Abstract
The expression levels of the p21Cip1 family CDK inhibitors (CKIs), p21Cip1, p27Kip1 and p57Kip2, play a pivotal role in the precise regulation of cyclin-dependent kinase (CDK) activity, which is instrumental to proper cell cycle progression. The stabilities of p21Cip1, p27Kip1 and p57Kip2 are all tightly and differentially regulated by ubiquitylation and proteasome-mediated degradation during various stages of the cell cycle, either in steady state or in response to extracellular stimuli, which often elicit site-specific phosphorylation of CKIs triggering their degradation.
Keywords: phosphorylation, ubiquitylation, proteasome, p21Cip1, p27Kip1, p57Kip2
Introduction
Ubiquitin (Ub)/proteasome-regulated proteolysis is a key mechanism for regulating many cellular and organismal processes. Conjugation of Ub to substrate proteins requires three enzymes: the Ub-activating enzyme (E1), a Ub-conjugating enzyme (E2), and a Ub ligase (E3). E1 activates Ub through the formation of a thiol-ester bond between the C-terminus of Ub and the active site cysteine (Cys). The activated Ub is then trans-esterified to a conserved Cys of an E2. The E3 ligase interacts with both E2 and the substrate and facilitates polyubiquitylation of the substrate.1 There are two distinct types of E3 ligases: the enzymatic HECT (homologous to E6-AP C-terminus) domain E3s and the adaptor E3s. HECT domain E3s form a thioester with Ub, which can then be transferred directly to the substrate. The adaptor E3s, containing a RING, SP-RING finger, variant RING/PHD (plant homeodomain) (also named LAP [leukemia-associated protein] domain), or a U box do not form a thioester with Ub but function as adaptors to facilitate the transfer of ubiquitin between a charged E2 and a substrates.2–5 E3s have a key role in defining the substrate specificity of ubiquitylation.
Cell cycle progression is governed by cyclin-dependent kinases (CDKs), which are activated by cyclin binding and inhibited by CDK inhibitors (CKIs). Although CDK protein levels do not change significantly, the dynamic activities of CDKs during the cell cycle are regulated through expression, ubiquitylation, and degradation of cyclins and CKIs, both temporally and spatially, in addition to phosphorylation and dephosphorylation.1 CKIs can be divided into two families. The Inhibitor of CDKs (INK4) family includes p15INK4B, p16INK4A, p18INK4C and p19INK4D; these CKIs specifically bind CDK4 and CDK6 and inhibit cyclin D association. The other family, the kinase inhibitor protein (KIP) or CDK-interacting protein (CIP) family, includes p21Cip1, p27Kip1 and p57Kip2; these CKIs bind and inhibit all cyclin-bound CDKs.6,7 The ubiquitylation and proteasome-mediated degradation of p16INK4A,8 and p19INK4D (which depends on binding to CDK4)9 has been reported. In this review, we discuss the ubiqutylation and degradation of mammalian p21Cip1, p27Kip1, including its analogues Sic1 and Far1 [in Saccharomyces (S.) cerevisiae] and Rum1 (in fission yeast) and p57Kip2.
The Skp1/cullin/F-box (SCF) complexes, functioning as E3 ligases, play a pivotal role in ubiquitylation of p21Cip1, p27Kip1 and p57Kip2. The SCF complexes consist of three invariant subunits—Skp1, CUL1 [cell division cycle (Cdc) 53p in yeast], and the RING finger protein RBX1 (RING box protein-1) (also known as regulator of cullins-1, Roc1)—as well as a variable component known as an F-box protein. F-box proteins bind to Skp1 through their F box and recognize substrates through other domains in the F-box protein.10 The human genome encodes 69 F-box proteins.11 Three classes of F-box proteins have been defined on the basis of their substrate recognition motifs: Fbw, F-box proteins containing WD40-repeat domains; Fbl, containing leucine-rich repeats (LRR) domains; and Fbx, containing other domains. The Fbw and Fbl classes of F-box proteins can recognize target phosphodegrons, specific sequences of amino acids in proteins that direct their degradation in a phosphorylation-dependent manner, through their WD40 or LRR domains.1 The CUL1 scaffold binds RBX1, a RING finger protein that recruits the charged E2, and Skp1. The human genome encodes eight Cullin proteins [CUL1-4A, 4B, 5, 7 and 9 (formerly PARC)] that form similar Cullin-RING Ligase (CRL) complexes.11 Neddylation of CUL1 is necessary for SCF activity as a Ub ligase, because unneddylated CUL1 holds RBX1 in a closed form bound by an inhibitory protein CAND1, thus preventing Skp1-F-box protein binding. Neddylation of CUL1 loosens the RBX1 structure and dissociates the CAND1 inhibitor, allowing binding of the Skp1-F-box protein complex. The reoriented RBX1 bridges the gap between the associated E2 and substrate bound to the F-box protein, facilitating both initial ubiquitylation and subsequent polyubiquitylation.11 SCF complexes, working in concert with the UBC3/ Cdc34 E2 (and other E2s, such as Ubc4), which binds to Rbx1, control G1-S progression and target CKIs (p21Cip1, p27Kip1 and p57Kip2 in mammals and Sic1 and Far1 in budding yeast) for degradation.1
p21Cip1
Expression of the p21Cip1 mammalian CKI is tightly regulated by signals that control cell division, and also in response to DNA damage, which activates cell cycle checkpoints. Shortly after its discovery, p21Cip1 was shown to be a short-lived protein that is degraded by the proteasome.12 Subsequent work has shown that p21Cip1 can be degraded through both ubiqiutin-dependent and independent mechanisms.
Ubiquitylation-dependent proteasomal degradation of p21Cip1
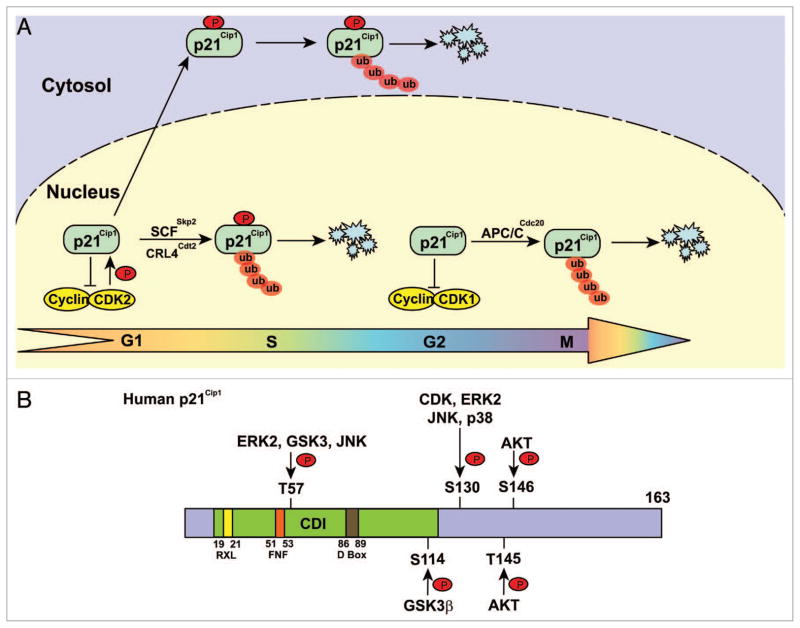
Proteolysis of p21Cip1 has been shown to require a functional Ub-activating enzyme (E1) and conjugation of the Ub-like protein, Nedd8, whose linkage to CULs is necessary for their Ub ligase activity.13,14 Consistent with a role for SCF complexes, p21Cip1 was shown to be ubiquitylated at four distinct Lys residues located in the C-terminal region by SCFSkp2.15 p21Cip1 interacts with and is phosphorylated on serine (S)130 by cyclin E/ CDK2, which promotes Skp2-dependent p21Cip1 degradation in S phase16,17 (Fig. 1). Mutation of all six Lys into Arg, disruption of the two nuclear export signal sequences in p21Cip1, or treatment with the nuclear-export inhibitor leptomycin B blocks H2O2-induced p21Cip1 degradation, implying that cytoplasmic localization is required for p21Cip1 degradation.18 Cytoplasmic p21Cip1 degradation is also induced by activation of extracellular signal-regulated kinase 2 (ERK2). ERK2 directly interacts with and phosphorylates p21Cip1 at threonine (T)57 and S130, promoting translocation of p21Cip1 into the cytoplasm and Ub-dependent degradation by unidentified E3 ligases, thereby resulting in cell cycle progression.19 In contrast, in transforming growth factor-β1 (TGFβ1) treated cells, p38 and JNK1 are activated and phosphorylate p21Cip1 at S130 leading to its stabilization. Whether p21Cip1 phosphorylation at S130 by different kinases somehow results in p21Cip1 being able to associate with distinct regulators that affect p21Cip1 stability differentially warrants further investigation. Similarly, T57 within the CDK binding domain of p21Cip1 is also a substrate of glycogen synthase kinase (GSK)3,20 or JNK,21 which result in degradation or stabilization of p21Cip1, respectively.
Figure 1.
Phosphorylated p21Cip1 is degraded by distinct E3 ligases. (A) E3 ligases involved in p21Cip1 degradation. p21Cip1 is ubiquitylated and degraded in late G1 and S phases by SCFSkp2 and CRL4Cdt2, and in G2 phase by APC/CCdc20 in the nucleus. A portion p21Cip1 is phosphorylated and translocated into the cytosol where it is ubiquitylated and degraded by proteasomes. (B) Schematic structure of p21Cip1 showing the regulatory phosphorylation sites and the cognate protein kinases. CDI: CDK inhibitor domain.
HER-2/Neu-activated AKT associates with p21Cip1 and phosphorylates it at T145, resulting in cytoplasmic localization of p21Cip1 and promotion of cell growth, whereas a nuclear p21Cip1 T145A mutant preferentially suppresses growth of transformed cells.22 AKT-dependent phosphorylation of p21Cip1 at T145 prevents complex formation of p21Cip1 with proliferating cell nuclear antigen (PCNA) and decreases the binding of CDK2 and CDK4 to p21Cip1, thereby attenuating the inhibitory activity of p21Cip1 on CDK and DNA replication.23,24 In addition to T145, S146 of p21Cip1 is also phosphorylated by AKT, and this phosphorylation is suggested to stabilize p21Cip1.24 How phosphorylation of two adjacent residues by AKT leads to opposite effects on p21Cip1 stability is not yet clear.
Ubiquitylation of p21Cip1 by the SCFSkp2 complex requires functional interaction between p21Cip1 and the cyclin E/CDK2 complex. Mutation of both the cyclin E recruitment motif (RXL) and the CDK2-binding motif (FNF) at the N-terminus of p21Cip1 abolishes its ubiquitylation by Skp2 (Fig. 1).15 p21Cip1 accumulates in Skp2−/− mouse embryonic fibroblasts during S phase, suggesting that SCFSkp2 plays a role in p21Cip1 degradation during the G1/S transition.25 In addition to Skp2, overexpression of p53-inducible RING-finger protein (p53RFP) causes degradation of p21Cip1,26 suggesting that multiple E3 ligases are involved in p21Cip1 ubiquitylation. Additional evidence shows that, in spite of regulation of p21Cip1 by SCFSkp2 during the G1/S transition, p21Cip1 reaccumulates during G2 and is degraded again in prometaphase through the interaction of the anaphase-promoting complex/cyclosome (APC/C)Cdc20 E3 ligase with the destruction box (D box) of p21Cip1 (Fig. 1). A D-box mutant of p21Cip1 is resistant to degradation by APC/CCdc20 E3 ligase, but not to degradation by SCFSkp2. Silencing of APC/CCdc20 induced accumulation and binding of p21Cip1 to CDK1, thereby inhibiting CDK1 activity during prometaphase, implying that p21Cip1 degradation by APC/CCdc20 is required for the full activation of CDK1 necessary for mitotic events.25 In contrast to the normal turnover of p21Cip1 mediated by the SCFSkp2 and APC/CCdc20 complexes at different phases of the cell cycle, ionizing radiation-induced Ub-dependent degradation of p21Cip1 requires the CRL4Cdt2 E3 ligase (composed of the Cul4A/B, DDB1, and the DCAF subunit Cdt2) and p21Cip1 binding to PCNA,27–30 suggesting that p21Cip1 stability is regulated by distinct mechanisms in response to different extracellular stimuli.
