive statistics to characterize whether Medicaid programs did or did not provide rosiglitazone coverage. We then used descriptive statistics to calculate monthly proportions of total prescriptions for each drug class and individual drug among all non-insulin, antihyperglycemic medications, categorized by state Medicaid program rosiglitazone coverage. We were unable to verify rosiglitazone coverage for all years in some states or for any years in two states, Arizona and Nevada. When this information was not available, we did not include data for that state/year in analyses.
The main analysis of interest was the impact of the May 2007 rosiglitazone safety warnings, which includes the published meta-analysis, FDA safety alert, and the surrounding media attention (12, 13), on prescribing of rosiglitazone among Medicaid programs that did and did not provide rosiglitazone coverage, and on prescribing of pioglitazone, metformin, and sulfonylureas. We examined the changes in proportional prescribing during the one year period after the safety warning was issued as well as longer term prescribing patterns.
The impact of this warning was determined using interrupted time-series regression analysis. This method is commonly used for evaluating effects of an “interruption” that occurs at a specific point in a time series — in this case, May 2007. The time series incorporated a total of 42 months, from January 2006 to June 2009, including 17 months before and 25 months after the safety warning was raised. This time frame should be adequate since the general recommendation is to include 12 observations before and after the interruption (23). However, because of this requirement, four states that changed rosiglitazone coverage during the study period were excluded from time-series analyses due to insufficient observation periods.
For analyses of each medication(s), interrupted time-series regression models were fit independently for state Medicaid programs that did and did not provide rosiglitazone coverage. After careful visual inspection of the data, we determined that rosiglitazone and pioglitazone prescribing was not linear over our observation period. To account for non-linearity, we adapted the regression models by separating the time period into three segments: before safety warning, one year after safety warning, and the subsequent observation period, estimated as follows. First, a linear trend was fitted for the pre-warnings time period from January 2006 to May 2007. Second, a quadratic trend was fitted for the 12 month post-warnings period of May 2007 to April 2008. Finally, a linear trend was fitted for the 12–24 month post-warnings time period of May 2008 to June 2009. This non-linear model provided a significantly better fit for estimating the impact of the safety warnings on prescribing for each medication(s). In addition, we conducted counterfactual analysis for each of these models, estimating the expected rates of prescribing after May 2007 had the rosiglitazone safety warnings not been issued, using the pre-warning prescribing trends (January 2006–May 2007). We compared the predicted utilization as of June 2009 to the observed utilization.
Because time-dependent data are often correlated, we investigated autocorrelation in the model residuals using the Durbin-Watson test statistic. If autocorrelation was detected, we adjusted it by adding autocorrelation parameters into the regression model. The parameter estimates from the complete regression models are provided in an Appendix. All analyses were conducted using Stata software version 11.0 (StataCorp LP, College Station, TX) and SAS 9.2 (SAS Institute, Inc., Cary, NC). P-values <0.05 were considered statistically significant.
LIMITATIONS
There are several limitations to consider. IMS Health provides aggregated data. We did not have access to patient or prescriber characteristics or clinical data, such as medical conditions or adverse reactions to non-TZD diabetes medications to determine whether rosiglitazone prescribing was clinically appropriate. In addition, we are unable to evaluate whether rosiglitazone was used as a first line or second line treatment. Finally, although greater rosiglitazone prescribing was found among state Medicaid programs that provided rosiglitazone coverage, we did not evaluate the association between rosiglitazone prescribing and patient outcomes, neither safety nor efficacy.
