Abstract
Background
Rates of relapse among cocaine-dependent patients are high, and new treatment approaches are needed. Clinical data demonstrate that a cocaine vaccine (TA-CD: Celtic Pharmaceutical) produces selective anti-cocaine antibodies, yet the impact of these antibodies on cocaine’s direct effects is unknown. The objective of this human laboratory study was to measure the relationship between antibody titers and the effects of smoked cocaine on ratings of intoxication, craving and cardiovascular effects.
Methods
Ten cocaine-dependent men not seeking drug treatment spent 2 nights per week for 13 weeks inpatient where the effects of cocaine (0, 25, 50 mg) were determined prior to vaccination and at weekly intervals thereafter. Two doses of TA-CD (82 µg, n=4; 360 µg, n=6) were administered at weeks 1, 3, 5 and 9.
Results
Peak plasma antibody levels, which were highly variable, significantly predicted cocaine’s effects. Those individuals in the upper half of antibody production had an immediate (within 4 minutes of cocaine smoking) and robust (55–81%) reduction in ratings of Good Drug Effect and Cocaine Quality, while those in the lower half showed only a nonsignificant attenuation (6–26%). Self-reported cocaine use while participants were outpatient tended to decrease as a function of antibody titer (p < 0.12). By contrast, higher antibody levels predicted significantly greater cocaine-induced tachycardia.
Conclusions
The TA-CD vaccine substantially decreased smoked cocaine’s intoxicating effects in those generating sufficient antibody. These data support further testing of cocaine immunotherapy as a treatment for cocaine dependence.
Keywords: Cocaine, immunotherapy, vaccination, antibody, addiction, dependence
Cocaine dependence continues to be a serious public health problem, with over 1.7 million individuals estimated to be dependent or abusing cocaine in the United States (1). Although behavioral treatments improve rates of cocaine abstinence compared to no treatment (2), relapse rates remain high. Unlike drugs such as heroin, alcohol or nicotine, there is no FDA-approved medication to facilitate the treatment of cocaine dependence, although over 60 medications have been tested for this indication (3,4). New treatment approaches are urgently needed.
The difficulty developing a medication to treat cocaine dependence may partly reflect its mechanism of action. Cocaine blocks the reuptake of several neurotransmitters, and modulating this effect is complex. An alternative to pharmacotherapy is immunotherapy, which sequesters cocaine in the peripheral circulation, rather than attempting to modulate cocaine effects in the CNS. Cocaine is too small a molecule to elicit an antibody response, yet antibodies can be elicited when cocaine is paired with a carrier protein. Over ten years ago, a cocaine vaccine (TA-CD; Celtic Pharmaceutical) was developed by binding a cocaine derivative (succinylnorcocaine) to a nontoxic subunit of recombinant cholera toxin, administered with an aluminum hydroxide adjuvant. Data in rats show that cocaine-specific antibodies generated in response to TA-CD reduced early cocaine distribution to the brain by 25–80% compared to controls (5).
Because the reinforcing effects of a drug are partly determined by how rapidly it reaches the brain (6,7), slowing the rate of drug entry is a potential treatment strategy (8). In rats, vaccination with TA-CD reduced the locomotor, discriminative stimulus and reinforcing effects of cocaine (9–11). The reinstatement of cocaine seeking following a low dose of cocaine was also blocked (12,13), suggesting that the vaccine may decrease relapse upon re-exposure to cocaine.
In individuals seeking treatment for cocaine use, Phase 1 and Phase II studies (14,15) testing a range of TA-CD doses (82–709 µg) and number of vaccinations (3–5) have shown that: (1) TA-CD was well tolerated and produced highly specific antibodies with low cross-reactivity with cocaine metabolites, (2) after 4–5 vaccinations over 8 weeks, antibody levels peaked between weeks 10–14 and were largely absent 6 months later, and (3) there is high individual variability in antibody production. A recent study in cocaine-and opioid-dependent patients showed that only 38% achieved serum antibody levels high enough to significantly reduce cocaine use (16).
An essential question is how antibody titers directly influence cocaine intoxication. Unlike clinical trials, human laboratory studies can directly assess cocaine effects using carefully-monitored procedures in individuals not seeking drug treatment (4). The objective of this study was to measure the subjective and cardiovascular effects of clinically-relevant doses of smoked ‘crack’ cocaine over 13 weeks: before, during and after a series of four vaccinations. Understanding the relationship between antibody levels and a range of cocaine effects is essential to the development of this novel treatment strategy.
Methods and Materials
Cocaine-dependent research volunteers who were explicitly not interested in treatment for their cocaine use signed a consent form approved by The New York State Psychiatric Institute Institutional Review Board which described the procedures and outlined the possible risks, including administration of an experimental vaccine and smoked cocaine. Volunteers were compensated for their participation.
Selection of participants
Fifteen participants, solicited primarily through newspaper advertisement, were enrolled from March 2003 to August 2005. Inclusion criteria included: age (21–45 years), cocaine dependence (DSM-IV criteria), inability to become pregnant, and route of cocaine administration (smoked). Baseline assessments included physical and psychiatric examination, electrocardiogram, urine toxicology, urinalysis, blood chemistry panels including HIV and complement depletion, pulmonary function (PFT), and ophthalmological testing. No volunteer could be HIV+, dependent on other drugs (except nicotine), be taking psychotropic or immunosuppressive medication, have clinically significant autoimmune disease, hypersensitivity to vaccines, have given blood within 3 months or have received any vaccine within 30 days.
Study Schedule
Table 1 portrays the study schedule. Participants spent two nights/week for 13 weeks on the Irving Center for Clinical Research at New York-Presbyterian Hospital. At each hospital visit, urine toxicology and a time-line followback questionnaire were completed to assess drug, alcohol and medication use since the previous visit. While inpatient, participants had access to television, radio, and videotaped movies, and cigarette smokers were permitted to smoke outside of laboratory sessions. No one was permitted to receive visitors, mail or leave the unit unescorted.
TABLE 1.
Study Schedule
| Study Week | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 26 | 39 | 52 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vaccination | x | x | x | x | ||||||||||||
| Cocaine Administration | x | x | x | x | x | x | x | x | x | x | x | x | x | |||
| Plasma Antibody Level | x | x | x | x | x | x | x | x | ||||||||
| Plasma Cocaine Level | x | x | x | x | x | |||||||||||
| Eye Exam | x | x | x | x | x | |||||||||||
| Blood Tests | x | x | x | x | x | |||||||||||
| Urinalysis | x | x | x | x | x | |||||||||||
| Physical Examination | x | x | x | x | x | x | x | x | ||||||||
| C3, C4 plasma levels | x | x | x | x | ||||||||||||
| PFT | x | x | ||||||||||||||
| Time-line Followback | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x |
| Urine Toxicology | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x |
| MD or RN Interview | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x |
Note: PFT: Pulmonary Function Test; Blood Tests: chemistry panel (electrolytes, BUN, creatinine), hepatic function (transaminases), and CBC.
