Abstract
Depressive symptoms and cognitive decline are associated in older age, but research is inconsistent about whether one condition influences the development of the other. We examined the directionality of relations between depressive symptoms and perceptual speed using bivariate dual change score models. Assessments of depressive symptoms and perceptual speed were completed by 1,206 nondemented older adults at baseline, and after two, eight, eleven, and fifteen years. After controlling for age, education, baseline general cognitive ability, and self-reported health, allowing depressive symptoms to predict subsequent change in perceptual speed provided the best fit. More depressive symptoms predicted subsequently stronger declines in perceptual speed over time lags of one year.
The coexistence of depressive symptoms and cognitive decline in older adulthood is well established, whereby greater depressive symptoms are associated with poorer cognitive functioning and cognitive decline (Dotson, Resnick, & Zonderman, 2008). However, the causal and temporal relations between depressive symptoms and cognition remain ill-defined.
There are suggestions that depressive symptoms may increase an individual's risk for subsequent cognitive decline. For example, there are reports that those with more baseline depressive symptoms had greater cognitive decline over time than those without depressive symptoms (Köhler et al., 2010; Yaffe et al., 1999). Another study found no increase in depressive symptoms during the prodromal phase of Alzheimer's disease (AD; Wilson, Arnold, Beck, Bienias, & Bennett, 2008), and concluded that depression acts to change cognition, and not the other way around. In contrast, lower cognitive functioning may increase the risk for depressive symptoms. Studies that have found depressive symptoms were unrelated to the rate of cognitive decline (Ganguli, Yangchun, Dodge, Ratcliff, & Chang, 2006; Vinkers, Gussekloo, Stek, Westendorp, & van der Mast, 2004) have consequently concluded that the alternative directional explanation (i.e., cognitive decline leads to depressive symptamotology) is more probable.
Thus the connection between aspects of depression and cognition remains equivocal. Further, it may not be best expressed by a simple unidirectional temporal pathway. Han and colleagues (2006) attributed the link between depressive symptoms and general cognitive functioning to short-term situational factors, in that the relation is concurrent or temporary, rather than leading to increased risk. Alternatively, Jorm (2000) noted various hypotheses concerning the common coexistence of depressive symptoms and dementia including other conceptualizations such that depression and dementia share common risk factors (e.g., vascular disease).
Nonetheless, we restrict our focus to the time-ordered relation between depressive symptoms and cognitive decline. Insight into the directionality of this association will aid in understanding whether and how their interrelation develops over time, and possibly provide key information into the disease process of dementia and major depression.
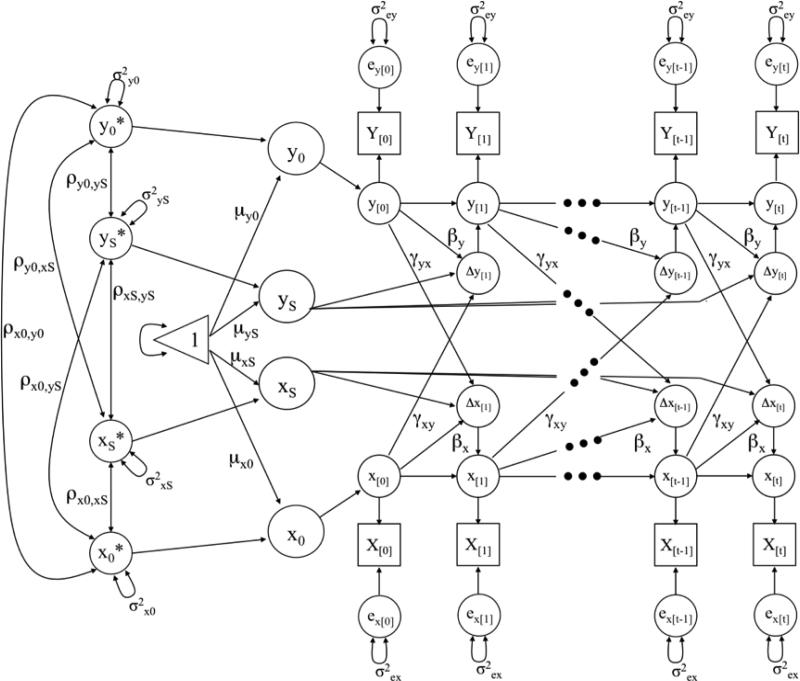
Although longitudinal analysis has informed our understanding of covariation between depressive symptoms and cognitive decline (Chen, Ganguli, Mulsant, & DeKosky, 1999; Ganguli et al., 2006; Han et al., 2006; Vinkers et al., 2004; Yaffe et al., 1999), there has been no direct test of the directionality of the relation whereby a dynamic association is allowed to occur across time, or where one variable is modeled as being related to change in the other variable over time. With recent advances in statistical modeling, the bivariate dual change score model (BDCSM, McArdle & Hamagami, 2001) indeed allows for such temporal comparisons. This model is based in a structural equation modeling framework, and builds upon the latent growth curve model which estimates latent intercept and slope factors at the population level. The BDCSM allows for examining time-ordered associations between two constructs, thereby testing whether one variable at a given point in time predicts subsequent change in the other variable (see Figure 1). This model has been successfully applied to empirically test associations among other psychological domains, including competing hypotheses about the nature of lead-lag associations between cognition and leisure activities (Lövdén, Ghisletta, & Lindenberger, 2005), cognition and well-being (Gerstorf, Lövdén, Röcke, Smith, & Lindenberger, 2007), and the dynamic associations of cognitive functioning between spouses (Gerstorf, Hoppmann, Anstey, & Luszcz, 2009).