RESULTS
Medicaid Program Rosiglitazone Coverage
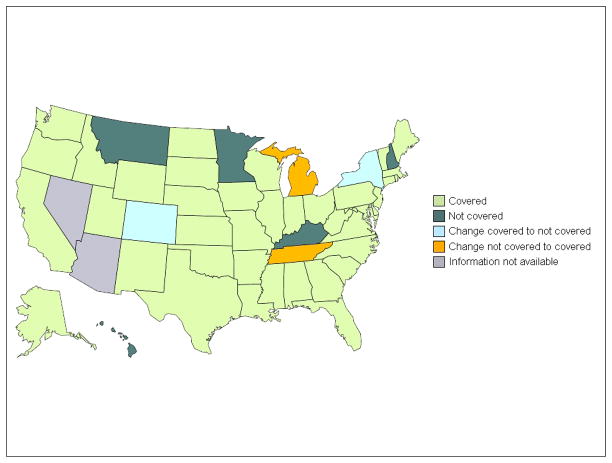
Nearly all state Medicaid programs provided rosiglitazone coverage from 2006 to 2009. In 2006, 42 of 49 states (86%) provided rosiglitazone coverage, either explicitly though a preferred drug list or implicitly through an open formulary (Figure 1). As of 2009, 42 (86%) states continued to provide rosiglitazone coverage. Between these two periods, two states switched from providing to not providing coverage (Colorado and New York) and two switched from not providing to providing coverage (Michigan and Tennessee; Figure 1). Similarly, in 2006, 48 of 49 (98%) states provided pioglitazone coverage, and in 2009, all 49 states provided pioglitazone coverage.
Figure 1.
State Medicaid program rosiglitazone coverage, 2006–2009.
Source: Authors’ survey of state Medicaid program preferred drug lists and prior authorization programs.
Antihyperglycemic Medication Prescribing
The overall volume of antihyperglycemic medication prescribing declined slightly over our study. In the 2006 calendar year, there were 4,658,549 antihyperglycemic medication prescriptions among Medicaid beneficiaries, 259,157 among states that provided coverage and 4,399,392 among states that did not. In the 2008 calendar year, there were 4,186,039 prescriptions, 263,634 and 3,922,405 respectively.
Rosiglitazone Prescribing
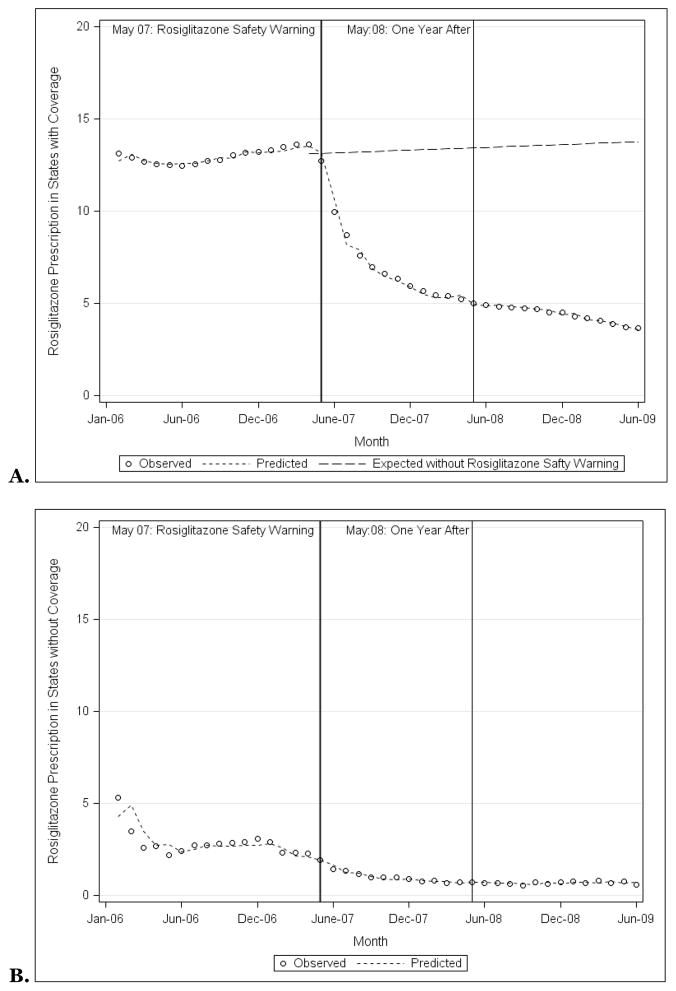
Overall, rosiglitazone prescribing was consistently higher among states with Medicaid programs that provided rosiglitazone coverage when compared with states with Medicaid programs that did not provide coverage. In January 2006, 13.1% of antihyperglycemic prescriptions were for rosiglitazone in states that provided coverage (Figure 2A), 5.3% in states that did not (p<0.001; Figure 2B). By June 2009, 3.7% of antihyperglycemic prescriptions were for rosiglitazone in states that provided coverage, 0.6% in states that did not (p=0.002).