Safety monitoring
Participants were vaccinated on weeks 1, 3, 5, and 9. Prior to each vaccination, we confirmed that there were no inflammatory or allergic reactions to the vaccine, to the presence of antibodies (e.g., serum sickness) or to cocaine. Blood tests (chemistry panel, CBC, complement depletion), urinalysis and a physical examination were conducted prior to each vaccination and at study termination (Table 1). Local and systemic reactions were monitored for 2 hours after vaccination and 2–4 days later. Each week, medical staff inquired about side effects and discussed the dangers of trying to override vaccine effects. Forced expiratory volume (FEV) tests were done following the first cocaine administration in each session. Prior to each vaccination and at study termination, participants underwent ophthalmologic examinations (tonometry, visual field and acuity, color vision, slit lamp examination, fundoscopy, inspection for retinal abnormalities). The FDA required these assessments due to preclinical evidence of retinal toxicity with a similar vaccine.
Follow-up assessments occurred at 6, 9, and 12 months after the first vaccination, and included vital signs, urine toxicology, self-reported use of drugs and alcohol within the past week, a physical examination, and a PFT (6-month visit).
Cocaine sessions
Three, 90-minute cocaine sessions occurred one day/week for 13 weeks (totalling 39 sessions). On each session day, three doses of cocaine were tested in counter-balanced order: one dose was administered twice in the morning, another dose was administered twice at noon, and a different dose was administered twice in the afternoon. Order of cocaine dosing was systematically varied between and within-subjects except, as a precaution, the lower dose (25 mg) preceded the higher dose (50 mg) in Week 1.
During a session, electrocardiograms were continuously monitored (MAC PC®, Marquette Electronics, Milwaukee, WI) while heart rate and blood pressure were recorded every two minutes (Sentry II-Model 6100, NBS Medical, Costa Mesa, CA) from 30-minutes prior to the first cocaine administration to 30-minutes following the second cocaine administration. Subjective effects and FEV were measured at baseline. The same dose of cocaine was administered twice, 20 minutes apart. Subjective effects were measured 4 minutes after each dose, and 15 and 30 minutes after the second cocaine administration. FEV was assessed 4 minutes after the first dose. During weeks 1, 3, 5, 9 and 13, plasma cocaine levels were assessed at baseline, 4 minutes after the first cocaine administration, and 4, 15 and 30 minutes after the second cocaine administration. Blood was withdrawn through an 18-gauge catheter (Quik-Cath®, Travenol Laboratories, Deerfield, IL) kept patent with physiological saline.
Research nurses monitored participants via a one-way mirror and communicated via intercom. Cocaine was not given if cardiovascular activity exceeded vital signs criteria for at least 6 minutes [SP > 160 mmHg, DP > 100 mmHg, or HR > (220-participant’s age)*0.85] or if changes on the electrocardiogram were deemed unsafe [e.g., > 10 premature ventricular contractions (PVCs)/session]. If two consecutive sessions were terminated due to vital signs, participants were counseled and discharged from the study.
Subjective-Effects Battery
A computerized subjective-effects questionnaire, comprising a series of 100-mm visual analog scales (VAS) labeled "Not at all" at one end and "Extremely" at the other, was completed 5 times per session. Cluster analysis of the 18 mood ratings (e.g., "I feel…"High"), yielded 4 clusters (17). The Good Drug Effect cluster: "Good Drug Effect," "High," "Stimulated," the Bad Drug Effect cluster: "Anxious," "Bad Drug Effect," "Confused," "Depressed," "Irritable," "Sedated," "Tired," the Social cluster: "Social," "Talkative", "Self-confident," "Alert," "Friendly," and the Focussed/Calm cluster: "Able to Concentrate," "Calm." An additional cluster comprised the 3 adjectives rating the quality of the dose. The Cocaine Quality cluster: "The choice was …"Good Quality," "Potent," "I Liked the Choice." Four VAS operationalized drug craving: "I want…" "Cocaine," "Heroin," "Alcohol," and "Tobacco." The last VAS asked how much participants would pay for the dose (0–$25).
Immunogenicity
As shown in Table 1, plasma samples were obtained at weeks 1, 3, 5, 9, 13 and at the 6, 9 and 12 month follow-up. Anti-cocaine antibody measures, specificity and binding capacity have been described in detail (14). In brief, plasma antibody titers were quantified using enzyme-linked immunosorbent assay (ELISA) and were compared to a standard pool of serum samples taken from a cohort of vaccinated cocaine abusers at their estimated peak antibody response (14, 15). The standard is defined as containing 100 units.
In order to replicate these measures, a second ELISA was done on a subset of samples (week 1 and 13; n=9) courtesy of Drs. Frank Orson and Thomas Kosten, Baylor College of Medicine (16). Cocaine was covalently bound to bovine serum albumin as the antigenic target. Bound human IgG anti-cocaine antibody was detected with horseradish peroxidase conjugated to a second antibody and appropriate substrate. Background antibody binding to the carrier alone was measured and subtracted to establish the quantity of antibody specific for cocaine in these sera. Each ELISA plate included wells with serially diluted polyclonal human IgG to provide an internal standard curve. The specificity of this ELISA was validated using the humanized monoclonal antibody to cocaine, 2E2 (18). Dilutions of this monoclonal antibody were also included on each ELISA plate as a second internal standard. Measurements of IgG antibody performed by this ELISA correlated significantly with the original antibody measurements (R2 = .856). Given that the second ELISA correlated well with the first, data are presented using the first ELISA analysis because a complete data set was available.
Plasma Cocaine Assay
Plasma cocaine was quantified using GC/MS (HP5988) with deuterated internal standards. Standard curves are linear throughout the entire range with r2 = 0.999+, intra and inter-assay RSD% less than 8% for all compounds at each QC level (20ng/ml, 75ng/ml and 300ng/ml) with the LDQ 1ng/ml.
Drugs
Cocaine
Cocaine base (25, 50 mg) was derived from cocaine hydrochloride (Mallinckrodt; 19). Participants were presented with cocaine in a glass pipe stem fitted with a smoke screen. A nurse held a flame on the cocaine and participants were instructed to take one large inhalation and hold it as long as they would outside the laboratory. Participants wore eye masks during cocaine administration to be blinded to the dose. The placebo condition was an empty pipe stem. Although an empty stem is not an ideal placebo, a subset of participants across our studies does not discern that there is no substance being inhaled, so this condition remains an important comparison to active cocaine.
