Abstract
The vestibulo-ocular reflex (VOR), which functions to stabilize gaze and ensure clear vision during everyday activities, shows impressive adaptation in response to environmental requirements. In particular, the VOR exhibits remarkable recovery following the loss of unilateral labyrinthine input as a result of injury or disease. The relative simplicity of the pathways that mediate the VOR, make it an excellent model system for understanding the changes (learning) that occur in the brain following peripheral vestibular loss to yield adaptive changes. This mini review considers the findings of behavioral, single unit recording and lesion studies of VOR compensation. Recent experiments have provided evidence that the brain makes use of multiple plasticity mechanisms (i.e., changes in peripheral as well as central processing) during course of vestibular compensation to accomplish the sensory-motor transformations required to accurately guide behavior.
Introduction
The vestibular system detects motion of the head-in-space and generates reflexes during our daily activities that result in stabilization of gaze head, and body posture (reviewed in: Angelaki and Cullen 2008). Because other sensory systems (i.e. visual, proprioceptive, and somatosensory) are concurrently activated during activities that stimulate the vestibular end organs, the significance of the vestibular system in our daily lives is often less obvious when compared to that of the “classical” five senses. Indeed, under normal conditions we are unaware of a distinct vestibular sensation. However, the vital role played by the vestibular system becomes immediately apparent following its loss. Patients with acute abnormal vestibular function have extreme difficulty in performing normal activities that we take for granted since even small head movements are accompanied by gaze instability and postural imbalance. In cases of complete vestibular loss, even the beating of our pulse can make the letters of a page appear to jump and become blurred. Such apparent movements of the visual field, called oscillopsia, become even more pronounced during essential daily activities such as walking. The loss of function also produces devastating symptoms such as dramatic, sudden onset of vertigo.
Fortunately, most cases of vestibular dysfunction are characterized by only partial loss of sensory input, and acute symptoms typically resolve over time, such that patients can resume most activities. While many studies have investigated the mechanisms underlying compensation over the past 70 years, many open questions remain. Notably, much recent effort has focused on understanding the mechanisms that improve the performance of the vestibulo-ocular reflex (VOR) following vestibular loss. The VOR functions to stabilize gaze and ensure clear vision during everyday activities, and the relative simplicity of the pathways mediating this reflex (as well as its impressive post-lesional plasticity/compensation) have made it an excellent model for understanding vestibular compensation at both behavioral and neuronal levels. However, despite intense research interest, the underlying mechanisms that mediate recovery of the VOR had remained unclear.
This minireview considers recent advances in this area, focusing on experiments in our laboratory that have studied the mechanisms that mediate the VOR compensation following unilateral peripheral vestibular loss. Specifically, the findings of recent behavioral and single unit recording studies are considered. We primarily focus on findings made using two different animal models: macaque monkeys and mice. Each preparation has its own benefits: while monkeys are phylogenetically closer to humans, mice have the advantage of providing genetically modifiable models and of allowing for direct comparison with a large part of in vitro work primarily conducted on rodents. This body of work is then discussed in relation to prior investigations, and will address the following questions: 1) How well does the VOR compensate over the physiologically relevant range of head movements? 2) Do significant changes at the level of the vestibular periphery mediate compensation, or is compensation exclusively mediated by central processes? and 3) What role does the vestibular cerebellum play in VOR compensation? We conclude by discussing the implications of these results in relation to the sensory-motor transformations through which vestibular signals are processed to guide behavior during self-motion.
Methods
Experiments were preformed on rhesus monkeys and mice (wild-type and the Lurcher (Lc/+) mutant). All animals we chronically implanted with a post for head restraint. In addition, monkeys were implanted with a recording chamber and scleral coils for eye movement recordings as has been previously described (Beraneck and Cullen 2007; Sadeghi et al. 2006). After recovery, we recorded VOR evoked eye movements in all animals (i.e. monkeys and both mouse strains), as well as the activity of single units in monkeys. All animal surgeries and experimental procedures were approved by the McGill University Animal care Committee and were in strict compliance with the guidelines of the Canadian Council on Animal Care.
Characterization of VOR compensation in Monkeys: Behavioral Experiments
To quantify the dynamics of VOR responses in macaque monkeys, we recorded eye and head movements using the search coil technique before and 1–39 days after unilateral labyrinthectomy (Sadeghi et al. 2006). Responses were characterized over the range of head movements encountered during natural activities. To this end, applied movements included transient rotations of the head-on-body with accelerations up to 12,000 °/s2, rapid rotations with velocities up to 500 °/s, as well as sinusoidal rotations with frequencies up to 15 Hz. In addition, rotations of the body under a stationary head were used to test whether neck proprioceptive inputs contributed to the recorded eye movement responses either before or after lesion.
Characterization of VOR compensation in Monkeys: Single Unit Experiments
To study the neural mechanisms underlying compensation in macaque monkeys, the responses of vestibular-nerve afferent fibers on the contralesional side were recorded before and after unilateral labyrinthectomy (Sadeghi et al. 2007). Afferent responses were characterized during passive whole-body rotations, passive head-on-body rotations and active head-on-body rotations. To verify the functionality of efferent vestibular pathways in alert behaving macaques (Sadeghi et al. 2009), we also recorded efferent-mediated responses in single afferent fibers of alert macaques during rotations with trapezoidsal velocity profiles (peak velocity, 320 °/s) in both directions of rotation, similar to those used by Plotnik et al. in anesthetized animals (2002).
VOR Compensation in Normal versus Cerebellar-Deficient Mice
Finally, to investigate the contribution of the cerebellum to the compensation process, we compared the recovery of VOR responses in wild-type (WT) versus cerebellar-deficient Lurcher (Lc/+) mice (Beraneck et al. 2008). The lurcher strain is characterized by the early degeneration of the cerebellar cortex within the first 3 post-natal weeks and mice show pronounced ataxia among various other motor skill deficits (for review see Vogel et al. 2007). Thus, the Lc/+ provides a model of cerebellar cortex ablation (Van Alphen et al. 2002). To compare VOR compensation in wild-type (WT) and Lurcher mice, eye movements were recorded using the noninvasive video-oculography method (Beraneck and Cullen 2007; Stahl et al. 2000). As for monkeys, the VOR was characterized over the range of head movements encountered during natural activities, including yaw rotations (0.2-4Hz; 40 °/s) and transient impulses (∼400 °/s; ∼2,000 °/s2). Wt and Lc/+ mice were tested before the labyrinthectomy to obtain pre-lesion VOR data as well as on days 1, 5, 10, and 20 following the lesion.
Results and Interpretations
How well does the VOR compensate over the physiologically relevant range of head movements?
Unilateral labyrinthectomy produces a complete loss of inputs from vestibular end organs on one side and leads to profound static symptoms as well as changes in both the dynamics and symmetry of the VOR. Acutely, the static symptoms include involuntary eye movements with slow components toward the lesioned side and fast components toward the intact side (i.e., spontaneous nystagmus), as well as head tilt and falling toward the lesioned side. These static symptoms usually resolve within the first week following lesion. The VOR also shows remarkable recovery following the loss of unilateral labyrinthine input. Notably, for lower frequencies and accelerations of head movement, VOR response dynamics recover to normal values within 1 week (Allum et al. 1988; Curthoys and Halmagyi 1995; Fetter and Zee 1988; Lasker et al. 2000; Paige 1983; Sadeghi et al. 2006).