Figure 2.
Observed and predicted rosiglitazone prescriptions as a proportion of all prescriptions for non-insulin antihyperglycemic medications among state Medicaid programs that did (A) and did not (B) provide coverage for rosiglitazone, 2006–2009, along with the prescription rates that would have been expected had the rosiglitazone safety warnings not been issued.
Source: Authors’ analyses based on IMS HEALTH Xponent™ database.
The rosiglitazone safety warnings were associated with decreased rosiglitazone prescribing only among states with Medicaid programs that provided rosiglitazone coverage, not among states that did not. Among states with Medicaid programs that provided coverage, before the safety warnings, rosiglitazone prescribing was stable (linear trend regression model slope of 0.03%; p=0.43). In the 12-months immediately afterwards, rosiglitazone prescribing decreased substantially (slope of −2.05%; p<0.001). Finally, in the subsequent 12–24 month period post-warnings, rosiglitazone prescribing continued to decline, although less substantially (slope of −0.13%; p<0.001). Using counterfactual analysis to estimate expected rates of rosiglitazone prescribing among states with Medicaid programs that provided rosiglitazone coverage had the rosiglitazone safety warnings not been issued, the safety warnings were associated with a 73.6% decline in rosiglitazone prescribing as of June 2009 (95% Confidence Interval (CI): −79.5%, −67.7%).
Among states with Medicaid programs that did not provide rosiglitazone coverage, before the safety warnings, rosiglitazone prescribing was steadily declining (slope of −0.16%; p=0.004). However, there was no significant decline in rosiglitazone prescribing in either the 12-month period or 12–24 month period after the safety warnings (slopes of −0.08%, p=0.74, and −0.15%, p=0.06, respectively). Counterfactual analysis among states that did not provide rosiglitazone coverage suggested that expected utilization would have reached zero percent had the rosiglitazone safety warning not been issued, lower than the 0.6% observed.
Pioglitazone Prescribing
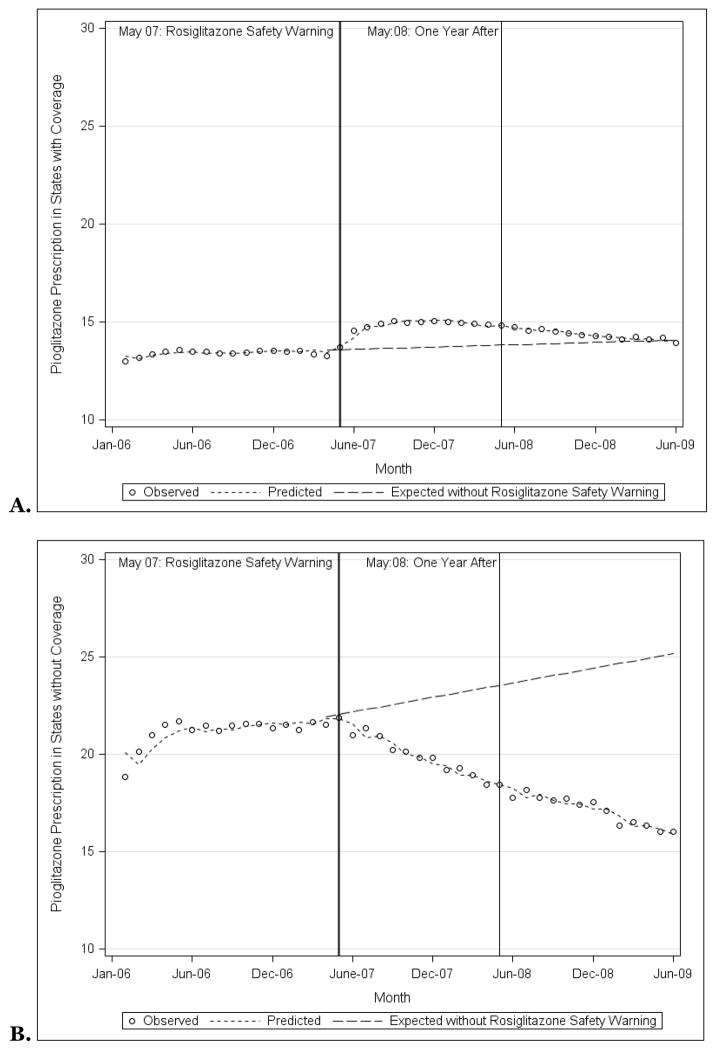