TA-CD
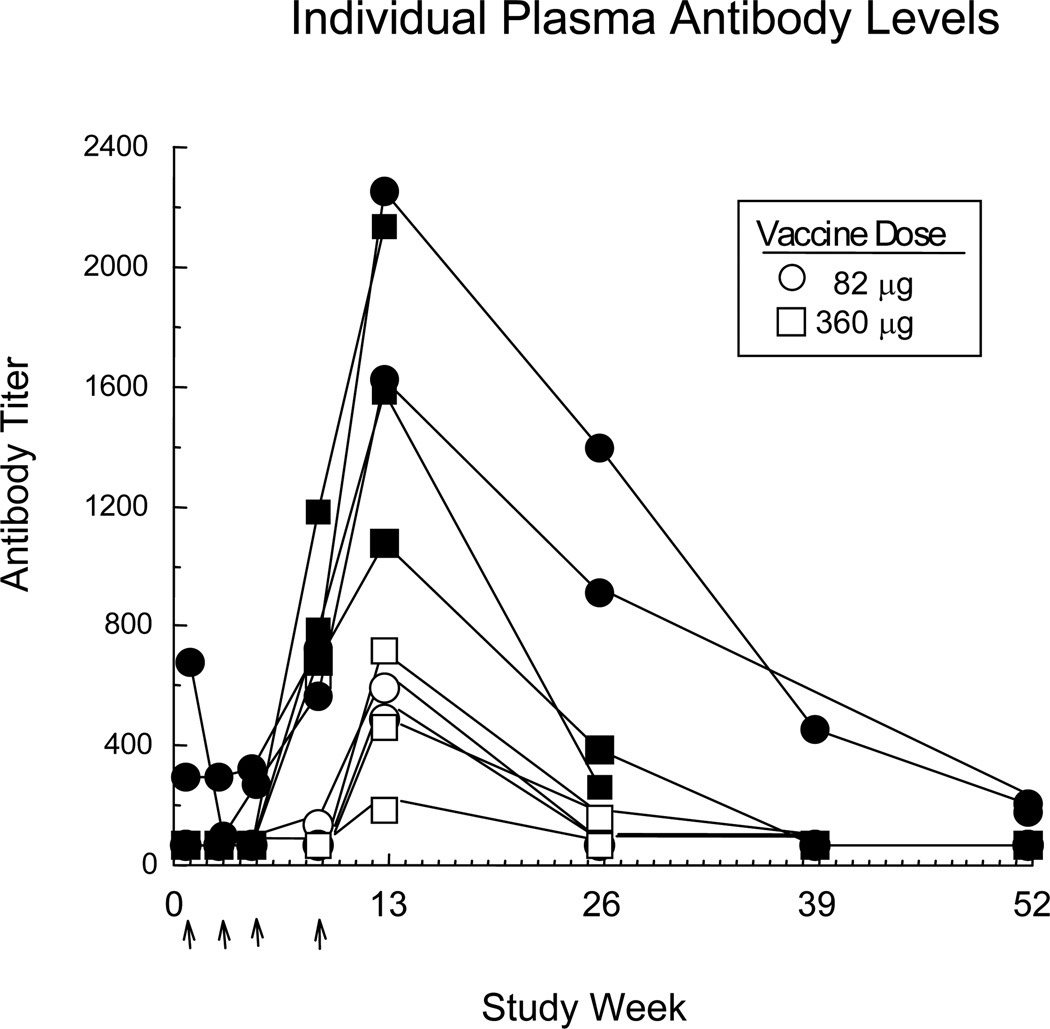
Formulated TA-CD suspension, provided by Celtic Pharmaceutical, contains protein conjugate adsorbed onto aluminum hydroxide gel adjuvant (1.0–1.7 mg/ml aluminum). The dose was administered intramuscularly (0.5 ml injection volume): Four participants received the low dose (82 µg) and six participants received the high dose (360 µg). Two doses were tested because the low dose expired mid-study. TA-CD dose was not treated as an independent variable because of the large individual variability in plasma antibody levels (regardless of dose) illustrated in Figure 1.
Figure 1.
Individual plasma antibody levels as a function of time. Open symbols indicate individuals assigned to the Low Antibody group and filled symbols indicate those assigned to the High Antibody group. Arrows indicate when vaccinations were given (weeks 1, 3, 5, 9). All participants contributed data points for the first 13 weeks, while only a subset returned to the laboratory at followup and thereby contributed to week 26 (n=9), week 39 (n=6), and week 52 (n=8).
Data Analysis
The primary outcome measure was cocaine effects on mood and drug ratings as a function of plasma antibody level using a generalized linear model for repeated outcomes. Weeks 3, 5, 9 and 13 were included in this analysis since these were the timepoints in which antibody levels were measured. Each outcome variable over time was modeled as a function of antibody level (in logarithmic scale) and cocaine dose (25, 50 mg). The interaction effect between antibody levels by cocaine dose was tested and included in the final model if statistically significant (p≤0.05). Baseline values of the outcome variables were included in the models as covariates when applicable.
Similar analyses were conducted to evaluate secondary outcome measures: Peak cocaine, alcohol and nicotine craving ratings, peak diastolic and systolic pressure, heart rate, timeline followback for cocaine and alcohol, and cocaine plasma levels. Data from all participants (n=10) were analyzed for each measure, except for plasma cocaine levels (n=9). Generalized estimating equations (GEE; 20) were used to estimate and test the models. The GEE methodology is able to handle correlated data arising from repeated measurements, requires no parametric distribution assumption, and provides robust inference with respect to misspecification of the within-subject correlation. PROC GENMOD in SAS 9.1.3 (SAS, 2003) was used to conduct the analyses. As an exploratory analysis of the study, all tests were conducted at a significance level of α= 0.05 without adjustment for multiple testing.
A secondary analysis was also conducted: When the GEE analysis indicated an influence of antibody on the response to cocaine, participants were evenly divided into two groups: High (n=5) and Low (n=5) antibody (AB) responders based on peak antibody levels at Week 13, and data were also analyzed using planned comparisons generated by a repeated measures Analysis of Variance (ANOVA) with two within-subjects factors: cocaine dose (0, 25, 50 mg) and study week (Week 3 vs. 13). Huynh-Feldt corrections were used when appropriate.
Results
Participant Characteristics
Table 2 portrays demographic data on the ten male research volunteers who completed the study. All met DSM-IV criteria for cocaine dependence (and tested positive for urinary benzoylecgonine during screening). No volunteer was interested in drug treatment, and none met criteria for dependence on other illicit drugs or alcohol or had a major affective illness, schizophrenia, hypertension, a significant history of heart disease or HIV. Five additional participants were enrolled but did not complete the study: one had a family emergency, two were unreliable and two were medically discharged (see below).
TABLE 2.
Demographic characteristics of study participants (n=10 men).
| Age (years) | 39.0 ± 1.9 |
| Race/Ethnicity (Black/White/Hispanic) | 6/3/1 |
| Smoked cocaine use (# days/wk) | 4.4 ± 1.1 |
| Dollars spent on cocaine/week* | $284 ± 97 |
| Years of smoked cocaine use | 12.7 ± 3.2 |
| Cigarettes Smokers (#) | 7 |
| Cigarettes/day | 10.3 ± 2.1 |
| Alcohol Drinkers (#) | 9 |
| Alcohol: Drinks/week | 11.9 ± 7.4 |
| Marijuana Smokers (#) | 4 |
| Marijuana cigarettes/week | 2.6 ± 1.1 |
| Education (yrs) | 12.3 ± 0.3 |
Note: Data are presented as means (± standard deviation) or as frequency.