Since p21Cip1 accumulates in cells harboring a temperature-sensitive mutation in Ub E1 at the restrictive temperature,13,14 degradation of p21Cip1 requires active ubiquitylation, but this does not have to be due to ubiquitylation of p21Cip1 itself. Although p21Cip1 ubiquitylation can be detected, there has been a lively debate about which residues in p21Cip1, if any, need to be ubiquitylated for its degradation, and this issue is complicated by the fact that p21Cip1 can be degraded by the proteasome in a ubiquitin-independent fashion (see below). Exogenously expressed p21Cip1 K6R, harboring mutations of all six internal Lys to Arg to prevent ubiquitylation, is still ubiquitylated.13 Moreover, p21Cip1 K6R is unstable, and its abundance also increases in response to proteasome inhibition indistinguishably from WT p21Cip1.31 These findings suggested that Lys-independent ubiquitylation and proteasomal degradation is involved in regulation of p21Cip1 stability, and this idea is supported by the evidence that N-terminally-tagged p21Cip1 can be ubiquitylated on its free α-NH2 group.13,14 However, this occurs because the N-terminal Tyr residue of the tag is not acetylated. In contrast, the majority of endogenous p21Cip1 is acetylated at its N-terminal Ser residue,32 meaning that N-terminal ubiquitylation of p21Cip1 is unlikely to be physiologically important. Nevertheless, with the recent discovery that N-terminally-acetylated residues, including N-acetyl Ser, can act as a degradation signal (degron) and specify a short half life for yeast proteins, the role of the acetylated N-terminus in p21Cip1 degradation needs further investigation.33 Such an AcN-degron might dictate the basal half life of p21Cip1, and involve ubiquitylation of internal Lys residues in p21Cip1 leading to its degradation by the proteasome.
p21Cip1 degradation can be inhibited by nucleophosmin (NPM)/B23, a multifunctional protein that binds p21Cip1. Actinomycin D stimulation increases the interaction and nucleoplasmic translocation of NPM and p21Cip1 and stabilizes p21Cip1.34 However, whether NPM-induced p21CIP1 stabilization is due to retention of p21Cip1 in nucleus thereby preventing p21Cip1 degradation in the cytosol remains unclear. p21Cip1 can also be stabilized by interaction with WISp39, a tetratricopeptide repeat (TPR) protein. The C-terminal TPR domain of WISp39 recruits Hsp90 and forms a trimeric complex to prevent proteasomal degradation of p21Cip1. Point mutations within the C-terminal TPR domain of WISp39, which abolish the binding of WISp39 to Hsp90 but not to p21Cip1, fail to stabilize p21, suggesting an essential role of Hsp90 in stabilization of p21Cip1.35
Ubiquitylation-independent proteasomal degradation of p21Cip1
Although the degradation of p21Cip1 is generally believed to depend on proteasomes, other observations suggest that proteasomal degradation of p21Cip1 does not obligatorily require its ubiquitylation. For instance, MDM2 promotes p21Cip1 degradation independently of ubiquitylation.36 Ubiquitylation- and Skp2-independent proteasomal degradation of p21Cip1 also occurs in response to ultraviolet irradiation, which is mediated by phosphorylation of p21Cip1 at S114 via GSK3β, a downstream effector of the ATR DNA damage signaling pathway.37,38 Further, the C8 α-subunit of the 20S proteasome has been shown to interact directly with the non-ubiquitylated C-terminus of p21Cip1 and thereby mediate the degradation of p21Cip1.39 The role of the C8 α-subunit of the 20S proteasome in p21Cip1 is also evidenced by Ras signaling-induced p21Cip1 stabilization. Ras activation promotes the formation of p21Cip1-cyclin D1 complexes and prevents p21Cip1 from associating with the C8 α-subunit of the 20S proteasome.40 This observation is further supported by the finding that degradation of unbound p21Cip1 by the 20S proteasome occurs in an ATP- and Ub-independent manner. This process is directly mediated by the proteasome activator REGγ, which binds and activates the 20S proteasome.41 Depletion of REGγ results in increased p21Cip1 protein levels and changes in cell cycling and proliferation.
Obviously, further detailed studies on the mechanisms by which p21Cip1 is regulated in quiescent and cycling cells will be required for a full understanding of the relative importance of the Ub-dependent and Ub-independent processes for degradation of p21Cip1.
p27Kip1, Sic1, Rum1 and Far1 p27Kip1
Regulation of p27Kip1 stability by phosphorylation of p27Kip1 at T187 by cyclin/CDK complexes
The most well-studied mammalian CKI is p27Kip1, which is abundant in quiescent and G1 cells and is downregulated in proliferating cells and in S- and G2-phase cells (Fig. 2). p27Kip1 acts in G0 and early G1 to inhibit G1 cyclin/CDK2 complexes, with the primary target being E-type cyclin/ CDK2.42–44 Before the onset of S-phase, cyclin E/CDK2 binds to and phosphorylates p27Kip1 at T187,45,46 with p27Kip1 complexed to cyclin E/CDK2 being a target for phosphorylation by a second, active cyclin E/CDK2 complex. Phosphorylation of T187 creates a phosphodegron that recruits Skp2 through LRR domain binding, resulting in p27Kip1 ubiquitylation by SCFSkp2 E3 ligase containing either the Rbx1/Roc1 RING finger protein47–49 or the Ro52 RING finger protein.50 In addition to using the CUL1-containing SCF complex, Skp2 interacts with CUL4A-DDB1-associated COP9 signalosomes to induce proteolysis of p27Kip1.51 Depletion of COP9 signalosome subunits 4 and 5 decreases the levels of Skp2 protein, stabilizes p27Kip1, and impairs cell proliferation, and this can be partially reversed by suppression of p27Kip1.52
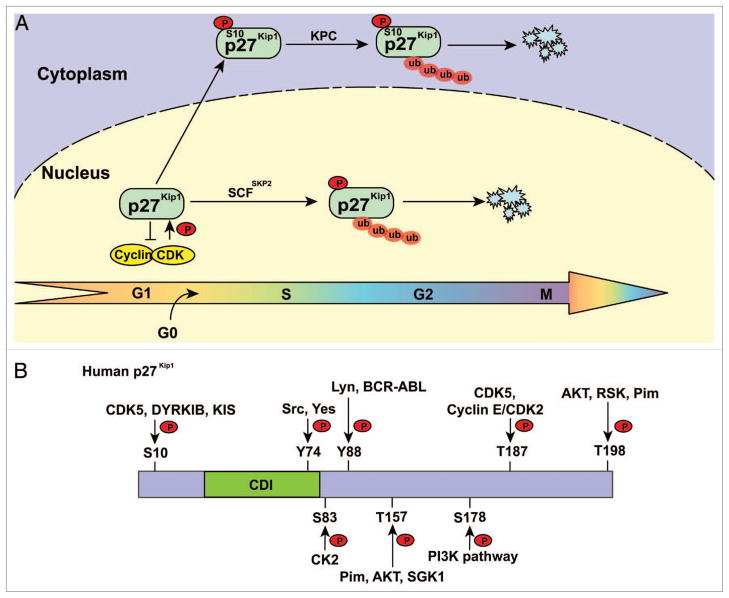
Figure 2.
Phosphorylated 27Kip1 is degraded by distinct E3 ligases. (A) E3 ligases involved in p27Kip1 degradation. p27Kip1 is ubiquitylated and degraded in late G1, S and G2 phases by SCFSkp2 in the nucleus. p27Kip1 phosphorylated at S10 is ubiquitylated by the KPC complex when exported to the cytoplasm. (B) Schematic structure of p27Kip1 showing the regulatory phosphorylation sites and the cognate protein kinases. CDI, CDK inhibitor domain.
The SCFSkp2-mediated ubiquitylation of p27Kip1 requires an additional factor, CDK subunit 1 (Cks1), which is a member of the highly conserved Suc1/Cks family of proteins that bind to some cyclin/CDK complexes and phosphorylated proteins. Cks1 binds to the LRR domain and C-terminal tail of Skp2,53 in which a negatively charged residue Asp331 is essential for the interaction.54 The formation of complexes between Cks1 and Skp2 causes conformational changes in both proteins and significantly stabilizes the interaction between Skp2 and Skp1 and enhances the binding of Skp2 to p27Kip1 phosphorylated at T187.53,55 p27Kip1 binds to both Cks1 and Skp2 by inserting the side chain of an invariant Glu185 into the interface between Skp2 and Cks1 and interacts with a Cks1 phosphate-binding site via the phosphorylated T187 side chain of p27Kip1.53 Phosphorylated p27Kip1, complexed to cyclin E/CDK2, binds to the SCFSkp2/Cks1 complex in a cooperative manner. Cyclin E/CDK2 contributes to p27Kip1 binding to the SCFSkp2/Cks1 by T187 phosphorylation as well as by a potential direct interaction between cyclin E/CDK2 and the SCFSkp2/Cks1 complex.56 In contrast, the interaction between phospho-T187 p27Kip1 and Cks1 is dramatically reduced by the cis-trans peptidylprolyl isomerase Pin1. Pin1 binds to the phosphorylated Thr187-Pro motif in p27Kip1 and can cause cis-trans isomerization of this bond altering the conformation of p27Kip1. p27Kip1 exhibits increased polyubiquitylation and has a shorter half life in Pin1−/− mouse embryonic fibroblasts than in wild-type cells.57
In addition to cyclin E, cyclin D and cyclin A are also involved in p27Kip1 degradation by distinct mechanisms. Depletion of cyclin D1 causes p27Kip1 accumulation, with a simultaneous decrease in CUL1 neddylation and increased binding to CAND1, which blocks the accessibility of CUL1 to Skp1 and Skp2, thus preventing the formation of the SCFSkp2 complex.58 How cyclin D1 is able to promote SCFSkp2 complex formation, however, remains unclear. Cyclin A/CDK2, but not cyclin B/CDK1, forms a stable complex with T187-phosphorylated p27Kip1 to stimulate p27Kip1 ubiquitylation.46,59 Distinct interaction regions in cyclin A separately bind to Skp2 and p27Kip1, and Skp2-cyclin A interaction directly protects cyclin A-CDK2 from inhibition by p27Kip1 through competitive binding. Disruption of cyclin A-Skp2 binding, which does not affect p27Kip1-cyclin A interaction, compromises Skp2’s proliferation stimulatory activity without affecting its ability to degrade p27Kip1 and p21Cip1.60
Although SCFSkp2 mediates the nuclear ubiquitylation of CDK2-bound p27Kip1 during S/G2 and M phases, when p27Kip1 is translocated into the cytoplasm its stability is controlled in an SCFSkp2-independent manner (see below).61–63 In addition, other E3 ligases, such as a HECT domain family ubiquitin ligase E6-AP,64 p53-inducible protein with RING-H2 domain (Pirh2),65 and CUL4A/CUL4B-based E3 ligases in response to active Wnt signaling66–68 are also reportedly involved in the ubiquitylation and proteasomal degradation of p27Kip1,64 in an extracellular stimuli- or cell type-specific manner.