1.
Graphical representation of a two-variable Dual Change Score Model (DCSM(McArdle & Hamagami, 2001)) for a system of two variables X and Y. Observed (manifest) variables are represented by squares, unobserved (latent) variables by circles, regression weights by one-headed arrows, variances and covariances by two-headed arrows, and the triangle represents a constant indicating means and intercepts. All unlabeled paths are set to 1.
In this study, we applied the BDCSM to 15-year longitudinal data of depressive symptoms and perceptual speed. Perceptual speed was selected as the marker of cognitive functioning, as it constitutes the limiting factor for other cognitive processes (Luszcz & Bryan, 1999) and is a highly reliable and sensitive marker of cognitive change throughout adulthood (Anstey, Hofer, & Luszcz, 2003; Salthouse, 2004). By simultaneously modeling longitudinal changes in depressive symptoms and cognition, we tested various hypotheses about their dynamic relation over time. First, we investigated both unidirectional hypotheses: whether the level of depressive symptoms at time [t] predicts subsequent change in perceptual speed between [t] and [t + 1], or whether the level of perceptual speed predicts changes in depressive symptomatology. Next, we analyzed bidirectional associations, including where both leading pathways exist and are of different magnitude, and where neither variable is associated with changes in the other. Finally, we covaried the effects of baseline age, education, general cognitive status, and number of medical conditions to ensure our findings do not simply reflect the influence of these variables on cognitive functioning and depressive symptoms (Bassuk, Berkman, & Wypij, 1998; Geerlings et al., 2000).
Method
Participants
The study sample was drawn from the Australian Longitudinal Study of Ageing (Luszcz et al., 2007). Potential participants resided in Adelaide, Australia, were 70 years or older, and lived in either community or residential care settings. Participants were recruited through the electoral roll, for which registration is compulsory for Australian citizens. Of the 2,703 residents eligible for study inclusion, 1477 (55%) agreed to participate. Spouses of participants who were over 65 years of age and co-residents over 70 were also invited to participate due to potential interest in investigating the dynamics among couples, and to increase the number of participants, respectively. These invitations resulted in a further 610 participants.
At baseline, the 2,087 participants were aged between 65 and 103 (M = 78.16, SD = 6.69), and approximately half of the sample was female (49.4%). The outcome measures pertinent to this study were collected at Waves 1 (September, 1992–February, 1993), 3 (September, 1994-February, 1995), 6 (September, 2000-February, 2001), 7 (September, 2003-April, 2004), and 9 (November, 2007-June, 2008), or at baseline and after approximately two, eight, eleven, and fifteen years. Data from these waves were collected in three formats: personal interview, self-completion questionnaire and clinical assessment, all collected in the participant's residence. The personal interview and self-completion questionnaire included a range of self-reported demographic, psychosocial, and health measures. The clinical assessment included objective measures of psychological and physical functioning and was completed approximately 2 weeks later (Luszcz, Bryan, & Kent, 1997).
Although the structural equation modeling framework permitted the inclusion of all participants regardless of attrition, we chose to include the requirement that participants needed to have valid baseline data on all variables of interest. Consequently, the final sample was reduced to 1,206, with a mean length of follow-up of 5.95 years (SD = 5.00).
Participants who completed all relevant Wave 1 measures were significantly younger (F (1, 2085) = 44.32, p<.001, η2 = .02; 77.88 years) and more likely to have stayed in school beyond age 14 (χ2 = 26.80, df=1, p<.001, η2 = .12; 48.3%) than those who did not have complete data for the first wave of measurement (79.84 years; 36.5%). They also had significantly fewer depressive symptoms (M=7.82) than those who did not have complete data (M=8.85; F(1, 1991= 9.33, p<.01, η2 = .01), but there was a wide range of depressive symptoms in the study sample (see Table 1). The vast majority of those who did not have complete Wave 1 data did not perform the perceptual speed task, thus preventing comparison on this variable, but those included in this report had higher Mini-Mental State Examination (MMSE; Folstein, Folstein, & McHugh, 1975) scores (F(1, 2042) = 94.24, p<.001, η2 = .04; M=27.61) than those who were not (M=25.82). There were no significant differences in relation to sex composition or medical conditions. Table 1 describes the baseline characteristics of the sample.
Table 1.
Descriptive statistics for measures entered into the bivariate dual change score model.
| Measure | n | M | SD | Raw Score Range |
|---|---|---|---|---|
| Depressive Symptoms | ||||
| Wave 1 | 1206 | 50.10 | 9.93 | 0 – 48 |
| Wave 3 | 955 | 49.31 | 9.34 | 0 – 42 |
| Wave 6 | 447 | 51.20 | 8.98 | 0 – 39 |
| Wave 7 | 301 | 50.21 | 9.96 | 0 – 40 |
| Wave 9 | 135 | 51.61 | 9.53 | 0 – 35 |
| Perceptual Speed | ||||
| Wave 1 | 1206 | 49.45 | 9.59 | 0 – 72 |
| Wave 3 | 859 | 51.16 | 10.11 | 2 – 72 |
| Wave 6 | 349 | 50.94 | 9.15 | 3 – 63 |
| Wave 7 | 262 | 49.07 | 9.72 | 0 – 67 |
| Wave 9 | 121 | 50.40 | 9.15 | 8 – 63 |
| Covariates | ||||
| Age | 1206 | 77.88 | 6.30 | 65 - 98 |
| Medical Conditions | 1206 | 2.47 | 1.66 | 0 - 10 |
| MMSE > 24 | 96 | 8.0% | ||
| Education < 15 years of age | 579 | 48.0% |
Note. Depressive symptoms and perceptual speed were assessed at baseline and after approximately two, eight, eleven and fifteen years. Depressive symptoms were assessed using the Center for Epidemiological Studies Depression Scale (Radloff, 1977). Depressive symptoms and perceptual speed scores were standardized to the T metric (M=50, SD =10) using the baseline sample. MMSE (Mini-mental Status Examination) was dichotomized as scoring less than 24, and 24 or more. Education information was limited to a dichotomized variable of leaving school before 15 years of age, or leaving at age 15 or later.