As a point of reference, the cost of street cocaine in New York City at the time of the study was $27–45/gram. U.S. Department of Justice. (2002). New York Drug Threat Assessment November 2002 (2002-S0378NY-001). Washington, DC: National Drug Intelligence Center.
Safety
There was no evidence of an allergic or inflammatory response. After the first vaccination, one participant reported soreness at the injection site, another had transient muscle pain (after heavy lifting), and a third reported transient paresthesia of the hand opposite to the vaccinated arm. On the last session in Week 13, one participant had an abnormal EKG (non-ischemic ST-T wave changes). The study cardiologist reviewed baseline and subsequent EKGs and troponin levels; all were normal. Two participants were discharged from the study (in Week 4 and in Week 11) because they had >10 PVCs/hour. Both had normal echocardiograms. All participants were encouraged to seek treatment for their cocaine use upon discharge.
Association between Antibody Level and Cocaine Effects
Figure 1, portraying antibody titers for individual participants over 52 weeks, demonstrates that (1) antibody levels did not begin to increase until after the third vaccination and appeared to peak at Week 13, and (2) there was wide individual variability. There was a significant effect of antibody levels on cluster ratings of Good Drug Effect (z =2.37, p=.0177) and Cocaine Quality (z =2.05, p=0.0399), such that the higher an individual’s antibody level, the lower the ratings. There was no significant interaction between cocaine dose and antibody level for these ratings. Further, there was no significant influence of antibody on the other mood clusters or on peak craving ratings for cocaine, nicotine or alcohol.
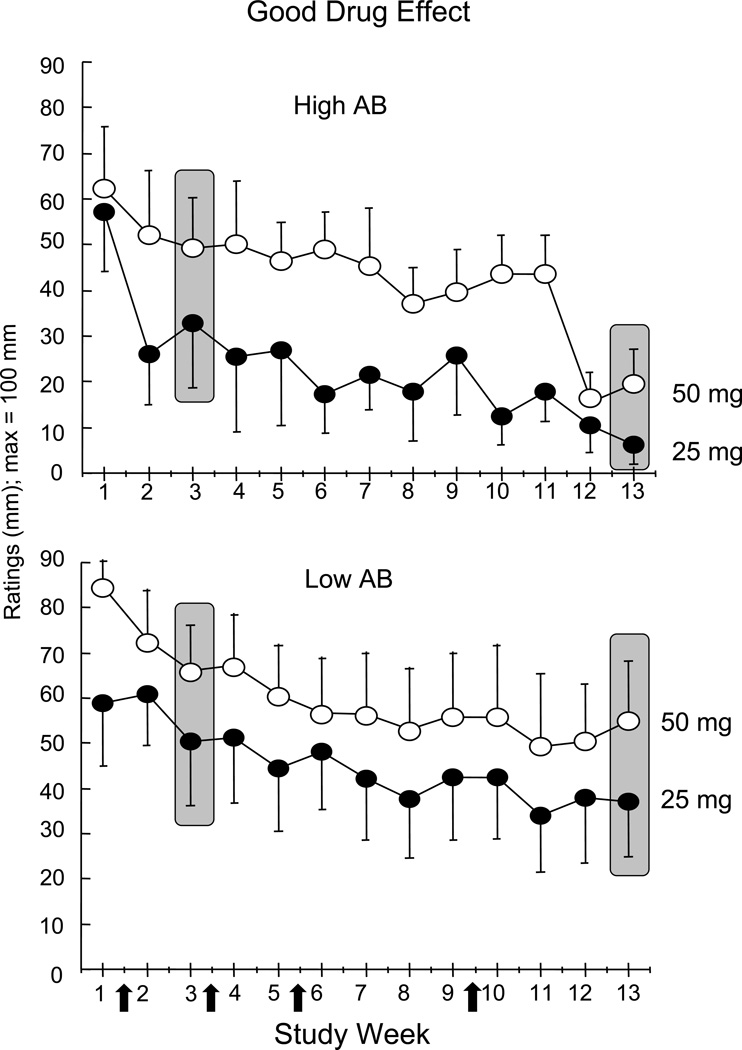
Figure 2, portraying peak mean ratings of Good Drug Effect as a function of cocaine dose and study week in High and Low antibody responders, illustrates that there tended to be an initial decrease in the response to cocaine following the first vaccination. It is unlikely that this decrease reflects antibody levels, which were low for the first 5 weeks, but may reflect a ‘placebo’ response to vaccination. Thus, to account for this placebo-like response and to provide a conservative comparison, all subsequent figures compare data from Week 3 to Week 13 for High and Low responders as a function of cocaine dose.
Figure 2.
Peak VAS ratings of the Good Drug Effect cluster over 13 weeks as a function of cocaine dose (open symbols: 50 mg; filled symbols: 25 mg). Participants (n=10) were evenly divided into High Antibody (AB) and Low Antibody (AB) groups based on their peak antibody levels at Week 13. Vertical bars indicate standard error (SEM). Arrows on x-axis indicate timing of TA-CD vaccination.
