Abstract
The cardinal manifestations of the pregnancy-specific disorder preeclampsia, new-onset hypertension and proteinuria that resolve with placental delivery, have been linked to an extracellular protein made by the placenta, sFlt1 (soluble fms-like tyrosine kinase 1), that injures the maternal vasculature. However, the mechanisms by which sFlt1, which is heavily matrix-bound, gains access to the systemic circulation remain unclear. Here we report that the preeclamptic placenta’s outermost layer, the syncytiotrophoblast, forms abundant “knots” that are enriched with sFlt1 protein. These syncytial knots easily detach from the syncytiotrophoblast, resulting in free, multinucleated aggregates (50–150 μm diameter) that are loaded with sFlt1 protein and mRNA, are metabolically active, and are capable of de novo gene transcription and translation. At least 25% of the measurable sFlt1 in 3rd trimester maternal plasma is bound to circulating placental microparticles. We conclude that detachment of syncytial knots from the placenta results in free, transcriptionally active syncytial aggregates that represent an autonomous source of sFlt1 delivery into the maternal circulation. The process of syncytial knot formation, shedding of syncytial aggregates, and appearance of placental microparticles in the maternal circulation appears to be greatly accelerated in preeclampsia and may contribute to the maternal vascular injury that characterizes this disorder.
Keywords: Syncytial knots, syncytial aggregates, microparticles, sFlt1, soluble VEGFR1, preeclampsia
Introduction
Preeclampsia (PE) affects 5–7% of all pregnancies and results in substantial morbidity both to the mother and the fetus 1, 2. While new-onset maternal hypertension, proteinuria, and edema are the hallmarks of this disorder, preeclampsia left unchecked can progress to seizures, acute liver injury and death. The only known treatment for PE is delivery of the placenta. Although the pathogenesis of PE remains incompletely understood, the rapid and complete resolution of this disease following delivery implicates the placenta as a critical factor for the pathogenesis of the disease.
We and others have described a marked elevation of soluble fms-like tyrosine kinase 1 (sFlt1 or sVEGFR1) in the circulation of women with PE that is both proportional to the severity of the disease and is antecedent to the clinical manifestations 3–7. Heterologous expression of sFlt1 in pregnant rodents is sufficient to recapitulate the major features of PE 6, 8, 9. sFlt1 may mediate maternal injury by binding and sequestering tropic growth factors such as VEGF and PlGF that are necessary for the maintenance of normal vascular endothelial function 10. Northern analysis of tissue arrays and measurement of its concentration in the uterine artery vs. vein have implicated the placenta as the major source for circulating sFlt1 during pregnancy 6, 11.
All the known sFlt1 isoforms contains heparin-binding domains in the 3rd and 4th immunoglobulin loops that fully account for its strong avidity to the extracellular matrix 12–15. This chemical property has raised questions about how sFlt1 made by placental cells can even gain access to the maternal circulation 16. Having observed that the outermost layer of the placenta, the syncytiotrophoblast, most strongly expresses sFlt1 17 and that placental material has been noted in the maternal circulation for decades18, we hypothesized that syncytial fragments shed into the maternal circulation are a significant source of circulating sFlt1 in preeclampsia. We performed experiments in preeclamptic placentae, 3rd trimester placental organ cultures, and 3rd trimester maternal plasma to test this hypothesis. To circumvent the well-described confusion in terms, we have used “syncytial knots” to describe multinucleated structures that are loosely attached to the tips of placental villi in situ, “syncytial aggregates” to describe detached multinuclear structures of 50–150 μm recovered from placental washes and organ cultures, and “microparticles” to describe products isolated by high-speed centrifugation of placental washes, culture medium, or maternal plasma19.
Material and Methods
Study population and sample collection protocols
Biological samples (plasma and placenta) were collected from normal and preeclamptic patients. Preeclampsia was defined as new onset of hypertension and proteinuria occurring after 20 weeks of gestation20. Diagnosis of preeclampsia was confirmed by an obstetrician after review of the medical records of the study participants. About 5 cc of blood was collected from subjects via venipuncture, centrifuged at 3500g for 10 minutes and the plasma was collected and stored at −80°C without thaw prior to analysis. For placental studies, several villous biopsies (2 cm3) were excised from the maternal surface midway between the chorionic and basal plates, within 30 minutes of delivery, and the decidual layer was carefully removed. A portion was flash frozen in liquid nitrogen for RNA and protein analysis and the remaining villous tissue collected was cut in to 0.5 cm3 and rinsed twice in 50 ml of ice-cold phosphate buffer saline (PBS) for two minutes. After rinsing, portion of the villous tissue was flash frozen and another portion of the villous tissue was used in explant cultures. The washes were combined (100 ml total) and filtered using a thin layer of gauze. The material in the filtrate was collected by centrifugation (800 g for 10 min), and then subjected to red blood cell (RBC) lysis (RBC lysis solution, Roche Applied Sciences, Mannheim, Germany). After centrifugation and re-suspension again in PBS, a portion of the pellet was used for microscopy and the remaining flash frozen in liquid nitrogen for mRNA and protein analyses. These human studies were approved by the institutional review boards at the Beth Israel Deaconess Medical Center, Boston and at the Magee Womens Research Institute, Pittsburgh and subjects gave informed consent.
Immunohistochemistry and Electron Microscopy (EM)
Expression of soluble Flt1 protein in formalin-fixed and paraffin-embedded sections of placenta was evaluated using an anti-human Flt1 antibody that recognizes the N-terminal region of Flt1/sFlt1 (catalog no. AF321; 1:200 dilution, R & D Systems, Minneapolis, MN) and an ImmPRESS anti-goat staining kit (catalog no. MP-7405; Vector Laboratories, Burlingame, CA) according to published protocols21. Evaluation of the H&E and sFlt1 staining was performed by a single pathologist (I.E.S.) in a blinded fashion. Grading of Flt1/sFlt1 staining was done using the semi-quantitative ordinal scale as follows: 1+ (focal trophoblast staining), 2+ (<50% of the villous trophoblast showing staining), 3+ (51–90% staining), and 4+ (>90% staining). Weak staining was considered negative (zero).
Contents of the placental washes were fixed in 10% neutral buffered formalin, and then washed with PBS. Samples were centrifuged at 800g for 10 min at 4C and were placed into Histogel specimen medium and then embedded into paraffin. 4μm sections were cut and stained for sFlt1 expression as described above. In addition, pellets of the placental washes were stained with Trypan blue and imaged at 10X and 40X magnifications using an Olympus–digital camera.
For EM studies, placental wash effluents were fixed in 3% formaldehyde 3 % glutaraldehyde in 0.1 M cacodylate buffer pH 7.35 (Tousismis Research Corporation Rockville,MD). The fixed cells/debris was processed in standard fashion (Epon embedded) for transmission electron microscopy and images were acquired using JEOL 1011 Transmission Electron Microscope, with a Hamamatsu Orca-HR Digital Camera (Advanced Microscopy Techniques, Woburn, MA).