Regulation of p27Kip1 stability by phosphorylation of p27Kip1 at S10
Stimulation by hepatocyte growth factor (HGF) increases p27Kip1 phosphorylation at S10 and induces nuclear export of p27Kip1,69 (Fig. 2). In keeping with this, ERK activation induces the cytoplasmic localization of p27Kip1,70 and p27Kip1 S10A is refractory to Ras-induced cytoplasmic translocation.71 p27Kip1 phosphorylated on S10 at the G0-G1 transition binds to the nuclear export protein CRM1, leading to its subsequent cytoplasmic translocation.62,63 The stability of p27Kip1 exported into the cytoplasm can be regulated by interaction with and ubiquitylation by the E3 complex KPC (Kip1 ubiquitylation-promoting complex), which consists of KPC1 and KPC2. KPC2 contains a Ub-like domain and two ubiquitin-associated (UBA) domains. KPC1 possesses a C-terminal RING-finger domain, and its N-terminal region is involved in the interaction with free p27Kip1. Thus, KPC may control the degradation of p27Kip1 exported from the nucleus in G1 phase.72–74 However, it remains unknown whether a direct modification of p27Kip1, such as phosphorylation at S10, is required for KPC-mediated p27Kip1 degradation. KPC1 itself can be regulated by the USP19 deubiquitinating enzyme, which interacts with and stabilizes KPC1, thereby modulating p27Kip1 levels and cell proliferation.75
p27Kip1 S10 phosphorylation can depend on both tissue and cell type as well as cell cycle stage. CDK5, an unconventional neuronal CDK that is activated in postmitotic neurons but not in proliferative cells, directly phosphorylates p27Kip1 at S10, which contributes to neuronal migration in the developing cerebral cortex.76 CDK5 is also able to phosphorylate T187 in vitro, but the physiological significance remains unclear.76 Other protein kinases can phosphorylate p27Kip1 at S10 at different stages of the cell cycle: Mirk/DYRK1B, which is maximally active in G0, phosphorylates and stabilizes p27Kip1 in quiescent cells;77 human kinase-interacting stathmin (KIS), which is activated by mitogens, binds the C-terminal domain of p27Kip1, phosphorylates S10 at the G0-G1 transition, and promotes its nuclear export to the cytoplasm.78 In addition, corneal endothelial cells treated with fibroblast growth factor (FGF)-2 possess distinct polyubiquitylation pathways for phospho-T187 and phospho-S10 p27Kip1; phospho-T187 p27Kip1 is ubiquitylated through nuclear SCFSkp2 during late G1 phase, whereas phospho-S10 p27Kip1 is ubiquitylated by cytosolic KPC during early G1 phase.79 These results further support the conclusion that p27Kip1 stability is regulated by phosphorylation at different residues at various stages of the cell cycle. While S10 phosphorylation stabilizes p27Kip1 in resting cells (G0 phase), this phosphorylation promotes p27Kip1 nuclear export and cytoplasmic degradation during early and late G1 phases.80 Consistently, the S10A p27Kip1 mutant has reduced stability compared with WT p27Kip1 in G0 phase whereas mutation of S10 into Asp or Glu to mimic phosphorylation stabilizes p27Kip1.80 A higher proportion of S10A p27Kip1 is found in association with cyclin/CDK complexes than WT p27Kip1, thus promoting p27Kip1 S10A assembly into cyclin-CDK complexes, which is, in turn, necessary for p27Kip1 turnover.71,81 p27Kip1 S10A knock-in mice have normal body size but exhibit organ-specific reductions in p27Kip1 expression in brain, thymus, spleen and testis.81 The reason for this organ specificity in the downregulation of p27Kip1 S10A remains an enigma.
Regulation of p27Kip1 stability by phosphorylation of p27Kip1 at tyrosines 74 and 88 and threonines 157 and 198
Distribution of p27Kip1 into cyclin/CDK complexes seems to be affected by phosphorylation of p27Kip1 at T198, an event that can be mediated by AKT or p90 ribosomal protein S6 kinases (RSK)82–84 (Fig. 2). Phosphorylation at this residue stabilizes free p27Kip1, whereas loss of this phosphorylation site promotes its binding to CDK2-containing complexes.84
Tyrosine (Y) 74 and Y88 of p27Kip1 can be phosphorylated by the Src-family kinases c-Src and Yes, whereas Lyn and the oncoprotein BCR-ABL appear to phosphorylate predominantly Y88. p27Kip1 phosphorylation at Y74/88 reduces its steady-state binding to cyclin E-CDK2, thus restoring partial CDK activity. The activated CDK2 phosphorylates p27Kip1 on T187, which in turn promotes SCFSkp2-dependent degradation of p27Kip1.85,86 Consistently, reduced p27Kip1 expression levels are observed in Src-activated breast cancer lines, correlating with Src activation in primary human breast cancers.85 In contrast, p27Kip1 phosphorylation at Y88/89 was also proposed to regulate CDK4 activity via a distinct mechanism. p27Kip1 associates with cyclin D-CDK4 constitutively. However, Y88 and Y89 phosphorylated preferentially in proliferating cells converts p27Kip1 to a non-inhibitor of cyclin D-CDK4 by dislodging p27Kip1 from the catalytic cleft of CDK4 to allow ATP binding.87,88
p27Kip1 T157 can be a substrate of multiple protein kinases. The Pim kinase family members (Pim1, Pim2 and Pim3) bind to and phosphorylate p27Kip1 at both T157 and T198. Pim-mediated phosphorylation induces p27Kip1 binding to 14-3-3 proteins, resulting in its nuclear export and proteasome-dependent degradation.89 Phosphorylation of p27Kip1 at T157 is also observed upon AKT and SGK1 activation, which interrupts association of p27Kip1 with importin-α, thus preventing re-entry of p27Kip1 into the nucleus.90,91 p27Kip1 phosphorylation at S83 by the protein kinase CK2,92 and at S178 downstream of the phosphoinositide 3-kinase pathway93 are also reported, although the biological significance of these phosphorylations remains unclear.
Significance of the regulation of p27Kip1 stability
The degradation of p27Kip1 is necessary for entry into S phase, as overexpression of a nondegradable p27Kip1 mutant (T187A), microjection of Skp2 antibody, or antisense oligonucleotides targeting Skp2 arrests cells in G1 phase.94,95 Cells derived from p27Kip1 T187A knock-in mice exhibit rising levels of p27Kip1 T187A in late G1/S/G2 cells and a defect in cell cycle progression and cell proliferation.96 Mice expressing this protein develop normally, and for unknown reasons, grow to be larger than WT mice. RBX1, which encodes an SCF subunit, is an essential gene for mouse embryogenesis, and disruption of RBX1 causes embryonic lethality due to reduced proliferation as a result of p27Kip1 accumulation. Simultaneous loss of p27Kip1 extends the life span of RBX1-deficient embryos from E6.5 to E9.5,97 indicating that a failure to downregulate p27Kip1 as well as other RBX1 substrates, is detrimental to embryonic development. Both Cks1- and Skp2-deficient mice are smaller than their WT littermates, and their cells tend to proliferate more slowly, which correlates with elevated levels of p27Kip1.98–100 Most of the cellular abnormalities apparent in Skp2−/− mice are not evident in Skp2−/−-p27Kip1−/− double-mutant mice, suggesting that p27Kip1 is a major physiological target of SCFSkp2.101 This is further supported by the fact that eliminating p27Kip1 phosphorylation on T187 in p27Kip1−/− T187A knock-in mice reproduces the effects of Skp2 knockout in preventing spontaneous tumorigenesis in Rb1+/− mice.102 In addition, the p27Kip1 T187A mutation inhibits progression of intestinal adenomas to carcinomas in a mouse model,103 and the absence of Skp2, which correlates with increased expression of p27Kip1, decreases the leukemogenicity of BCR-ABL in a murine model of chronic myelogenous leukemia (CML).104 Furthermore, Skp2 deficiency restricts tumorigenesis induced by inactivation of Pten or Arf, which is mediated by induction of cellular senescence resulting from concomitant upregulation of p27Kip1, p21Cip1 and Atf4.105 These results provide additional in vivo evidence that p27Kip1 regulation by Skp2 contributes to the development of some tumor types. However, in certain tumor types p27Kip1 T187 phosphorylation-induced protein degradation might not be the primary mechanism for regulation of p27Kip1 stability. For instance, expression of p27Kip1 T187A is not higher than that of WT p27Kip1, and the expression of both WT and the T187A mutant of p27Kip1 is downregulated at the transcriptional level during development of activated K-Ras-induced non-small cell lung cancers in mice.103 Moreover, regulation of the p27Kip1 transcript levels in human breast cancers has been reported.103
In agreement with the requirement for phosphorylation by and association with cyclins/CDKs for Skp2-mediated p27Kip1 degradation, the expression levels of p27CK− in p27CK− knock-in cells, which carry point mutations in protein interaction domains that abolish p27Kip1 binding to cyclins and CDKs and consequently cannot inhibit cyclins/CDKs, are increased in various tissues and mouse embryonic fibroblasts.71 As expected, an increased growth rate and body size and general organomegaly are observed in p27CK− knock-in mice as well as in p27Kip1−/− mice. p27Kip1−/− mice also exhibit sterility (in females) and disrupted retinal architecture and develop multiorgan hyperplasia and pituitary tumors.106–108 Furthermore, a decline or loss of p27Kip1 protein, with an associated increase in Skp2 protein levels, has been detected in many human cancers and is of prognostic significance.7 In addition, indirect regulation of p27Kip1 may also contribute to human cancer development. For instance, inactivating mutations in the Fbw7 F-box protein (also known as hCdc4 and archipelago) in a number of different human cancers, which result in loss of SCFFbw7-mediated cyclin E ubiquitylation and accumulation of cyclin E,109,110 may enhance p27Kip1 degradation indirectly by contributing to p27Kip1 binding to SCFSkp2.56
The Sic1p budding yeast CKI