Measures
Depressive symptoms were assessed using the 20-item Center for Epidemiological Studies Depression Scale (Radloff, 1977). Perceptual speed was measured by the Digit Symbol Substitution test (Wechsler, 1981). Participants were presented with a coding key pairing numbers 1-9 with nine symbols. The score was the number of items correctly coded in 90 sec.
Regarding the covariates, we chose to control for the effects of age, education, general cognitive status, and number of medical conditions because of their well-established association with cognitive impairment and dementia (Williams, Plassman, Burke, Holsinger, & Benjamin, 2010). Baseline age was calculated to the nearest day and centered near sample mean at 78 years old. Education was assessed by a binary measure of the age participants left formal schooling: before age 15 (n = 579), or at age 15 or older (n = 627). General cognitive status was determined by Wave 1 MMSE scores and coded as a binary variable using the clinical dementia cutoff of less than 24 (n = 96), and 24 or more (n = 1110) . Medical conditions were based on the number of self-reported current chronic conditions from a comprehensive list of 37 diseases (e.g., arthritis, heart disease, cancer).
Data Preparation & Statistical Analysis
To enable comparison across measures, depressive symptoms and perceptual speed were standardized to a T metric (M = 50, SD =10), using the mean scores of the baseline sample as the reference. The intra-class correlations derived from the random-intercept only models revealed that that there was substantial between-person and within-person variance in the data (depressive symptoms: 0.488; perceptual speed: 0.616), justifying the use of the BDCSM to describe these changes.
Figure 1 shows a graphical representation of the BDCSM, with depressive symptom scores defined by X, and perceptual speed scores defined by Y. The X[0] to X[t] and Y[0] to Y[t] represent the assessments of depressive symptoms and perceptual speed. By adding unmeasured ‘node’ variables for occasions on which a given variable was not assessed, we simplified the interpretation of model parameters and guaranteed an equal-interval, time-invariant scaling of approximately one year in-between occasion.
The latent scores x[t] (or y[t]) are defined as the unit-weighted sum of the latent score x[t-1] (or y[t-1]), or the latent score at the previous assessment, plus the latent difference score Δx[t] (or Δy[t]), resulting in the latent, reliable change between x[t-1] and x[t] (or y[t-1]) and y[t]) (McArdle & Nesselroade, 1994). The intercepts (x0, y0) and slope factors (xs, ys) represent scores at baseline and linear change score, respectively, and are estimated at the population level (μx0, μxS; μy0, μys). They are allowed to vary (σ2x0, σ2xS ; σ2y0; σ2ys), and to covary with one another (ρx0xS; ρy0yS; ρx0y0; ρxSyS; ρx0yS; ρy0xS). Error terms (ex, ey) are assumed to be normally distributed with a mean of zero, have a time-invariant variance, and be uncorrelated with other components.
As an extension of typical linear Latent Growth Curve models, the difference scores Δx[t] and Δy[t] are defined as the unit-weighted sum of the linear component of change within the target variable plus two additional influences. First, the auto-proportion parameter (βx, βy) indicates the effect of the target variable at time t-1 on change in the same variable between t-1 and t (e.g., the effect that level of depressive symptoms has on change in depressive symptoms). Second, an intervariable, cross-lagged dynamics parameter (γxy; γyx) estimates the effect of one variable (e.g, X) at time t-1 on subsequent change in the other variable (e.g., Y) between t-1 and t (e.g., the effect that level of depressive symptoms has on subsequent change in perceptual speed). Both β and γ parameters are assumed to be time-invariant. Further information on the assumptions of the BDCSM can be found elsewhere (Ferrer & McArdle, 2004; McArdle & Hamagami, 2001).
The major point of interest in our study is the cross-lagged γ or coupling parameters, which model the predictive effects of depressive symptoms on subsequent change in perceptual speed (γDep→Speed), and the opposite predictive effect of perceptual speed on subsequent change in depressive symptoms (γSpeed→Dep). With slight variations to these γ parameters, we can directly compare the goodness-of-fit indices of four statistically nested models. First, a model that freely estimated both coupling parameters γDep→Speed and γSpeed→Dep was referred to as the full coupling model. This model was the most complex model tested and served as reference by which to judge all other models that estimated fewer parameters. Two of the models tested unidirectional hypotheses: in coupling γDep→Speed, only the predictive effects of depressive symptoms to change in perceptual speed were estimated; conversely, in coupling γSpeed→Dep, only the prediction of perceptual speed for depressive symptom change was estimated. The final model predicted that neither variable predicted change in the other by setting both parameters to 0 (no coupling). Models were fit using Mplus (Muthén & Muthén, 1998-2007).