A main goal of research in our laboratory has been to understand how well the VOR compensates for peripheral loss over the full range of movements naturally encountered during daily life. For example, previous work by our lab and other laboratories has shown that the frequency content of head movements made during common activities such as running or making orienting head movements contain significant power up to frequencies of 20 Hz for both humans and monkeys (Armand and Minor 2001; Grossman et al. 1988; Huterer and Cullen 2002). Moreover, during these same movements head accelerations and velocities can easily reach 25,000 °/s2 and 400-500 °/s, respectively (Armand and Minor 2001; Huterer and Cullen 2002). Similarly, we have shown that the head rotations produced by mice during natural exploratory behaviors have frequency content of up to 20Hz, and reach accelerations of 10,000 °/s2 and velocities of up to 400 °/s (Beraneck et al. 2008).
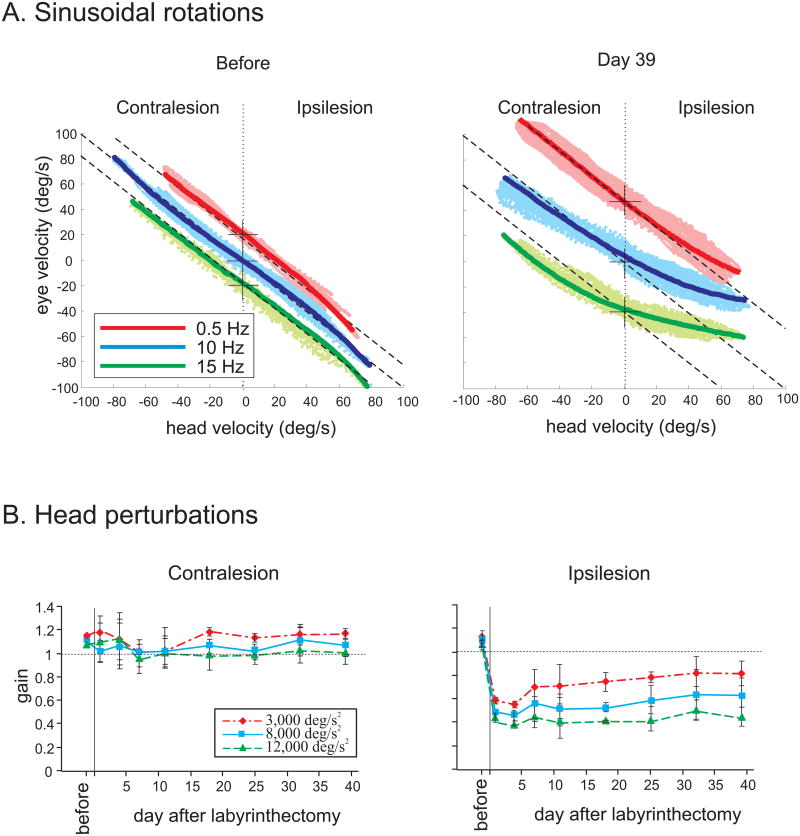
Under normal conditions (i.e., where vestibular function is fully intact), we have further shown that the VOR produces compensatory responses (i.e., gain of unity) over the full range of head movements encountered in daily life in both monkeys (Sadeghi et al. 2006) and mice (Beraneck et al. 2008). In contrast, following unilateral lesion in macaque monkeys, we found that although responses to sinusoidal rotations with low frequencies and velocities recovered in ∼1 month, compensation was not complete for the most challenging stimuli (Fig 1A) and response gains only reached values of ∼ 0.7 and 0.3 for contralesionally versus ipsilesionally-directed rotations, respectively (Fig 1B). Interestingly, this was the case regardless of the type of stimulus that was applied; responses with comparable gains were evoked by transient head-on-body perturbations with accelerations of ∼12,000 °/s2, sinusoidal whole body rotations with velocities of ∼500 °/s, and sinusoidal head-on-body rotations at 15 Hz with a peak velocity of 100 °/s. Moreover, lack of eye movements during body-under-head rotations showed that no neck inputs contributed to the generation of eye movements during head-on-body rotations. For these challenging stimuli, no significant compensation was observed in the responses to contralesionally or ipsilesionally-directed rotations beyond the first day that followed the lesion. This finding is illustrated for the VOR evoked after lesion by transient head-on-body perturbations in Fig 1B.
Figure 1.
Compensation in the VOR response following unilateral labyrinthectomy in macaque monkeys. A. Response to sinusoidal rotations at 0.5 (red), 10 (blue), and 15 (green) Hz (peak velocity of 40 °/s) before (left) and 39 days (right) after lesion. Traces are offset from their zero eye velocity (cross) for clearer presentation. Light colors show data points and the dark line is fitted to the points using the 3rd order equation. Dashed lines have a slope of -1 (i.e., perfect VOR response). Note that even at this late stage after labyrinthectomy, responses gains become increasingly attenuated with rising frequency for ipsilesional half-cycles. B. Mean VOR gain elicited by transient head perturbations before and at different days after lesion. Head perturbations reached peak accelerations of 3,000, 8,000, and 12,000 °/s2. Responses reach steady-state levels by around day 18. Error bars represent standard error of the mean.
The results of our parallel experiments in mice revealed even more pronounced deficits in VOR performance immediately following unilateral lesion. Notably, responses to ipsilaterally directed rotations were reduced by nearly 75% on day 1, and this value was constant across the physiologically-relevant range of head motion such that it did not improve when more moderate (i.e. less transient, lower acceleration/frequency) stimuli were applied (Beraneck et al. 2008). As in rhesus monkeys, the VOR evoked by contralesional responses was relatively less affected on day 1 (∼20% reduction), and recovery was nearly complete one week following lesion. Over the longer term, mice showed similar compensation to rhesus monkeys. Response gains recovered to ∼80% of control values for ipsilaterally directed rotations and showed close-to-normal response gains for contralesionally directed movements when tested with frequencies up to 4Hz at velocities of 40 °/s. However, when rapid impulses were applied to test responses to more dynamically challenging stimuli (∼2000 °/s2), we found limited recovery for contralesional, characterized by VOR gains of ∼0.7 rotations (Beraneck et al. 2008).
Thus our findings show that the vestibular system is not able to fully compensate following unilateral labyrinthectomy - particularly for higher frequency/acceleration rotations towards the contralesional side. We furthered this by testing whether a single model formulation of the response dynamics of the VOR pathway could predict the responses measured over our complete range of stimuli. To do this, we adapted a previously published model of the squirrel monkey VOR (Lasker et al. 2000) to our rhesus monkey data. Notably, a single parameter set could be used to account for VOR responses across the range of sinusoidal and transient head rotations that were applied indicating that there are no fundamental differences between responses to transient head rotations and sinusoidal head movements. Moreover, modeling the VOR compensation required not only changes in the properties of central cells, but also an increase in the gain of the inputs from the phasic peripheral pathway (i.e. irregular afferents). One possible mechanism that could underlie this latter change is a drive from the vestibular efferent system. This possibility is further discussed in the next section below.
Do changes at the level of the vestibular periphery mediate compensation or is compensation exclusively mediated by central processes?