Northern blot analysis
Total RNA was isolated from archived placental wash-pellets using RNAwiz (Ambion, Austin, TX) and Northern blot analysis was performed using total RNA (20 μg) isolated from the washes as described previously 22. Two regions of the FLT1 mRNA (gene bank accession # X51602) spanning the region 250–881 (5′ ATGGTCAGCTACTGG-GACACCGGGGTC and 5′ ACTGTTGCTTCACAGGTCAGAAGC, respectively) and 2300 to 3300 (5′ CTAATGGTGTCCCCGAGCCT and 5′ CCATTTGTACTCCTGGGTA-TGG) were amplified using PCR and were used as probes in the northern blots using published protocols.
Villous Explant Cultures and Isolation of Syncytial aggregates
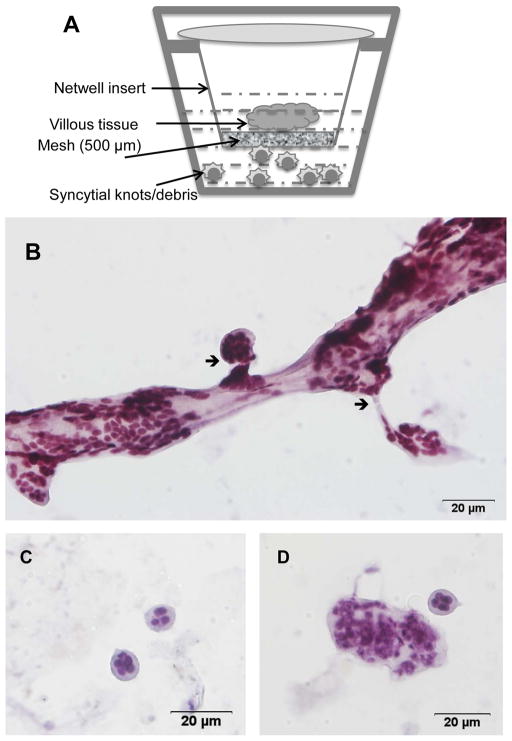
Placental villous explant preparation and culture was carried out according to published protocols23. Explants were incubated at 37°C for 24 hrs on an orbital shaker (60 rpm, Belly Dancer, Stovall Life Science Inc., Greensboro, NC) under standard tissue culture conditions in a cell culture incubator (Napco Series 8000 WJ, Thermo Scientific, Marietta, OH). At the end of the incubation period, the explants were removed, blotted with sterile cotton gauze to remove any excess media, and flash frozen in liquid nitrogen and stored at −80° C. To isolate syncytial aggregates, we cultured villous explants on Netwells (15mm Netwell insert with 500 μm mesh size, Corning) as described 24. To evaluate for shed products, the placental material collected in the lower chamber was concentrated by centrifugation at 800g for 5 min and used in further experiments.
Ultracentrifugation of Plasma and Explant Culture Medium
We subjected plasma samples obtained from normal pregnant (n=12) and preeclamptic women (n=16) to ultra-centrifugation (100,000 rpm for 90 min, ~415,000 g) after diluting them ten times with ELISA assay calibration buffer (R&D systems, MN) or PBS. Similarly the villous explant conditioned medium was centrifuged for 5 min at 800g to remove larger particles and diluted ten times with PBS and processed for ultracentrifugation.
Heparin-agarose enrichment of sFlt1 and Western Blot Analyses
Soluble Flt1 in human plasma samples and conditioned medium from explant cultures was concentrated by heparin-agarose affinity chromatography using published protocols25 and western blots performed as previously described22. Briefly, the 100K pellets were resuspended in 1ml of PBS and were incubated with 25 μl of heparin-agarose beads (Sigma Chemical company, St Louis, MO) at 4°C for 1 hr with continuous mixing. The heparin-agarose/sFlt1 conjugate was then centrifuged and the pellet washed three times with PBS buffer. After the final wash, the beads were re-suspended in minimal volume of 1X Laemmli’s solution and western blots performed using mouse monoclonal VEGFR1 antibody (V4262; Sigma Chemical, St. Louis, MO) that recognizes the amino acid terminus epitope present in both Flt1 and sFlt1.
Enzyme-linked immunosorbent assay (ELISA) and Placental Alkaline Phosphatase (PlAP) colorimetric assay
Soluble Flt1 in culture medium and in maternal plasma, pre and post 100K centrifugation, was measured by Enzyme-linked immunosorbent assay (ELISA) using the human VEGFR1 Quantakine kit from R&D systems (R&D Systems, Minneapolis, MN) following manufacturer’s instructions. Sensitivity of the assay was 5.01 pg/ml, with an intra-assay coefficient of variation of 2.6 – 3.8% and an inter assay coefficient of variation of 7.0 – 8.1%. PlAP activity in the 100K pellet fraction (same fraction as used in Western blot) was estimated using a kit from Abcam (# ab83369-500, Abcam, Cambridge, MA) according to manufacturers instructions. The PlAP activity was expressed as μmole of p-nitrophenol release.
Adenoviral expression studies
Placental explants were incubated for 24 to 48 hrs on a Netwell (Cat # 29442-134, Corning Life Sciences, Pittston, PA) that facilitates any cells and debris to move in to the lower chamber24. The medium was collected and centrifuged at 800 x g in order to harvest the debris. After RBC lysis the concentrated debris were transduced with 2 μl of 1x1010 PFU/ml adenovirus carrying GFP (Vector BioLabs, Eagleville, PA) and assessed for GFP expression after 12 to 24hr following published protocols24. Briefly, the debris was collected by centrifugation, resuspended in 30 to 50 μl of PBS and spread on a microscopic slide and let air-dry. The slides were dipped in ice-cold acetone for 30 min for fixation and PBS and the nuclei were stained with DAPI blue (Molecular Probes Inc., Eugene, OR). The slides were mounted using Gelvetol and fluorescence microscopy was done using an Olympus–digital camera and the images were processed using DP2-BSW program. In another experiment the debris was tranduced with adenovirus expressing truncated mouse sFlt1 protein 26 for 48 hrs. The medium was subjected to heparin-agarose enrichment and probed for sFlt1 expression using Western blots as described above.