Results
The results are presented in three main parts. First, we report a comparison of the four models used to evaluate the various accounts of the relation between depressive symptoms and perceptual speed. Next, we focus on the full coupling model to illustrate the effects of the differential magnitude of the coupling parameters over time. Finally, we investigate whether controlling for baseline age, MMSE score, education, and chronic health conditions had an effect on the dynamic relations between depressive symptoms and cognition.
Comparison of Various Coupling Models
The goodness-of-fit model statistics from the four different versions of the BDCSM are presented in Table 2. Compared to the full coupling model, the only model which did not result in a significant loss of model fit was the γDep→Speed model, which permitted cross-lagged effects from depressive symptoms to perceptual speed, but did not allow predictive effects of perceptual speed for depressive symptoms. This means that we can reject the unidirectional hypothesis that perceptual speed predicts change in depressive symptoms. In contrast, fixing the predictive effect of depressive symptoms for change in perceptual speed to zero, and only estimating the opposite pathway (i.e., γSpeed→Dep; perceptual speed predicting change in depressive symptoms) resulted in a highly significant loss in model fit. In addition, the no coupling model described the data less precisely than the full coupling model suggesting that we also cannot accept this model. We obtained similar results with the other goodness-of-fit statistics. Both the root mean square error of approximation and the comparative fit index were comparable between the full coupling and the γDep→Speed model, but provided noticeably poorer fit for the two other models. Overall, nested model comparisons suggest that depressive symptoms predict changes in perceptual speed, whereas we found no evidence for the hypotheses that perceptual speed predicts subsequent changes in depressive symptoms or that the associations do not exist.
Table 2.
Goodness-of-fit statistics for alternative bivariate dual change score models of depressive symptoms and perceptual speed.
| Model | Goodness-of-fit indices |
|||
|---|---|---|---|---|
| χ2 (df) | Δ χ2 (df) | RMSEA | CFI | |
| Unidirectional | ||||
| γ Dep→Speed | 82.87 (46) | 1.98 (1) | .026 | .981 |
| YSpeed→Dep | 130.27 (46) | 49.38 (1)**** | .039 | .957 |
| Bidirectional | ||||
| Full coupling | 80.89 (45) | - | .026 | .982 |
| No coupling | 130.70 (47) | 49.81 (2)**** | .038 | .957 |
| Covarying age, education, cognitive status and medical conditions |
||||
|---|---|---|---|---|
| Unidirectional | ||||
| YDep→Speed | 121.28 (70) | 3.28 (1) | .025 | .980 |
| YSpeed→Dep | 171.85 (70) | 53.85 (1)**** | .035 | .961 |
| Bidirectional | ||||
| Full coupling | 118.00 (69) | - | .024 | .981 |
| No coupling | 171.88 (71) | 53.88 (2)**** | .034 | .961 |
Note. Significance refers to loss in χ2 assuming the full coupling model to be correct. RMSEA = root mean square error of approximation; CFI = comparative fit index.
p<.0001
Differential Magnitude of Depressive Symptoms-Perceptual Speed Couplings
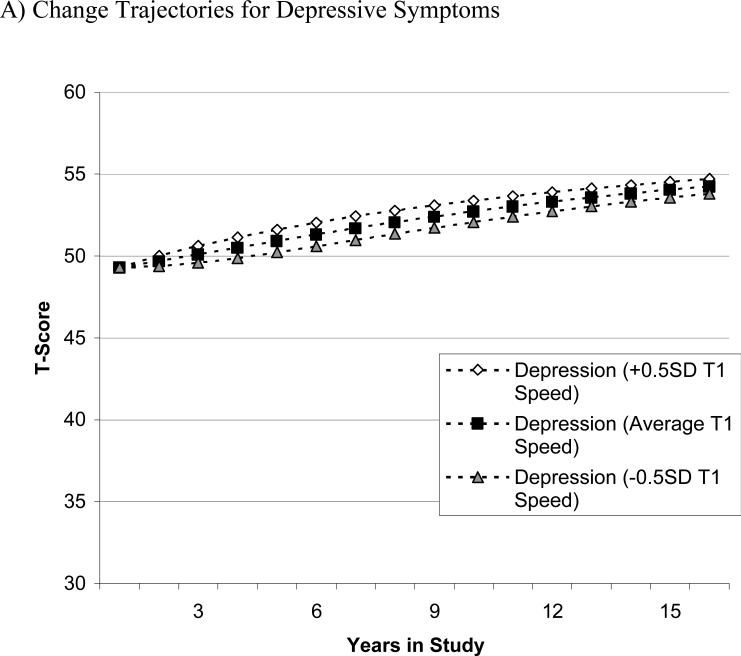
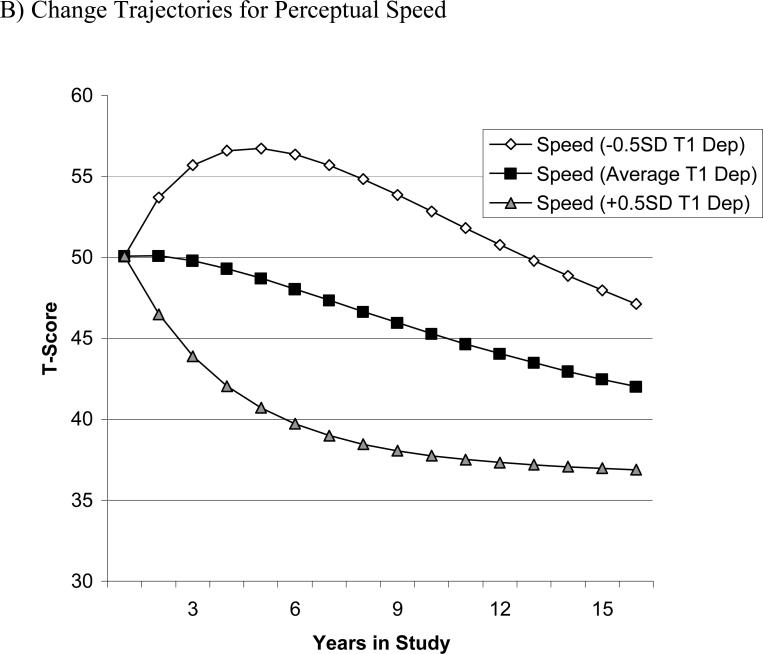
To appreciate the size of predictive effects of depressive symptoms for subsequent changes in perceptual speed, we present a series of graphical examples. To illustrate the differential magnitude of the coupling parameters and their effects over time, we varied the baseline sample means for one target variable by half a standard deviation (i.e., 5 T-score units) while keeping the baseline sample means for the other variable constant (Lövdén et al., 2005). This hypothetical scenario demonstrates how the coupling parameter of one variable predicts the estimated mean longitudinal trajectories of the other variable. The figures were produced using estimates from the full coupling model and the formulas x[t] = 1 × Xs + (1 + βx) × x[t-1] + γyx × y[t-1] and y[t] = 1 × Ys + (1 + βy) × y[t-1] + γxy × x[t-1]. Panel A of Figure 2 shows the model-implied change in depressive symptoms over 15 years, in the hypothetical case that all participants showed the same amount of depressive symptoms at baseline, but differed in their initial perceptual speed performance. As can be seen, varying initial levels of perceptual speed performance does little to alter the subsequent changes in depressive symptoms across time. In contrast, panel B shows the model-implied change of perceptual speed when the initial sample mean of depressive symptoms is varied by 0.5 SD, but the initial performance in perceptual speed is kept constant. There are clear differences as a result of varying the amount of baseline depressive symptoms. Individuals with more depressive symptoms at Wave 1 (+0.5 SD) showed steeper decline in perceptual speed, whereas individuals with fewer initial depressive symptoms (-0.5 SD) experienced shallower declines in perceptual speed.