In S. cerevisiae, the CDK inhibitor Sic1p, which is functionally and structurally analogous to the mammalian p27Kip1,111 must be degraded for the onset of B-type cyclin/CDK (Clb/Cdc28) activity and consequent DNA replication.111 Sic1p is phosphorylated in late G1 phase by G1 cyclin/CDK (Cln/Cdc28) activity, and the phosphorylated Sic1p binds to the WD40 repeat domain of the Cdc4 F-box protein.112–114 Cdc4 dimerization via its D domain, which facilitates Ub conjugation but not substrate recognition, is required for SCFCdc4 function and Sic1p ubiquitylation.115,116 Six N-terminal lysines of Sic1p serve as the major ubiquitylation sites.117 Sic1p has nine suboptimal Cdc4 phosphodegrons (CPDs);118,119 Sic1p mutants that lack multiple CDK phosphorylation sites are stabilized, and hence arrest cells in G1 phase.112 The nine Sic1p phosphorylation sites form three separate Cdc4-recognition phosphodegrons (T2/5/9, T33/45/48 and S69/76/80), and each degron contains two essential phosphorylated residues and binds to Cdc4 with similar affinities. Double phosphorylation of a single degron is necessary and sufficient for binding to Skp1-Cdc4.116 The nine phosphorylation sites can be replaced by a single high affinity CPD, leading to the proposal that multiple suboptimal CPDs in Sic1p serve as a mechanism for setting a threshold of cyclin/CDK phosphorylation before Sic1p degradation can be triggered.119 Kinetic analysis reveals that Sic1p ubiquitylation has an initiation step, which is a rate-limiting attachment of the first Ub followed by the acidic tail loop of Cdc34 (an E2)-dependent rapid synthesis of K48-linked Ub chains.120 Subsequently, polyubiquitin chains are built on SCF substrates by sequential transfers of single ubiquitins.121 Protein kinase CK2-mediated phosphorylation of Cdc34 on the acidic tail domain (S207/216 in yeast and S203/222/231 in humans) stimulates Cdc34-SCFCdc4 ubiquitylation activity toward Sic1p and cell cycle progression.122 The multiubiquitin chain binding proteins (MCBPs) Rad23 and Rpn10 contribute to recruitment of ubiquitylated Sic1p to the 26S proteasome, where Rpn11 metalloprotease is essential for deubiquitylation and degradation of Sic1p.123,124 Intriguingly, analysis using an in vitro biochemical reconstitution system shows that Sic1p degradation is essential for triggering the ATP hydrolysis-dependent dissociation and disassembly of the 19S regulatory particles from the 26S proteasome, implying that this is a general process for degradation of other proteins.125
During sporulation, Sic1p degradation is independent of Cdc28 but requires Ime2 protein kinase activity.126,127 The meiosis-specific kinase Ime2 phosphorylates only a subset of the Sic1p sites corresponding to CDK sites, which, by itself, is insufficient to promote Sic1p binding to Cdc4 and Sic1 degradation128 The identity of the other kinase(s) that phosphorylate Sic1 in meiosis to promote Sic1p degradation in combination with Ime2 remains obscure. Intriguingly, Ime2p kinase is degraded by SCFGrr1p upon glucose stimulation, which increases Sic1p levels, resulting in blockage of meiotic DNA replication.129
The Rum1 fission yeast CKI
Rum1, the sole CKI in fission yeast, is essential for proper regulation of the G1/S transition, inhibiting Cdc13/Cdc2 complex activity. Rum1, the fission yeast analogue of p27Kip1 and Sic1p,130 is a substrate of SCF complexes containing the fission yeast orthologues of Cdc4, Pop1 and Pop2.131–134 Rum1 degradation requires phosphorylation of Rum1 at T58 and T62 by Cig1/Cdc2 (cyclin/CDK) and covalent attachment of NEDD8 to the Cul-family proteins Pcu1 and Pcu4 (Cul-1 and Cul-4A/Cul-4B orthologues, respectively).135,136 Pop1 interacts with the N-terminal domain of Pop2 and forms heterooligomeric complexes, which bind and direct polyubiquitylation of Rum1.137,138
The Far1 budding yeast CKI
In addition to Sic1p, a second CKI is present in budding yeast, Far1. While Sic1 specifically inhibits Clb/Cdc28 kinases,111 Far1 inhibits Cln1,2/Cdc28 and Cln3/Cdc28 complexes during the pheromone response.139,140 Despite the functional similarities between Far1 and p27Kip1, these proteins share only a very small amount of amino acid identity.43,69,141 Far1 is required to arrest the cell cycle of S. cerevisiae in response to mating factor. Far1 is phosphorylated at S87 by Cln2/Cdc28 and degraded by SCFCdc4 in the nucleus, through recognition of the pS87 phosphodegron by the Cdc4 WD40 repeat domain.142 In response to mating pheromone, a fraction of Far1 is stabilized after it is exported into the cytoplasm by Ste21/ Msn5, whereas blockage of nuclear export destabilizes Far1.143
p57Kip2
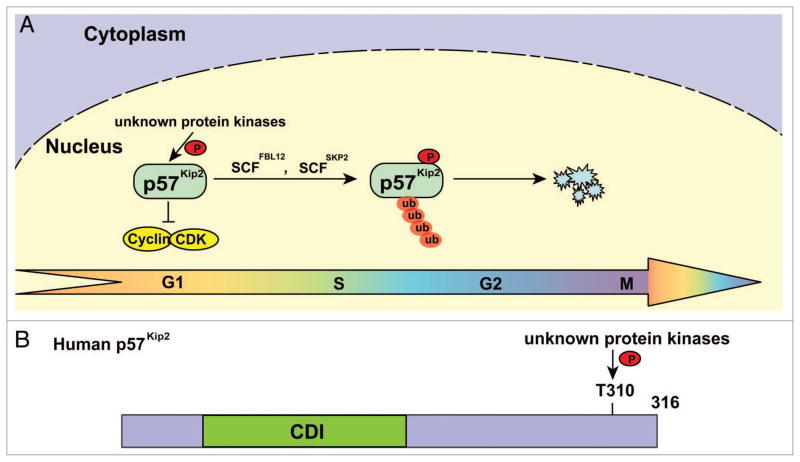
p57Kip2, the third member of the p21Cip1 family of CKIs, is most closely related to p27Kip.1 p57Kip2 is primarily expressed in terminally differentiated cells and associated with G1 CDKs, and this can cause cell cycle arrest in the G1 phase.144 p57Kip2, which accumulates following serum starvation, causing cell cycle arrest of osteoblastic cells, is rapidly degraded upon transforming growth factor (TGF)β1 stimulation.145 TGFβ1-stimulated ubiquitylation and proteasomal degradation of p57K1p2 does not influence the levels of p21Cip1 and p27Kip1 proteins, indicating that p57Kip2 degradation in response to TGFβ1 is mediated by a distinct mechanism. One specific mechanism of p57Kip2 degradation is mediated through TGFβ1-activated, Smad-dependent transcription of the gene for the F-box protein FBL12,146,147 (Fig. 3). FBL12 forms an SCFFBL12 complex that binds to and ubiquitylates mouse p57K1p2 phosphorylated at T329 (equivalent to human p57Kip2 T310), which is conserved between the COOH-terminal QT domains of p57Kip2 and p27Kip1. Inhibition of FBL12 suppresses TGFβ-induced degradation of p57Kip2, increases the steady-state level of p57Kip2, and promotes the differentiation of primary osteoblasts.147
Figure 3.
Phosphorylated p57Kip2 is degraded by distinct E3 ligases. (A) E3 ligases involved in p57Kip2 degradation. p57KIP2 phosphorylated at T329 is ubiquitylated and degraded in late G1 and S phases by SCFFBL12 and SCFSkp2. (B) Schematic structure of p57Kip2 showing the single regulatory phosphorylation site. CDI, CDK inhibitor domain.
SCFSkp2 is another E3 ligase responsible for regulating the cellular level of p57Kip2 by targeting it for ubiquitylation and proteolysis.148 Overexpression of WT Skp2 promotes degradation of p57Kip2, whereas expression of a dominant-negative mutant of Skp2 prolongs the half-life of p57Kip2. p57Kip2 interacts with Skp2, and mutation of T310 in human p57Kip2 abrogates Skp2-induced p57Kip2 degradation, suggesting that phosphorylation at this site is required for SCFSkp2-mediated ubiquitylation. Similar to the role of cyclin/CDK in p27KIP1 ubiquitylation, purified recombinant SCFSkp2 complex ubiquitinates p57Kip2 and this is dependent on the presence of the cyclin E/CDK2 complex. Skp2−/− cells have abnormal accumulation of p57Kip2,148 suggesting that SCFFBL12 cannot compensate for the deficiency of Skp2 in the ubiquitylation and degradation of p57Kip2.
Whereas the lack of p21Cip1 or p27Kip1 does not show gross defects in embryonic development,149 most p57Kip2-null mice die after birth and display severe developmental defects resulting from increased apoptosis and delayed differentiation.144,150 Most of the developmental defects apparent in tissues of the p57Kip2 knockout mouse are corrected by replacing the p57Kip2 gene with the p27Kip1 gene, although the fact that a few developmental defects remain suggests that p57Kip2 also has specific functions.151
Conclusion
The precise regulation of CDK activity is instrumental to cell cycle progression. Unlike the activity of many other protein kinases, which are often themselves regulated by direct ubiquitylation and degradation of the protein kinase itself,1 CDK activity is controlled by regulation of cyclins and CKIs. The stability of p21Cip1, p27Kip1 and p57Kip2 are tightly and differentially regulated by the Ub/proteasome system, in a manner that depends on many factors including the nature of extracellular stimuli, cell cycle stage, differences in subcellular context in different tissues and cells, interaction of CKIs with other regulatory proteins, such as Cks1 for p27Kip1 and NPM for p21Cip1, involvement of distinct E3 ligases, phosphorylation by distinct protein kinases, and a distinct subcellular compartment for degradation.
p21Cip1, p27Kip1 and p57Kip2 are all targeted by more than one E3 ligase for ubiquitylation. The function of E3 ligases can be overlapping, as illustrated by p21Cip1 degradation, where the Cul4-DDB1 and the SCFSkp2 E3 ligases are redundant with each other in promoting the degradation of p21Cip1 during an unperturbed S phase of the cell cycle.29 However, these CKIs exhibit specifically regulated ubiquitylation by distinct E3 ligases in response to different extracellular stimuli, such as mitotic stimulation, ionizing radiation, or stress signaling. Under many conditions, phosphorylation of p21Cip1, p27Kip1 and p57Kip2 is important for their degradation. Many of the regulatory phosphorylation sites lie outside the CDI region in the unique domains of these related proteins, allowing specific regulation of stability and subcellular localization by phosphorylation. Phosphorylation of p27Kip1 at S10 or T187 will determine the localization and stages of the cell cycle where degradation of p27Kip1 is mediated by distinct E3 ligases, whereas phosphorylation of p21Cip1 at T57, S130 or S114 by CDK2, ERK2 or GSK3β will determine its degradation by a Ub-dependent or Ub-independent proteasomal system in cells with or without various stimuli. Given that CKI inactivation by accelerated degradation and mislocalization occurs in cancers,152 interrupting degradation of p21Cip1, p27Kip1 and p57Kip2 by targeting the different regulatory steps could open new avenues for cancer therapy.
Acknowledgments
We thank Tamara Locke for critical reading of this manuscript and Weiwei Yang for the construction of figures. This work was supported by National Cancer Institute grant 5R01CA109035 (Z.L.), American Cancer Society Research Scholar Award RSG-09-277-01-CSM (Z.L.), a Brain Tumor Society research grant (Z.L.), and an institutional research grant from The University of Texas MD Anderson Cancer Center (Z.L.). T.H. is supported by National Cancer Institute grants CA80100, CA82683 and CA116402. T.H. is a Frank and Else Schilling American Cancer Society Professor.