Pioglitazone prescribing was consistently lower among states with Medicaid programs that provided rosiglitazone coverage when compared with states with Medicaid programs that did not, although these differences narrowed over time. In January 2006, 13.0% of antihyperglycemic prescriptions were for pioglitazone in states that provided rosiglitazone coverage (Figure 3A), 18.8% in states that did not (p<0.001; Figure 3B). By June 2009, 13.9% and 16.0% of prescriptions were for pioglitazone respectively (p<0.001).
Figure 3.
Observed and predicted pioglitazone prescriptions as a proportion of all prescriptions for non-insulin antihyperglycemic medications among state Medicaid programs that did (A) and did not (B) provide coverage for rosiglitazone, 2006–2009, along with the prescription rates that would have been expected had the rosiglitazone safety warnings not been issued.
Source: Authors’ analyses based on IMS HEALTH Xponent™ database.
The rosiglitazone safety warnings were associated with decreased pioglitazone prescribing only among states with Medicaid programs that did not provide rosiglitazone coverage, not among states that did provide coverage. Among states with Medicaid programs that provided rosiglitazone coverage, before the safety warnings, pioglitazone prescribing was declining minimally (slope of −0.02%; p=0.06). In the 12-months immediately afterwards, pioglitazone prescribing increased (slope of 0.40%; p<0.001). Finally, in the subsequent 12–24 month period post-warnings, pioglitazone again declined (slope of −0.08%; p<0.001). Counterfactual analysis among states with Medicaid programs that provided rosiglitazone coverage demonstrated that the safety warnings were not associated with a significant change in pioglitazone prescribing (−0.9%, 95% CI: −5.7%, 3.9%).
In contrast, among states with Medicaid programs that did not provide rosiglitazone coverage, before the safety warnings, pioglitazone prescribing was steadily increasing (slope of 0.12%; p=0.006). In the 12-months immediately afterwards, pioglitazone prescribing decreased substantially (slope of −0.55%; p=0.02). Finally, in the subsequent 12–24 month period post-warnings, pioglitazone prescribing continued to decline, although less substantially (slope of −0.31%; p<0.001). Among states with Medicaid programs that did not provide rosiglitazone coverage, counterfactual analysis demonstrated that the safety warnings were associated with a 36.4% decline in pioglitazone prescribing compared to what would have been expected as of June 2009 (95% CI: −44.0%, −28.9%).
