Abstract
Objective
The aim was to identify factors affecting recruitment of eligible subjects in pharmacogenetic studies at a large Midwestern pediatric academic medical center. Objectives were to evaluate recruitment success of ongoing trials and ascertain contributors to differential recruitment rates. We hypothesized studies with good recruitment of eligible subjects would share characteristics not present in studies with lower than anticipated recruitment. The goal was to better understand barriers to good recruitment in pharmacogenetic studies to help inform future trial and infrastructure design.
Study Design
Investigators designed a survey with proposed elements of success, which was then completed by lead/site investigators of all pharmacogenetics studies at the institution. Results were evaluated using an investigator-developed likelihood of success scoring system.
Results
Two studies recruited over 95% of eligible patients approached, four studies were consistent with investigator-anticipated recruitment (>50%), and one study did not meet expected recruitment. A study's total score on the investigator-devised scoring tool correlated well with the proportion of approached patients recruited (Pearson correlation, r = 0.82; P<0.001). Multiple factors impact successful recruitment into these pharmacogenetic studies. Features of studies with successful recruitment included standardized clinical care, an ongoing team/patient relationship, severe/life-threatening outcome measure, study coordinator with experience in clinical research, a study medication with few or no alternative treatment options, and active involvement of the research team in clinical care.
Conclusions
A scoring system for study characteristics may be useful to calculate the risk of failure for successful recruitment, allow discrimination among characteristics contributing to the risk, and permit study design alterations to improve likelihood of successful recruitment in pediatric pharmacogenetic studies.
Keywords: pharmacogenetics, pharmacogenomics, pediatrics, clinical trial, recruitment
Introduction
Pharmacogenetics, the study of how genetic differences influence variability in drug response, is increasingly an area of interest in pediatrics.1 The potential large clinical impact of pharmacogenetic directed therapeutics rests on its ability to help clinicians select medication, adjust doses, and predict responses with greater certainty. Such tailoring is expected to improve outcomes and reduce the incidence of adverse events and costs.2
Clinical studies have established, for a variety of medications, links between specific genetic variations and patients' differential metabolizing capacity, transporter function, and receptor sensitivity.3 More specifically, numerous clinical studies have identified associations among differential metabolizing capacities, medication levels, and clinical response.4 Thus, for some clinical scenarios, knowledge of a patient's genotype for a specific gene of interest could be instrumental in drug and/or dose selection to optimize clinical response. For example, warfarin pharmacogenetic studies in adults have shown the potential for improved dosing.5 However, there exists a gap between demonstrating the impact of pharmacogenetics on drug-related outcomes and the application of pharmacogenetics in clinical practice, especially for children.
In order to realize the potential for individualized prescribing, prospective studies of patients in “real world” clinical care treatment situations are necessary. Little published research has explored the effectiveness of pharmacogenetic information with regard to pediatric treatment, especially in the context of routine clinical care.1 There are both epidemiological and practical reasons for the dearth of pediatric studies. The epidemiology of pediatric diseases is different from adults.8 Whereas a small number of conditions affects large numbers of adults, children are affected by a large number of lower-prevalence conditions, making it difficult and expensive to accrue large enough pediatric research samples.9 Further, routine genotyping of patients is not a part of standard clinical practice at most medical centers, making it difficult to identify potential cohorts for observational pharmacogenetic studies. These issues highlight the need to explore feasible means of accruing an appropriate study cohort to help fill the knowledge gap between pharmacogenetic contributions to drug response and clinical application in the pediatric population.
Central challenges in clinical trials include public participation in research, developing information systems, adequate workforce training, and funding. Recruitment problems are a leading barrier to the success of clinical trials, with only 5% of individuals responding for initial screening completing trials; these recruitment and retention difficulties frequently result from a combination of design and study personnel issues. Among eligible patients, there is a lack of awareness of the opportunity to participate in research studies; those who are aware must consider benefit, cost, potential complications, and inconvenience. Hence, recruitment is difficult, costly, and time consuming.10 Additional issues limiting recruitment in pediatric trials include a relatively small eligible population, the need for consent from parents, inadequate research infrastructure, suitable eligibility criteria and outcome measures, and determination of appropriate payments for research participation.7,11
The aim of this study was to determine the factors affecting recruitment in clinical pharmacogenetic studies at a large Midwestern pediatric medical center. Our objectives were to evaluate recruitment success of ongoing pharmacogenetics trials and ascertain factors associated with differential recruitment rates. We hypothesized studies with good recruitment of eligible patients would share characteristics not present in studies with lower than anticipated recruitment. The goal of this study was to better understand recruitment challenges in pharmacogenetic studies to help inform future trial and infrastructure design.