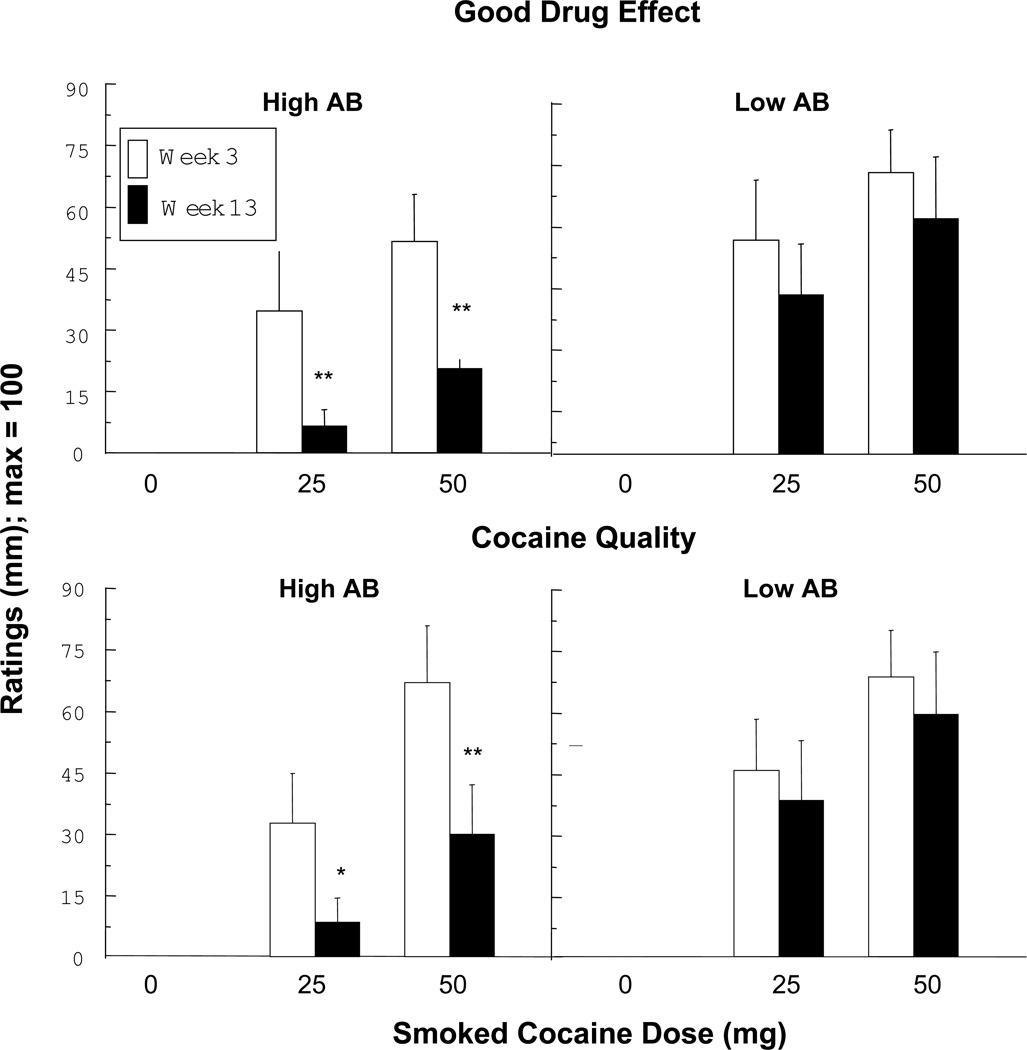
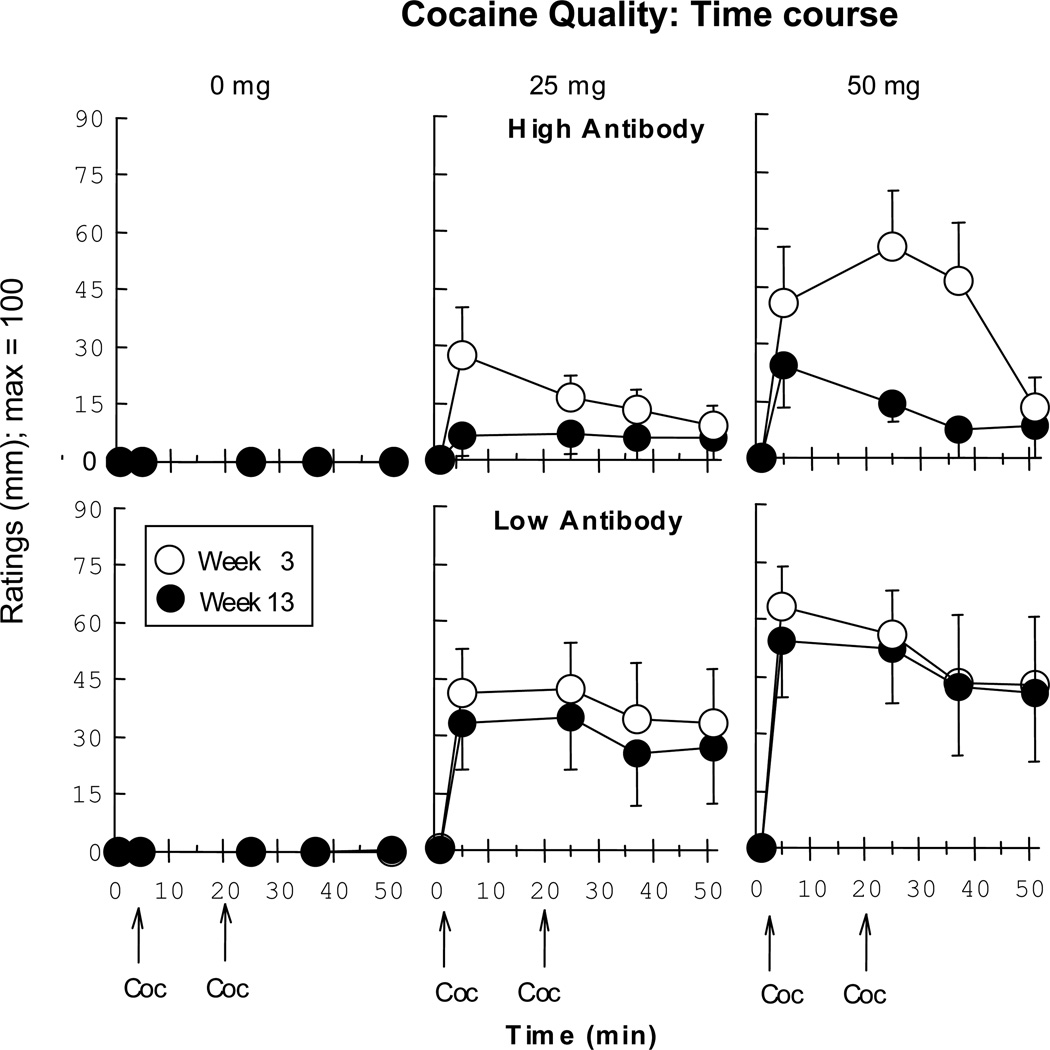
Figure 3 illustrates that the High AB group had significantly lower ratings of Good Drug Effect (p < 0.001) and Cocaine Quality (p < 0.03) in Week 13 compared to Week 3 for both active cocaine doses, while there was no significant decline in these ratings for the Low AB group. Figure 4 portrays the time course of Cocaine Quality ratings at Week 3 and at Week 13. The subjective effects of cocaine were apparent immediately after smoking, yet by Week 13 these effects were blunted in the High AB group but not the Low AB group. The Good Drug Effect time course showed a closely similar pattern (data not portrayed). This figure demonstrates that antibodies do not simply delay the peak onset of cocaine effects but blunt cocaine’s effects cocaine immediately and at each timepoint thereafter.
Figure 3.
Peak VAS ratings of the Good Drug Effect and Cocaine Quality clusters in Week 3 (open bar) and Week 13 (filled bar) as a function of cocaine dose in High and Low AB groups. Vertical bars indicate standard error (SEM). Asterisks indicate a significant difference between Week 3 and Week 13 (* p < 0.05; ** p < 0.01).
Figure 4.
VAS ratings of time course within a cocaine session for the Cocaine Quality cluster in Week 3 (open symbols) and Week 13 (filled circles) as a function of cocaine dose. Vertical bars indicate standard error (SEM).
Outpatient Drug Use
Participants continued their use of cocaine and other drugs during the 5 days/week when they were outpatient. At each visit, they gave a urine sample and reported their drug use since the last visit. The influence of antibody levels on self-reported cocaine use approached significance (p=0.1166), with higher antibody levels associated with less reported cocaine use. Thus, in Week 3, the high AB group reported spending $89 ± 50 on cocaine, while in Week 13, they averaged $40 ±12. The low antibody group averaged $113 ± 35 on cocaine in Week 3 and $108 ± 63 on Week 13. Cocaine metabolites were present in urine for 90–100% of the participants throughout the 13 weeks.