Results
Soluble Flt1 is highly expressed in syncytial knots within placentae of women with preeclampsia
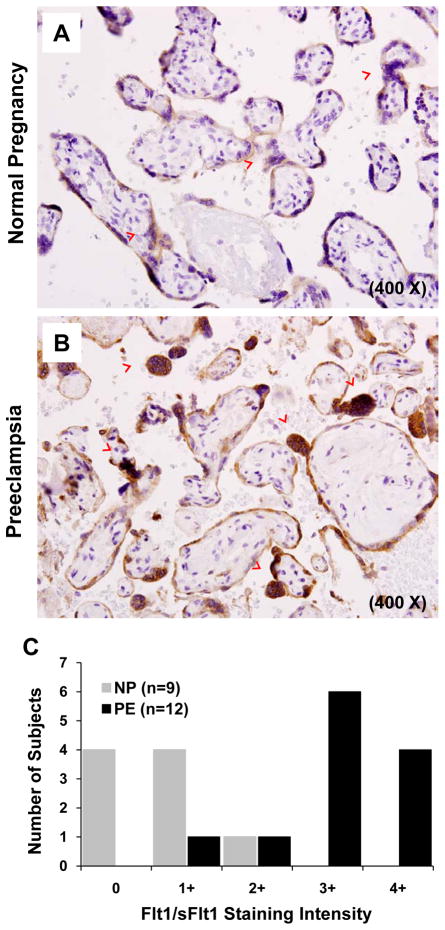
Compared to normal 3rd trimester placentae (N=9), Flt1/sFlt1 expression was significantly increased in the syncytial layer of preeclamptic placentae (N=12), with pronounced over-expression in syncytial knots (Figure 1A and B). Furthermore, the abundance of such knots was increased in preeclamptic placentae. Blinded scoring confirmed that Flt1/sFlt1 staining was markedly enhanced in the syncytial layer of preeclamptic placentae (Figure 1C, p<0.001).
Figure 1. Immunohistochemistry for sFlt1 expression in normal and preeclamptic placentae.
Immunohistochemical staining and analysis of placental tissues from normal pregnancy (n=9) and preeclampsia (n=12) for Flt/sFlt1 expression were performed. Panel A and B show a representative staining of normal and preeclamptic placenta, at term, respectively. The red arrowheads represent syncytial knots. Magnification 400X. Panel C shows a graphical representation of the quantitation of the Flt/sFlt1 staining.
Washing of preeclamptic placentae releases syncytial aggregates
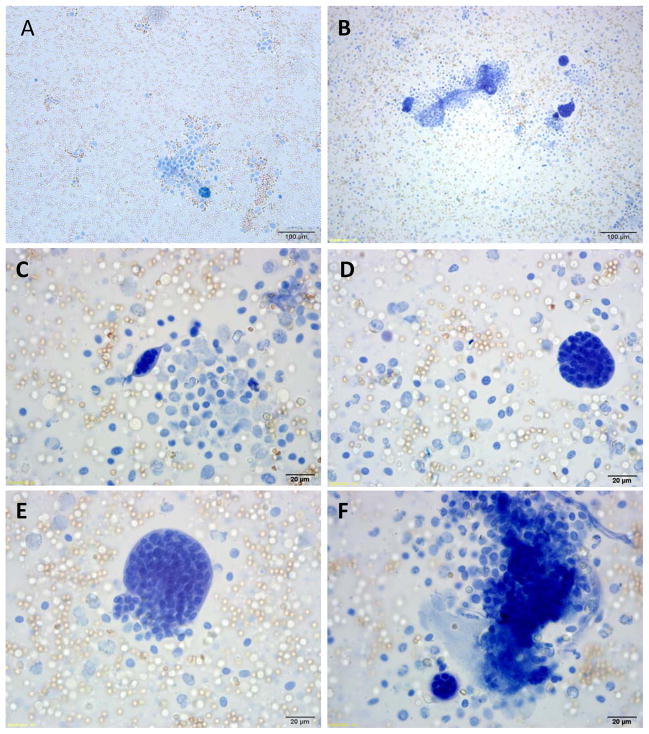
Based on the anatomical orientation of the syncytial knots at the tips of placental villi, we hypothesized that they may easily detach from this outermost layer of the placenta. To test this, we gently flushed placentae from preeclamptic pregnancies with PBS and collected the effluent. The effluent contained large structures whose size of 50–150 μm was consistent with detached syncytial knots (Figure 2). Closer examination demonstrated that these structures were always membrane-bound and always multinucleated, again consistent with a syncytial origin (Figure 2B–F). We repeated this experiment in placentae from normal term pregnancies and observed very few multi-nucleated structures in the effluent (Figure 2A). This data showed that the abundant syncytial knots in preeclamptic placentae might easily detach from the syncytial layer to become free aggregates of syncytial origin.
Figure 2. Analyses of placental washes from normal and preeclamptic placentae.
Representative photomicrograph of Trypan blue staining of the contents of the placental washes obtained from a normal (panel A) and preeclamptic women (panel B). Panels C – F show different sized syncytial aggregates in preeclamptic placental effluents. Red blood cells and leukocytes can be seen in the background.
Soluble Flt1 mRNA and protein are elevated in preeclamptic placental effluents
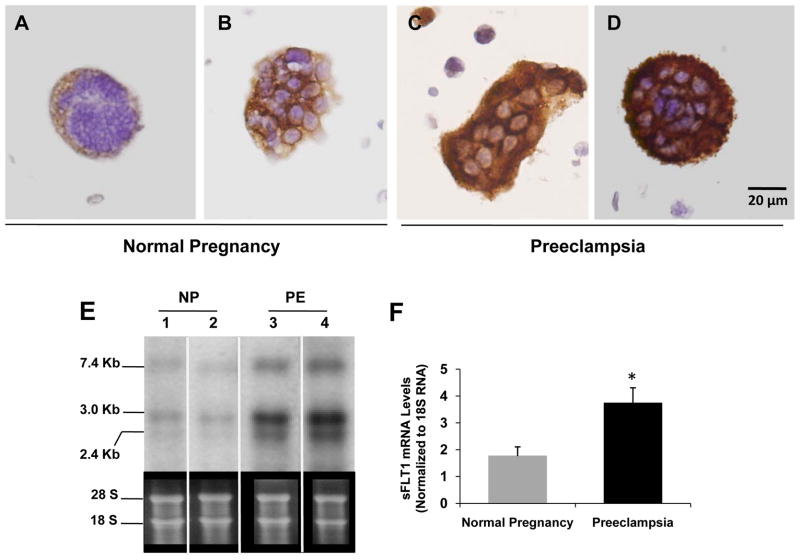
Having previously observed that gentle washing of preeclamptic placentae reduced the abundance of sFlt1 mRNA 22, we now asked whether the released syncytial aggregates in the effluent contained sFlt1. To test this, we first performed immunohistochemistry on syncytial aggregates isolated by low speed spin (800 g) of placental washes from normal and preeclamptic third-trimester placentae and observed that the latter stained much stronger for sFlt1 (Figure 3A–D) and the related angiogenic protein endoglin (see http://hyper.ahajournals.org – Supplementary Figure S1).