Metformin Prescribing
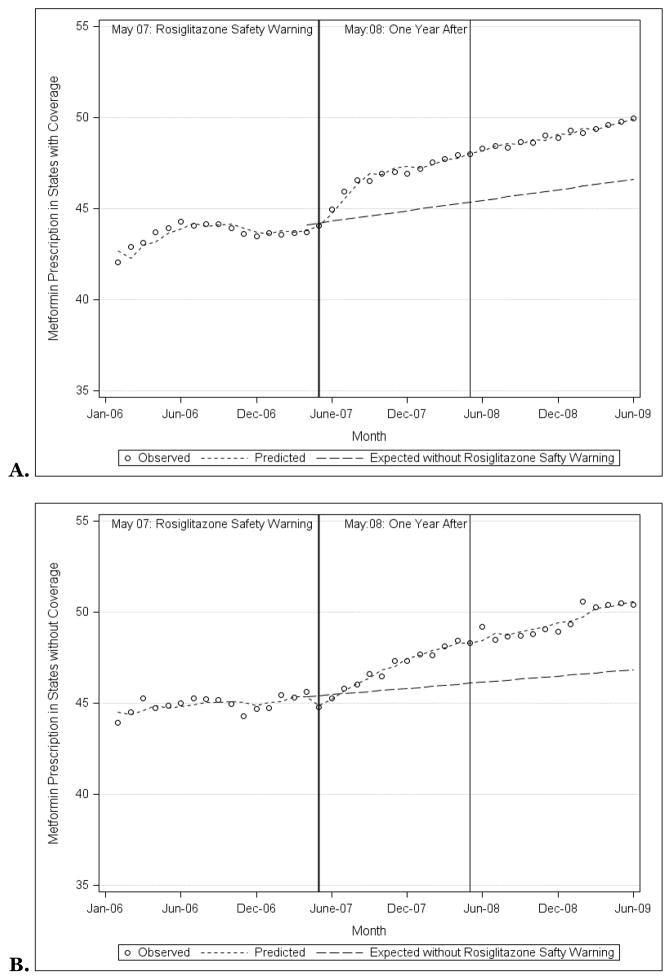
Metformin prescribing became increasingly similar among states with Medicaid programs that did and did not provide rosiglitazone coverage. In January 2006, 42.1% of antihyperglycemic prescriptions were for metformin in states that provided rosiglitazone coverage, 43.9% in states that did not (p=0.08; Figure 3A). By June 2009, 50.0% and 50.4% of prescriptions were for metformin respectively (p=0.21). Counterfactual analysis demonstrated that the safety warnings were associated with 7.1% (95% CI: 0.1%, 14.1%) and 7.9% (95% CI: 4.0%, 11.9%) increases in metformin prescribing compared to what would have been expected among states that did and did not provide rosiglitazone coverage respectively.
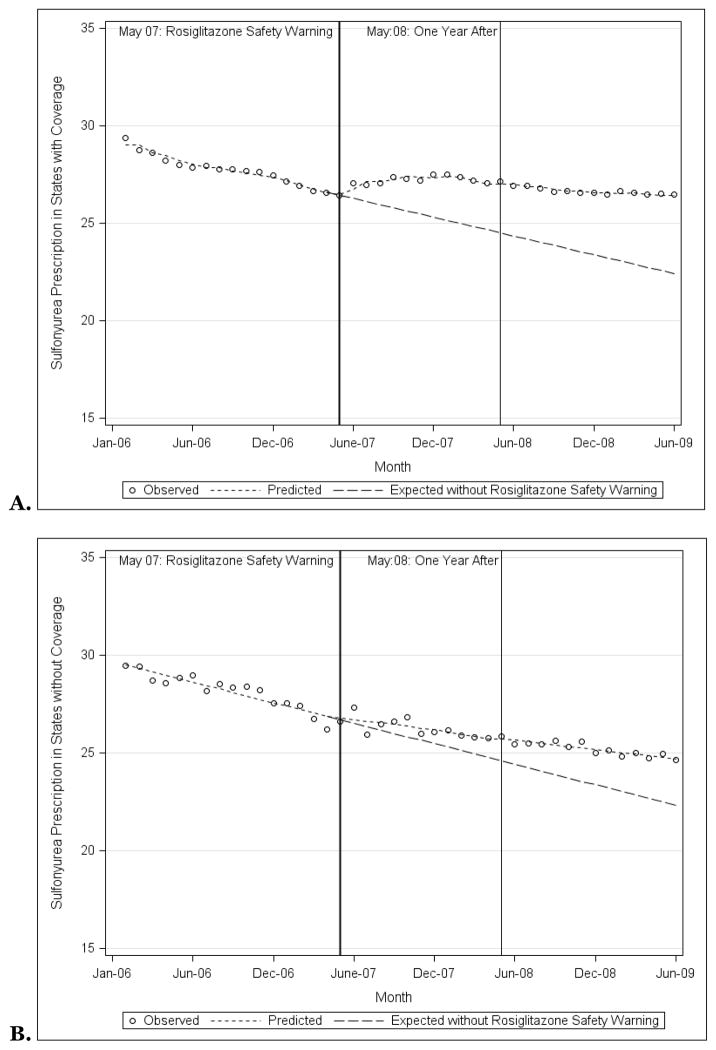
Sulfonylurea Prescribing
Sulfonylurea prescribing decreased similarly among states with Medicaid programs that did and did not provide rosiglitazone coverage. In January 2006, 29.4% of antihyperglycemic prescriptions were for sulfonylureas in states that provided rosiglitazone coverage, 29.5% in states that did not (p=0.85; Figure 3B). By June 2009, 26.5% and 24.7% of prescriptions were for sulfonylureas respectively (p<0.001). Counterfactual analysis demonstrated that the safety warnings mitigated the decline in sulfonylurea prescribing such that the warnings were associated with 17.7% (95% CI: 12.8%, 22.7%) and 10.7% (95% CI: 5.0%, 16.3%) increases in sulfonylurea prescribing compared to what would have been expected among both states that did and did not provide rosiglitazone coverage respectively Therefore, there was a significant slowing in the decline of sulfonylurea prescribing.
DISCUSSION
Our examination of the response of state Medicaid programs to the 2007 rosiglitazone safety warnings found very few programs changed rosiglitazone coverage. In fact, whereas two programs discontinued coverage, another two began providing coverage for the drug, despite not providing coverage before the safety warnings were raised. However, these state Medicaid coverage decisions played key roles in rosiglitazone prescribing, as states that provided coverage had consistently higher proportional use of rosiglitazone, both before and after the 2007 safety warnings. Medicaid preferred drug lists and prior authorization programs represent a potential mechanism to promote higher-quality prescribing that should be utilized more consistently.