Nine participants reported alcohol use, averaging 5–12 drinks/week throughout the study; antibody level had no significant influence on this measure. Occasional marijuana use was reported by a subset of participants (n=6; average 0–8 marijuana cigarettes/week). The percentage of individuals testing positive for cannabis each week ranged from 10–50%.
Cardiovascular Measures
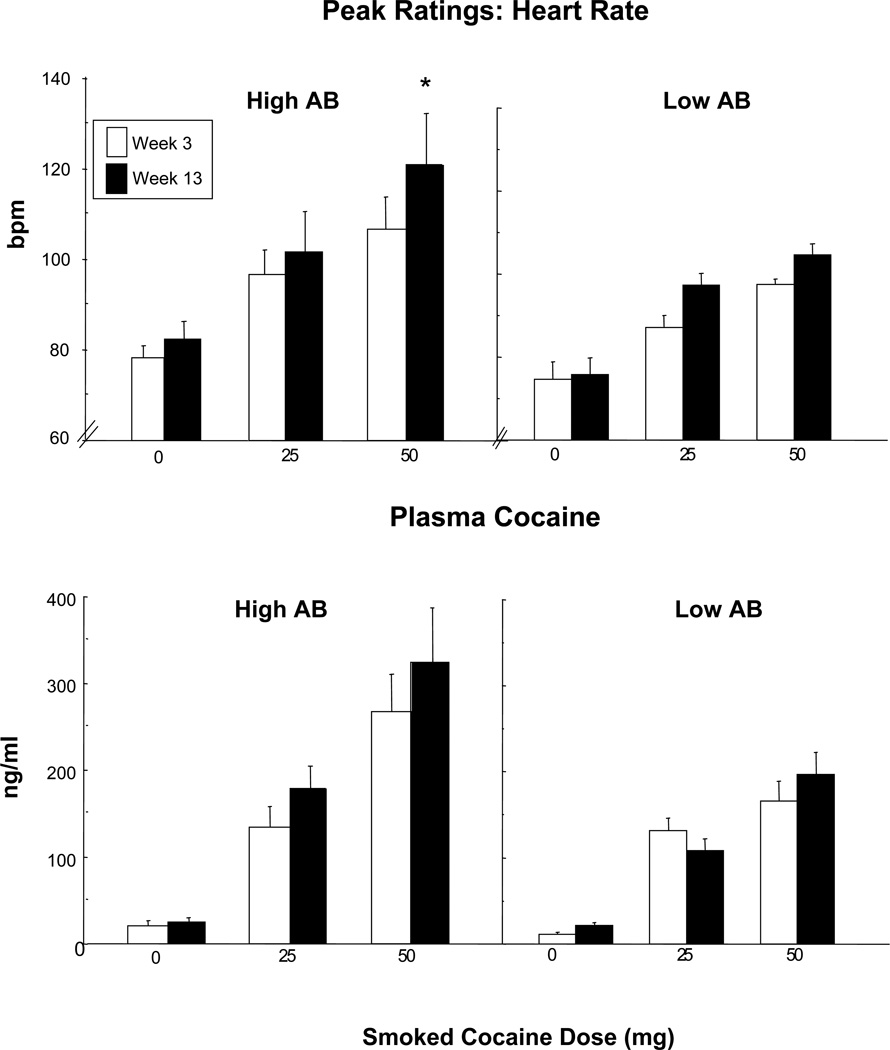
There was a significant effect of antibody titers on peak heart rate (z = 4.21, p<.0001): higher plasma antibody levels were associated with higher peak heart rate. Figure 5, portrays heart rate as a function of cocaine dose in Weeks 3 and 13 in the two AB groups. In Week 13, the High AB group had a significantly greater heart rate following cocaine (50 mg) than the same individuals in Week 3 (p < 0.05). Differences were not significant for the Low AB group. There was no significant effect of antibody level on cocaine’s pressor effects (data not shown).
Figure 5.
Peak heart rate and plasma cocaine levels in Week 3 (open bar) and Week 13 (filled bar) as a function of cocaine dose in High and Low AB groups. Vertical bars indicate standard error (SEM). Asterisks indicate a significant difference between Week 3 and Week 13 (* p < 0.05). Note that the GEE analysis of the effect of antibody levels on plasma cocaine levels did not achieve significance (p=0.16).
Plasma Cocaine
Plasma levels of cocaine (reflecting both free and bound cocaine) were not significantly influenced by antibody levels (p=0.16). Although the effects of antibody were not significant, Figure 5 portrays plasma cocaine levels in Week 3 and Week 13 in order to demonstrate how the results mirror the cardiovascular effects of cocaine. In fact, plasma cocaine levels following high dose cocaine (50 mg) significantly correlated with peak heart rate in Week 13 (p < 0.05).
Discussion
These findings show that antibody production following vaccination with TA-CD effectively blunted the positive subjective effects of smoked cocaine. There was considerable individual variability in antibody production. Dividing participants evenly into two groups based on peak antibody titers illustrates that those in the upper half of antibody production (High AB group) showed an immediate (within 4 minutes) and robust (55–81%) decrease in the intoxicating effects of cocaine, while those in the lower half (Low AB group) did not show a significant attenuation of cocaine effects (6–26%). These data are the first to confirm both the magnitude and the rapidity by which anti-cocaine antibodies attenuate cocaine subjective effects.
In contrast to blunted intoxication, higher plasma antibodies were associated with an increase in cocaine’s tachycardiac effects. The mechanism by which antibodies presumably decrease cocaine intoxication is by binding a fraction of the circulating cocaine and slowing the rise in brain cocaine levels (21, 22). Given that cocaine primarily increases heart rate by peripheral rather than central activation of the sympathetic nervous system (23), higher plasma cocaine levels in the peripheral circulation could explain the enhanced heart rate in the high antibody group. Although antibody-related increases in plasma cocaine levels were not significant, plasma cocaine levels significantly correlated with heart rate at Week 13, suggesting that increased plasma cocaine contributed to the greater cocaine-induced tachycardia in the high antibody group levels. We hypothesize that increased peripheral sympathetic activity reflects free cocaine in the plasma that is dynamically bound and re-bound by antibody (9, 24).
Although heart rate did not exceed our criteria for continued enrollment for any participant, cocaine was only administered twice per session. The potential risk of enhanced cardiovascular effects in concert with blunted subjective effects needs to be evaluated when cocaine is smoked repeatedly in a short time-frame, mimicking the binge pattern of use in the natural ecology. Acute tolerance develops rapidly to the heart rate-inducing effects of cocaine (25), which may mitigate the risk of negative cardiovascular consequences. In fact, in a clinical study, some patients with elevated antibody appeared to try and overcome the vaccine’s effects by using large amounts of cocaine, yet there was no increase in cocaine-related adverse events (overdose, hospitalization; 16). Nonetheless, the potential cardiovascular risk of cocaine immunotherapy needs careful investigation.