Vestibular compensation is a distributed process which most likely includes changes not only at the level of the vestibular-related structures located throughout the brain, but also at the level of the vestibular periphery (i.e. hair cells and associated afferent fibers). Accordingly, the recovery in VOR which follows a unilateral labyrinthectomy is likely associated with modifications in i) the peripheral signals received by neurons in the vestibular nuclei, ii) the response dynamics of these same neurons, as well as iii) inputs from other central vestibular neurons including those in the cerebellum – a structure long known to play a critical role in VOR plasticity. In this and the following sections, we present recent experimental findings which establish the nature of changes that occur at the level of vestibular afferents/efferents and which document the vital role of the cerebellum in the vestibular compensation process.
The peripheral vestibular organs have long been known to receive a bilateral efferent innervation via a group of neurons located in the brainstem neighboring the abducens nucleus (Gacek and Lyon 1974; Goldberg and Fernandez 1980; Rasmussen and Gacek 1958). Prior neurophysiological studies have confirmed the integrity of these projections via electrical activation and rotational stimulation of efferents in anesthetized, decerebrate animals (Bricout-Berthout et al. 1984; Goldberg and Fernandez 1980; Plotnik et al. 2001; 2002) as well as alert fish (Boyle et al. 1991; Boyle and Highstein 1990; Highstein and Baker 1985). Furthermore, previous studies have provided evidence for compensatory changes in Scarpa's ganglion neuronal proteins following unilateral labyrinthectomy (Kitahara et al. 2007). Nevertheless, the functional role of the vestibular efferent system however has remained poorly understood. Accordingly, in our studies we tested whether the vestibular efferent system might play a role in the long-term changes associated with compensation following unilateral labyrinthectomy over the range of natural head movements. For example, an efferent-mediated increase in the resting discharge and a decrease in the sensitivity of afferents could theoretically result in an increase in the working range of the vestibular afferents in the intact side (see also: Cullen and Minor 2002).
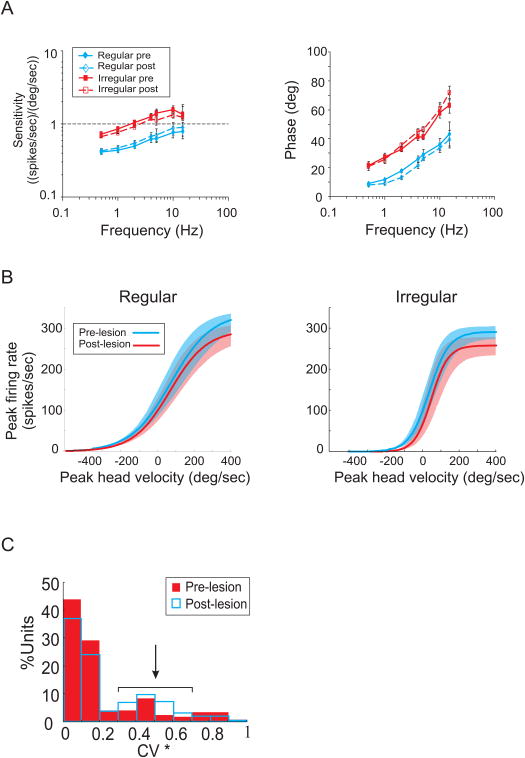
We first demonstrated that efferent-mediated rotational responses could be obtained from vestibular-nerve fibers innervating the semicircular canals in alert behaving macaque monkeys (Sadeghi et al. 2009). Notably, we found that such responses were larger in irregular afferents (CV*>0.1, Goldberg et al. 1984) compared to regular afferents (CV*<0.1, Goldberg et al. 1984), were excitatory, and were of vestibular origin. Next, to address whether efferent inputs might mediate compensatory changes in the activity of afferents following vestibular lesion we investigated the long-term changes associated with compensation following unilateral labyrinthectomy. Afferent responses on the contralesional side were recorded before and after unilateral labyrinthectomy for stimulation over the frequency range where compensation is the most complete (i.e. < 4 Hz), as well as for more challenging (i.e., higher frequency/acceleration) stimulation (Sadeghi et al. 2007). We found that response sensitivities and phases were comparable before and after lesion regardless of the stimulation that was applied. This finding is illustrated in Fig 2A; the responses of regular and C irregular afferents to sinusoidal head-on-body rotations are comparable before and after lesion (t-test, p> 0.4 for all groups). Furthermore, we established that all classes of vestibular-nerve afferents were driven into inhibitory cutoff or excitatory saturation at similar velocities before and after lesion (t-test, p> 0.8 for all groups) (Fig 2B).
Figure 2.
Response of vestibular-nerve afferents innervating the semicircular canals before and after unilateral labyrinthectomy in macaque monkeys. A. Response of afferents as a function of frequency. Sensitivity and phase lead(relative to velocity) of responses during sinusoidal rotations with frequencies of 0.5 to 15 Hz (±50°/s) before (continuous lines, filled symbols) and after (dashed lines, empty symbols) labyrinthectomy. B. Average response of irregular and regular afferents during rotations with velocities of up to 500 °/s, before (blue lines) and after lesion (red lines). Light colored areas represent the SE of the responses. Differences in each group were not significant under the two conditions. C. Comparison of the distribution of afferents based on the regularity of the resting discharge (measured by the normalized coefficient of variation, CV*) before (filled red bars) and after (empty blue bars) lesion. There was a slight increase in the percentage of irregular afferents following lesion.
The findings of prior investigations in frog had suggested that extravestibular signals (e.g., somatosensory, proprioceptive, and/or motor efference copy signals) transmitted through efferent neurons can be used to change afferent responses under specific behavioral conditions (Bricout-Berthout et al. 1984; Caston and Bricout-Berthout 1984; Precht et al. 1971; Schmidt 1963). Thus, we also tested this possibility by characterizing afferent responses to such extravestibular signals during passive stimulation of neck somatosensory and proprioceptive inputs, as well as during active head movements during which a command to move the head was produced (Sadeghi et al. 2007). We found that in primates, unlike frog, the efferent system does not encode extravestibular information that influences the response of vestibular afferents. The responses of afferents on the contralesional side were completely insensitive to each of the aforementioned extravestibular signals both before and after unilateral labyrinthectomy.
Strikingly, however, we did observe a change in the overall distribution of afferent types after unilateral labyrinthectomy (Sadeghi et al. 2007). This change was characterized by an increase in the proportion of irregular afferents (z statistics, p< 0.001) and a decrease in the proportion of regular afferents (z statistics, p< 0.01) (Fig 2C). Given that irregular afferents have more phasic responses than do regular afferents, this result suggests a re-weighting of phasic versus tonic input from the periphery to the central pathways after lesion. The most plausible mechanism underlying this change is a modification of the responses of regular dimorphic afferents, which contact both type I and type II hair cells peripherally and comprise the majority of regular afferents in intact animals (Baird et al. 1988). This is supported by recent studies showing that the discharge regularity of afferents can be modulated through activation of GABAB receptors (Holstein et al. 2004a; Holstein et al. 2004b), which are predominately expressed in the efferent nerve endings (Kitahara et al. 1994; Kong et al. 1998a; b; Usami et al. 1987).
The role of the cerebellum in the VOR compensation - lessons from cerebellar-deficient mice: What role does the vestibular cerebellum play in VOR compensation?