Methods
Cincinnati Children's Hospital Medical Center (CCHMC), a large Midwestern pediatric academic medical center, established in 2004 a Pharmacogenetics Working Group, a multidisciplinary collaborative effort focused on both fundamental and translational pediatric pharmacogenetic research. This group welcomed geneticists, clinical pharmacologists, pharmacists, statisticians, basic scientists, and physician scientists from divisions throughout the medial center, and today provides intellectual resources for those involved in pharmacogenetic research (http://gps.cchmc.org). From among its efforts, seven large trials were identified with a scientific aim related to pharmacogenetics. Most were clinical studies with a pharmacogenetic component. The pharmacogenetic components of all studies were minimal risk. Only the methylphenidate study allowed subjects to opt-out of the pharmacogenetics component. All included studies were approved by the CCHMC Institutional Review Board (IRB). Consent was always required, and all studies included IRB-directed assent. The IRB also approved this current study.
Prior to identifying ongoing pharmacogenetics studies at the institution, the Pharmacogenetics Working Group designed a structured survey focused on elements potentially involved with differential recruitment success. The survey consisted of 21 study characteristics that were considered to potentially impact on the likelihood of successful recruitment. These characteristics were in three categories (Table 1): global infrastructure, general study characteristics, patient population characteristics.
Table 1.
Twenty one factors assessed in the survey
| Global infrastructure (n=9) |
| Availability of Department/Divisional research resources* |
| (0 = shared/rented; 1 = dedicated) |
| Experience of lead/site Investigator* |
| (0 = inexperienced; 1 = experienced) |
| Experience of Study Coordinator* |
| (0 = inexperienced; 1 = experienced) |
| Clinician champion with protected time for research* |
| (0 = no; 1 = yes) |
| Mentors with clinical research experience* |
| (0 = contribute as needed; 1 = active on study) |
| Relationship between research and clinical care team* |
| (0 = research is passive team; 1 = research is clinical team) |
| Source of study funding |
| (there were no unfunded or departmentally funded studies) |
| External funding for clinician champion |
| (all studies had clinician champion with external funding) |
| Biostatistician involvement |
| (all studies had biostatistician consult/part of team from start) |
| General Studv characteristics (n=6) |
| Study design* |
| (0 = interventional; 1 = observational) |
| Clinical treatment alternatives* |
| (0 = multiple alternatives; 1 = no/few alternatives) |
| Study outcome is severe or life-threatening* |
| (0 = no; 1 = yes) |
| Time to reach study outcome* |
| (0 = >7 days; 1 = <7 days) |
| Disease or drug specific study design |
| (all studies were disease or drug specific) |
| Objective versus subjective outcome measure |
| (0 = subjective; 1 = objective) |
| Patient copulation (n=6) |
| Amount of standardization of clinical care during study* |
| (0 = none; 1 = standardized clinical pathway) |
| Ongoing relationship between study team and patient/subject* |
| (0 = no; 1 = yes) |
| Demographics |
| (no study restricted eligibility by age, race, ethnicity, or sex) |
| Concomitant medications |
| (allowed in all studies; could affect outcome measures) |
| Location of study patients (inpatient or outpatient) |
| (all studies outpatient or inpatient/outpatient; no inpatient only) |
| Method of subject identification |
| (no studies had identical methods to identify potential subjects) |
characteristics that differentially occurred among the seven studies
The survey was sent by electronic mail to the lead/site investigators for these seven pharmacogenetics studies. All investigators completed and returned the surveys. Investigators were salaried employees paid by the research, and in part by the hospital (if clinicians). All coordinators were employed by the research foundation.
Nine characteristics that were found to be either uniformly present/absent across all seven studies or unique to individual studies were dropped from further analysis (e.g. biostatistician involvement, objective outcome measures). A scoring system for the remaining 12 characteristics was developed that assigned each characteristic a value of 1 (present) or 0 (absent). For the studies where a characteristic was present, the mean of the proportion of consented subjects was calculated. Similarly, a mean of the proportion was calculated for those studies where the characteristic was reported as absent. A positive difference of at least 25% in these mean of the proportions was defined as indicating a potentially beneficial characteristic.
Additionally, the likelihood a study with a given characteristic had a higher percentage consent rate than a study without that characteristic was examined graphically. As such, bars above the 0.50 reference line were defined as indicating a beneficial characteristic for recruitment (Figure 1). A simple linear regression12 analysis was conducted, with the proportion of subjects consenting representing the response variable as a function of the total of the number of characteristics present (maximum of 12). This association between number of characteristics present and the percent of subjects consenting was described by a regression coefficient and a Pearson correlation coefficient.
Figure 1.