Regarding other safety concerns, the TA-CD vaccine was well tolerated throughout the study. There was no evidence of hypersensitivity or immune complex disease, and there were no complications related to combining vaccine and cocaine. This is noteworthy, as perhaps the chief concern with this vaccine is the issue of surmountability. This was particularly worrisome in the present study because the volunteers were not seeking treatment and continued to use cocaine while outpatient. Further, antibody levels had no apparent effect on craving for cocaine or for any other drug used by this group. Yet, rather than an override, those in the High AB group reported less cocaine use as outpatients when antibody levels were peaking. Anecdotally, they reported trying cocaine but not wasting their money if they did not like the effects. Because of our concerns, the medical staff repeatedly warned participants, orally and in writing, of the potential dangers of attempting to surmount a blunted cocaine effect. The fact that drug use decreased in the High AB group suggests that these warnings were effective and should be an important component of immunotherapy trials (26).
In addition to the issue of override, there are other factors to consider with the vaccine approach to cocaine treatment. In terms of adherence, patients must follow a vaccination schedule lasting about 2 months, so strategies are needed to ensure that they complete the immunizations. Vaccine efficacy is not affected by cocaine exposure (10, 27), so it may be possible to vaccinate prior to abstinence initiation, or to combine immunization with psychosocial treatments or pharmacotherapy. An additional issue is the individual variation in antibody formation. Strategies to improve antibody production, such as manipulating the adjuvant or optimizing the frequency and inter-dose interval of vaccination are needed (16, 22, 24).
In terms of weaknesses with this study design, plasma antibody levels appeared to be peaking precisely when the cocaine challenges were terminated (Week 13). The time of peak antibody production was not known when the study was designed in 2001, but the current results are consistent with the clinical data published since then. Additionally, there was no placebo TA-CD condition, due to the substantial study demands. Yet, the poor immunogenicity in at least half of the participants resulted in a de facto placebo condition, and allowed us to assess the impact a range of antibody titers.
To conclude, this small, intensive human laboratory study supports the clinical potential of TA-CD immunotherapy. Individuals who developed sufficient antibody levels had a significant and immediate blunted subjective response to cocaine, an outcome that was more robust than any medication tested in combination with cocaine using similar laboratory procedures (4). There are clear caveats to the vaccine approach (24), particularly regarding the potential for increased cocaine use to overcome antibody effects, that indicate this strategy should be pursued cautiously. Yet the production of sufficient quantities of anti-cocaine antibodies that last for several weeks or months might increase the likelihood that motivated treatment seekers would engage in treatment, and not relapse even if they use some cocaine. Thus, the TA-CD vaccine has the potential to substantially improve options for treating cocaine dependence.
TABLE 3.
Summary of Results
| THC | Lofexidine | THC/Lofexidine | |
|---|---|---|---|
|
Marijuana Withdrawal |
⇑ Good Drug Effect, Mellow, Talkative, Irritable ⇑ Capsule Strength, Liking, Take again ⇑ Food intake ⇑ Psychomotor task performance |
⇑ Sedation ⇑ Capsule Strength ⇑ Sleep (objective and subjective) |
⇑ Good Drug Effect, Sedated ⇑ Capsule Strength ⇑ Sleep (objective and subjective) ⇑ Psychomotor task performance |
| ⇓ Restless, Chills ⇓ Sleep (objective) ⇓ Time spent Talking |
⇓ Restless, Chills, Upset Stomach ⇓ Food intake ⇓ Time spent Talking |
⇓ Restless, Chills, Upset Stomach, Marijuana Craving, Cigarette Craving, Social, Talkative ⇓ Cigarette smoking ⇓ Time spent Talking |
|
| ⇑⇓ Psychomotor task performance | |||
|
Marijuana Relapse |
No Change | Decrease | Decrease |
Acknowledgements
This research was supported by NIDA SPIRCAP 1U19DA10946 (Thomas Kosten, M.D.), and participants resided on the Irving Center for Clinical Research of The New York-Presbyterian Medical Center, supported by Grant No. MOI-RR-00645 from the National Institutes of Health. Celtic Pharmaceutical is gratefully acknowledged for supplying the vaccine, measuring plasma antibody titers, and for funding the ophthalmological exams required by the FDA. We are grateful to Dr. Kosten and his colleague, Dr. Frank Orson and their team at Baylor College of Medicine (Yan Wu, Dr. Berma Kinsey) for their generous help in analyzing antibody levels. The authors would also like to thank Dr. Edward Nunes for his assistance in statistical analysis. The nurses, physicians and research assistants working in the Marian W. Fischman Cocaine Research Laboratory are greatly appreciated, as are our fellow investigators, Drs. Carl L. Hart, and Suzanne Vosburg. Finally, we thank Dr. Amie S. Ward and our late mentor, Dr. Marian W. Fischman, who initially designed this protocol.
Footnotes
Financial Disclosures
Dr. Haney received consultant fees from Celtic Pharmaceuticals in 2007 (<$4000 total), and is a co-investigator on a NIDA-funded multi-site clinical trial to test TA-CD in cocaine-dependent patients. Dr. Haney has also received funding from Bristol-Meyers Squibb to conduct a study assessing the influence of aripiprazole on cocaine self-administration in humans. Dr. Foltin currently receives research support for an investigator-initiated protocol from Aztra Zeneca. Drs. Gunderson, Jiang, and Collins reported no biomedical financial interests or potential conflicts of interest.
Contributor Information
Margaret Haney, College of Physicians and Surgeons of Columbia University New York State Psychiatric Institute.
Erik W. Gunderson, University of Virginia
Huiping Jiang, New York State Psychiatric Institute
Eric D. Collins, New York State Psychiatric Institute New York Presbyterian Hospital.
Richard W. Foltin, College of Physicians and Surgeons of Columbia University New York State Psychiatric Institute.