Figure 3. Expression of sFlt1 mRNA and protein in syncytial debris obtained from placental washes.
Panels A to D show sFlt1 staining by IHC of the syncytial knots obtained from placental washes from two normal pregnant women (A and B) and from two preeclamptic women (C and D). Scale bar 20 μm in all the panels. RNA obtained from placental washes of normal pregnant (NP) (n=5) and preeclamptic women (PE) (n=6) was analyzed by Northern blot. A representative blot from two samples of each category is shown in panel E and the quantitation in a graph in panel F. *p=<0.05 by ANOVA.
Next, we performed Northern analysis to quantify the relative amount of sFlt1 mRNA associated with this liberated placental material. Both normal and preeclamptic placental effluents demonstrated the 7.4 kb band that corresponds to membrane-bound Flt1 and the two smaller bands (3.0 and 2.4 kb) corresponding to alternatively spliced products encoding soluble forms of Flt1 (Figure 3E), but the relative amounts of sFlt1-encoding bands was markedly higher in preeclamptic placental effluents (Figure 3F). Finally, sFlt1-encoding mRNA was not significantly altered in peripheral blood mononuclear cells obtained from preeclamptic subjects, suggesting that loss of peripheral blood cells was unlikely to account for the reduction in sFlt1 mRNA following placental washes (see http://hyper.ahajournals.org – Supplementary Figure S2).
Soluble Flt1 is associated with microparticles in villous explant culture medium
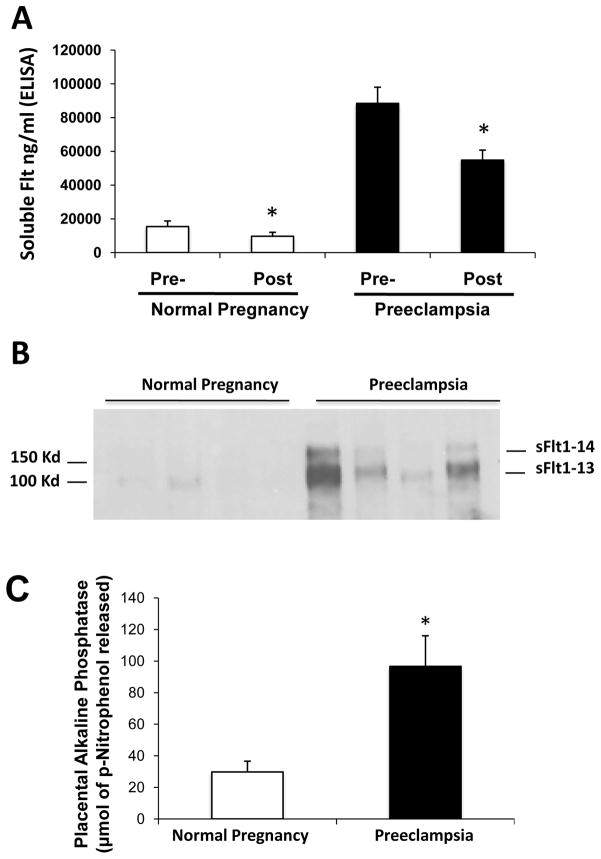
To rule out the possibility that the observed syncytial aggregates were artifacts of physical manipulation, we next placed villous explants from preeclamptic and normal term placentae in organ culture and collected the medium at 48 hrs for further analysis. While sFlt1 protein has previously been demonstrated in the culture medium using this technique3, it has generally been assumed that this solely represents free secreted protein in solution. We asked whether sFlt1 was also bound to microparticles. Centrifugation at 100,000 rpm (~415,000 g) for 90 minutes reduced the amount of sFlt1 by 30% in the conditioned media for both normal pregnancy and preeclamptic villous explants (Figure 4A). To demonstrate that the reduction of sFlt1 following centrifugation was related to placental microparticle-bound sFlt1, we solubilized the pellet and assayed for sFlt1 and placental alkaline phosphatase. Pellets obtained from culture medium from preeclamptic women showed significantly higher amounts of sFlt1 (Figure 4B), and higher placental alkaline phosphatase than their normal counterparts (Figure 4C). These results show that shedding of placental microparticle material can occur spontaneously rather than being a simple artifact of aggressive flushing and that this release is exaggerated in preeclamptic placentae. Moreover, these data suggest that approximately one-third of the secreted sFlt1 is associated with released microparticles.
Figure 4. Microparticle associated sFlt1 in the culture medium of placental villous explants.
Placental villous explants were cultured as described in materials and methods and supernatant was analyzed for sFlt1 expression. High-speed centrifugation reduced sFlt1 by ~30% in placental explant conditioned medium. The pre- and post-spin sFlt1 levels measured by ELISA are shown in Panel A. sFlt1 in 100K pellets was detected by Western blot analyses (Panel B). The quantitation of placental alkaline phosphatase (PlAP) by densitometry from the Western blots is shown in panel C. *p<0.05 by ANOVA.
Ex vivo organ culture recapitulates syncytial knot formation and spontaneous syncytial aggregate release
To evaluate further the nature of syncytial material being released by the preeclamptic placenta, we performed 3rd trimester placental organ cultures on Netwell inserts and collected the culture medium below the mesh for further characterization (Figure 5A). This technique has previously only been used for the isolation of microparticles from 1st trimester placental organ culture24. Cytological analysis of the culture medium below the mesh revealed membrane-bound knot-like structures containing multiple nuclei that appear to be “budding” off a main branch (Figure 5B). We also observed free membrane-bound particles 50–150 μm in diameter containing multiple nuclei (Figure 5C–D). Both the size and composition of these aggregates exactly mirrored the contents of effluents from placentae (Figure 2) and the spontaneously released material in villous explant cultures of preeclamptic placentae (Figure 3). These results therefore suggest that ex vivo culture of 3rd-trimester placentae on Netwell results provides an efficient method for studying the spontaneous release of syncytial aggregates.
Figure 5. Characterization of placental syncytial aggregates using ex vivo organ cultures.
Panel A is a schematic of the ex vivo placental organ cultures on Netwell inserts. Panel B shows the generation of syncytial knots of different sizes from the main villous tissue. Arrow shows the break point of multinucleated aggregates from the villous tissue. Panel C–D shows representative high power images of individual multi-nucleated aggregates that have separated from the villous tissue.