Abbreviations
- CDKs
cyclin-dependent kinases
- CKIs
CDK inhibitors
- KIP
kinase inhibitor protein
- CIP
CDK interacting protein
- Ub
ubiquitin
- E1
the Ub-activating enzyme
- E2
Ub-conjugating enzyme
- E3
Ub ligase
- HECT
homologous to E6-AP C-terminus
- PHD
plant homeodomain
- LAP
leukemia-associated protein
- SCF
Skp1/cullin/F-box
- LRR
leucine-rich repeats
- CRL
cullin-RING ligase
- Cks1
CDK subunit 1
- Pirh2
protein with RING-H2 domain
- HGF
hepatocyte growth factor
- UBA domain
ubiquitin-associated domain
- FGF
fibroblast growth factor
- MCBP
multiubiquitin chain binding protein
- ERK
extracellular signal-regulated kinase
- PCNA
proliferating cell nuclear antigen
- APC/C
anaphase-promoting complex/cyclosome
- GSK
glycogen synthase kinase
- TGF
transforming growth factor
- D box
destruction box
- KPC
Kip1 ubiquitylation-promoting complex
- RSK
p90 ribosomal protein S6 kinase
References
- 1.Lu Z, Hunter T. Degradation of activated kinases by ubiquitination. Annu Rev Biochem. 2009;78:435–75. doi: 10.1146/annurev.biochem.013008.092711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jackson PK, Eldridge AG, Freed E, Furstenthal L, Hsu JY, Kaiser BK, et al. The lore of the RINGs: substrate recognition and catalysis by ubiquitin ligases. Trends Cell Biol. 2000;10:429–39. doi: 10.1016/s0962-8924(00)01834-1. [DOI] [PubMed] [Google Scholar]
- 3.Hatakeyama S, Yada M, Matsumoto M, Ishida N, Nakayama KI. U box proteins as a new family of ubiquitin-protein ligases. J Biol Chem. 2001;276:33111–20. doi: 10.1074/jbc.M102755200. [DOI] [PubMed] [Google Scholar]
- 4.Coscoy L, Sanchez DJ, Ganem D. A novel class of herpesvirus-encoded membrane-bound E3 ubiquitin ligases regulates endocytosis of proteins involved in immune recognition. J Cell Biol. 2001;155:1265–73. doi: 10.1083/jcb.200111010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boname JM, Stevenson PG. MHC class I ubiquitination by a viral PHD/LAP finger protein. Immunity. 2001;15:627–36. doi: 10.1016/s1074-7613(01)00213-8. [DOI] [PubMed] [Google Scholar]
- 6.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–12. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 7.Bloom J, Pagano M. Deregulated degradation of the cdk inhibitor p27 and malignant transformation. Semin Cancer Biol. 2003;13:41–7. doi: 10.1016/s1044-579x(02)00098-6. [DOI] [PubMed] [Google Scholar]
- 8.Ben-Saadon R, Fajerman I, Ziv T, Hellman U, Schwartz AL, Ciechanover A. The tumor suppressor protein p16(INK4a) and the human papillomavirus oncoprotein-58 E7 are naturally occurring lysine-less proteins that are degraded by the ubiquitin system. Direct evidence for ubiquitination at the N-terminal residue. J Biol Chem. 2004;279:41414–21. doi: 10.1074/jbc.M407201200. [DOI] [PubMed] [Google Scholar]
- 9.Thullberg M, Bartek J, Lukas J. Ubiquitin/proteasome-mediated degradation of p19INK4d determines its periodic expression during the cell cycle. Oncogene. 2000;19:2870–6. doi: 10.1038/sj.onc.1203579. [DOI] [PubMed] [Google Scholar]
- 10.DeSalle LM, Pagano M. Regulation of the G1 to S transition by the ubiquitin pathway. FEBS Lett. 2001;490:179–89. doi: 10.1016/s0014-5793(01)02121-4. [DOI] [PubMed] [Google Scholar]
- 11.Skaar JR, Pagano M. Control of cell growth by the SCF and APC/C ubiquitin ligases. Current opinion in cell biology. 2009;21:816–24. doi: 10.1016/j.ceb.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blagosklonny MV, Wu GS, Omura S, el-Deiry WS. Proteasome-dependent regulation of p21WAF1/CIP1 expression. Biochem Biophys Res Commun. 1996;227:564–9. doi: 10.1006/bbrc.1996.1546. [DOI] [PubMed] [Google Scholar]
- 13.Bloom J, Amador V, Bartolini F, DeMartino G, Pagano M. Proteasome-mediated degradation of p21 via N-terminal ubiquitinylation. Cell. 2003;115:71–82. doi: 10.1016/s0092-8674(03)00755-4. [DOI] [PubMed] [Google Scholar]
- 14.Coulombe P, Rodier G, Bonneil E, Thibault P, Meloche S. N-Terminal ubiquitination of extracellular signal-regulated kinase 3 and p21 directs their degradation by the proteasome. Mol Cell Biol. 2004;24:6140–50. doi: 10.1128/MCB.24.14.6140-6150.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang W, Nacusi L, Sheaff RJ, Liu X. Ubiquitination of p21Cip1/WAF1 by SCFSkp2: substrate requirement and ubiquitination site selection. Biochemistry. 2005;44:14553–64. doi: 10.1021/bi051071j. [DOI] [PubMed] [Google Scholar]
- 16.Bornstein G, Bloom J, Sitry-Shevah D, Nakayama K, Pagano M, Hershko A. Role of the SCFSkp2 ubiquitin ligase in the degradation of p21Cip1 in S phase. J Biol Chem. 2003;278:25752–7. doi: 10.1074/jbc.M301774200. [DOI] [PubMed] [Google Scholar]
- 17.Bendjennat M, Boulaire J, Jascur T, Brickner H, Barbier V, Sarasin A, et al. UV irradiation triggers ubiquitin-dependent degradation of p21(WAF1) to promote DNA Repair. Cell. 2003;114:599–610. doi: 10.1016/j.cell.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Hwang CY, Kim IY, Kwon KS. Cytoplasmic localization and ubiquitination of p21(Cip1) by reactive oxygen species. Biochem Biophys Res Commun. 2007;358:219–25. doi: 10.1016/j.bbrc.2007.04.120. [DOI] [PubMed] [Google Scholar]
- 19.Hwang CY, Lee C, Kwon KS. Extracellular signal-regulated kinase 2-dependent phosphorylation induces cytoplasmic localization and degradation of p21Cip1. Mol Cell Biol. 2009;29:3379–89. doi: 10.1128/MCB.01758-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rossig L, Badorff C, Holzmann Y, Zeiher AM, Dimmeler S. Glycogen synthase kinase-3 couples AKT-dependent signaling to the regulation of p21Cip1 degradation. J Biol Chem. 2002;277:9684–9. doi: 10.1074/jbc.M106157200. [DOI] [PubMed] [Google Scholar]
- 21.Densham RM, O’Neill E, Munro J, Konig I, Anderson K, Kolch W, et al. MST kinases monitor actin cytoskeletal integrity and signal via c-Jun N-terminal kinase stress-activated kinase to regulate p21Waf1/Cip1 stability. Mol Cell Biol. 2009;29:6380–90. doi: 10.1128/MCB.00116-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou BP, Liao Y, Xia W, Spohn B, Lee MH, Hung MC. Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neuoverexpressing cells. Nat Cell Biol. 2001;3:245–52. doi: 10.1038/35060032. [DOI] [PubMed] [Google Scholar]
- 23.Rossig L, Jadidi AS, Urbich C, Badorff C, Zeiher AM, Dimmeler S. Akt-dependent phosphorylation of p21(Cip1) regulates PCNA binding and proliferation of endothelial cells. Mol Cell Biol. 2001;21:5644–57. doi: 10.1128/MCB.21.16.5644-5657.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y, Dowbenko D, Lasky LA. AKT/PKB phosphorylation of p21Cip/WAF1 enhances protein stability of p21Cip/WAF1 and promotes cell survival. J Biol Chem. 2002;277:11352–61. doi: 10.1074/jbc.M109062200. [DOI] [PubMed] [Google Scholar]
- 25.Amador V, Ge S, Santamaria PG, Guardavaccaro D, Pagano M. APC/C(Cdc20) Controls the Ubiquitin-Mediated Degradation of p21 in Prometaphase. Mol Cell. 2007;27:462–73. doi: 10.1016/j.molcel.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ng CC, Arakawa H, Fukuda S, Kondoh H, Nakamura Y. p53RFP, a p53-inducible RING-finger protein, regulates the stability of p21WAF1. Oncogene. 2003;22:4449–58. doi: 10.1038/sj.onc.1206586. [DOI] [PubMed] [Google Scholar]
- 27.Stuart SA, Wang JY. Ionizing radiation induces ATM-independent degradation of p21Cip1 in transformed cells. J Biol Chem. 2009;284:15061–70. doi: 10.1074/jbc.M808810200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim Y, Starostina NG, Kipreos ET. The CRL4Cdt2 ubiquitin ligase targets the degradation of p21Cip1 to control replication licensing. Genes Dev. 2008;22:2507–19. doi: 10.1101/gad.1703708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abbas T, Sivaprasad U, Terai K, Amador V, Pagano M, Dutta A. PCNA-dependent regulation of p21 ubiquitylation and degradation via the CRL4Cdt2 ubiquitin ligase complex. Genes Dev. 2008;22:2496–506. doi: 10.1101/gad.1676108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishitani H, Shiomi Y, Iida H, Michishita M, Takami T, Tsurimoto T. CDK inhibitor p21 is degraded by a proliferating cell nuclear antigen-coupled Cul4-DDB1Cdt2 pathway during S phase and after UV irradiation. J Biol Chem. 2008;283:29045–52. doi: 10.1074/jbc.M806045200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheaff RJ, Singer JD, Swanger J, Smitherman M, Roberts JM, Clurman BE. Proteasomal turnover of p21Cip1 does not require p21Cip1 ubiquitination. Mol Cell. 2000;5:403–10. doi: 10.1016/s1097-2765(00)80435-9. [DOI] [PubMed] [Google Scholar]
- 32.Chen X, Chi Y, Bloecher A, Aebersold R, Clurman BE, Roberts JM. N-acetylation and ubiquitin-independent proteasomal degradation of p21(Cip1) Mol Cell. 2004;16:839–47. doi: 10.1016/j.molcel.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 33.Hwang CS, Shemorry A, Varshavsky A. N-terminal acetylation of cellular proteins creates specific degradation signals. Science. 327:973–7. doi: 10.1126/science.1183147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiao J, Zhang Z, Chen GG, Zhang M, Ding Y, Fu J, et al. Nucleophosmin/B23 interacts with p21WAF1/CIP1 and contributes to its stability. Cell Cycle. 2009;8:889–95. doi: 10.4161/cc.8.6.7898. [DOI] [PubMed] [Google Scholar]
- 35.Jascur T, Brickner H, Salles-Passador I, Barbier V, El Khissiin A, Smith B, et al. Regulation of p21(WAF1/CIP1) stability by WISp39, a Hsp90 binding TPR protein. Mol Cell. 2005;17:237–49. doi: 10.1016/j.molcel.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 36.Jin Y, Lee H, Zeng SX, Dai MS, Lu H. MDM2 promotes p21waf1/cip1 proteasomal turnover independently of ubiquitylation. EMBO J. 2003;22:6365–77. doi: 10.1093/emboj/cdg600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee JY, Yu SJ, Park YG, Kim J, Sohn J. Glycogen synthase kinase 3beta phosphorylates p21WAF1/CIP1 for proteasomal degradation after UV irradiation. Mol Cell Biol. 2007;27:3187–98. doi: 10.1128/MCB.01461-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee H, Zeng SX, Lu H. UV Induces p21 rapid turnover independently of ubiquitin and Skp2. J Biol Chem. 2006;281:26876–83. doi: 10.1074/jbc.M605366200. [DOI] [PubMed] [Google Scholar]