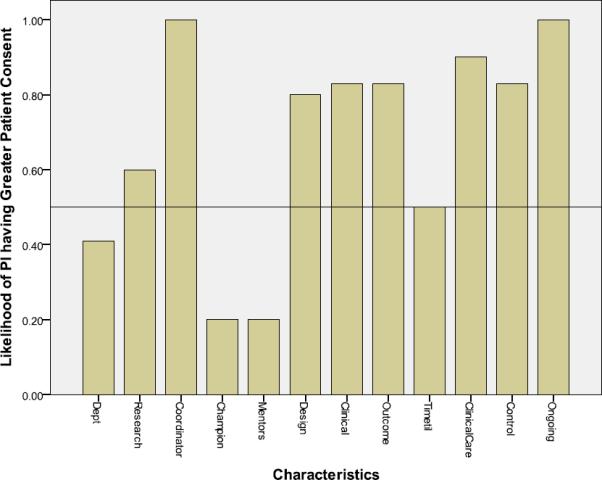
Likelihood of Principal Investigator (PI) having higher patient consent rate as a function of study characteristics
Likelihood a study with a given characteristic will have a higher percent consent rate than a study without that characteristic. Bars above the 0.50 reference line indicate the characteristic is beneficial for recruitment
Characteristics: Dept: Department/Divisional research resources; Research: Principal Investigator experienced in clinical research; Coordinator: Study coordinator experienced in clinical research; Champion: Clinician champion with protected time for research; Mentors: Mentors with clinical research experience; Observational design (absent=interventional design); Clinical: No/few clinical treatment alternatives (absent=multiple alternatives); Outcome: Severe/life-threatening outcome measure (absent=outcome not severe); Timetil: Time to reach outcome: <7 days (absent= >7 days); ClinicalCare: Standardized clinical care (during study) adequate for study; Control: Research team is the clinical team, can control patient care; Ongoing: Ongoing relationship between study team and patient/subject
Results
Seven research studies were identified for analysis. A brief description of each study follows:
Study 1
Mycophenolate mofetil (MMF) is one of the most commonly prescribed medications for immunosuppression in pediatric kidney transplant recipients and the only one that does not significantly increase the renal or cardiovascular risk profile of patients. Yet, MMF-associated side effects, including leukopenia and diarrhea in up to 50% of patients often prompt dose reduction or discontinuation in children,13–15 increasing their reliance on more toxic medications and placing them at greater risk for organ rejection.16 Genetic variants in the genes encoding MMF's primary drug metabolizing enzymes, uridine diphosphate-glucuronosyl transferases (UGTs) 1A9 and 2B7, result in variability in drug exposure and risk for sub- or supratherapeutic effects.17 This study aims to identify genetic variants that increase risk for MMF-related leukopenia in order to inform personalized drug monitoring and dosing regimens.
Study 2
The calcineurin inhibitors (CIs) comprise another class of drugs that form the back-bone of post-liver transplant immunosuppression. While introduction of these drugs (cyclosporine and tacrolimus) revolutionized solid organ transplantation by decreasing acute allograft rejection and early graft loss, the adverse effects associated with long-term use of the CIs are significant. The most common and potentially devastating of these is CI induced nephrotoxicity, which affects up to one third of organ transplant recipients.18 Neither drug dose nor trough drug concentration reliably predicts the toxicity of these medications, which display complex pharmacokinetics and pharmacodynamics.19,20 Therefore, genetic variability is likely to influence individual response. This study examined the role of genetic variability in determining post-transplant renal dysfunction, focusing on key genes involved in CI exposure and response. These genes included: ABCB1 (controls intestinal uptake of CIs), CYP3A4/5 (controls CI metabolism), and TGF-β1 (a key mediator of CI induced nephrotoxicity).21–25
Study 3
Morphine, the most commonly used “gold standard” opioid for managing perioperative pain in children, has a narrow therapeutic index and a large inter-patient variability in response. Frequent and wide variations in analgesic response are clinically significant with inadequate pain relief at one end of the spectrum of responses and serious side effects such as respiratory depression at the other end. Variants in the genes encoding proteins involved in pain perception (COMT), opioid transport (ABCB1), opioid receptor signaling (OPRM1) and codeine biotransformation to morphine (CYP2D6) have been implicated in response variability.26,27 This is a prospective, genotype-blinded observational study to determine the extent to which these genetic factors contribute to variation in morphine's and codeine's analgesia and adverse effects in children who have a tonsillectomy without or with adenoidectomy (T/TA).
Study 4
Childhood Absence Epilepsy (CAE) is characterized by very frequent absence seizures in an otherwise normal child. The annual incidence of CAE is 6.3 to 8 per 100,000 children under 15 years of age.28 Three antiepileptic drugs (AEDs), ethosuximide (ETX), lamotrigine (LTG), and valproic acid (VPA), are commonly used as initial monotherapy for CAE, with efficacy in the 50 to 70% range. However, current treatment for CAE remains largely empiric, as no single drug is the universally accepted first choice.29–33 A randomized, double blind, comparative trial of ETX, LTG, and VPA as initial monotherapy for children with CAE was conducted across 32 centers. Factors potentially predictive for the most common treatment limitations of each AED were studied, including the pharmacogenetics, pharmacokinetics, and clinical profiles of patients developing LTG associated rash, VPA induced weight gain or ETX associated gastrointestinal side effects. The contribution of genetic polymorphisms in 17 candidate genes (AED receptors, transporters, and metabolizing enzymes) to the marked interpatient variability in AED efficacy and toxicity was investigated.