The vestibulo-cerebellar pathway effectively provides a parallel inhibitory side loop which can modulate the gain of the direct VOR pathway (Fukuda et al. 1972; Highstein 1973; Ito et al. 1977; Sato et al. 1988). However, while the vestibular cerebellum's role in motor leaning is well established (reviewed in: Broussard and Kassardjian 2004), only a few investigations have explicitly addressed whether this region plays a comparable role in the VOR compensation process following peripheral lesions. The findings of lesion studies in cats (Courjon et al. 1982; Haddad et al. 1977) and rats (Kim et al. 1997a; b; Kitahara et al. 2000; Kitahara et al. 1997; Kitahara et al. 1998) suggest a role of the cerebellum in the resolution of static symptoms and initiation of behavioral recovery. However, the contribution of the vestibular cerebellum to the VOR recovery across the full range of physiologically significant head movements, its implication in restoration of VOR during ipsi- versus contralesionally directed movements, and the precise time course of its contribution had, until recently, remained open questions (reviewed in: Darlington and Smith 2000).
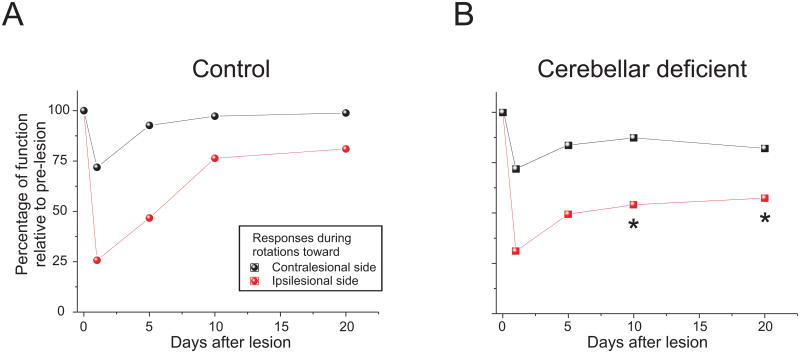
To investigate these issues, we recently took advantage of a well characterized strain of cerebellar deficient mice (i.e. the Lurcher (Lc/+) mouse; (Aleisa et al. 2007; Beraneck et al. 2008). In normal (i.e. wild-type (WT) mice), most of the VOR recovery observed during compensation was achieved by day 10 after unilateral labyrinthectomy such that ∼80% of pre-lesion gain was restored for ipsilesionally directed movements and ∼100 % for contralesionally directed movements (Fig 3A). While the VOR of Lurcher mice was similar to that of WT before lesion, we found that recovery following unilateral labyrinthectomy was compromised (Fig 3B). Notably, recovery in Lurcher mice followed the same time course as that of WT for the first 5 days but then plateaued in response to ipsilesionally directed movements, remaining at less than 60% of pre-lesion values (p<0.05 at day 10 and 20). In response to contralesionally directed rotations, Lurcher mice showed up to ∼80% recovery in VOR gain such that relative improvement was comparable to that observed for WT mice (p>0.3 for all tested time points and conditions). Taken together, our findings show that cerebellar pathways are critical for the long-term restoration of the VOR during head movements directed toward the lesioned side, whereas non-cerebellar pathways are sufficient to restore proper gaze stabilization during contralesionally directed head movements in cerebellar-deficient mice.
Figure 3.
Compensation in the VOR response following unilateral labyrinthectomy in mice during sinusoidal rotations. A. Normalized VOR gain (re. prelesion value) in control mice. For both contra- and ipsilesional rotations, VOR responses showed compensation, reaching normal values in about 10 days. B. Normalized VOR gain (re. prelesion value) in cerebellar deficient mice. While contralesional VOR responses reached normal values in ∼10 days, ipsilesional responses did not show an improvement after the first week and remained lower than normal. Asterisks on B show significant differences between control and cerebellar deficient mice (p< 0.05).
The results of previous investigations of vestibular compensation in cerebellar mutant mice differ from our study in several aspects (Faulstich et al. 2006; Funabiki et al. 1995; Kitahara et al. 1998; Murai et al. 2004). First, Faulstich et al. (2006) proposed that the vestibular compensation process initially depends on an intact cerebellar circuitry. However, in their experiments these investigators were only able to quantify recovery of VOR during the first 5 days following unilateral vestibular damage (see Faulstich et al. 2006; Beraneck et al. 2008 for a discussion). In particular, their use of scleral search coils, which are known to reduce the gain and timing of the reflex in mouse (Van Alphen et al. 2002), made longer duration experiments unfeasible. In contrast, in our studies we used video-oculography and thus were able to follow vestibular compensation in WT and Lc/+ over a ∼3 week period thereby allowing the characterization of chronic as well as acute compensation processes. While, the difference between the two studies could partly be related to the different types of lesion performed, our results suggest that the cerebellum is more instrumental for the long term recovery of the VOR.
Second, our study was the first to describe the asymmetry inherent to the recovery of VOR responses following labyrinthectomy. Previous studies had reported response gains which were calculated by averaging across ipsilesionally- and contralesionally-directed rotations – a process which would have effectively reduced the apparent differences between strains (Faulstich et al. 2006; Funabiki et al. 1995; Kitahara et al. 1998; Murai et al. 2004). Third, another important consideration is the important differences between the mutant strains that have been tested across studies. Notably, while Murai et al. (2004) described delayed but complete recovery of VOR in the cerebellar deficient GLURδ2 (δ2 -/-) mutant, it is important to note that GLURδ2 mice do not lack Purkinje cells (as the Lurcher mutant). Instead GLURδ2 mice have defects in Purkinje cell synapse formation. This difference most likely underlies the apparent discrepancy between our results and those from studies of GLURδ2 mice.
Finally, it is noteworthy that the plateau in recovery that we reported for VOR in Lc/+ mice during ipsilesionally directed movements was also qualitatively similar to that reported for the vestibulo-spinal reflex studied in another cerebellar deficient strain (the GLURδ2 knock-out mouse; δ2 -/-; Funabiki et al. 1995). This correspondence suggests that cerebellar regions (e.g. nodulus uvula) implicated in other vestibular related reflexes (Barmack 2003) could play a role in the compensation of balance similar to that of flocculus in VOR recovery. Note that other extravestibular pathways, such as proprioceptive inputs from the spinal cord (Straka and Dieringer 1995), could potentially also contribute to the behavioral compensation observed following vestibular lesions.
General Discussion
VOR compensation following unilateral lesion is relatively complete within the first month after lesion and provides a simple model for studying post lesional plasticity and its neural correlates. Prior investigations had characterized VOR compensation in a variety of mammalian species including humans, monkeys, cats, and guinea pigs (Fetter and Zee 1988; Galiana et al. 2001; Lasker et al. 2000; Maioli et al. 1983; Paige 1983; Smith and Curthoys 1989). These studies showed that while the VOR is compensated for rotations towards the contralesional side, it remains incomplete for rotations towards the lesioned side. Important differences across studies had led to the suggestion that deficits in the VOR are more pronounced in response to impulses than to sinusoids (see: Gilchrist et al. 1998). However, our recent behavioural results in monkeys and mice indicate that this is not the case. In our studies we were able to address these apparent discrepancies by applying an extensive range of stimuli, with dynamics that extended over the full range of natural head movements. In monkeys, long term deficits occurred at the higher end of accelerations, velocities, or frequencies of rotation, independent of the type of stimulus. In mouse our findings were even more striking; the time course of recovery was relatively consistent across the range of stimuli that were tested.