Released syncytial aggregates are viable and metabolically active
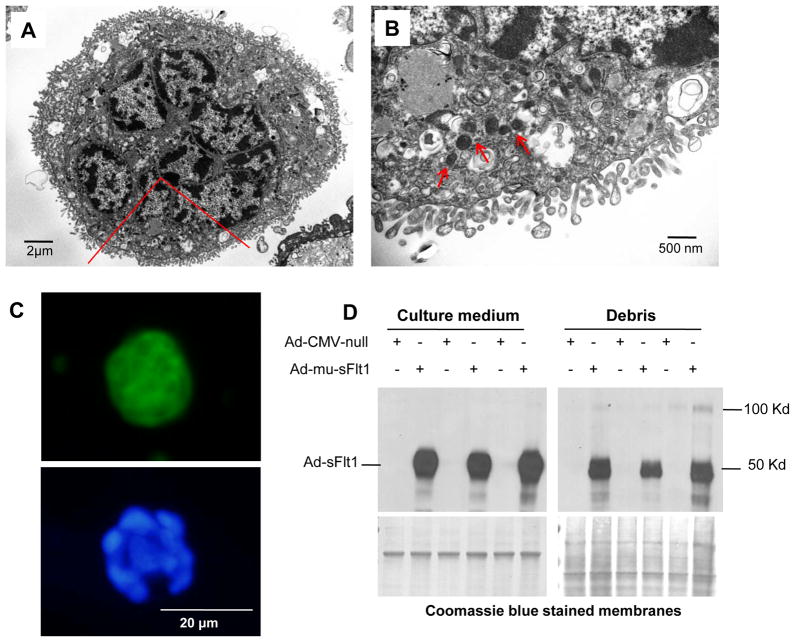
We applied the Netwell method on 3rd-trimester placental organ culture to collect syncytial aggregates for further study. Several previous reports have termed these structures “debris” 27, implying that they are dead or non-viable material. Transmission electron microscopy of these structures showed that the plasma membrane was organized into a microvillous structure and that the cytoplasm contained several nuclei, numerous mitochondria, and other organelles (Figure 6A, B). The internal composition of these aggregates and the fact that we had detected both sFlt1 mRNA and protein in them (Figures 3 and 4) suggested to us that these structures could have gene expression capacity. To test this, we infected aggregates with GFP-encoding adenovirus (Figure 6C). Aggregates were fluorescent for more than 48 hours after infection, demonstrating that they possess both transcriptional and translational activity and the energetic capacity required for these processes. We repeated this experiment with sFlt1-adenovirus and obtained a comparable result (Figure 6D). Finally, to confirm the ability of these microparticles to synthesize sFlt1 protein from endogenous sFlt1 mRNA, we performed a pulse-chase experiment with 35S, immunoprecipitated the conditioned media with anti-Flt1, and confirmed the presence of new protein by autoradiography (see http://hyper.ahajournals.org – Supplementary Figure S3). These results show that spontaneously released syncytial aggregates from late-pregnancy placentae possess a spectrum of biological capacities, including the ability to synthesize sFlt1 protein from endogenous stores of its mRNA.
Figure 6. Syncytial aggregates exhibit transcriptional and translational capacities.
(A–B). Electron micrograph of multinucleated aggregate shows of microvillous cell membrane and abundant cytoplasmic organelles (Panel A). A region marked in red is magnified to show mitochondria (Panel B) with red arrowheads. Panel C. GFP expression in a syncytial aggregate infected with GFP adenovirus is shown in the upper panel. Nuclear co-localization was done with DAPI blue, bottom panel. Panel D. Syncytial aggregates were infected with adenovirus carrying truncated sFlt1 protein and the conditioned medium was analyzed by Western blot for expression of sFlt1 protein.
Released microparticles show anti-angiogenic properties
Pellets obtained by ultra-centrifugation of the culture medium from the placental explant cultures were resuspended in growth medium and were employed in endothelial tube formation assays, a standard tool for assessing angiogenic activity3. Preeclampsia suspensions showed significant inhibition of endothelial tube formation (see http://hyper.ahajournals.org – Supplementary Figure S4A–B) that was reversed by the addition of exogenous VEGF (see http://hyper.ahajournals.org – Supplementary Figure S4C). Quantitation of the tube lengths are presented in Supplementary Figure S4D (see http://hyper.ahajournals.org).
Circulating microparticles of syncytial origin contribute at least 25% of the soluble Flt1 in the plasma of normal pregnancy and preeclamptic subjects
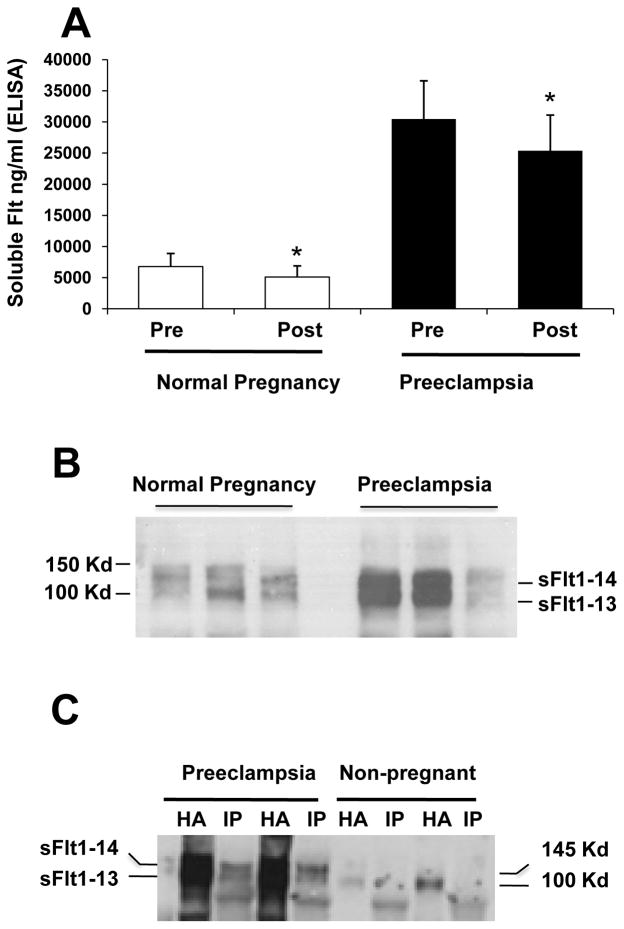
Having performed a series of ex vivo experiments with 3rd-trimester human placentae to establish structural and functional features of shed trophoblast material, we returned to the maternal circulation to ask how much shed microparticles of syncytial origin contribute to circulating levels of sFlt1 in normal and preeclamptic pregnancies. After a low-speed spin to remove cellular components, we centrifuged plasma at 100,000 rpm (~415,000 g) for 90 minutes to collect microparticles into a pellet. We measured the sFlt1 concentration in plasma before and after centrifugation. In plasma from 3rd-trimester normal pregnancies (N=12 subjects), these values were 6779 ± 1458 pg/ml and 5116 ± 1228 pg/ml, respectively. In plasma from preeclamptic subjects (N=15), centrifugation reduced the sFlt1 concentration from 30486 ± 6108 pg/ml to 25381 ± 5710 pg/ml. Therefore, in both clinical settings, free-microparticle-associated sFlt1 accounted for approximately 25% of the total sFlt1 concentration in plasma samples from both groups (Figure 7A).