Study 5
Warfarin, a narrow therapeutic index medication widely prescribed throughout the US, is responsible for more iatrogenic adverse events in outpatients than almost any other drug34 but is the only oral anticoagulant available for long-term use in children. The objective of this study was to develop a dosing algorithm for children that incorporates clinical data as well as CYP2C9 (drug metabolizing enzyme) and VKORC1 (site of drug action) genotypes.
Study 6
Although methylphenidate (MPH), a psychostimulant medication, improves symptoms in children with ADHD with generally large effect sizes,35 noted variability exists in optimal dosage, duration of effect, and tolerability. Several genes are hypothesized to play a role in MPH response, including those that encode for the sites of drug action (dopamine and norepinephrine transporters) as well as other catecholamine pathway proteins (adrenergic α2A receptor, dopamine D4 receptor, catechol-O-methyltransferase, and synaptosomal-associated protein 25).36,37 The aims of the MPH in ADHD study were to improve understanding of MPH response and its predictors, with the ultimate goal of constructing a MPH algorithm for treatment of children with predominantly inattentive-type ADHD.
Study 7
Risperidone is an atypical antipsychotic used to treat bipolar disorder, autism spectrum disorders, and disruptive behavior in children.38,39 Variants in the gene encoding risperidone's primary drug metabolizing enzyme, CYP2D6,40 influence enzyme function and the rate of drug metabolism. Individuals with reduced or absent CYP2D6 function may experience a greater than expected effect from a given dose compared with the majority of the population, and are at increased risk for ADRs,2 such as weight gain, insulin resistance, dyslipidemia, hyperprolactinemia, involuntary movements, and sedation.41 An observational study was conducted in psychiatry inpatients, for whom routine CYP2D6 and CYP2C19 genotyping was performed after admission. The objective was to determine the feasibility of enrolling risperidone-treated patients with specific CYP2D6 genotype-predicted metabolizer phenotypes during a one-year period.
Six of the seven research studies were meeting or exceeding anticipated recruitment (Table 1). The MMF/kidney transplant and CNI/liver transplant studies recruited greater than 95% of eligible patients. The MPH/ADHD, warfarin, epilepsy, and morphine/codeine in T/TA studies were on track with investigator-specified planned enrollment, all with recruitment of greater than 50% of eligible patients. In contrast, the risperidone/CYP2D6 study did not meet expected recruitment. All trials, except the epilepsy and risperidone/CYP2D6 studies, continue to enroll patients.
Six characteristics were considered beneficial based on having at least a 25% difference in consent rates (rank order in parentheses): (1) experienced (had previous clinical trial involvement) study coordinator, (2) standardized clinical care, (3) ongoing team/patient relationship, (4 - tie) research team is the clinical team, (4 - tie) no/few clinical treatment alternatives, (6) outcome measure severe/life-threatening (Table 3). Each of these six characteristics also showed increases in the proportion of patients consenting when the characteristic was present (Figure 1).
Table 3.
Characteristics and influences on pharmacogenetic study recruitment success related to consent
| Characteristics of the Research Study | Mean % Consented if Present | Mean % Consented if Absent | Difference in Consent Rates* |
|---|---|---|---|
| Department/Divisional research resources | 71.7 | 60.8 | 10.9 |
| Lead/site Investigator experienced in clinical research | 65.4 | 65.5 | −0.1 |
| Study coordinator experienced in clinical research | 76.3 | 0.0 | 76.3 |
| Clinician champion with protected time for research | 59.5 | 67.8 | −8.3 |
| Mentors with clinical research experience | 59.5 | 67.8 | −8.3 |
| Observational design (absent=interventional design) | 67.8 | 59.5 | 8.3 |
| No/few clinical treatment alternatives (absent=multiple alternatives) | 81.8 | 43.7 | 38.1 |
| Severe/life-threatening outcome measure (absent=outcome not severe) | 81.0 | 53.8 | 27.2 |
| Time to reach outcome: <7 days (absent= >7 days) | 70.0 | 62.0 | 8.0 |
| Standardized clinical care (during study) adequate for study | 78.8 | 32.0 | 46.8 |
| Research team is the clinical team, can control patient care | 81.8 | 43.7 | 38.1 |
| Ongoing relationship between study team and patient/subject | 90.7 | 46.5 | 44.2 |
Positive number indicates increase (benefit) in % patients consented if characteristic. A positive difference of at least 25% was defined as indicating a beneficial characteristic and are in bold font.
Figure 1 identifies two additional characteristics: lead/site investigator experience (greater than two years of active clinical research) and observational study design that increased the likelihood of successful recruitment but did not reach the 25% difference mark in Table 3. In contrast, four characteristics did not show at least a 25% likelihood that a study with the characteristic would have a higher percent consent rate than a study without that characteristic (Figure 1). The four characteristics not showing a benefit related to consent were department/divisional research resources (dedicated departmental resources for fully funded studies versus resources shared among departments or only partially available for non-fully funded studies), clinician champion with protected time, mentors with clinical research experience, and time to reach the study outcome (less than 7 days or more than 7 days).