While changes at the level of the central vestibular system are known to contribute to VOR compensation following unilateral labyrinthectomy (see review: Smith and Curthoys 1989), whether changes at the level of the vestibular periphery also contribute to compensation had remained unclear. Our single unit recording results suggest a small contribution at the level of the vestibular periphery with an increase in the proportion of irregular afferents compared to regular afferents on the contralesional side. These findings are consistent with the predictions of previous studies, which have proposed an enhancement of the phasic properties of both the afferents and central vestibular neurons located on the contralesional side after lesion. The resulting modification of the pathway response dynamics is likely to be an important component of the long term compensation process for high frequency/acceleration movements. Interestingly, similar changes to the proportion of irregular/regular afferents parallel the changes observed in central vestibular neurons recorded in slices one month after unilateral labyrinthectomy: synaptic and intrinsic membrane properties of central neurons co-adapted in a way to produce more phasic discharge properties in the contralesional vestibular nuclei (Beraneck and Cullen 2007; Beraneck et al. 2004; Pfanzelt et al. 2008; Straka et al. 2005).
Our results in cerebellar deficient Lurcher mice further indicate that vestibular pathways through the cerebellum also have a significant role in the long term restoration of VOR observed following unilateral lesions. However, while recovery was impaired in Lurcher mice, compensation was not completely abolished demonstrating that cerebellar-independent mechanisms play an important role in the recovery of the VOR. As such, we suggest that the adaptive changes during motor learning as well as compensatory mechanisms following UL are generated independently of the cerebellum, but the intensity of these modifications is cerebellar dependant. Support for this idea also comes from studies by Porras-Garcia and colleagues (2005) using classical conditioning eye blinks in Lurcher mice, which were still capable of motor learning, but with reduced performances of the generated conditioned response.
Prior work had shown substantial changes in the intrinsic membrane properties of central vestibular neurons (Beraneck et al. 2003; Beraneck et al. 2004; Cameron and Dutia 1997; 1999; Darlington et al. 2002; Him and Dutia 2001; Ris et al. 2002; Straka et al. 2005), and synaptic reorganization of the brainstem vestibular pathways (Dieringer 2003; Goto et al. 2000; 2002; 2001; Johnston et al. 2001; Vibert et al. 2000; Yamanaka et al. 2000) following unilateral labyrinthectomy. Notably, Johnston and colleagues (2002) have suggested that the increase in intrinsic excitability that develops in ipsilesional vestibular nuclei neurons during vestibular compensation is cerebellar-dependent, while the concurrent down-regulation of functional efficacy of GABA receptors is not. Because the most dramatic changes in intrinsic membrane properties occur more than one week after unilateral labyrinthectomy (Beraneck et al. 2003; Beraneck et al. 2004; Vibert et al. 1999), we propose that the plateau we observe in the Lurcher's recovery (i.e., after week 1) reflects the absence of floccular-mediated effects on the intrinsic membrane properties of the deafferented neurons.
Conclusion
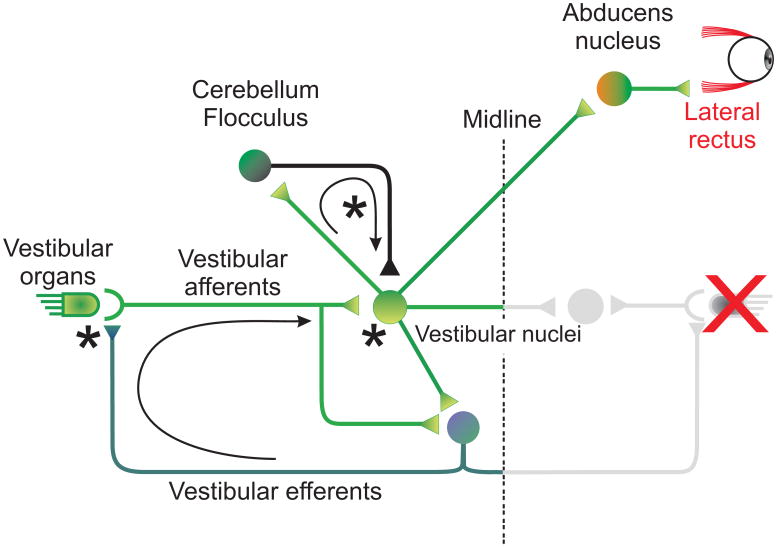
Here, we focused on the contributions of the peripheral vestibular system (vestibular nerve afferents and efferents) and the vestibular-cerebellar pathway to the recovery of the VOR following unilateral labyrinthectomy. Sites in which we demonstrate the neural substrates underlying vestibular compensation are indicated by stars in schematic shown in Figure 4. Our studies were carried out using two different animal models: macaque monkeys and mice, and provided evidence that the loss of vestibular nerve input triggers a cascade of events along the VOR pathway to facilitate compensation over the physiologically relevant range of head movements. We first provided evidence that the VOR does not fully compensate over the physiologically relevant range of head movements - responses to rotations towards the side of the lesion can remain substantially attenuated in both monkeys and mice. Next, we addressed the question of whether significant changes at the level of the vestibular periphery mediate compensation, or if instead compensation is exclusively mediated by central processes. Our findings provide evidence that, after lesion, the distribution of vestibular afferents shifts such that, on average, afferents have more phasic properties. We suggest that this change is mediated through the efferent vestibular system and may contribute to driving concurrent changes that have been measured in the intrinsic membrane properties of central vestibular neurons (reviewed in: Straka et al. 2005). In turn the altered response dynamics of the VOR pathway are likely to support of the long term compensation during high frequency /acceleration rotations. Finally, we considered the role of the vestibular cerebellum in VOR compensation and showed that compensation is very limited in cerebellar-deficient mice. Overall, the results of our recent investigations indicate that vestibular compensation is a process consisting of plastic changes at several locations in the VOR pathway – notably, changes at both peripheral and central levels are likely to make important contributions to the recovery of the VOR that is observed in behavioral and clinical studies.
Figure 4.
Direct and indirect VOR pathways. Vestibular nuclei receive direct inputs from the vestibular-nerve afferents, as well as indirect inputs from the cerebellum and contralateral vestibular nuclei. Signals cross the midline to the abducens neurons on the other side, which are then transmitted to the eye muscles. A feedback loop carries signals through the vestibular efferents from the vestibular nuclei to the periphery.
Acknowledgments
We thank Diana Mitchell and Mohsen Jamali for critically reading the manuscript. This work was supported by grants from the Canadian Institutes of Health Research (CIHR) to KEC and NIH R01 DC02390 to KEC and LBM. MB was supported by the Centre National d'Etudes Spatiales.