Within state Medicaid programs, the rosiglitazone safety warnings had a clear impact on nearly all diabetes medications. The safety warnings were associated with a substantial decline in rosiglitazone prescribing among states that provided rosiglitazone coverage, but no change among states that did not. In contrast, the safety warnings were not associated with a change in pioglitazone prescribing among states that provided rosiglitazone coverage, but a substantial decline among states that did not. Finally, among all states, the rosiglitazone safety warnings were associated with substantially greater than would have been expected metformin and sulfonylurea prescribing. Reassuringly, TZD use appears to have been substituted with safer available alternatives. However, we cannot be certain of what caused the changes we observed. Most likely, our findings reflect changing behavior among physicians, shifting prescribing away from rosiglitazone to other antihyperglycemic medications. Alternatively, our findings may reflect changing behavior among patients as they discontinued filling their prescriptions for rosiglitazone, perhaps in response to the media attention these safety warnings generated.
Safety concerns arise frequently among prescription medications after they are approved by the FDA and rosiglitazone is just one recent example. While disseminating new safety information to patients and clinicians is challenging, payors can play a critical role in promoting safer and more effective prescribing. Medicaid is an especially important payor given the sheer number of beneficiaries covered, geographic focus, economic implications of Medicaid costs on state budgets, and vulnerability of beneficiaries. For most Medicaid programs, medications are reviewed for inclusion or exclusion on preferred drug lists once a year, meaning most programs have annual opportunities to change coverage. In this case, only two state programs changed their pharmaceutical benefit design to discontinue rosiglitazone coverage after safety concerns were raised by both independent investigators and by the FDA.
By continuing to provide coverage for a potentially unsafe medication when equally effective, safer, and cheaper alternative medications were available, Medicaid programs missed an important opportunity to maximize the minimization of harm. We found substantially different rates of proportional prescribing of rosiglitazone among Medicaid programs that did and did not provide coverage, on the order of three to five times greater among programs providing coverage. In fact, even though both state programs that did and did not provide rosiglitazone coverage had substantially fewer rosiglitazone prescriptions after the safety warning, proportional rosiglitazone prescribing remained higher among state Medicaid programs that did provide coverage as compared with those that did not. The impact of the FDA’s risk evaluation and mitigation strategy to restrict the drug’s availability (14, 15) on those who continue to be prescribed rosiglitazone will require future monitoring.