The number of positive characteristics in a study is related to the proportion of patients recruited: each positive characteristic in a study, on average, increased likelihood of consent by 10.8% (95% C.I. 2.1–19.4; p=0.024; r=0.82).
Discussion
Numerous characteristics of a pharmacogenetic study contribute to its success, and in this study, we identified several potential elements of successful recruitment in pediatric pharmacogenetics trials. Twelve items varied among the studies (Table 3).
Six characteristics were considered beneficial based on having at least a 25% difference in consent rates (Table 3) and association with increased proportion of patients consenting (Figure 1) were (1) experienced study coordinator, (2) standardized clinical care, (3) ongoing team/patient relationship, (4 - tie) research team is the clinical team, (4 - tie) no/few clinical treatment alternatives, and (6) severe/life-threatening outcome measure.
Clinical environments with research integration appear to promote positive perceptions and attitudes among clinicians, patients, and families, and encourage participation and dedication to the study. Yet even in these motivated settings, with a large number of potentially eligible patients, clinician investigators with time limitations and clinical responsibilities may not be able to lead these efforts alone. In fact, the factor most closely tied to recruitment success in this study was the presence of an experienced study coordinator, which endorses the crucial involvement of clinical research coordinators.42,43
Sufficient means to identify and approach patients and families is critical, highlighting the importance of an ongoing relationship between researchers and patients/families and stressing the importance of trained personnel to coordinate recruitment. When the research team is also the clinical team who cares for the patient and interacts with the family, the established relationship and trust appeared to enhance the family's willingness be an important part of the clinician's research. Likewise, when the outcome is potentially severe or life-threatening, and families feel a study may help their child, or their participation in a study may help improve therapy for other patients and families battling the condition in the future, there is a higher rate of participation.
Three items appeared to contribute to successful recruitment but did not reach this study's pre-determined significance of 25% difference: access to or availability of department / divisional resources, observational (rather than interventional) study design, and time to reach outcome of fewer than 7 days (versus 7 or more days). Although investigators presumed a shorter time to reach and measure the outcome of interest may increase recruitment due to a more timely availability of treatment outcome measures for patients and families, this item was not associated with a meaningful increase in consent rate. Although the observational study design centered on allowing clinical care to proceed as usual with minimal added burden, this factor did not substantially contribute to successful recruitment. Each factor was associated with a positive impact (8.0%–10.9%), thus was not unfavorable, but none of these items alone contributed to a meaningful increase in recruitment.
Three items were not advantageous, but were not detrimental (Table 3) to recruitment in pediatric pharmacogenetic studies: clinician champion with protected time for research, mentors with clinical research experience, and lead/site investigator experienced in clinical research. While these features may influence other pre-study elements, they did not result in meaningful increases in recruitment in the seven trials evaluated as part of the present study.
One limitation to this study is exclusion of factors common to all studies (biostatistician, objective outcome measures, external funding of clinician champion) from analyses, some of which, such as biostatistician involvement from the planning stages, have been demonstrated to be beneficial for trial feasibility and securing funding.42 Consequently, the benefit (or detriment) to having these characteristics on recruitment rates was not determined and cannot be presumed. Additionally, the survey was not able to investigate all factors which may influence recruitment, including lack of examination of socioeconomic status or subjects' and families prior involvement in research, as these data were not consistently collected across studies.
Another potential limitation is that there was not weighting of means; each study was equally weighted, regardless of the number of subjects approached. The study, not the number of subjects, was the experimental unit, hence the rationale for this approach. An alternative to our method of weighting studies equally, a weighting of means by the number of subjects, could be performed and may be more appropriate when investigators want to capture important factors by subjects rather than studies.
Overall, there were small numbers of trials examined, investigators surveyed, and subjects recruited in pharmacogenetic studies. The single study with poor recruitment may owe enrollment difficulty more to study design, specifically restrictive exclusion criteria, than to the factors assessed in the survey. When we remove this study from the analysis, a positive correlation (r=0.45) is still observed between a study's total score on the investigator-devised scoring tool and the proportion of patients recruited. However, given only a sample size of 6, this moderate correlation coefficient is not statistically significant with a p-value of 0.37. This suggests an effect of additional study design features on recruitment.
A weakness of this study is the inability of the survey to address the differential impact of race and ethnicity on pharmacogenetic study recruitment. Only one study recruited regionally and one nationally, the remainder recruited from the Cincinnati, Ohio area (a 13 county region in Ohio, Kentucky, and Indiana). In this region 59.7% of people are Caucasian, 19.1% black, 16.9% other, 2% multiracial, 1.3% Hispanic, 0.8% Asian/Pacific Islander, and 0.1% American Indian/Alaska native. If there are racial or ethnic effects on recruitment, this survey would not have been able to detect it.