- 39.Touitou R, Richardson J, Bose S, Nakanishi M, Rivett J, Allday MJ. A degradation signal located in the C-terminus of p21WAF1/CIP1 is a binding site for the C8 alpha-subunit of the 20S proteasome. EMBO J. 2001;20:2367–75. doi: 10.1093/emboj/20.10.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coleman ML, Marshall CJ, Olson MF. Ras promotes p21(Waf1/Cip1) protein stability via a cyclin D1-imposed block in proteasome-mediated degradation. EMBO J. 2003;22:2036–46. doi: 10.1093/emboj/cdg189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li X, Amazit L, Long W, Lonard DM, Monaco JJ, O’Malley BW. Ubiquitin- and ATP-independent proteolytic turnover of p21 by the REGgamma-proteasome pathway. Mol Cell. 2007;26:831–42. doi: 10.1016/j.molcel.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 42.Slingerland J, Pagano M. Regulation of the cdk inhibitor p27 and its deregulation in cancer. J Cell Physiol. 2000;183:10–7. doi: 10.1002/(SICI)1097-4652(200004)183:1<10::AID-JCP2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 43.Polyak K, Lee MH, Erdjument-Bromage H, Koff A, Roberts JM, Tempst P, et al. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994;78:59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- 44.Coats S, Flanagan WM, Nourse J, Roberts JM. Requirement of p27Kip1 for restriction point control of the fibroblast cell cycle. Science. 1996;272:877–80. doi: 10.1126/science.272.5263.877. [DOI] [PubMed] [Google Scholar]
- 45.Vlach J, Hennecke S, Amati B. Phosphorylation-dependent degradation of the cyclin-dependent kinase inhibitor p27. EMBO J. 1997;16:5334–44. doi: 10.1093/emboj/16.17.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Montagnoli A, Fiore F, Eytan E, Carrano AC, Draetta GF, Hershko A, et al. Ubiquitination of p27 is regulated by Cdk-dependent phosphorylation and trimeric complex formation. Genes Dev. 1999;13:1181–9. doi: 10.1101/gad.13.9.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carrano AC, Eytan E, Hershko A, Pagano M. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat Cell Biol. 1999;1:193–9. doi: 10.1038/12013. [DOI] [PubMed] [Google Scholar]
- 48.Tsvetkov LM, Yeh KH, Lee SJ, Sun H, Zhang H. p27(Kip1) ubiquitination and degradation is regulated by the SCF(Skp2) complex through phosphorylated Thr187 in p27. Curr Biol. 1999;9:661–4. doi: 10.1016/s0960-9822(99)80290-5. [DOI] [PubMed] [Google Scholar]
- 49.Sutterluty H, Chatelain E, Marti A, Wirbelauer C, Senften M, Muller U, et al. p45SKP2 promotes p27Kip1 degradation and induces S phase in quiescent cells. Nat Cell Biol. 1999;1:207–14. doi: 10.1038/12027. [DOI] [PubMed] [Google Scholar]
- 50.Sabile A, Meyer AM, Wirbelauer C, Hess D, Kogel U, Scheffner M, et al. Regulation of p27 degradation and S-phase progression by Ro52 RING finger protein. Mol Cell Biol. 2006;26:5994–6004. doi: 10.1128/MCB.01630-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bondar T, Kalinina A, Khair L, Kopanja D, Nag A, Bagchi S, et al. Cul4A and DDB1 associate with Skp2 to target p27Kip1 for proteolysis involving the COP9 signalosome. Mol Cell Biol. 2006;26:2531–9. doi: 10.1128/MCB.26.7.2531-2539.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Denti S, Fernandez-Sanchez ME, Rogge L, Bianchi E. The COP9 signalosome regulates Skp2 levels and proliferation of human cells. J Biol Chem. 2006;281:32188–96. doi: 10.1074/jbc.M604746200. [DOI] [PubMed] [Google Scholar]
- 53.Hao B, Zheng N, Schulman BA, Wu G, Miller JJ, Pagano M, Pavletich NP. Structural basis of the Cks1-dependent recognition of p27(Kip1) by the SCF(Skp2) ubiquitin ligase. Mol Cell. 2005;20:9–19. doi: 10.1016/j.molcel.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 54.Wang W, Ungermannova D, Chen L, Liu X. A negatively charged amino acid in Skp2 is required for Skp2-Cks1 interaction and ubiquitination of p27Kip1. J Biol Chem. 2003;278:32390–6. doi: 10.1074/jbc.M305241200. [DOI] [PubMed] [Google Scholar]
- 55.Yao ZP, Zhou M, Kelly SE, Seeliger MA, Robinson CV, Itzhaki LS. Activation of ubiquitin ligase SCF(Skp2) by Cks1: insights from hydrogen exchange mass spectrometry. J Mol Biol. 2006;363:673–86. doi: 10.1016/j.jmb.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 56.Xu S, Abbasian M, Patel P, Jensen-Pergakes K, Lombardo CR, Cathers BE, et al. Substrate recognition and ubiquitination of SCFSkp2/Cks1 ubiquitin-protein isopeptide ligase. J Biol Chem. 2007;282:15462–70. doi: 10.1074/jbc.M610758200. [DOI] [PubMed] [Google Scholar]
- 57.Zhou W, Yang Q, Low CB, Karthik BC, Wang Y, Ryo A, et al. Pin1 catalyzes conformational changes of Thr-187 in p27Kip1 and mediates its stability through a polyubiquitination process. J Biol Chem. 2009;284:23980–8. doi: 10.1074/jbc.M109.022814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jonason JH, Gavrilova N, Wu M, Zhang H, Sun H. Regulation of SCF(SKP2) ubiquitin E3 ligase assembly and p27(KIP1) proteolysis by the PTEN pathway and cyclin D1. Cell Cycle. 2007;6:951–61. doi: 10.4161/cc.6.8.4104. [DOI] [PubMed] [Google Scholar]
- 59.Zhu XH, Nguyen H, Halicka HD, Traganos F, Koff A. Noncatalytic requirement for cyclin A-cdk2 in p27 turnover. Mol Cell Biol. 2004;24:6058–66. doi: 10.1128/MCB.24.13.6058-6066.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ji P, Goldin L, Ren H, Sun D, Guardavaccaro D, Pagano M, et al. Skp2 contains a novel cyclin A binding domain that directly protects cyclin A from inhibition by p27Kip1. J Biol Chem. 2006;281:24058–69. doi: 10.1074/jbc.M603105200. [DOI] [PubMed] [Google Scholar]
- 61.Tomoda K, Kubota Y, Kato J. Degradation of the cyclin-dependent-kinase inhibitor p27Kip1 is instigated by Jab1. Nature. 1999;398:160–5. doi: 10.1038/18230. [DOI] [PubMed] [Google Scholar]
- 62.Rodier G, Montagnoli A, Di Marcotullio L, Coulombe P, Draetta GF, Pagano M, et al. p27 cytoplasmic localization is regulated by phosphorylation on Ser10 and is not a prerequisite for its proteolysis. EMBO J. 2001;20:6672–82. doi: 10.1093/emboj/20.23.6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ishida N, Hara T, Kamura T, Yoshida M, Nakayama K, Nakayama KI. Phosphorylation of p27Kip1 on serine 10 is required for its binding to CRM1 and nuclear export. J Biol Chem. 2002;277:14355–8. doi: 10.1074/jbc.C100762200. [DOI] [PubMed] [Google Scholar]
- 64.Mishra A, Godavarthi SK, Jana NR. UBE3A/E6-AP regulates cell proliferation by promoting proteasomal degradation of p27. Neurobiol Dis. 2009;36:26–34. doi: 10.1016/j.nbd.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 65.Hattori T, Isobe T, Abe K, Kikuchi H, Kitagawa K, Oda T, et al. Pirh2 promotes ubiquitin-dependent degradation of the cyclin-dependent kinase inhibitor p27Kip1. Cancer Res. 2007;67:10789–95. doi: 10.1158/0008-5472.CAN-07-2033. [DOI] [PubMed] [Google Scholar]
- 66.Miranda-Carboni GA, Krum SA, Yee K, Nava M, Deng QE, Pervin S, et al. A functional link between Wnt signaling and SKP2-independent p27 turnover in mammary tumors. Genes Dev. 2008;22:3121–34. doi: 10.1101/gad.1692808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li B, Jia N, Kapur R, Chun KT. Cul4A targets p27 for degradation and regulates proliferation, cell cycle exit, and differentiation during erythropoiesis. Blood. 2006;107:4291–9. doi: 10.1182/blood-2005-08-3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Higa LA, Yang X, Zheng J, Banks D, Wu M, Ghosh P, et al. Involvement of CUL4 ubiquitin E3 ligases in regulating CDK inhibitors Dacapo/p27Kip1 and cyclin E degradation. Cell Cycle. 2006;5:71–7. doi: 10.4161/cc.5.1.2266. [DOI] [PubMed] [Google Scholar]
- 69.McAllister SS, Becker-Hapak M, Pintucci G, Pagano M, Dowdy SF. Novel p27(kip1) C-terminal scatter domain mediates Rac-dependent cell migration independent of cell cycle arrest functions. Mol Cell Biol. 2003;23:216–28. doi: 10.1128/MCB.23.1.216-228.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Foster JS, Fernando RI, Ishida N, Nakayama KI, Wimalasena J. Estrogens downregulate p27Kip1 in breast cancer cells through Skp2 and through nuclear export mediated by the ERK pathway. J Biol Chem. 2003;278:41355–66. doi: 10.1074/jbc.M302830200. [DOI] [PubMed] [Google Scholar]
- 71.Besson A, Gurian-West M, Chen X, Kelly-Spratt KS, Kemp CJ, Roberts JM. A pathway in quiescent cells that controls p27Kip1 stability, subcellular localization and tumor suppression. Genes Dev. 2006;20:47–64. doi: 10.1101/gad.1384406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kamura T, Hara T, Matsumoto M, Ishida N, Okumura F, Hatakeyama S, et al. Cytoplasmic ubiquitin ligase KPC regulates proteolysis of p27(Kip1) at G1 phase. Nat Cell Biol. 2004;6:1229–35. doi: 10.1038/ncb1194. [DOI] [PubMed] [Google Scholar]
- 73.Kotoshiba S, Kamura T, Hara T, Ishida N, Nakayama KI. Molecular dissection of the interaction between p27 and Kip1 ubiquitylation-promoting complex, the ubiquitin ligase that regulates proteolysis of p27 in G1 phase. J Biol Chem. 2005;280:17694–700. doi: 10.1074/jbc.M500866200. [DOI] [PubMed] [Google Scholar]
- 74.Hara T, Kamura T, Kotoshiba S, Takahashi H, Fujiwara K, Onoyama I, et al. Role of the UBL-UBA protein KPC2 in degradation of p27 at G1 phase of the cell cycle. Mol Cell Biol. 2005;25:9292–303. doi: 10.1128/MCB.25.21.9292-9303.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lu Y, Adegoke OA, Nepveu A, Nakayama KI, Bedard N, Cheng D, et al. USP19 deubiquitinating enzyme supports cell proliferation by stabilizing KPC1, a ubiquitin ligase for p27Kip1. Mol Cell Biol. 2009;29:547–58. doi: 10.1128/MCB.00329-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kawauchi T, Chihama K, Nabeshima Y, Hoshino M. Cdk5 phosphorylates and stabilizes p27kip1 contributing to actin organization and cortical neuronal migration. Nat Cell Biol. 2006;8:17–26. doi: 10.1038/ncb1338. [DOI] [PubMed] [Google Scholar]