2.
Graphical illustration of the differential magnitude of the coupling parameters and their effects over time. Model-implied means from the full coupling model were varied by half a standard deviation for one variable while the other was kept constant. Panel A shows change in depressive symptoms based on different initial perceptual speed scores. Panel B shows change in perceptual speed based on different initial depressive symptoms. Under the assumption of comparable perceptual speed performance at T1, Panel B shows that participants with few initial depressive symptoms (–0.5 SD T1 Dep) showed relatively shallow perceptual speed decline, whereas those with more depressive symptoms initially (+0.5 SD T1 Dep) showed relatively steep perceptual speed decline subsequently. In contrast, Panel A shows that depressive symptoms trajectories of change over time were minimally changed as a function of different initial levels of perceptual speed performance at T1.
The Role of Age, Education, Cognitive Status, and Medical Conditions
In a final step, we investigated whether individual differences in baseline age, education, cognitive status and chronic health conditions would have an effect on dynamic associations between depressive symptoms and perceptual speed. The additional variables were included in the model as time-invariant covariates of the latent intercept and slope factors, thereby residualizing all model parameters for the covariates.
The pattern of results remained the same (see lower half of Table 2), with the coupling γDep→Speed model not significantly differing from the full coupling model. Conversely, the two other models resulted in a significantly poorer fit to the data compared to the full coupling model. The directionality of the effects was also reflected by the coupling parameters in the full coupling model: The dynamic effect of depressive symptoms for perceptual speed change was -0.98 (SE = .25, p < .01), whereas the opposite coupling parameter γSpeed→Dep was modest and not significant (0.09, SE = .06, p > .10). Further parameter estimates are shown in Table 3. Although inclusion of the covariates did not substantively alter the dynamics reported, they contributed significant proportions of overall explained variance to the model parameters (e.g., the level and change of perceptual speed, and level and change of depressive symptoms were: R2 = .22, .06, .03, .12 with age only covaried vs. R2 = .34, .12, .15, .20 with all four covariates).
Table 3.
Parameter Estimates from a Bivariate Dual Change Score Model (Full Dynamics) Between Depressive Symptoms and Perceptual Speed With Covariates.
| Depressive Symptoms | Perceptual Speed | |||||
|---|---|---|---|---|---|---|
| Parameter | Estimate | SE | Estimate | SE | Estimate | SE |
| Fixed effects | ||||||
| Intercept mean (μ0) | 46.24*** | 0.50 | 49.77*** | 0.51 | ||
| Slope mean (μS) | –9.73 | 8.30 | 67.72*** | 18.04 | ||
| Proportion (β) | 0.13 | 0.11 | -0.87*** | 0.14 | ||
| Random effects | ||||||
| Intercept variance (σ20) | 42.06*** | 2.82 | 51.54*** | 3.13 | ||
| Slope variance (σ2S) | 0.76 | 1.32 | 40.35 | 20.88 | ||
| Error variance (σ2e) | 42.90*** | 1.50 | 20.96*** | 1.14 | ||
| Covariance / correlation | ||||||
| Intercept <--> slope | -4.32/ -.63 | 4.30 | 9.61* / .16* | 4.18 | ||
| Dynamics | ||||||
| γ Dep→Speed | -0.98*** | 0.25 | ||||
| γ Speed→Dep | 0.09 | 0.06 | ||||
| Covariance / correlation | ||||||
| Dep intercept – Speed slope | 34.38***/ .72*** | 9.77 | ||||
| Speed intercept – Dep slope | -1.86/ -.22 | 1.40 | ||||
| Dep intercept – Speed intercept | -13.03***/ -.21*** | 2.04 | ||||
| Dep slope – Speed slope | -5.45/ -.82 | 6.11 | ||||
Note. Depressive symptoms and perceptual speed were standardized to the T metric using the T1 ALSA sample as the reference. Estimates are residualized for age, education, cognitive status and medical conditions. Mean = 50, SD = 10. All estimates are unstandardized. Model fit statistics: χ2 (69) = 118.00, CFI = .981; RMSEA = .024.