Figure 7. Microparticle associated sFlt1 in plasma of pregnant women.
Panel A. Plasma samples from normal pregnant (n=12) and preeclamptic women (n=15) at term show an approximate 25% reduction in circulating sFlt1 levels after ultracentrifugation. Panel B is a representative western blot analyses for sFlt1 protein expression in the 100K pellets of the plasma obtained from normal and preeclamptic women. Panel C demonstrates that sFlt1 containing microparticles also express syncytin. Plasma samples from preeclamptic patients (n=2) and non-pregnant women (n=2) were subjected to ultracentrifugation. The 100K pellet was precipitated using heparin-agarose (HA) or using syncytin antibody (IP) and Western blot performed with antibody directed against the N-terminus of Flt1. * p< 0.05 by ANOVA.
Next, we performed Western analysis on the centrifuged pellets and observed that preeclamptic plasma was enriched for sFlt1 bound to these microparticles (Figure 7B). Finally, to confirm that the sFlt1 in these pellets was of syncytiotrophoblast origin, we performed co-immunoprecipitation by pulling down the syncytiotrophoblast marker syncytin-1 and blotting for sFlt1 (Figure 7C).
Discussion
Our results show that 3rd trimester placentae from preeclamptic women have more syncytial knots that are more heavily loaded with sFlt1 protein compared to those from normal pregnancies. Gentle flushing of these placentae selectively releases more trophoblast mass in the form of syncytial aggregates from the preeclamptic placentae than their normal counterparts. Liberation of syncytial aggregates could not be attributed to aggressive handling as preeclamptic placentae in organ culture spontaneously released aggregates of identical size and multinuclear composition into the medium. This shed material contained both sFlt1 protein and mRNA. Placement of 3rd trimester placental explants over a 500 μm mesh in organ culture not only enabled efficient isolation of syncytial aggregates, but also suggested that this shed material may arise by syncytial sprouting and fission from the underlying syncytium. Isolated aggregates were multinuclear, rich in cytoplasmic organelles, and capable of de novo gene expression, demonstrating that these structures were not only viable, but also biologically active. Finally, we returned to the 3rd trimester maternal circulation, where we could demonstrate that at least 25% of plasma sFlt1 was associated with microparticles. While placental heparanase upregulation has been recently implicated as one factor that may contribute to the release of sFlt1 into systemic circulation16, our data suggests that release of syncytial microparticles may be an important additional factor that contributes to the elevated sFlt1 in human preeclampsia.
Based on these results, we speculate that 3rd-trimester placentae spontaneously form living syncytial spouts/knots (Figure 1) that detach from placental villi through fission (Figure 5), liberating membrane-bound multinuclear structures (Figures 2 and 3) that we called syncytial aggregates that possess critical biological capacities (Figure 6), including the ability to synthesize sFlt1 protein from endogenous stores of mRNA. Since each phase of this sequence is exaggerated in preeclampsia—in situ knots (Figure 1) followed by liberated sFlt1-expressing syncytial aggregates (Figure 3) that then perhaps further disaggregate to sFlt1 associated microparticles (Figures 7)—we also speculate that accelerated knot/sprout formation within the placenta may be an early event in preeclampsia that enhances the delivery of sFlt1 into the maternal circulation.
Normal pregnancy is characterized by trophoblast turnover and shedding, as evidenced by the detection of trophoblastic microparticles in the maternal circulation throughout pregnancy28–32 and by the appearance of detached syncytial aggregates with euchromatic nuclei—labeled “syncytial sprouts”—in term human placentae33. Based on the current results, we speculate that 3rd-trimester placentae spontaneously form syncytial sprouts/knots (Figure 1) that detach from placental villi through fission (Figure 5), liberating membrane-bound multinuclear structures (Figures 2 and 3), termed syncytial aggregates, that possess critical biological capacities (Figure 6), including the ability to synthesize sFlt1 protein from endogenous stores of mRNA. Therefore, while apoptotic, necrotic, or “aponecrotic” placental material34 loaded with sFlt1 protein may well be released into the maternal circulation, our data suggest that term placentae deport or shed biologically active syncytial aggregates that, in turn, may release smaller microparticles with similar biological capacities into the maternal circulation, akin to the release of platelets from megakaryocytes residing in the bone marrow.
In addition to demonstrating the biological capacity of 3rd trimester shed trophoblast material, the current findings also add to the existing literature in other ways. First, deportation of living placental material, followed by de novo translation of pre-existing mRNA, may be a new mechanism by which sFlt1 is delivered into the maternal circulation. Second, while sFlt1 protein has been identified on circulating placental particles35 and an increase in circulating particles has been associated with PE 18, no explanation has been proposed for how these particles are formed. This is perhaps because late-pregnancy particles in the maternal circulation have been assumed to be dead material. To our knowledge, ours are the first data that physically connect circulating microparticles to syncytial knots by suggesting that shed syncytial aggregates are the intermediary form. Third, if syncytial knots give rise to circulating sFlt1-expressing microparticles through the shed aggregates, and if these phenomena are quantitatively stronger in preeclampsia, our data suggest that accelerated syncytial knot formation is a proximal event in the pathogenesis of preeclampsia, in agreement with prior reports 16. Regardless, it is unlikely that syncytial knots within the intact placenta simply represent an artifact of tangential sectioning, as has also been proposed 36.
Important questions remain for future investigation. Metabolically active microparticles appear to begin forming in the first trimester 24. Does PE, therefore, represent the same process, but accelerated? If so, what fraction of peripheral sFlt1 protein is contributed by viable sFlt1 expressing microparticles versus dead/inactive microparticles that are already pre-loaded with sFlt1 protein? Also, what PE-specific mechanisms drive the induction of knot formation, deportation of syncytial aggregates, and microparticle generation? Conversely, could enhanced sFlt1 production somehow trigger syncytial knot formation? Second, we present novel evidence that living syncytial aggregates arise from syncytial sprouting and fission, but the molecular apparatus of nuclear aggregation and cytokinesis remain to be described. Third, different mechanisms for sFlt1 export from the placenta have been described, including the shedding of dead syncytial material 37 and the release of matrix-bound sFlt1 by matrix-dissolving enzymes such as heparanase16. While upregulation or accumulation of heparanase has not been shown to occur in the preeclamptic human placenta16, it will be of interest to determine the relative contributions of these processes to maternal sFlt1 exposure. Fourth, we have established some of the biological capacity of syncytial aggregates by demonstrating de novo gene expression, but other functions, including regulation of inflammation and immunity, may also be important 38. It would also be important to determine whether pro-inflammatory stimuli and other factors such as angiotensin autoantibodies that have been linked with preeclampsia pathogenesis may induce syncytial knot and microparticle formation. Finally, it has been suggested that shed syncytial aggregates get trapped in the capillary beds of lung tissue, where they further undergo disaggregation or apoptosis/necrosis to release the smaller microparticles into the systemic circulation39–41. The relative contribution of these processes to the formation of trophoblast microparticles within the maternal circulation remains to be determined.