CONCLUSIONS
The number of positive characteristics in a study was related to the proportion of subjects consented: each positive characteristic in a study on average increased consent by 10.8%. These prominent characteristics indicated a solid clinical and research infrastructure is necessary for successfully recruiting patients for pediatric pharmacogenetics studies. Future prospective studies may aim to characterize the importance of these characteristics and their contribution to recruitment.
Table 2.
Study progress
| Warfarin | MMF/Kidney | CNI/Liver | CAE | Morphine/T/TA | MPH/ADHD | Risperidone | |
|---|---|---|---|---|---|---|---|
| Current duration of study (start to date data provided) | |||||||
| 0–6 months | |||||||
| 6–12 months | × | × | |||||
| 12–18 months | × | ||||||
| 18–24 months | × | ||||||
| 24–36 months | × | ||||||
| > 36 months | × | × | |||||
| Recruitment progress | |||||||
| Number screened | 158 | 64 | 225 | 1188 | 775 | 435 | 637 |
| Number eligible | 76 | 64 | 218 | 820 | 708 | 288 | 4 |
| % eligible | 42 | 100 | 97 | 69 | 91 | 66 | 0.6 |
| Number approached/contacted | 76 | 64 | 218 | 820 | 274 | 193 | 1 |
| Number consented | 60 | 62 | 210 | 453 | 183 | 124 | 0 |
| % consented | 79 | 97 | 96 | 55 | 67 | 64 | 0 |
Acknowledgements
The authors would like to thank Carole M. Lannon, M.D., M.P.H., and Robert Kahn, M.D., M.P.H. for their advice and guidance throughout this study as well as critically reviewing the manuscript. The authors are also grateful to Drew H. Barzman, M.D., and Sergio V. Delgado, M.D., for their leadership on the risperidone/CYP2D6 study.
Source of funding: Agency for Healthcare Research and Quality grant 18-HS016957-01 and NIH grants 5U10HD037249 (SNS, AAV) and K23MH083881 (TEF). The sponsors had no role in the study design; data collection, analysis, or interpretation; writing the manuscript or the decision to submit it for publication. The first draft was written by Dr. Saldaña and all co-authors; no form of payment was given to any individual to produce this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: Dr. Glauser is a consultant for UCB Pharma, Ortho-McNeil Neurologics, Questcor, Lunbeck, and Eisai Pharmaceuticals; Drs. Saldaña, Hooper, Froehlich, Campbell, Sadhasivam, Nick, Seid, and Vinks, and Ms. Prows have no conflicts of interest or financial relationships to disclose.
References
- 1.Leeder JS, Kearns GL, Spielberg SP, van den Anker J. Understanding the relative roles of pharmacogenetics and ontogeny in pediatric drug development and regulatory science. Journal of clinical pharmacology. 2010 Dec;50(12):1377–1387. doi: 10.1177/0091270009360533. [DOI] [PubMed] [Google Scholar]
- 2.Phillips KA, Veenstra DL, Oren E, Lee JK, Sadee W. Potential role of pharmacogenomics in reducing adverse drug reactions: a systematic review. Jama. 2001 Nov 14;286(18):2270–2279. doi: 10.1001/jama.286.18.2270. [DOI] [PubMed] [Google Scholar]
- 3.Grant SF, Hakonarson H. Recent development in pharmacogenomics: from candidate genes to genome-wide association studies. Expert review of molecular diagnostics. 2007 Jul;7(4):371–393. doi: 10.1586/14737159.7.4.371. [DOI] [PubMed] [Google Scholar]
- 4.Gardiner SJ, Begg EJ. Pharmacogenetics, drug-metabolizing enzymes, and clinical practice. Pharmacological reviews. 2006 Sep;58(3):521–590. doi: 10.1124/pr.58.3.6. [DOI] [PubMed] [Google Scholar]
- 5.Schelleman H, Limdi NA, Kimmel SE. Ethnic differences in warfarin maintenance dose requirement and its relationship with genetics. Pharmacogenomics. 2008 Sep;9(9):1331–1346. doi: 10.2217/14622416.9.9.1331. [DOI] [PubMed] [Google Scholar]
- 6.Leeder JS, Kearns GL, Spielberg SP, van den Anker J. Understanding the Relative Roles of Pharmacogenetics and Ontogeny in Pediatric Drug Development and Regulatory Science. Journal of clinical pharmacology. Feb 11; doi: 10.1177/0091270009360533. [DOI] [PubMed] [Google Scholar]
- 7.Macrae D. Conducting clinical trials in pediatrics. Crit Care Med. 2009 Jan;37(1 Suppl):S136–139. doi: 10.1097/CCM.0b013e318192101f. [DOI] [PubMed] [Google Scholar]