p < .05
** p < .01
p < .001.
Discussion
We examined time-ordered relations between depressive symptoms and perceptual speed across 15 years using models that allowed for the comparison of competing hypotheses of directionality. Our data best fit the hypothesis that depressive symptoms predict subsequent changes in perceptual speed. This finding is consistent with studies suggesting that depression leads cognitive decline in older age (Jorm, 2001; Wilson et al., 2008; Yaffe et al., 1999), and demonstrates that a temporal relation exists even amongst relatively healthy older adults. It is worthwhile to note that the same pattern of results was found when participants with possible dementia at any wave across the 15 years were removed from the analyses (n = 218). Therefore, the importance of affect on normal cognitive aging should not be underestimated.
However, the mechanisms through which depressive symptoms relate to increased decline in perceptual speed are uncertain, and either end of the functioning spectrum may be driving the effect. Rather than more depressive symptoms acting as a risk for cognitive decline, it may be that having few depressive symptoms serves as a protective factor against decline. Regarding the highly prevalent comorbidity of depression and dementia (Forsell & Windblad, 1998), Jorm (2001) stated that the most likely explanation for why depression increases dementia risk involves all or one of three hypotheses: depression can be an early prodrome of dementia; depression brings forth the clinical manifestation of dementing diseases; or depression leads to hippocampal damage through a glucocorticoid cascade (Sapolsky, Krey, & McEwen, 1986). Further, Butters and colleagues (2008) proposed a reserve threshold theory whereby both depression and cognitive impairment can act to decrease cognitive reserve, thus increasing an individual's risk of developing other disorders. The coexistence of late-life depression and dementia may be the result of a number of possible pathways, beginning with either AD neuropathology, depression, or cerebrovascular disease, all of which can reduce reserve, and consequently result in the earlier expression of another disease. Therefore, although we found a unidirectional relation whereby higher depressive scores predicted lower processing speed, the exact cause of this leading relation is unknown. It is possible that similar investigations of entire samples with clinical depression or diagnosed dementia might elucidate the temporal association further. Finally, there are clearly other factors that influence the presence of both depression and dementia which further complicate any causal inferences that can be drawn.
Compared to other cognitive domains, processing speed undergoes the longest period of decline before dementia diagnosis (Thorvaldsson et al., 2010). Consequently, the temporal pathways associated with depressive symptoms may vary depending on the cognitive domain, and more importantly, the degree of cognitive impairment of the sample.
Similar to the research on depressive symptoms and cognitive decline, there have been inconsistent findings in the literature examining the directionality of the relation between depression and dementia (Brommelhoff et al., 2009; Chen et al., 1999; but see Jorm, 2001; Ownby, Crocco, Acevedo, John, & Loewenstein, 2006), and no study has directly compared competing temporal hypotheses linking depression and dementia. The present findings that depressive symptoms appear to lead changes in perceptual speed might hence be valuable to understanding the development of the link between these clinical conditions.
Our findings must be interpreted in light of a number of methodological limitations. First, sample attrition across time resulted in a relatively small sample at later waves, and both greater depressive symptoms and cognitive decline have been linked with study dropout and incomplete data in this sample (Anstey & Luszcz, 2002a, 2002b). Although the covariates included are typically informative of attrition and thus should have accounted for a good portion of this concern, the amount of cognitive decline and depressive symptoms are likely underestimated. Given this possible attenuation of the range of scores, it is all the more compelling that the observed relationship is genuine. However, whether the non-random missingness led to an over-or under-estimation of the effect sizes of the across-domain couplings is unclear as this is dependent on which end of the functioning spectrum is driving the relation (i.e., risk versus protective factor). Next, we did not obtain a clinical diagnosis of depression, nor do we have clinical data on cardiovascular disease and other risk factors that have been linked to both depression and dementia (Anstey, von Sanden, Sargent-Cox, & Luszcz, 2007). However, the unidirectional pathway of depressive symptoms predicting declines in perceptual speed remained even when the number of medical conditions, including a range of cardiovascular health problems, was taken into account. Finally, there are a number of different factors that may have contributed to inconsistent findings between past studies, including variations in time frame, measurement lag, sample, measurement of depressive symptoms and cognition, and statistical analysis. Consequently, as all studies are, the present results are limited to the variables and factors presented.
From a methodological perspective, further caveats should be noted. Despite the ability of the bivariate dual change score model to permit a dynamic association to occur across time, very strict statistical assumptions were required. The relation between each variable at time [t] and change in the other variable between [t] and [t + 1] was assumed to be linear and identical across all participants and through time, and also limited to time-lags across one measurement occasion (i.e., it did not include lags over longer time intervals). Further, the best-fitting model was a function of the sample and the specific times of measurements, indicating that a different model might have been a better fit if the occasions were closer together. Most importantly, the demonstration of time-ordered association between the two variables does not equal causation.