References
- Aleisa M, Zeitouni AG, Cullen KE. Vestibular compensation after unilateral labyrinthectomy: normal versus cerebellar dysfunctional mice. J Otolaryngol. 2007;36:315–321. [PubMed] [Google Scholar]
- Allum JH, Yamane M, Pfaltz CR. Long-term modifications of vertical and horizontal vestibuloocular reflex dynamics in man. I. After acute unilateral peripheral vestibular paralysis. Acta Otolaryngol. 1988;105:328–337. doi: 10.3109/00016488809097015. [DOI] [PubMed] [Google Scholar]
- Angelaki DE, Cullen KE. Vestibular system: the many facets of a multimodal sense. Annu Rev Neurosci. 2008;31:125–150. doi: 10.1146/annurev.neuro.31.060407.125555. [DOI] [PubMed] [Google Scholar]
- Armand M, Minor LB. Relationship between time- and frequency-domain analyses of angular head movements in the squirrel monkey. J Comput Neurosci. 2001;11:217–239. doi: 10.1023/a:1013771014232. [DOI] [PubMed] [Google Scholar]
- Baird RA, Desmadryl G, Fernandez C, Goldberg JM. The vestibular nerve of the chinchilla. II. Relation between afferent response properties and peripheral innervation patterns in the semicircular canals. J Neurophysiol. 1988;60:182–203. doi: 10.1152/jn.1988.60.1.182. [DOI] [PubMed] [Google Scholar]
- Barmack NH. Central vestibular system: vestibular nuclei and posterior cerebellum. Brain Res Bull. 2003;60:511–541. doi: 10.1016/s0361-9230(03)00055-8. [DOI] [PubMed] [Google Scholar]
- Beraneck M, Cullen KE. Activity of vestibular nuclei neurons during vestibular and optokinetic stimulation in the alert mouse. J Neurophysiol. 2007;98:1549–1565. doi: 10.1152/jn.00590.2007. [DOI] [PubMed] [Google Scholar]
- Beraneck M, Hachemaoui M, Idoux E, Ris L, Uno A, Godaux E, Vidal PP, Moore LE, Vibert N. Long-term plasticity of ipsilesional medial vestibular nucleus neurons after unilateral labyrinthectomy. J Neurophysiol. 2003;90:184–203. doi: 10.1152/jn.01140.2002. [DOI] [PubMed] [Google Scholar]
- Beraneck M, Idoux E, Uno A, Vidal PP, Moore LE, Vibert N. Unilateral labyrinthectomy modifies the membrane properties of contralesional vestibular neurons. J Neurophysiol. 2004;92:1668–1684. doi: 10.1152/jn.00158.2004. [DOI] [PubMed] [Google Scholar]
- Beraneck M, McKee JL, Aleisa M, Cullen KE. Asymmetric recovery in cerebellar-deficient mice following unilateral labyrinthectomy. J Neurophysiol. 2008;100:945–958. doi: 10.1152/jn.90319.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle R, Carey JP, Highstein SM. Morphological correlates of response dynamics and efferent stimulation in horizontal semicircular canal afferents of the toadfish, Opsanus tau. J Neurophysiol. 1991;66:1504–1521. doi: 10.1152/jn.1991.66.5.1504. [DOI] [PubMed] [Google Scholar]
- Boyle R, Highstein SM. Efferent vestibular system in the toadfish: action upon horizontal semicircular canal afferents. J Neurosci. 1990;10:1570–1582. doi: 10.1523/JNEUROSCI.10-05-01570.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricout-Berthout A, Caston J, Reber A. Influence of stimulation of auditory and somatosensory systems on the activity of vestibular nuclear neurons in the frog. Brain Behav Evol. 1984;24:21–34. doi: 10.1159/000121302. [DOI] [PubMed] [Google Scholar]
- Broussard DM, Kassardjian CD. Learning in a simple motor system. Learn Mem. 2004;11:127–136. doi: 10.1101/lm.65804. [DOI] [PubMed] [Google Scholar]
- Cameron SA, Dutia MB. Cellular basis of vestibular compensation: changes in intrinsic excitability of MVN neurones. Neuroreport. 1997;8:2595–2599. doi: 10.1097/00001756-199707280-00035. [DOI] [PubMed] [Google Scholar]
- Cameron SA, Dutia MB. Lesion-induced plasticity in rat vestibular nucleus neurones dependent on glucocorticoid receptor activation. J Physiol. 1999;518(Pt 1):151–158. doi: 10.1111/j.1469-7793.1999.0151r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caston J, Bricout-Berthout A. Responses to somatosensory input by afferent and efferent neurons in the vestibular nerve of the frog. Brain Behav Evol. 1984;24:135–143. doi: 10.1159/000121311. [DOI] [PubMed] [Google Scholar]
- Courjon JH, Flandrin JM, Jeannerod M, Schmid R. The role of the flocculus in vestibular compensation after hemilabyrinthectomy. Brain Res. 1982;239:251–257. doi: 10.1016/0006-8993(82)90847-2. [DOI] [PubMed] [Google Scholar]
- Cullen KE, Minor LB. Semicircular canal afferents similarly encode active and passive head-on-body rotations: implications for the role of vestibular efference. J Neurosci. 2002;22:RC226. doi: 10.1523/JNEUROSCI.22-11-j0002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curthoys IS, Halmagyi GM. Vestibular compensation: a review of the oculomotor, neural, and clinical consequences of unilateral vestibular loss. J Vestib Res. 1995;5:67–107. [PubMed] [Google Scholar]
- Darlington CL, Dutia MB, Smith PF. The contribution of the intrinsic excitability of vestibular nucleus neurons to recovery from vestibular damage. Eur J Neurosci. 2002;15:1719–1727. doi: 10.1046/j.1460-9568.2002.02024.x. [DOI] [PubMed] [Google Scholar]
- Darlington CL, Smith PF. Molecular mechanisms of recovery from vestibular damage in mammals: recent advances. Prog Neurobiol. 2000;62:313–325. doi: 10.1016/s0301-0082(00)00002-2. [DOI] [PubMed] [Google Scholar]
- Dieringer N. Activity-related postlesional vestibular reorganization. Ann N Y Acad Sci. 2003;1004:50–60. [PubMed] [Google Scholar]
- Faulstich M, van Alphen AM, Luo C, du Lac S, De Zeeuw CI. Oculomotor plasticity during vestibular compensation does not depend on cerebellar LTD. J Neurophysiol. 2006;96:1187–1195. doi: 10.1152/jn.00045.2006. [DOI] [PubMed] [Google Scholar]
- Fetter M, Zee DS. Recovery from unilateral labyrinthectomy in rhesus monkey. J Neurophysiol. 1988;59:370–393. doi: 10.1152/jn.1988.59.2.370. [DOI] [PubMed] [Google Scholar]
- Fukuda J, Highstein SM, Ito M. Cerebellar inhibitory control of the vestibulo-ocular reflex investigated in rabbit 3rd nucleus. Exp Brain Res. 1972;14:511–526. doi: 10.1007/BF00236593. [DOI] [PubMed] [Google Scholar]
- Funabiki K, Mishina M, Hirano T. Retarded vestibular compensation in mutant mice deficient in delta 2 glutamate receptor subunit. Neuroreport. 1995;7:189–192. [PubMed] [Google Scholar]
- Gacek RR, Lyon M. The localization of vestibular efferent neurons in the kitten with horseradish peroxidase. Acta Otolaryngol. 1974;77:92–101. doi: 10.3109/00016487409124603. [DOI] [PubMed] [Google Scholar]
- Galiana HL, Smith HL, Katsarkas A. Modelling non-linearities in the vestibulo-ocular reflex (VOR) after unilateral or bilateral loss of peripheral vestibular function. Exp Brain Res. 2001;137:369–386. doi: 10.1007/s002210000667. [DOI] [PubMed] [Google Scholar]