- 77.Deng X, Mercer SE, Shah S, Ewton DZ, Friedman E. The cyclin-dependent kinase inhibitor p27Kip1 is stabilized in G(0) by Mirk/dyrk1B kinase. J Biol Chem. 2004;279:22498–504. doi: 10.1074/jbc.M400479200. [DOI] [PubMed] [Google Scholar]
- 78.Boehm M, Yoshimoto T, Crook MF, Nallamshetty S, True A, Nabel GJ, et al. A growth factor-dependent nuclear kinase phosphorylates p27(Kip1) and regulates cell cycle progression. EMBO J. 2002;21:3390–401. doi: 10.1093/emboj/cdf343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee JG, Kay EP. Involvement of two distinct ubiquitin E3 ligase systems for p27 degradation in corneal endothelial cells. Invest Ophthalmol Vis Sci. 2008;49:189–96. doi: 10.1167/iovs.07-0855. [DOI] [PubMed] [Google Scholar]
- 80.Vervoorts J, Luscher B. Post-translational regulation of the tumor suppressor p27(KIP1) Cell Mol Life Sci. 2008;65:3255–64. doi: 10.1007/s00018-008-8296-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kotake Y, Nakayama K, Ishida N, Nakayama KI. Role of serine 10 phosphorylation in p27 stabilization revealed by analysis of p27 knock-in mice harboring a serine 10 mutation. J Biol Chem. 2005;280:1095–102. doi: 10.1074/jbc.M406117200. [DOI] [PubMed] [Google Scholar]
- 82.Fujita N, Sato S, Katayama K, Tsuruo T. Akt-dependent phosphorylation of p27Kip1 promotes binding to 14-3-3 and cytoplasmic localization. J Biol Chem. 2002;277:28706–13. doi: 10.1074/jbc.M203668200. [DOI] [PubMed] [Google Scholar]
- 83.Fujita N, Sato S, Tsuruo T. Phosphorylation of p27Kip1 at threonine 198 by p90 ribosomal protein S6 kinases promotes its binding to 14-3-3 and cytoplasmic localization. J Biol Chem. 2003;278:49254–60. doi: 10.1074/jbc.M306614200. [DOI] [PubMed] [Google Scholar]
- 84.Kossatz U, Vervoorts J, Nickeleit I, Sundberg HA, Arthur JS, Manns MP, et al. C-terminal phosphorylation controls the stability and function of p27kip1. EMBO J. 2006;25:5159–70. doi: 10.1038/sj.emboj.7601388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chu I, Sun J, Arnaout A, Kahn H, Hanna W, Narod S, et al. p27 phosphorylation by Src regulates inhibition of cyclin E-Cdk2. Cell. 2007;128:281–94. doi: 10.1016/j.cell.2006.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Grimmler M, Wang Y, Mund T, Cilensek Z, Keidel EM, Waddell MB, et al. Cdk-inhibitory activity and stability of p27Kip1 are directly regulated by oncogenic tyrosine kinases. Cell. 2007;128:269–80. doi: 10.1016/j.cell.2006.11.047. [DOI] [PubMed] [Google Scholar]
- 87.James MK, Ray A, Leznova D, Blain SW. Differential modification of p27Kip1 controls its cyclin D-cdk4 inhibitory activity. Mol Cell Biol. 2008;28:498–510. doi: 10.1128/MCB.02171-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ray A, James MK, Larochelle S, Fisher RP, Blain SW. p27Kip1 inhibits cyclin D-cyclin-dependent kinase 4 by two independent modes. Mol Cell Biol. 2009;29:986–99. doi: 10.1128/MCB.00898-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Morishita D, Katayama R, Sekimizu K, Tsuruo T, Fujita N. Pim kinases promote cell cycle progression by phosphorylating and downregulating p27Kip1 at the transcriptional and posttranscriptional levels. Cancer Res. 2008;68:5076–85. doi: 10.1158/0008-5472.CAN-08-0634. [DOI] [PubMed] [Google Scholar]
- 90.Hong F, Larrea MD, Doughty C, Kwiatkowski DJ, Squillace R, Slingerland JM. mTOR-raptor binds and activates SGK1 to regulate p27 phosphorylation. Mol Cell. 2008;30:701–11. doi: 10.1016/j.molcel.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 91.Shin I, Rotty J, Wu FY, Arteaga CL. Phosphorylation of p27Kip1 at Thr-157 interferes with its association with importin alpha during G1 and prevents nuclear re-entry. J Biol Chem. 2005;280:6055–63. doi: 10.1074/jbc.M412367200. [DOI] [PubMed] [Google Scholar]
- 92.Tapia JC, Bolanos-Garcia VM, Sayed M, Allende CC, Allende JE. Cell cycle regulatory protein p27KIP1 is a substrate and interacts with the protein kinase CK2. J Cell Biochem. 2004;91:865–79. doi: 10.1002/jcb.20027. [DOI] [PubMed] [Google Scholar]
- 93.Brandts CH, Bilanges B, Hare G, McCormick F, Stokoe D. Phosphorylation-independent stabilization of p27kip1 by the phosphoinositide 3-kinase pathway in glioblastoma cells. J Biol Chem. 2005;280:2012–9. doi: 10.1074/jbc.M408348200. [DOI] [PubMed] [Google Scholar]
- 94.Zhang H, Kobayashi R, Galaktionov K, Beach D. p19Skp1 and p45Skp2 are essential elements of the cyclin A-CDK2 S phase kinase. Cell. 1995;82:915–25. doi: 10.1016/0092-8674(95)90271-6. [DOI] [PubMed] [Google Scholar]
- 95.Sheaff RJ, Groudine M, Gordon M, Roberts JM, Clurman BE. Cyclin E-CDK2 is a regulator of p27Kip1. Genes Dev. 1997;11:1464–78. doi: 10.1101/gad.11.11.1464. [DOI] [PubMed] [Google Scholar]
- 96.Malek NP, Sundberg H, McGrew S, Nakayama K, Kyriakides TR, Roberts JM. A mouse knock-in model exposes sequential proteolytic pathways that regulate p27Kip1 in G1 and S phase. Nature. 2001;413:323–7. doi: 10.1038/35095083. [DOI] [PubMed] [Google Scholar]
- 97.Tan M, Davis SW, Saunders TL, Zhu Y, Sun Y. RBX1/ROC1 disruption results in early embryonic lethality due to proliferation failure, partially rescued by simultaneous loss of p27. Proc Natl Acad Sci USA. 2009;106:6203–8. doi: 10.1073/pnas.0812425106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ganoth D, Bornstein G, Ko TK, Larsen B, Tyers M, Pagano M, Hershko A. The cell cycle regulatory protein Cks1 is required for SCF(Skp2)-mediated ubiquitinylation of p27. Nat Cell Biol. 2001;3:321–4. doi: 10.1038/35060126. [DOI] [PubMed] [Google Scholar]
- 99.Spruck C, Strohmaier H, Watson M, Smith AP, Ryan A, Krek TW, Reed SI. A CDK-independent function of mammalian Cks1: targeting of SCF(Skp2) to the CDK inhibitor p27Kip1. Mol Cell. 2001;7:639–50. doi: 10.1016/s1097-2765(01)00210-6. [DOI] [PubMed] [Google Scholar]
- 100.Nakayama K, Nagahama H, Minamishima YA, Matsumoto M, Nakamichi I, Kitagawa K, et al. Targeted disruption of Skp2 results in accumulation of cyclin E and p27(Kip1), polyploidy and centrosome overduplication. EMBO J. 2000;19:2069–81. doi: 10.1093/emboj/19.9.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nakayama K, Nagahama H, Minamishima YA, Miyake S, Ishida N, Hatakeyama S, et al. Skp2-mediated degradation of p27 regulates progression into mitosis. Dev Cell. 2004;6:661–72. doi: 10.1016/s1534-5807(04)00131-5. [DOI] [PubMed] [Google Scholar]
- 102.Wang H, Bauzon F, Ji P, Xu X, Sun D, Locker J, et al. Skp2 is required for survival of aberrantly proliferating Rb1-deficient cells and for tumorigenesis in Rb1+/− mice. Nat Genet. 2010;42:83–8. doi: 10.1038/ng.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Timmerbeul I, Garrett-Engele CM, Kossatz U, Chen X, Firpo E, Grunwald V, et al. Testing the importance of p27 degradation by the SCFskp2 pathway in murine models of lung and colon cancer. Proc Natl Acad Sci USA. 2006;103:14009–14. doi: 10.1073/pnas.0606316103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Agarwal A, Bumm TG, Corbin AS, O’Hare T, Loriaux M, VanDyke J, et al. Absence of SKP2 expression attenuates BCR-ABL-induced myeloproliferative disease. Blood. 2008;112:1960–70. doi: 10.1182/blood-2007-09-113860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lin HK, Chen Z, Wang G, Nardella C, Lee SW, Chan CH, et al. Skp2 targeting suppresses tumorigenesis by Arf-p53-independent cellular senescence. Nature. 464:374–9. doi: 10.1038/nature08815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nakayama K, Ishida N, Shirane M, Inomata A, Inoue T, Shishido N, et al. Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia and pituitary tumors. Cell. 1996;85:707–20. doi: 10.1016/s0092-8674(00)81237-4. [DOI] [PubMed] [Google Scholar]
- 107.Kiyokawa H, Kineman RD, Manova-Todorova KO, Soares VC, Hoffman ES, Ono M, et al. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27(Kip1) Cell. 1996;85:721–32. doi: 10.1016/s0092-8674(00)81238-6. [DOI] [PubMed] [Google Scholar]
- 108.Fero ML, Rivkin M, Tasch M, Porter P, Carow CE, Firpo E, et al. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27(Kip1)-deficient mice. Cell. 1996;85:733–44. doi: 10.1016/s0092-8674(00)81239-8. [DOI] [PubMed] [Google Scholar]
- 109.Moberg KH, Bell DW, Wahrer DC, Haber DA, Hariharan IK. Archipelago regulates Cyclin E levels in Drosophila and is mutated in human cancer cell lines. Nature. 2001;413:311–6. doi: 10.1038/35095068. [DOI] [PubMed] [Google Scholar]
- 110.Strohmaier H, Spruck CH, Kaiser P, Won KA, Sangfelt O, Reed SI. Human F-box protein hCdc4 targets cyclin E for proteolysis and is mutated in a breast cancer cell line. Nature. 2001;413:316–22. doi: 10.1038/35095076. [DOI] [PubMed] [Google Scholar]
- 111.Barberis M, De Gioia L, Ruzzene M, Sarno S, Coccetti P, Fantucci P, et al. The yeast cyclin-dependent kinase inhibitor Sic1 and mammalian p27Kip1 are functional homologues with a structurally conserved inhibitory domain. Biochem J. 2005;387:639–47. doi: 10.1042/BJ20041299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Verma R, Annan RS, Huddleston MJ, Carr SA, Reynard G, Deshaies RJ. Phosphorylation of Sic1p by G1 Cdk required for its degradation and entry into S phase. Science. 1997;278:455–60. doi: 10.1126/science.278.5337.455. [DOI] [PubMed] [Google Scholar]
- 113.Feldman RM, Correll CC, Kaplan KB, Deshaies RJ. A complex of Cdc4p, Skp1p and Cdc53p/cullin catalyzes ubiquitination of the phosphorylated CDK inhibitor Sic1p. Cell. 1997;91:221–30. doi: 10.1016/s0092-8674(00)80404-3. [DOI] [PubMed] [Google Scholar]
- 114.Skowyra D, Craig KL, Tyers M, Elledge SJ, Harper JW. F-box proteins are receptors that recruit phosphorylated substrates to the SCF ubiquitin-ligase complex. Cell. 1997;91:209–19. doi: 10.1016/s0092-8674(00)80403-1. [DOI] [PubMed] [Google Scholar]