In conclusion, we found that very few state Medicaid programs changed their coverage for rosiglitazone after the 2007 rosiglitazone safety warnings. However, these coverage decisions play a key role in prescribing as states that provided coverage had consistently higher rates of rosiglitazone prescribing, both before and after the 2007 safety warnings. Incentive formulary designs such as preferred drug lists and prior authorization programs have the potential to profoundly influence prescribing, not just by limiting high-cost prescribing, but also by promoting safer, more effective prescribing. Both opportunities were missed by most Medicaid programs.
Supplementary Material
Figure 4.
Observed and predicted metformin prescriptions as a proportion of all prescriptions for non-insulin antihyperglycemic medications among state Medicaid programs that did (A) and did not (B) provide coverage for rosiglitazone, 2006–2009, along with the prescription rates that would have been expected had the rosiglitazone safety warnings not been issued.
Source: Authors’ analyses based on IMS HEALTH Xponent™ database.
Figure 5.
Observed and predicted sulfonylurea prescriptions as a proportion of all prescriptions for non-insulin antihyperglycemic medications among state Medicaid programs that did (A) and did not (B) provide coverage for rosiglitazone, 2006–2009, along with the prescription rates that would have been expected had the rosiglitazone safety warnings not been issued.
Source: Authors’ analyses based on IMS HEALTH Xponent™ database.
Acknowledgments
The statements, findings, conclusions, views, and opinions contained and expressed in this article are based in part on data obtained under license from the following IMS Health Incorporated information service(s): Xponent™ database (2005–2009) IMS Health Incorporated. All Rights Reserved. The statements, findings, conclusions, views, and opinions contained and expressed herein are not necessarily those of IMS Health Incorporated or any of its affiliated or subsidiary entities.
References
- 1.Centers for Medicare & Medicaid Services. [Accessed March 14, 2011];Medicaid Program -- General Overview. 2011 February 23; Available at: https://www.cms.gov/MedicaidGenInfo/
- 2.Kaiser Commission on Medicaid and the Uninsured. [Accessed June 14, 2011.];Headed for A Crunch: An update on Medicaid spending, coverage, and policy heading into an economic downturn. Results from a 50-state Medicaid budget survey for state fiscal years 2008 and 2009. 2008 September; Available at: http://www.kff.org/medicaid/upload/7815.pdf.
- 3.Fischer MA, Choudhry NK, Winkelmayer WC. Impact of Medicaid prior authorization on angiotensin-receptor blockers: can policy promote rational prescribing? Health Aff (Millwood) 2007;26(3):800–7. doi: 10.1377/hlthaff.26.3.800. [DOI] [PubMed] [Google Scholar]
- 4.Fischer MA, Schneeweiss S, Avorn J, Solomon DH. Medicaid prior-authorization programs and the use of cyclooxygenase-2 inhibitors. N Engl J Med. 2004;351(21):2187–94. doi: 10.1056/NEJMsa042770. [DOI] [PubMed] [Google Scholar]
- 5.Virabhak S, Shinogle JA. Physicians’ prescribing responses to a restricted formulary: the impact of Medicaid preferred drug lists in Illinois and Louisiana. Am J Manag Care. 2005;11(Spec No):SP14–20. [PubMed] [Google Scholar]
- 6.Soumerai SB. Benefits and risks of increasing restrictions on access to costly drugs in Medicaid. Health Aff (Millwood) 2004;23(1):135–46. doi: 10.1377/hlthaff.23.1.135. [DOI] [PubMed] [Google Scholar]
- 7.Polinski JM, Wang PS, Fischer MA. Medicaid’s prior authorization program and access to atypical antipsychotic medications. Health Aff (Millwood) 2007;26(3):750–60. doi: 10.1377/hlthaff.26.3.750. [DOI] [PubMed] [Google Scholar]
- 8.Shah ND, Montori VM, Krumholz HM, Tu K, Alexander GC, Jackevicius CA. Responding to an FDA warning--geographic variation in the use of rosiglitazone. N Engl J Med. 2011;363(22):2081–4. doi: 10.1056/NEJMp1011042. [DOI] [PubMed] [Google Scholar]
- 9.Lipska KJ, Ross JS. Switching from rosiglitazone: thinking outside the class. JAMA. 2011;305(8):820–1. doi: 10.1001/jama.2011.193. [DOI] [PubMed] [Google Scholar]
- 10.Montori VM, Shah ND. What have we learnt from the rosiglitazone saga? BMJ. 2011;342:d1354. doi: 10.1136/bmj.d1354. [DOI] [PubMed] [Google Scholar]
- 11.U.S. Food and Drug Administration. [Accessed June 14, 2011.];FDA Drug Safety Communication: Information for Healthcare Professionals: Pioglitazone HCl (marketed as Actos, Actoplus Met, and Duetact) 2007 August; Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm124178.htm.