- Gilchrist DP, Curthoys IS, Cartwright AD, Burgess AM, Topple AN, Halmagyi M. High acceleration impulsive rotations reveal severe long-term deficits of the horizontal vestibulo-ocular reflex in the guinea pig. Exp Brain Res. 1998;123:242–254. doi: 10.1007/s002210050566. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Fernandez C. Efferent vestibular system in the squirrel monkey: anatomical location and influence on afferent activity. J Neurophysiol. 1980;43:986–1025. doi: 10.1152/jn.1980.43.4.986. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Smith CE, Fernandez C. Relation between discharge regularity and responses to externally applied galvanic currents in vestibular nerve afferents of the squirrel monkey. J Neurophysiol. 1984;51:1236–1256. doi: 10.1152/jn.1984.51.6.1236. [DOI] [PubMed] [Google Scholar]
- Goto F, Straka H, Dieringer N. Expansion of afferent vestibular signals after the section of one of the vestibular nerve branches. J Neurophysiol. 2000;84:581–584. doi: 10.1152/jn.2000.84.1.581. [DOI] [PubMed] [Google Scholar]
- Goto F, Straka H, Dieringer N. Gradual and reversible central vestibular reorganization in frog after selective labyrinthine nerve branch lesions. Exp Brain Res. 2002;147:374–386. doi: 10.1007/s00221-002-1266-7. [DOI] [PubMed] [Google Scholar]
- Goto F, Straka H, Dieringer N. Postlesional vestibular reorganization in frogs: evidence for a basic reaction pattern after nerve injury. J Neurophysiol. 2001;85:2643–2646. doi: 10.1152/jn.2001.85.6.2643. [DOI] [PubMed] [Google Scholar]
- Grossman GE, Leigh RJ, Abel LA, Lanska DJ, Thurston SE. Frequency and velocity of rotational head perturbations during locomotion. Exp Brain Res. 1988;70:470–476. doi: 10.1007/BF00247595. [DOI] [PubMed] [Google Scholar]
- Haddad GM, Friendlich AR, Robinson DA. Compensation of nystagmus after VIIIth nerve lesions in vestibulo-cerebellectomized cats. Brain Res. 1977;135:192–196. doi: 10.1016/0006-8993(77)91066-6. [DOI] [PubMed] [Google Scholar]
- Highstein SM. Synaptic linkage in the vestibulo-ocular and cerebello-vestibular pathways to the VIth nucleus in the rabbit. Exp Brain Res. 1973;17:301–314. doi: 10.1007/BF00234668. [DOI] [PubMed] [Google Scholar]
- Highstein SM, Baker R. Action of the efferent vestibular system on primary afferents in the toadfish, Opsanus tau. J Neurophysiol. 1985;54:370–384. doi: 10.1152/jn.1985.54.2.370. [DOI] [PubMed] [Google Scholar]
- Him A, Dutia MB. Intrinsic excitability changes in vestibular nucleus neurons after unilateral deafferentation. Brain Res. 2001;908:58–66. doi: 10.1016/s0006-8993(01)02600-2. [DOI] [PubMed] [Google Scholar]
- Holstein GR, Martinelli GP, Henderson SC, Friedrich VL, Jr, Rabbitt RD, Highstein SM. Gamma-aminobutyric acid is present in a spatially discrete subpopulation of hair cells in the crista ampullaris of the toadfish Opsanus tau. J Comp Neurol. 2004a;471:1–10. doi: 10.1002/cne.11025. [DOI] [PubMed] [Google Scholar]
- Holstein GR, Rabbitt RD, Martinelli GP, Friedrich VL, Jr, Boyle RD, Highstein SM. Convergence of excitatory and inhibitory hair cell transmitters shapes vestibular afferent responses. Proc Natl Acad Sci U S A. 2004b;101:15766–15771. doi: 10.1073/pnas.0402824101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huterer M, Cullen KE. Vestibuloocular reflex dynamics during high-frequency and high-acceleration rotations of the head on body in rhesus monkey. J Neurophysiol. 2002;88:13–28. doi: 10.1152/jn.2002.88.1.13. [DOI] [PubMed] [Google Scholar]
- Ito M, Nisimaru N, Yamamoto M. Specific patterns of neuronal connexions involved in the control of the rabbit's vestibulo-ocular reflexes by the cerebellar flocculus. J Physiol. 1977;265:833–854. doi: 10.1113/jphysiol.1977.sp011747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston AR, Him A, Dutia MB. Differential regulation of GABA(A) and GABA(B) receptors during vestibular compensation. Neuroreport. 2001;12:597–600. doi: 10.1097/00001756-200103050-00033. [DOI] [PubMed] [Google Scholar]
- Johnston AR, Seckl JR, Dutia MB. Role of the flocculus in mediating vestibular nucleus neuron plasticity during vestibular compensation in the rat. J Physiol. 2002;545:903–911. doi: 10.1113/jphysiol.2002.024281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MS, Jin BK, Chun SW, Lee MY, Lee SH, Kim JH, Park BR. Effect of MK801 on cFos-like protein expression in the medial vestibular nucleus at early stage of vestibular compensation in uvulonodullectomized rats. Neurosci Lett. 1997a;231:147–150. doi: 10.1016/s0304-3940(97)00550-8. [DOI] [PubMed] [Google Scholar]
- Kim MS, Jin BK, Chun SW, Lee MY, Lee SH, Kim JH, Park BR. Role of vestibulocerebellar N-methyl-D-aspartate receptors for behavioral recovery following unilateral labyrinthectomy in rats. Neurosci Lett. 1997b;222:171–174. doi: 10.1016/s0304-3940(97)13371-7. [DOI] [PubMed] [Google Scholar]
- Kitahara T, Fukushima M, Takeda N, Saika T, Kubo T. Effects of pre-flocculectomy on Fos expression and NMDA receptor-mediated neural circuits in the central vestibular system after unilateral labyrinthectomy. Acta Otolaryngol. 2000;120:866–871. doi: 10.1080/000164800750061741. [DOI] [PubMed] [Google Scholar]
- Kitahara T, Horii A, Kizawa K, Maekawa C, Kubo T. Changes in mitochondrial uncoupling protein expression in the rat vestibular nerve after labyrinthectomy. Neurosci Res. 2007;59:237–242. doi: 10.1016/j.neures.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Kitahara T, Takeda N, Ohno K, Araki T, Kubo T, Kiyama H. Expression of GABAA receptor gamma 1 and gamma 2 subunits in the peripheral vestibular system of the rat. Brain Res. 1994;650:157–160. doi: 10.1016/0006-8993(94)90220-8. [DOI] [PubMed] [Google Scholar]
- Kitahara T, Takeda N, Saika T, Kubo T, Kiyama H. Role of the flocculus in the development of vestibular compensation: immunohistochemical studies with retrograde tracing and flocculectomy using Fos expression as a marker in the rat brainstem. Neuroscience. 1997;76:571–580. doi: 10.1016/s0306-4522(96)00374-0. [DOI] [PubMed] [Google Scholar]
- Kitahara T, Takeda N, Uno A, Kubo T, Mishina M, Kiyama H. Unilateral labyrinthectomy downregulates glutamate receptor delta-2 expression in the rat vestibulocerebellum. Brain Res Mol Brain Res. 1998;61:170–178. doi: 10.1016/s0169-328x(98)00228-9. [DOI] [PubMed] [Google Scholar]
- Kong WJ, Hussl B, Thumfart WF, Schrott-Fischer A. Ultrastructural localization of GABA-like immunoreactivity in the human utricular macula. Hear Res. 1998a;119:104–112. doi: 10.1016/s0378-5955(98)00034-3. [DOI] [PubMed] [Google Scholar]