Perspectives
The present studies show that syncytial knots in the 3rd trimester placentae may give rise to biologically active microparticles that can translate packaged sFlt1 mRNA into protein. In turn, this process may be a novel means by which sFlt1 and other toxic proteins such as soluble endoglin may be delivered into the maternal circulation, where they mediate the major manifestations of preeclampsia. Our findings not only have direct implications for the care of women and unborn children with this disease, but may also advance our understanding of fundamental cell biological processes. Future work on the basic biology of syncytialization may shed clues on the molecular defect in preeclampsia 42, 43.
Supplementary Material
Acknowledgments
The authors wish to thank Dawn McCullough and Saira Sallaludhin for their help in sample procurement and Saumya Hegde and Poorna Natarajan for technical assistance.
Sources of Funding
Funded by NIH RO3 HD055219-03 to A.R.; NIH P01 HD030367 to C.A.H and A.J. S.R is supported by Harvard Diversity and Community Partnership Faculty Fellowship Award. The Gulbenkian Programme for Advanced Medical Education is sponsored by Fundação Calouste Gulbenkian, Fundação Champalimaud, Ministério da Saúde e Fundação para a Ciência e Tecnologia, Portugal. S.A.K. is an investigator of the Howard Hughes Medical Institute.
Footnotes
Disclosures
Dr. Karumanchi is a co-inventor of multiple patents related to angiogenic proteins for the diagnosis and therapy of preeclampsia. These patents have been licensed to multiple companies. Dr. Karumanchi reports having served as a consultant to Roche and Beckman Coulter and has financial interest in Aggamin LLC. The remaining authors report no conflicts.
BIBLIOGRAPHY
- 1.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 2.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–799. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 3.Ahmad S, Ahmed A. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ Res. 2004;95:884–891. doi: 10.1161/01.RES.0000147365.86159.f5. [DOI] [PubMed] [Google Scholar]
- 4.Chaiworapongsa T, Romero R, Espinoza J, Bujold E, Mee Kim Y, Goncalves LF, Gomez R, Edwin S. Evidence supporting a role for blockade of the vascular endothelial growth factor system in the pathophysiology of preeclampsia. Am J Obstet Gynecol. 2004;190:1541–1547. doi: 10.1016/j.ajog.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 5.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 6.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaiworapongsa T, Romero R, Kim YM, Kim GJ, Kim MR, Espinoza J, Bujold E, Goncalves L, Gomez R, Edwin S, Mazor M. Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre- eclampsia. J Matern Fetal Neonatal Med. 2005;17:3–18. doi: 10.1080/14767050400028816. [DOI] [PubMed] [Google Scholar]
- 8.Bergmann A, Ahmad S, Cudmore M, Gruber AD, Wittschen P, Lindenmaier W, Christofori G, Gross V, Gonzalves A, Grone HJ, Ahmed A, Weich HA. Reduction of circulating soluble Flt-1 alleviates preeclampsia-like symptoms in a mouse model. J Cell Mol Med. 2010;14:1857–1867. doi: 10.1111/j.1582-4934.2009.00820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu F, Longo M, Tamayo E, Maner W, Al-Hendy A, Anderson GD, Hankins GD, Saade GR. The effect of over-expression of sFlt-1 on blood pressure and the occurrence of other manifestations of preeclampsia in unrestrained conscious pregnant mice. Am J Obstet Gynecol. 2007;196:396, e1–7. doi: 10.1016/j.ajog.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 10.Powe CE, Levine RJ, Karumanchi SA. Preeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular disease. Circulation. 2011;123:2856–2869. doi: 10.1161/CIRCULATIONAHA.109.853127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bujold E, Romero R, Chaiworapongsa T, Kim YM, Kim GJ, Kim MR, Espinoza J, Goncalves LF, Edwin S, Mazor M. Evidence supporting that the excess of the sVEGFR-1 concentration in maternal plasma in preeclampsia has a uterine origin. J Matern Fetal Neonatal Med. 2005;18:9–16. doi: 10.1080/14767050500202493. [DOI] [PubMed] [Google Scholar]
- 12.Holash J, Davis S, Papadopoulos N, Croll SD, Ho L, Russell M, Boland P, Leidich R, Hylton D, Burova E, Ioffe E, Huang T, Radziejewski C, Bailey K, Fandl JP, Daly T, Wiegand SJ, Yancopoulos GD, Rudge JS. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A. 2002;99:11393–11398. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci U S A. 1993;90:10705–10709. doi: 10.1073/pnas.90.22.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park M, Lee ST. The fourth immunoglobulin-like loop in the extracellular domain of FLT-1, a VEGF receptor, includes a major heparin-binding site. Biochem Biophys Res Commun. 1999;264:730–734. doi: 10.1006/bbrc.1999.1580. [DOI] [PubMed] [Google Scholar]
- 15.Sela S, Itin A, Natanson-Yaron S, Greenfield C, Goldman-Wohl D, Yagel S, Keshet E. A novel human-specific soluble vascular endothelial growth factor receptor 1: cell-type-specific splicing and implications to vascular endothelial growth factor homeostasis and preeclampsia. Circ Res. 2008;102:1566–1574. doi: 10.1161/CIRCRESAHA.108.171504. [DOI] [PubMed] [Google Scholar]
- 16.Sela S, Natanson-Yaron S, Zcharia E, Vlodavsky I, Yagel S, Keshet E. Local retention versus systemic release of soluble VEGF receptor-1 are mediated by heparin-binding and regulated by heparanase. Circ Res. 2011;108:1063–1070. doi: 10.1161/CIRCRESAHA.110.239665. [DOI] [PubMed] [Google Scholar]
- 17.Nevo O, Soleymanlou N, Wu Y, Xu J, Kingdom J, Many A, Zamudio S, Caniggia I. Increased expression of sFlt-1 in in vivo and in vitro models of human placental hypoxia is mediated by HIF-1. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1085–1093. doi: 10.1152/ajpregu.00794.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Redman CW, Sargent IL. Circulating microparticles in normal pregnancy and pre-eclampsia. Placenta. 2008;29(Suppl A):S73–77. doi: 10.1016/j.placenta.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 19.Askelund KJ, Chamley LW. Trophoblast deportation part I: Review of the evidence demonstrating trophoblast shedding and deportation during human pregnancy. Placenta. 2011;32(10):716–723. doi: 10.1016/j.placenta.2011.07.081. [DOI] [PubMed] [Google Scholar]