- 12.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356(24):2457–71. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 13.U.S. Food and Drug Administration. [Accessed June 14, 2011];FDA Issues Safety Alert on Avandia. 2007 May 21; Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2007/ucm108917.htm.
- 14.U.S. Food and Drug Administration. [Accessed June 14, 2011];FDA Drug Safety Communication: FDA Drug Safety Communication: Avandia (rosiglitazone) labels now contain updated information about cardiovascular risks and use in certain patients. 2011 February 3; Available at: http://www.fda.gov/Drugs/DrugSafety/ucm241411.htm.
- 15.U.S. Food and Drug Administration. [Accessed June 14, 2011.];FDA Drug Safety Communication: Avandia (rosiglitazone): REMS - risk of cardiovascular events, includes Avandia, Avandamet, and Avandaryl. 2011 May 18; Available at: http://www.fda.gov/safety/medwatch/safetyinformation/safetyalertsforhumanmedicalproducts/ucm226994.htm.
- 16.Cohen A, Rabbani A, Shah N, Alexander GC. Changes in glitazone use among office-based physicians in the U.S. 2003–2009. Diabetes Care. 2010;33(4):823–5. doi: 10.2337/dc09-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.George J, Hannah St, Lang CC. Thiazolidinediones and the influence of media adverse reporting on prescribing attitudes in practice (TZD-IMPACT) study. Cardiovasc Ther. 2009;27(2):83–8. doi: 10.1111/j.1755-5922.2009.00083.x. [DOI] [PubMed] [Google Scholar]
- 18.Morrow RL, Carney G, Wright JM, Bassett K, Sutherland J, Dormuth CR. Impact of rosiglitazone meta-analysis on use of glucose-lowering medications. Open Med. 2010;4(1):e50–9. [PMC free article] [PubMed] [Google Scholar]
- 19.Starner CI, Schafer JA, Heaton AH, Gleason PP. Rosiglitazone and pioglitazone utilization from January 2007 through May 2008 associated with five risk-warning events. J Manag Care Pharm. 2008;14(6):523–31. doi: 10.18553/jmcp.2008.14.6.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen M. An overview of Medicaid enrollees with Diabetes in 2003. [Accessed June 14, 2011.];Kaiser Commission on Medicaid and the Uninsured. 2007 October; Available at: http://www.kff.org/medicaid/upload/7700.pdf.
- 21.Bennett WL, Maruthur NM, Singh S, Segal JB, Wilson LM, Chatterjee R, et al. Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2-drug combinations. Ann Intern Med. 2011;154(9):602–13. doi: 10.7326/0003-4819-154-9-201105030-00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belongia EA, Sullivan BJ, Chyou PH, Madagame E, Reed KD, Schwartz B. A community intervention trial to promote judicious antibiotic use and reduce penicillin-resistant Streptococcus pneumoniae carriage in children. Pediatrics. 2001;108(3):575–83. doi: 10.1542/peds.108.3.575. [DOI] [PubMed] [Google Scholar]
- 23.Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27(4):299–309. doi: 10.1046/j.1365-2710.2002.00430.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.