The present study is the first to apply models which directly test the lead-lag relations between depressive symptoms and cognitive ability across time. We found higher levels of depressive symptoms predicted more rapid decline in perceptual speed, whereas there was no reliable predictive effect of perceptual speed on change in depressive symptoms. This pattern remained after controlling for age, education, baseline general cognitive status, and health. Therefore, depressive symptoms may be a risk factor for cognitive decline, rather than a prodrome of it.
Acknowledgements
The Australian Longitudinal Study of Ageing is conducted by the Flinders Centre for Ageing Studies, Flinders University, Adelaide.
Funding: A. A. M. Bielak was supported by a postdoctoral research fellowship from the Canadian Institutes of Health Research. D. Gerstorf is grateful for the support provided by the National Institute on Aging (Grants NIA R21-AG032379 and NIA R21-AG033109). M.A. Luszcz and the ALSA are presently primarily funded by ARC-DP 0879152. K. J. Anstey was supported by National Health and Medical Research Council (NHMRC) Fellowship No. 366756. Past funding sources for ALSA include: NIA (No AG08523), NHMRC (229922), Australian Research Council (ARC-DP 0879152; ARC-LP 0669272; ARC-LP 100200413), the Flinders University Research Grants Scheme, and the South Australian Department of Families and Communities.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/pag
References
- Anstey KJ, Hofer SM, Luszcz MA. A latent growth curve analysis of late-life sensory and cognitive function over 8 years: Evidence for specific and common factors underlying change. Psychology and Aging. 2003;18:714–726. doi: 10.1037/0882-7974.18.4.714. [DOI] [PubMed] [Google Scholar]
- Anstey KJ, Luszcz MA. Mortality risk varies according to gender and change in depressive status in very old adults. Psychosomatic Medicine. 2002a;64:880–888. doi: 10.1097/01.psy.0000028827.64279.60. [DOI] [PubMed] [Google Scholar]
- Anstey KJ, Luszcz MA. Selective non-response to clinical assessment in the longitudinal study of aging: implications for estimating population levels of cognitive function and dementia. International Journal of Geriatric Psychiatry. 2002b;17:704–709. doi: 10.1002/gps.651. [DOI] [PubMed] [Google Scholar]
- Anstey KJ, von Sanden C, Sargent-Cox K, Luszcz MA. Prevalence and risk factors for depression in a longitudinal, population-based study including individuals in the community and residential care. American Journal of Geriatric Psychiatry. 2007;15:497–505. doi: 10.1097/JGP.0b013e31802e21d8. [DOI] [PubMed] [Google Scholar]
- Bassuk S, Berkman L, Wypij D. Depressive symptomatology and incident cognitive decline in an elderly community sample. Archives of General Psychiatry. 1998;55:1073–1081. doi: 10.1001/archpsyc.55.12.1073. [DOI] [PubMed] [Google Scholar]
- Brommelhoff JA, Gatz M, Johansson B, McArdle JJ, Fratiglioni L, Pedersen NL. Depression as a risk factor or prodromal feature for dementia? Findings in a population-based sample of Swedish twins. Psychology and Aging. 2009;24:373–384. doi: 10.1037/a0015713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butters MA, Young JB, Lopez O, Aizenstein HJ, Mulsant BH, Reynolds CFI, et al. Pathways linking late-life depression to persistent cognitive impairment and dementia. Dialogues in Clinical Neuroscience. 2008;10(3):345–357. doi: 10.31887/DCNS.2008.10.3/mabutters. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Ganguli M, Mulsant BH, DeKosky ST. The temporal relationship between depressive symptoms and dementia: A community-based prospective study. Archives of General Psychiatry. 1999;56:261–266. doi: 10.1001/archpsyc.56.3.261. [DOI] [PubMed] [Google Scholar]
- Dotson VM, Resnick SM, Zonderman AB. Differential association of concurrent, baseline, and average depressive symptoms with cognitive decline in older adults. American Journal of Geriatric Psychiatry. 2008;16(4):318–330. doi: 10.1097/JGP.0b013e3181662a9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer E, McArdle JJ. An experimental analysis of dynamic hypotheses about cognitive abilities and achievement from childhood to early adulthood. Developmental Psychology. 2004;40:935–952. doi: 10.1037/0012-1649.40.6.935. [DOI] [PubMed] [Google Scholar]
- Folstein M, Folstein S, McHugh PR. Mini-Mental State: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Forsell Y, Windblad B. Major depression in a population of demented and nondemented older people: Prevalence and correlates. Journal of the American Geriatrics Society. 1998;46:27–30. doi: 10.1111/j.1532-5415.1998.tb01009.x. [DOI] [PubMed] [Google Scholar]
- Ganguli M, Yangchun D, Dodge HH, Ratcliff GG, Chang C-CH. Depressive symptoms and cognitive decline in late life: A prospective epidemiological study. Archives of General Psychiatry. 2006;63(153-160) doi: 10.1001/archpsyc.63.2.153. [DOI] [PubMed] [Google Scholar]
- Geerlings M, Schoevers R, Beekman A, Jonker C, Deeg D, Schmand B, et al. Depression and risk of cognitive decline and Alzheimer's disease. British Journal of Psychiatry. 2000;176(568-575) doi: 10.1192/bjp.176.6.568. [DOI] [PubMed] [Google Scholar]