- 8.Forrest CB. Outcomes research on children, adolescents, and their families: directions for future inquiry. Medical care. 2004 Apr;42(4 Suppl):III19–23. doi: 10.1097/01.mlr.0000119394.40098.43. [DOI] [PubMed] [Google Scholar]
- 9.Forrest CB, Simpson L, Clancy C. Child health services research. Challenges and opportunities. Jama. 1997 Jun 11;277(22):1787–1793. [PubMed] [Google Scholar]
- 10.Sung NS, Crowley WF, Jr., Genel M, et al. Central challenges facing the national clinical research enterprise. Jama. 2003 Mar 12;289(10):1278–1287. doi: 10.1001/jama.289.10.1278. [DOI] [PubMed] [Google Scholar]
- 11.Tishler CL, Reiss NS. Pediatric drug-trial recruitment: enticement without coercion. Pediatrics. 2011 May;127(5):949–954. doi: 10.1542/peds.2010-2585. [DOI] [PubMed] [Google Scholar]
- 12.Harrell FE. Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. Springer-Verlag; New York, New York: 2001. [Google Scholar]
- 13.Butani L, Palmer J, Baluarte HJ, Polinsky MS. Adverse effects of mycophenolate mofetil in pediatric renal transplant recipients with presumed chronic rejection. Transplantation. 1999 Jul 15;68(1):83–86. doi: 10.1097/00007890-199907150-00016. [DOI] [PubMed] [Google Scholar]
- 14.Goebel JLB, Maseck D, Derotte M, Tarabishi R, Vinks S. Pharmacokinetic, -dynamic and clinical variability of mycophenolate mofetil in pediatric kidney transplantation. Pediatr Transplant. 2007;11(59) Abstract #106. [Google Scholar]
- 15.Roberti I, Reisman L. A comparative analysis of the use of mycophenolate mofetil in pediatric vs. adult renal allograft recipients. Pediatr Transplant. 1999 Aug;3(3):231–235. doi: 10.1034/j.1399-3046.1999.00041.x. [DOI] [PubMed] [Google Scholar]
- 16.Knoll GA, MacDonald I, Khan A, Van Walraven C. Mycophenolate mofetil dose reduction and the risk of acute rejection after renal transplantation. J Am Soc Nephrol. 2003 Sep;14(9):2381–2386. doi: 10.1097/01.asn.0000079616.71891.f5. [DOI] [PubMed] [Google Scholar]
- 17.Prausa SE, Fukuda T, Maseck D, et al. UGT genotype may contribute to adverse events following medication with mycophenolate mofetil in pediatric kidney transplant recipients. Clinical pharmacology and therapeutics. 2009 May;85(5):495–500. doi: 10.1038/clpt.2009.3. [DOI] [PubMed] [Google Scholar]
- 18.Ojo AO, Held PJ, Port FK, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003 Sep 4;349(10):931–940. doi: 10.1056/NEJMoa021744. [DOI] [PubMed] [Google Scholar]
- 19.Bartosh SM, Alonso EM, Whitington PF. Renal outcomes in pediatric liver transplantation. Clin Transplant. 1997 Oct;11(5 Pt 1):354–360. [PubMed] [Google Scholar]
- 20.Berg UB, Ericzon BG, Nemeth A. Renal function before and long after liver transplantation in children. Transplantation. 2001 Aug 27;72(4):631–637. doi: 10.1097/00007890-200108270-00012. [DOI] [PubMed] [Google Scholar]
- 21.Campistol JM, Sacks SH. Mechanisms of nephrotoxicity. Transplantation. 2000 Jun 27;69(12 Suppl):SS5–10. doi: 10.1097/00007890-200006271-00002. [DOI] [PubMed] [Google Scholar]
- 22.Hesselink DA, van Schaik RH, van der Heiden IP, et al. Genetic polymorphisms of the CYP3A4, CYP3A5, and MDR-1 genes and pharmacokinetics of the calcineurin inhibitors cyclosporine and tacrolimus. Clinical pharmacology and therapeutics. 2003 Sep;74(3):245–254. doi: 10.1016/S0009-9236(03)00168-1. [DOI] [PubMed] [Google Scholar]
- 23.Ishikawa T, Tsuji A, Inui K, et al. The genetic polymorphism of drug transporters: functional analysis approaches. Pharmacogenomics. 2004 Jan;5(1):67–99. doi: 10.1517/phgs.5.1.67.25683. [DOI] [PubMed] [Google Scholar]
- 24.Khanna AK, Hosenpud JS, Plummer MS, Hosenpud JD. Analysis of transforming growth factor-beta and profibrogenic molecules in a rat cardiac allograft model treated with cyclosporine. Transplantation. 2002 May 27;73(10):1543–1549. doi: 10.1097/00007890-200205270-00005. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Benet LZ. The gut as a barrier to drug absorption: combined role of cytochrome P450 3A and P-glycoprotein. Clin Pharmacokinet. 2001;40(3):159–168. doi: 10.2165/00003088-200140030-00002. [DOI] [PubMed] [Google Scholar]
- 26.Lotsch J, von Hentig N, Freynhagen R, et al. Cross-sectional analysis of the influence of currently known pharmacogenetic modulators on opioid therapy in outpatient pain centers. Pharmacogenetics and genomics. 2009 Jun;19(6):429–436. doi: 10.1097/fpc.0b013e32832b89da. [DOI] [PubMed] [Google Scholar]