References
- 1.Substance Abuse and Mental Health Services Administration. Results from the 2006 National Survey on Drug Use and Health: National findings. Rockville, Maryland: Office of Applied Studies; 2007. NSUDH Series H-32, DHHS Publication No. SMA 07-4293. [Google Scholar]
- 2.Knapp WP, Soares B, Farrell M, Silva de Lima M. Psychosocial interventions for cocaine and psychostimulant amphetamines related disorders. Cochrane Database of Systematic Reviews. 2007;(Issue 3) doi: 10.1002/14651858.CD003023.pub2. Art. No.: CD003023. [DOI] [PubMed] [Google Scholar]
- 3.Karila L, Gorelick D, Weinstein A, Noble F, Benyamina A, Coscas S, Blecha L, Lowenstein W, Martinot JL, Reynaud M, Lépine JP. New Treatments for Cocaine Dependence: A Focused Review. Int J Neuropsychopharmacology. 2008;11:425–438. doi: 10.1017/S1461145707008097. [DOI] [PubMed] [Google Scholar]
- 4.Haney M, Spealman R. Controversies in Translational Research: Drug Self-Administration. Psychopharmacology. 2008;199:403–419. doi: 10.1007/s00213-008-1079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carrera MR, Ashley JA, Parsons LH, Wirsching P, Koob GF, Janda KD. Suppression of Psychoactive Effects of Cocaine by Active Immunization. Nature. 1995;378:727–730. doi: 10.1038/378727a0. [DOI] [PubMed] [Google Scholar]
- 6.Balster RL, Schuster CR. Fixed-Interval Schedule of Cocaine Reinforcement: Effect of Dose and Infusion Duration. J Exp Anal Behav. 1973;20:119–129. doi: 10.1901/jeab.1973.20-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abreu ME, Bigelow GE, Fleisher L, Walsh SL. Effect of Intravenous Injection Speed on Responses to Cocaine and Hydromorphone in Humans. Psychopharmacology. 2001;154:76–84. doi: 10.1007/s002130000624. [DOI] [PubMed] [Google Scholar]
- 8.Orson FM, Kinsey BM, Singh RA, Wu Y, Gardner T, Kosten TR. The Future of Vaccines in the Management of Addictive Disorders. Curr Psychiatry Rep. 2007;9:381–387. doi: 10.1007/s11920-007-0049-z. [DOI] [PubMed] [Google Scholar]
- 9.Fox BS. Development of a Therapeutic Vaccine for the Treatment of Cocaine Addiction. Drug Alcohol Depend. 1997;48:153–158. doi: 10.1016/s0376-8716(97)00121-x. [DOI] [PubMed] [Google Scholar]
- 10.Kantak KM, Collins SL, Bond J, Fox BS. Time Course of Changes in Cocaine Self-Administration Behavior in Rats During Immunization With the Cocaine Vaccine IPC-1010. Psychopharmacology. 2001;153:334–340. doi: 10.1007/s002130000555. [DOI] [PubMed] [Google Scholar]
- 11.Johnson MW, Ettinger RH. Active Cocaine Immunization Attenuates the Discriminative Properties of Cocaine. Exp Clin Psychopharmacol. 2001;8:163–167. doi: 10.1037//1064-1297.8.2.163. [DOI] [PubMed] [Google Scholar]
- 12.Carrera MR, Ashley JA, Zhou B, Wirsching P, Koob GF, Janda KD. Cocaine Vaccines: Antibody Protection Against Relapse in a Rat Model. Proc Natl Acad Sci. 2000;97:6202–6206. doi: 10.1073/pnas.97.11.6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kantak KM, Collins SL, Lipman EG, Bond J, Giovanoni K, Fox BS. Evaluation of Anti-Cocaine Antibodies and a Cocaine Vaccine in a Rat Self-Administration Model. Psychopharmacology. 2000;148:251–262. doi: 10.1007/s002130050049. [DOI] [PubMed] [Google Scholar]
- 14.Kosten TR, Rosen M, Bond J, Settles M, Roberts JS, Shields J, Jack L, Fox B. Human Therapeutic Cocaine Vaccine: Safety and Immunogenicity. Vaccine. 2002;20:1196–1204. doi: 10.1016/s0264-410x(01)00425-x. [DOI] [PubMed] [Google Scholar]
- 15.Martell BA, Mitchell E, Poling J, Gonsai K, Kosten TR. Vaccine Pharmacotherapy for the Treatment of Cocaine Dependence. Biol Psychiatry. 2005;58:158–164. doi: 10.1016/j.biopsych.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 16.Martell BA, Orson FM, Poling J, Mitchell E, Rossen RD, Gardner T, Kosten TR. Cocaine Vaccine for the Treatment of Cocaine Dependence in Methadone Maintained Patients: A Randomized Double-Blind Placebo-Controlled Efficacy Trial. Arch Gen Psychiatry. doi: 10.1001/archgenpsychiatry.2009.128. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans SM, Haney M, Foltin RW. The Effects of Smoked Cocaine During the Follicular and Luteal Phases of the Menstrual Cycle in Women. Psychopharmacology. 2002;159:397–406. doi: 10.1007/s00213-001-0944-7. [DOI] [PubMed] [Google Scholar]
- 18.Paula S, Tabet MR, Farr CD, Norman AB, Ball WJ., Jr. Three-Dimensional Quantitative Structure-Activity Relationship Modeling of Cocaine Binding by a Novel Human Monoclonal Antibody. J Med Chem. 2004;47:133–142. doi: 10.1021/jm030351z. [DOI] [PubMed] [Google Scholar]
- 19.Foltin RW, Fischman MW, Nestadt G, Stromberger H, Cornell EE, Pearlson GD. Demonstration of Naturalistic Methods for Cocaine Smoking by Human Volunteers. Drug Alcohol Depend. 1990;26:145–154. doi: 10.1016/0376-8716(90)90121-t. [DOI] [PubMed] [Google Scholar]
- 20.Diggle PJ, Liang K-Y, Zeger SL. Analysis of Longitudinal Data. New York: Oxford University Press; 1994. [Google Scholar]
- 21.Pentel PR, Keyler DE. Vaccines to treat drug addiction. In: Levine MM, Kaper JB, Rappuoli R, Liu MA, Good MF, editors. New Generation Vaccines. 3rd ed. New York, NY: Marcel Dekker Publications; 2004. pp. 1057–1066. [Google Scholar]
- 22.Kosten T, Owens SM. Immunotherapy for the Treatment of Drug Abuse. Pharmacol Ther. 2005;108:76–85. doi: 10.1016/j.pharmthera.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 23.Dickerson LW, Rodak DJ, Kuhn FE, Wahlstrom SK, Tessel RE, Visner MS, Schaer GL, Gillis RA. Cocaine-Induced Cardiovascular Effects: Lack of Evidence for a Central Nervous System Site of Action Based on Hemodynamic Studies With Cocaine Methiodide. J Cardiovasc Pharmacol. 1999;33:36–42. doi: 10.1097/00005344-199901000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Koetzner L, Deng S, Sumpter TL, Weisslitz M, Abner RT, Landry DW, Woods JH. Titer-Dependent Antagonism of Cocaine Following Active Immunization in Rhesus Monkeys. J Pharmacol Exp Ther. 2001;296:789–796. [PubMed] [Google Scholar]
- 25.Foltin RW, Fischman MW. Smoked and Intravenous Cocaine in Humans: Acute Tolerance, Cardiovascular and Subjective Effects. J Pharmacol Exp Ther. 1991;257:247–261. [PubMed] [Google Scholar]
- 26.Haney M, Kosten TR. Therapeutic Vaccines for Substance Dependence. Expert Rev Vaccines. 2004;3:11–18. doi: 10.1586/14760584.3.1.11. [DOI] [PubMed] [Google Scholar]
- 27.Byrnes-Blake KA, Carroll FI, Abraham P, Owens SM. Generation of Anti-(+)Methamphetamine Antibodies is Not Impeded by (+)Methamphetamine Administration During Active Immunization of Rats. Int Immunopharmaco. 2001;1:329–338. doi: 10.1016/s1567-5769(00)00019-9. [DOI] [PubMed] [Google Scholar]