- Gerstorf D, Hoppmann CA, Anstey KJ, Luszcz M. Dynamic links of cognitive functioning among married couples: Longitudinal evidence from the Australian Longitudinal Study of Ageing. Psychology and Aging. 2009;24:296–309. doi: 10.1037/a0015069. [DOI] [PubMed] [Google Scholar]
- Gerstorf D, Lövdén M, Röcke C, Smith J, Lindenberger U. Well-being affects changes in perceptual speed in advanced old age: Longitudinal evidence for a dynamic link. Developmental Psychology. 2007;43(705-718) doi: 10.1037/0012-1649.43.3.705. [DOI] [PubMed] [Google Scholar]
- Han L, McCusker J, Abrahamowicz M, Cole M, Capek R. The temporal relationship between depression symptoms and cognitive functioning in older medical patients - Prospective or concurrent? Journal of Gerontology: Medical Sciences. 2006;61A:1319–1323. doi: 10.1093/gerona/61.12.1319. [DOI] [PubMed] [Google Scholar]
- Jorm AF. Is depression a risk factor for dementia or cognitive decline? Gerontology. 2000;46:219–227. doi: 10.1159/000022163. [DOI] [PubMed] [Google Scholar]
- Jorm AF. History of depression as a risk factor for dementia: An updated review. Australian and New Zealand Journal of Psychiatry. 2001;35:776–781. doi: 10.1046/j.1440-1614.2001.00967.x. [DOI] [PubMed] [Google Scholar]
- Köhler S, van Boxtel MPJ, van Os J, Thomas AJ, O'Brien JT, Jolles J, et al. Depressive syptoms and cognitive decline in community-dwelling older adults. Journal of the American Geriatrics Society. 2010;58:873–879. doi: 10.1111/j.1532-5415.2010.02807.x. [DOI] [PubMed] [Google Scholar]
- Lövdén M, Ghisletta P, Lindenberger U. Social participation attenuates decline in perceptual speed in old and very old age. Psychology and Aging. 2005;20:423–434. doi: 10.1037/0882-7974.20.3.423. [DOI] [PubMed] [Google Scholar]
- Luszcz MA, Bryan J. Toward understanding age-related memory loss in late adulthood. Gerontology. 1999;45:2–9. doi: 10.1159/000022048. [DOI] [PubMed] [Google Scholar]
- Luszcz MA, Bryan J, Kent P. Predicting episodic memory performance of very old men and women: Contributions from age, depression, activity, cognitive ability, and speed. Psychology and Aging. 1997;12(2):340–351. doi: 10.1037//0882-7974.12.2.340. [DOI] [PubMed] [Google Scholar]
- Luszcz MA, Giles L, Eckermann S, Edwards P, Browne-Yung K, Hayles C. The Australian Longitudinal Study of Ageing: 15 Years of Ageing in South Australia. South Australian Department of Families and Communities; 2007. [Google Scholar]
- McArdle JJ, Hamagami F. Latent difference score structural models for linear dynamic analyses with incomplete longitudinal data. In: Collins LM, Sayer AG, editors. New Methods for the Analysis of Change. American Psychological Association; Washington, D.C.: 2001. pp. 137–176. [Google Scholar]
- McArdle JJ, Nesselroade JR. Using multivariate data to structure developmental change. In: Cohen SH, Reese HW, editors. Life-span developmental psychology: Methodological innovations. Erlbaum; Hillsdale, NJ: 1994. pp. 223–267. [Google Scholar]
- Muthén LK, Muthén BO. Mplus User's Guide. 4 ed. Muthén & Muthén; Los Angeles, CA: 1998-2007. [Google Scholar]
- Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D. Depression and risk for Alzheimer Disease: Systematic review, meta-analysis, and metaregression analysis. Archives of General Psychiatry. 2006;63:530–538. doi: 10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- Salthouse T. What and when of cognitive aging. Current Directions in Psychological Science. 2004;13:140–144. [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocrine Reviews. 1986;7:284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- Thorvaldsson V, MacDonald SWS, Fratiglioni L, Winblad B, Kivipelto M, Jonsson Laukka E, et al. Onset and rate of cognitive change before dementia diagnosis: Findings from two Swedish population-based longitudinal studies. Journal of the International Neuropsychological Society. 2010 doi: 10.1017/S1355617710001372. Submitted. [DOI] [PubMed] [Google Scholar]
- Vinkers DJ, Gussekloo J, Stek ML, Westendorp RGJ, van der Mast RC. Temporal relation between depression and cognitive impairment in old age: Prospective population based study. British Medical Journal. 2004;329:881. doi: 10.1136/bmj.38216.604664.DE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale - Revised. The Psychological Corporation; New York: 1981. [Google Scholar]
- Williams JW, Plassman BL, Burke J, Holsinger T, Benjamin S. Preventing Alzheimer's disease and cognitive decline. 2010 [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Arnold SE, Beck TL, Bienias JL, Bennett DA. Change in depressive symptoms during the prodromal phase of Alzheimer Disease. Archives of General Psychiatry. 2008;65(4):439–436. doi: 10.1001/archpsyc.65.4.439. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Blackwell T, Gore R, Sands L, Reus V, Browner WS. Depressive symptoms and cognitive decline in nondemented elderly women: A prospective study. Archives of General Psychiatry. 1999;56:425–430. doi: 10.1001/archpsyc.56.5.425. [DOI] [PubMed] [Google Scholar]