- 115.Tang X, Orlicky S, Lin Z, Willems A, Neculai D, Ceccarelli D, et al. Suprafacial orientation of the SCFCdc4 dimer accommodates multiple geometries for substrate ubiquitination. Cell. 2007;129:1165–76. doi: 10.1016/j.cell.2007.04.042. [DOI] [PubMed] [Google Scholar]
- 116.Hao B, Oehlmann S, Sowa ME, Harper JW, Pavletich NP. Structure of a Fbw7-Skp1-cyclin E complex: multisite-phosphorylated substrate recognition by SCF ubiquitin ligases. Mol Cell. 2007;26:131–43. doi: 10.1016/j.molcel.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 117.Petroski MD, Deshaies RJ. Context of multiubiquitin chain attachment influences the rate of Sic1 degradation. Mol Cell. 2003;11:1435–44. doi: 10.1016/s1097-2765(03)00221-1. [DOI] [PubMed] [Google Scholar]
- 118.Orlicky S, Tang X, Willems A, Tyers M, Sicheri F. Structural basis for phosphodependent substrate selection and orientation by the SCFCdc4 ubiquitin ligase. Cell. 2003;112:243–56. doi: 10.1016/s0092-8674(03)00034-5. [DOI] [PubMed] [Google Scholar]
- 119.Nash P, Tang X, Orlicky S, Chen Q, Gertler FB, Mendenhall MD, et al. Multisite phosphorylation of a CDK inhibitor sets a threshold for the onset of DNA replication. Nature. 2001;414:514–21. doi: 10.1038/35107009. [DOI] [PubMed] [Google Scholar]
- 120.Petroski MD, Deshaies RJ. Mechanism of lysine 48-linked ubiquitin-chain synthesis by the cullin-RING ubiquitin-ligase complex SCF-Cdc34. Cell. 2005;123:1107–20. doi: 10.1016/j.cell.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 121.Pierce NW, Kleiger G, Shan SO, Deshaies RJ. Detection of sequential polyubiquitylation on a millisecond timescale. Nature. 2009;462:615–9. doi: 10.1038/nature08595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sadowski M, Mawson A, Baker R, Sarcevic B. Cdc34 C-terminal tail phosphorylation regulates Skp1/cullin/ F-box (SCF)-mediated ubiquitination and cell cycle progression. Biochem J. 2007;405:569–81. doi: 10.1042/BJ20061812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Verma R, Oania R, Graumann J, Deshaies RJ. Multiubiquitin chain receptors define a layer of substrate selectivity in the ubiquitin-proteasome system. Cell. 2004;118:99–110. doi: 10.1016/j.cell.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 124.Verma R, Aravind L, Oania R, McDonald WH, Yates JR, 3rd, Koonin EV, et al. Role of Rpn11 metalloprotease in deubiquitination and degradation by the 26S proteasome. Science. 2002;298:611–5. doi: 10.1126/science.1075898. [DOI] [PubMed] [Google Scholar]
- 125.Babbitt SE, Kiss A, Deffenbaugh AE, Chang YH, Bailly E, Erdjument-Bromage H, et al. ATP hydrolysis-dependent disassembly of the 26S proteasome is part of the catalytic cycle. Cell. 2005;121:553–65. doi: 10.1016/j.cell.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 126.Benjamin KR, Zhang C, Shokat KM, Herskowitz I. Control of landmark events in meiosis by the CDK Cdc28 and the meiosis-specific kinase Ime2. Genes Dev. 2003;17:1524–39. doi: 10.1101/gad.1101503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dirick L, Goetsch L, Ammerer G, Byers B. Regulation of meiotic S phase by Ime2 and a Clb5,6-associated kinase in Saccharomyces cerevisiae. Science. 1998;281:1854–7. doi: 10.1126/science.281.5384.1854. [DOI] [PubMed] [Google Scholar]
- 128.Sedgwick C, Rawluk M, Decesare J, Raithatha S, Wohlschlegel J, Semchuk P, et al. Saccharomyces cerevisiae Ime2 phosphorylates Sic1 at multiple PXS/T sites but is insufficient to trigger Sic1 degradation. Biochem J. 2006;399:151–60. doi: 10.1042/BJ20060363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Purnapatre K, Gray M, Piccirillo S, Honigberg SM. Glucose inhibits meiotic DNA replication through SCFGrr1p-dependent destruction of Ime2p kinase. Mol Cell Biol. 2005;25:440–50. doi: 10.1128/MCB.25.1.440-450.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sanchez-Diaz A, Gonzalez I, Arellano M, Moreno S. The Cdk inhibitors p25rum1 and p40SIC1 are functional homologues that play similar roles in the regulation of the cell cycle in fission and budding yeast. J Cell Sci. 1998;111:843–51. doi: 10.1242/jcs.111.6.843. [DOI] [PubMed] [Google Scholar]
- 131.Kominami K, Ochotorena I, Toda T. Two F-box/ WD-repeat proteins Pop1 and Pop2 form hetero- and homo-complexes together with cullin-1 in the fission yeast SCF (Skp1-Cullin-1-F-box) ubiquitin ligase. Genes Cells. 1998;3:721–35. doi: 10.1046/j.1365-2443.1998.00225.x. [DOI] [PubMed] [Google Scholar]
- 132.Kominami K, Toda T. Fission yeast WD-repeat protein pop1 regulates genome ploidy through ubiquitin-proteasome-mediated degradation of the CDK inhibitor Rum1 and the S-phase initiator Cdc18. Genes Dev. 1997;11:1548–60. doi: 10.1101/gad.11.12.1548. [DOI] [PubMed] [Google Scholar]
- 133.Jallepalli PV, Tien D, Kelly TJ. sud1(+) targets cyclin-dependent kinase-phosphorylated Cdc18 and Rum1 proteins for degradation and stops unwanted diploidization in fission yeast. Proc Natl Acad Sci USA. 1998;95:8159–64. doi: 10.1073/pnas.95.14.8159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Maekawa H, Kitamura K, Shimoda C. The Ste16 WD-repeat protein regulates cell cycle progression under starvation through the Rum1 protein in Schizosaccharomyces pombe. Current genetics. 1998;33:29–37. doi: 10.1007/s002940050305. [DOI] [PubMed] [Google Scholar]
- 135.Benito J, Martin-Castellanos C, Moreno S. Regulation of the G1 phase of the cell cycle by periodic stabilization and degradation of the p25rum1 CDK inhibitor. EMBO J. 1998;17:482–97. doi: 10.1093/emboj/17.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Osaka F, Saeki M, Katayama S, Aida N, Toh EA, Kominami K, et al. Covalent modifier NEDD8 is essential for SCF ubiquitin-ligase in fission yeast. EMBO J. 2000;19:3475–84. doi: 10.1093/emboj/19.13.3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Seibert V, Prohl C, Schoultz I, Rhee E, Lopez R, Abderazzaq K, et al. Combinatorial diversity of fission yeast SCF ubiquitin ligases by homo- and heterooligo-meric assemblies of the F-box proteins Pop1p and Pop2p. BMC Biochem. 2002;3:22. doi: 10.1186/1471-2091-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wolf DA, McKeon F, Jackson PK. F-box/WD-repeat proteins pop1p and Sud1p/Pop2p form complexes that bind and direct the proteolysis of cdc18p. Curr Biol. 1999;9:373–6. doi: 10.1016/s0960-9822(99)80165-1. [DOI] [PubMed] [Google Scholar]
- 139.Peter M, Gartner A, Horecka J, Ammerer G, Herskowitz I. FAR1 links the signal transduction pathway to the cell cycle machinery in yeast. Cell. 1993;73:747–60. doi: 10.1016/0092-8674(93)90254-n. [DOI] [PubMed] [Google Scholar]
- 140.Tyers M, Futcher B. Far1 and Fus3 link the mating pheromone signal transduction pathway to three G1-phase Cdc28 kinase complexes. Mol Cell Biol. 1993;13:5659–69. doi: 10.1128/mcb.13.9.5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Toyoshima H, Hunter T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell. 1994;78:67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- 142.Henchoz S, Chi Y, Catarin B, Herskowitz I, Deshaies RJ, Peter M. Phosphorylation- and ubiquitin-dependent degradation of the cyclin-dependent kinase inhibitor Far1p in budding yeast. Genes Dev. 1997;11:3046–60. doi: 10.1101/gad.11.22.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Blondel M, Galan JM, Chi Y, Lafourcade C, Longaretti C, Deshaies RJ, Peter M. Nuclear-specific degradation of Far1 is controlled by the localization of the F-box protein Cdc4. EMBO J. 2000;19:6085–97. doi: 10.1093/emboj/19.22.6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yan Y, Frisen J, Lee MH, Massague J, Barbacid M. Ablation of the CDK inhibitor p57Kip2 results in increased apoptosis and delayed differentiation during mouse development. Genes Dev. 1997;11:973–83. doi: 10.1101/gad.11.8.973. [DOI] [PubMed] [Google Scholar]
- 145.Urano T, Yashiroda H, Muraoka M, Tanaka K, Hosoi T, Inoue S, et al. p57(Kip2) is degraded through the proteasome in osteoblasts stimulated to proliferation by transforming growth factor beta1. J Biol Chem. 1999;274:12197–200. doi: 10.1074/jbc.274.18.12197. [DOI] [PubMed] [Google Scholar]
- 146.Nishimori S, Tanaka Y, Chiba T, Fujii M, Imamura T, Miyazono K, et al. Smad-mediated transcription is required for transforming growth factor-beta1-induced p57(Kip2) proteolysis in osteoblastic cells. J Biol Chem. 2001;276:10700–5. doi: 10.1074/jbc.M007499200. [DOI] [PubMed] [Google Scholar]
- 147.Kim M, Nakamoto T, Nishimori S, Tanaka K, Chiba T. A new ubiquitin ligase involved in p57KIP2 proteolysis regulates osteoblast cell differentiation. EMBO Rep. 2008;9:878–84. doi: 10.1038/embor.2008.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kamura T, Hara T, Kotoshiba S, Yada M, Ishida N, Imaki H, et al. Degradation of p57Kip2 mediated by SCFSkp2-dependent ubiquitylation. Proc Natl Acad Sci USA. 2003;100:10231–6. doi: 10.1073/pnas.1831009100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Vidal A, Koff A. Cell cycle inhibitors: three families united by a common cause. Gene. 2000;247:1–15. doi: 10.1016/s0378-1119(00)00092-5. [DOI] [PubMed] [Google Scholar]
- 150.Zhang P, Liegeois NJ, Wong C, Finegold M, Hou H, Thompson JC, et al. Altered cell differentiation and proliferation in mice lacking p57KIP2 indicates a role in Beckwith-Wiedemann syndrome. Nature. 1997;387:151–8. doi: 10.1038/387151a0. [DOI] [PubMed] [Google Scholar]
- 151.Susaki E, Nakayama K, Yamasaki L, Nakayama KI. Common and specific roles of the related CDK inhibitors p27 and p57 revealed by a knock-in mouse model. Proc Natl Acad Sci USA. 2009;106:5192–7. doi: 10.1073/pnas.0811712106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chu IM, Hengst L, Slingerland JM. The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat Rev Cancer. 2008;8:253–67. doi: 10.1038/nrc2347. [DOI] [PubMed] [Google Scholar]