- 20.NIH. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183:S1–S22. [PubMed] [Google Scholar]
- 21.Silasi M, Rana S, Powe C, Cohen B, Lim KH, Zsengeller ZK, Karumanchi SA, Stillman IE. Placental expression of angiogenic factors in Trisomy 13. Am J Obstet Gynecol. 2011;204:546. doi: 10.1016/j.ajog.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 22.Rajakumar A, Michael HM, Rajakumar PA, Shibata E, Hubel CA, Karumanchi SA, Thadhani R, Wolf M, Harger G, Markovic N. Extra-placental expression of vascular endothelial growth factor receptor-1, (Flt-1) and soluble Flt-1 (sFlt-1), by peripheral blood mononuclear cells (PBMCs) in normotensive and preeclamptic pregnant women. Placenta. 2005;26:563–573. doi: 10.1016/j.placenta.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Rajakumar A, Doty K, Daftary A, Harger G, Conrad KP. Impaired oxygen-dependent reduction of HIF-1alpha and −2alpha proteins in pre-eclamptic placentae. Placenta. 2003;24:199–208. doi: 10.1053/plac.2002.0893. [DOI] [PubMed] [Google Scholar]
- 24.Abumaree MH, Stone PR, Chamley LW. An in vitro model of human placental trophoblast deportation/shedding. Mol Hum Reprod. 2006;12:687–694. doi: 10.1093/molehr/gal073. [DOI] [PubMed] [Google Scholar]
- 25.Rajakumar A, Powers RW, Hubel CA, Shibata E, von Versen-Hoynck F, Plymire D, Jeyabalan A. Novel soluble Flt-1 isoforms in plasma and cultured placental explants from normotensive pregnant and preeclamptic women. Placenta. 2009;30:25–34. doi: 10.1016/j.placenta.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuo CJ, Farnebo F, Yu EY, Christofferson R, Swearingen RA, Carter R, von Recum HA, Yuan J, Kamihara J, Flynn E, D’Amato R, Folkman J, Mulligan RC. Comparative evaluation of the antitumor activity of antiangiogenic proteins delivered by gene transfer. Proc Natl Acad Sci U S A. 2001;98:4605–4610. doi: 10.1073/pnas.081615298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Redman CW, Sargent IL. Placental debris, oxidative stress and pre-eclampsia. Placenta. 2000;21:597–602. doi: 10.1053/plac.2000.0560. [DOI] [PubMed] [Google Scholar]
- 28.Aharon A, Brenner B. Microparticles and pregnancy complications. Thromb Res. 2011;127(Suppl 3):S67–71. doi: 10.1016/S0049-3848(11)70019-6. [DOI] [PubMed] [Google Scholar]
- 29.Germain SJ, Sacks GP, Sooranna SR, Sargent IL, Redman CW. Systemic inflammatory priming in normal pregnancy and preeclampsia: the role of circulating syncytiotrophoblast microparticles. J Immunol. 2007;178:5949–5956. doi: 10.4049/jimmunol.178.9.5949. [DOI] [PubMed] [Google Scholar]
- 30.Kumpel B, King MJ, Sooranna S, Jackson D, Eastlake J, Cheng R, Johnson M. Phenotype and mRNA expression of syncytiotrophoblast microparticles isolated from human placenta. Ann N Y Acad Sci. 2008;1137:144–147. doi: 10.1196/annals.1448.017. [DOI] [PubMed] [Google Scholar]
- 31.Than NG, Abdul Rahman O, Magenheim R, Nagy B, Fule T, Hargitai B, Sammar M, Hupuczi P, Tarca AL, Szabo G, Kovalszky I, Meiri H, Sziller I, Rigo J, Jr, Romero R, Papp Z. Placental protein 13 (galectin-13) has decreased placental expression but increased shedding and maternal serum concentrations in patients presenting with preterm pre-eclampsia and HELLP syndrome. Virchows Arch. 2008;453:387–400. doi: 10.1007/s00428-008-0658-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Post JA, Lok CA, Boer K, Sturk A, Sargent IL, Nieuwland R. The functions of microparticles in pre-eclampsia. Semin Thromb Hemost. 2011;37:146–152. doi: 10.1055/s-0030-1270342. [DOI] [PubMed] [Google Scholar]
- 33.Burton GJ. Deportation of syncytial sprouts from the term human placenta. Placenta. 2011;32:96–98. doi: 10.1016/j.placenta.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 34.Chamley LW, Chen Q, Ding J, Stone PR, Abumaree M. Trophoblast deportation: just a waste disposal system or antigen sharing? J Reprod Immunol. 2011;88:99–105. doi: 10.1016/j.jri.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Guller S, Tang Z, Ma YY, Di Santo S, Sager R, Schneider H. Protein composition of microparticles shed from human placenta during placental perfusion: Potential role in angiogenesis and fibrinolysis in preeclampsia. Placenta. 2011;32:63–69. doi: 10.1016/j.placenta.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huppertz B. IFPA Award in Placentology Lecture: Biology of the placental syncytiotrophoblast--myths and facts. Placenta. 2010;31(Suppl):S75–81. doi: 10.1016/j.placenta.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 37.Guller S. Role of the syncytium in placenta-mediated complications of preeclampsia. Thromb Res. 2009;124:389–392. doi: 10.1016/j.thromres.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Redman CW, Sargent IL. Pre-eclampsia, the placenta and the maternal systemic inflammatory response--a review. Placenta. 2003;24(Suppl A):S21–27. doi: 10.1053/plac.2002.0930. [DOI] [PubMed] [Google Scholar]
- 39.Attwood HD, Park WW. Embolism to the lungs by trophoblast. J Obstet Gynaecol Br Commonw. 1961;68:611–617. doi: 10.1111/j.1471-0528.1961.tb02778.x. [DOI] [PubMed] [Google Scholar]
- 40.Johansen M, Redman CW, Wilkins T, Sargent IL. Trophoblast deportation in human pregnancy--its relevance for pre-eclampsia. Placenta. 1999;20:531–539. doi: 10.1053/plac.1999.0422. [DOI] [PubMed] [Google Scholar]
- 41.Lapaire O, Holzgreve W, Oosterwijk JC, Brinkhaus R, Bianchi DW. Georg Schmorl on trophoblasts in the maternal circulation. Placenta. 2007;28:1–5. doi: 10.1016/j.placenta.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 42.Fox H. The Significance of Villous Syncytial Knots in the Human Placenta. J Obstet Gynaecol Br Commonw. 1965;72:347–355. doi: 10.1111/j.1471-0528.1965.tb01469.x. [DOI] [PubMed] [Google Scholar]
- 43.Huppertz B. Placental origins of preeclampsia: challenging the current hypothesis. Hypertension. 2008;51:970–975. doi: 10.1161/HYPERTENSIONAHA.107.107607. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.