- 27.Williams DG, Patel A, Howard RF. Pharmacogenetics of codeine metabolism in an urban population of children and its implications for analgesic reliability. Br J Anaesth. 2002 Dec;89(6):839–845. doi: 10.1093/bja/aef284. [DOI] [PubMed] [Google Scholar]
- 28.Pavone P, Bianchini R, Trifiletti RR, Incorpora G, Pavone A, Parano E. Neuropsychological assessment in children with absence epilepsy. Neurology. 2001 Apr 24;56(8):1047–1051. doi: 10.1212/wnl.56.8.1047. [DOI] [PubMed] [Google Scholar]
- 29.Buoni S, Grosso S, Fois A. Lamotrigine in typical absence epilepsy. Brain Dev. 1999 Jul;21(5):303–306. doi: 10.1016/s0387-7604(99)00023-6. [DOI] [PubMed] [Google Scholar]
- 30.Callaghan N, O'Hare J, O'Driscoll D, O'Neill B, Daly M. Comparative study of ethosuximide and sodium valproate in the treatment of typical absence seizures (petit mal) Dev Med Child Neurol. 1982 Dec;24(6):830–836. doi: 10.1111/j.1469-8749.1982.tb13703.x. [DOI] [PubMed] [Google Scholar]
- 31.Frank LM, Enlow T, Holmes GL, et al. Lamictal (lamotrigine) monotherapy for typical absence seizures in children. Epilepsia. 1999 Jul;40(7):973–979. doi: 10.1111/j.1528-1157.1999.tb00805.x. [DOI] [PubMed] [Google Scholar]
- 32.Santavuori P. Absence seizures: valproate or ethosuximide? Acta Neurol Scand Suppl. 1983;97:41–48. doi: 10.1111/j.1600-0404.1983.tb01534.x. [DOI] [PubMed] [Google Scholar]
- 33.Sato S, White BG, Penry JK, Dreifuss FE, Sackellares JC, Kupferberg HJ. Valproic acid versus ethosuximide in the treatment of absence seizures. Neurology. 1982 Feb;32(2):157–163. doi: 10.1212/wnl.32.2.157. [DOI] [PubMed] [Google Scholar]
- 34.Budnitz DS, Pollock DA, Weidenbach KN, Mendelsohn AB, Schroeder TJ, Annest JL. National surveillance of emergency department visits for outpatient adverse drug events. Jama. 2006 Oct 18;296(15):1858–1866. doi: 10.1001/jama.296.15.1858. [DOI] [PubMed] [Google Scholar]
- 35.Clinical practice guideline: treatment of the school-aged child with attention-deficit/hyperactivity disorder. Pediatrics. 2001 Oct;108(4):1033–1044. doi: 10.1542/peds.108.4.1033. [DOI] [PubMed] [Google Scholar]
- 36.Froehlich TE, McGough JJ, Stein MA. Progress and promise of attention-deficit hyperactivity disorder pharmacogenetics. CNS Drugs. 2010 Feb 1;24(2):99–117. doi: 10.2165/11530290-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stein MA, McGough JJ. The pharmacogenomic era: promise for personalizing attention deficit hyperactivity disorder therapy. Child Adolesc Psychiatr Clin N Am. 2008 Apr;17(2):475–490. xi–xii. doi: 10.1016/j.chc.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kowatch RA, Fristad M, Birmaher B, Wagner KD, Findling RL, Hellander M. Treatment guidelines for children and adolescents with bipolar disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2005 Mar;44(3):213–235. doi: 10.1097/00004583-200503000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Myers SM, Johnson CP. Management of children with autism spectrum disorders. Pediatrics. 2007 Nov;120(5):1162–1182. doi: 10.1542/peds.2007-2362. [DOI] [PubMed] [Google Scholar]
- 40.He H, Richardson JS. A pharmacological, pharmacokinetic and clinical overview of risperidone, a new antipsychotic that blocks serotonin 5-HT2 and dopamine D2 receptors. Int Clin Psychopharmacol. 1995 Mar;10(1):19–30. doi: 10.1097/00004850-199503000-00003. [DOI] [PubMed] [Google Scholar]
- 41.McConville BJ, Sorter MT. Treatment challenges and safety considerations for antipsychotic use in children and adolescents with psychoses. The Journal of clinical psychiatry. 2004;65(Suppl 6):20–29. [PubMed] [Google Scholar]
- 42.Dainty K, Karlsson J. Factors to consider in determining the feasibility of randomized clinical trials. Arthroscopy. 2003 Oct;19(8):882–884. doi: 10.1016/s0749-8063(03)00707-2. [DOI] [PubMed] [Google Scholar]
- 43.Yanagawa H, Kishuku M, Akaike M, Azuma H, Irahara M. View of physicians on and barriers to patient enrollment in a multicenter clinical trial: experience in a Japanese rural area. Int Arch Med. 2010;3:7. doi: 10.1186/1755-7682-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