- Kong WJ, Hussl B, Thumfart WF, Schrott-Fischer A. Ultrastructural localization of GABA-like immunoreactivity in the vestibular periphery of the rat. Acta Otolaryngol. 1998b;118:90–95. doi: 10.1080/00016489850155198. [DOI] [PubMed] [Google Scholar]
- Lasker DM, Hullar TE, Minor LB. Horizontal vestibuloocular reflex evoked by high-acceleration rotations in the squirrel monkey. III. Responses after labyrinthectomy. J Neurophysiol. 2000;83:2482–2496. doi: 10.1152/jn.2000.83.5.2482. [DOI] [PubMed] [Google Scholar]
- Maioli C, Precht W, Ried S. Short- and long-term modifications of vestibulo-ocular response dynamics following unilateral vestibular nerve lesions in the cat. Exp Brain Res. 1983;50:259–274. doi: 10.1007/BF00239190. [DOI] [PubMed] [Google Scholar]
- Murai N, Tsuji J, Ito J, Mishina M, Hirano T. Vestibular compensation in glutamate receptor delta-2 subunit knockout mice: dynamic property of vestibulo-ocular reflex. Eur Arch Otorhinolaryngol. 2004;261:82–86. doi: 10.1007/s00405-003-0644-5. [DOI] [PubMed] [Google Scholar]
- Paige GD. Vestibuloocular reflex and its interactions with visual following mechanisms in the squirrel monkey. II. Response characteristics and plasticity following unilateral inactivation of horizontal canal. J Neurophysiol. 1983;49:152–168. doi: 10.1152/jn.1983.49.1.152. [DOI] [PubMed] [Google Scholar]
- Pfanzelt S, Rossert C, Rohregger M, Glasauer S, Moore LE, Straka H. Differential dynamic processing of afferent signals in frog tonic and phasic second-order vestibular neurons. J Neurosci. 2008;28:10349–10362. doi: 10.1523/JNEUROSCI.3368-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnik M, Marlinski V, Goldberg JM. Efferent-mediated binaural interactions between the vestibular end-organs in the chinchilla. Ann N Y Acad Sci. 2001;942:479–481. doi: 10.1111/j.1749-6632.2001.tb03774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnik M, Marlinski V, Goldberg JM. Reflections of efferent activity in rotational responses of chinchilla vestibular afferents. J Neurophysiol. 2002;88:1234–1244. doi: 10.1152/jn.2002.88.3.1234. [DOI] [PubMed] [Google Scholar]
- Porras-Garcia E, Cendelin J, Dominguez-del-Toro E, Vozeh F, Delgado-Garcia JM. Purkinje cell loss affects differentially the execution, acquisition and prepulse inhibition of skeletal and facial motor responses in Lurcher mice. Eur J Neurosci. 2005;21:979–988. doi: 10.1111/j.1460-9568.2005.03940.x. [DOI] [PubMed] [Google Scholar]
- Precht W, Llinas R, Clarke M. Physiological responses of frog vestibular fibers to horizontal angular rotation. Exp Brain Res. 1971;13:378–407. doi: 10.1007/BF00234338. [DOI] [PubMed] [Google Scholar]
- Rasmussen GL, Gacek RR. Concerning the question of the efferent fiber component of the vestibular nerve of the cat. Anat Rec. 1958;130:361–362. [Google Scholar]
- Ris L, Hachemaoui M, Godaux E. Effect of labyrinthectomy on the spike generator of vestibular neurons in the guinea pig. Neuroreport. 2002;13:1875–1879. doi: 10.1097/00001756-200210280-00009. [DOI] [PubMed] [Google Scholar]
- Sadeghi SG, Goldberg JM, Minor LB, Cullen KE. Efferent-mediated responses in vestibular nerve afferents of the alert macaque. J Neurophysiol. 2009;101:988–1001. doi: 10.1152/jn.91112.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi SG, Minor LB, Cullen KE. Dynamics of the horizontal vestibuloocular reflex after unilateral labyrinthectomy: response to high frequency, high acceleration, and high velocity rotations. Exp Brain Res. 2006;175:471–484. doi: 10.1007/s00221-006-0567-7. [DOI] [PubMed] [Google Scholar]
- Sadeghi SG, Minor LB, Cullen KE. Response of vestibular-nerve afferents to active and passive rotations under normal conditions and after unilateral labyrinthectomy. J Neurophysiol. 2007;97:1503–1514. doi: 10.1152/jn.00829.2006. [DOI] [PubMed] [Google Scholar]
- Sato Y, Kanda K, Kawasaki T. Target neurons of floccular middle zone inhibition in medial vestibular nucleus. Brain Res. 1988;446:225–235. doi: 10.1016/0006-8993(88)90881-5. [DOI] [PubMed] [Google Scholar]
- Schmidt RS. Frog labyrinthine efferent impulses. Acta Otolaryngol. 1963;56:51–64. doi: 10.3109/00016486309127679. [DOI] [PubMed] [Google Scholar]
- Smith PF, Curthoys IS. Mechanisms of recovery following unilateral labyrinthectomy: a review. Brain Res Brain Res Rev. 1989;14:155–180. doi: 10.1016/0165-0173(89)90013-1. [DOI] [PubMed] [Google Scholar]
- Stahl JS, van Alphen AM, De Zeeuw CI. A comparison of video and magnetic search coil recordings of mouse eye movements. J Neurosci Methods. 2000;99:101–110. doi: 10.1016/s0165-0270(00)00218-1. [DOI] [PubMed] [Google Scholar]
- Straka H, Dieringer N. Spinal plasticity after hemilabyrinthectomy and its relation to postural recovery in the frog. J Neurophysiol. 1995;73:1617–1631. doi: 10.1152/jn.1995.73.4.1617. [DOI] [PubMed] [Google Scholar]
- Straka H, Vibert N, Vidal PP, Moore LE, Dutia MB. Intrinsic membrane properties of vertebrate vestibular neurons: function, development and plasticity. Prog Neurobiol. 2005;76:349–392. doi: 10.1016/j.pneurobio.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Usami S, Igarashi M, Thompson GC. GABA-like immunoreactivity in the chick vestibular end organs. Brain Res. 1987;418:383–387. doi: 10.1016/0006-8993(87)90108-9. [DOI] [PubMed] [Google Scholar]
- Van Alphen AM, Schepers T, Luo C, De Zeeuw CI. Motor performance and motor learning in Lurcher mice. Ann N Y Acad Sci. 2002;978:413–424. doi: 10.1111/j.1749-6632.2002.tb07584.x. [DOI] [PubMed] [Google Scholar]
- Vibert N, Babalian A, Serafin M, Gasc JP, Muhlethaler M, Vidal PP. Plastic changes underlying vestibular compensation in the guinea-pig persist in isolated, in vitro whole brain preparations. Neuroscience. 1999;93:413–432. doi: 10.1016/s0306-4522(99)00172-4. [DOI] [PubMed] [Google Scholar]
- Vibert N, Beraneck M, Bantikyan A, Vidal PP. Vestibular compensation modifies the sensitivity of vestibular neurones to inhibitory amino acids. Neuroreport. 2000;11:1921–1927. doi: 10.1097/00001756-200006260-00023. [DOI] [PubMed] [Google Scholar]
- Vogel MW, Caston J, Yuzaki M, Mariani J. The Lurcher mouse: fresh insights from an old mutant. Brain Res. 2007;1140:4–18. doi: 10.1016/j.brainres.2005.11.086. [DOI] [PubMed] [Google Scholar]
- Yamanaka T, Him A, Cameron SA, Dutia MB. Rapid compensatory changes in GABA receptor efficacy in rat vestibular neurones after unilateral labyrinthectomy. J Physiol. 2000;523(Pt 2):413–424. doi: 10.1111/j.1469-7793.2000.t01-1-00413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]