Abstract
The integration of neck proprioceptive and vestibular inputs underlies the generation of accurate postural and motor control. Recent studies have shown that central mechanisms underlying the integration of these sensory inputs differ across species. Notably, in rhesus monkey (macaca mulata), an Old World monkey, neurons in the vestibular nuclei are insensitive to passive stimulation of neck proprioceptors. In contrast, in squirrel monkey, a New World monkey, stimulation produces robust modulation. This has led to the suggestion that there are differences in how sensory information is integrated during self motion in old versus New World monkeys. To test this hypothesis, we recorded from neurons in the vestibular nuclei of another species in the macaca genus (i.e., macaca fascicularis (cynomolgus monkey)). Recordings were made from vestibular-only (VO) and position-vestibular-pause (PVP) neurons. The majority (53%) of neurons in both groups were sensitive to neck proprioceptive and vestibular stimulation during passive body-under-head and whole body rotation, respectively. Furthermore, responses during passive rotations of the head-on-body were well predicted by the linear summation of vestibular and neck responses (which were typically antagonistic). During active head movement, the responses of VO and PVP neurons were further attenuated (relative to a model based on linear summation) for the duration of the active head movement or gaze shift, respectively. Taken together, our findings show that the brain's strategy for the central processing of sensory information can vary even within a single genus. We suggest that similar divergence may be observed in other areas in which multimodal integration occurs.
Keywords: vestibular, neck proprioceptive, active, passive, VCR, VO, PVP
Introduction
The vestibular system plays an essential role during daily activities by contributing to spatial orientation, gaze stabilization and accurate motor control. Vestibular sensory organs transmit information to the vestibular nuclei by way of afferents of the vestibular nerve. The integration of vestibular with extra-vestibular information at this first stage of central processing is a hallmark of the vestibular system (reviewed in Angelaki and Cullen 2008). For example, during every day activities, the stimulation of neck proprioceptive afferents will occur whenever the head is rotated relative to the body. Projections from the cerebellum, brain stem, as well as many cortical areas (for review see, Fukushima 1997) carry neck proprioceptive information to the vestibular nuclei. In turn, the integration of vestibular and proprioceptive information allows the brain to compute the movement of the head relative to the body as well as to space - an ability that is critical for the generation of accurate postural and motor control. In this way the vestibular system both senses and controls self motion.
Our understanding of how the human brain processes information from different modalities is largely based on the results of single unit recording experiments in nonhuman primates. For example, to characterize the vestibular system, researchers have carried out experiments in both old and New World monkeys; two groups of primates that evolutionarily diverged approximately 30 million years ago from the Simiiformes infraorder (Ackermann and Cheverud 2004). The results of recent studies have suggested divergence in the way the two groups integrate sensory information. Notably, rotation sensitive neurons in the vestibular nuclei of saimiri sciureus (i.e. squirrel monkey), a type of New World monkey, are modulated by both vestibular and neck proprioceptive inputs (Gdowski and McCrea 2000). In contrast, such neurons in the vestibular nuclei of the rhesus monkey (macaca mulatta), a type of Old World monkey, only respond to vestibular stimulation (Roy and Cullen 2001a; b; 2002). These differences may have developed to meet the requirements of the behavioral repertoire of each group - Old World monkeys are more terrestrial, while New World monkeys are mostly arboreal (Fleagle 1978).
Currently, the macaque monkey (a genus of the Old World monkeys) is the most widely studied animal model used to understand the higher level processing of sensory inputs at the level of single neurons. The ability to then compare human and macaque data using fMRI has further allowed us to extend our understanding of neural mechanism underlying human central processing based on neurophysiological studies in monkeys. While the rhesus macaque (macaca mulatta) is the most widely used species of Old World monkeys, other species within the macaque monkey genus, such as the macaca fascicularis (cynomolgus monkey) and macaca fuscata (Japanese macaque), have also been extensively used. The question we aimed to answer was whether these two species within a single genus (macaca mulatta and fascicularis) use different strategies for central processing of sensory information. Specifically, we asked whether they might utilize different neural substrates for vestibular and neck proprioceptive integration. To address this question, we recorded from neurons in the vestibular nuclei of the macaca fascicularis, and compared responses to those recorded previously in comparable studies in macaca mulatta. Recordings were made from 2 functional classes of neurons during passive head and body rotations as well as during voluntarily generated head movements: (i) Vestibular-only (VO) neurons which have been implicated in vestibular spinal reflexes and also most likely send projections to higher-order structures within the cerebellum and cortex, and (ii) Position-vestibular-pause (PVP) neurons which are responsible for mediating the vestibulo-ocular reflex (reviewed in Cullen and Roy 2004). Our results suggest that there are significant differences in how the vestibular system integrates sensory information early in sensory processing. These results have important implications regarding the choice of a model species for comparing human data directly with an animal model and suggest the need form more detailed neurophysiological and neuroanatomical experiments.
Materials and Methods
Two cynomolgus monkeys were prepared for chronic extracellular recordings using aseptic surgical techniques. All experimental procedures were approved by McGill University Animal Care Committee and followed the guidelines and of the Canadian Council on Animal Care.
Surgical procedures
Surgeries were performed as described by Sylvestre and Cullen (1999). Briefly, anesthesia was achieved by the use of isoflurane gas (2-3% initially) and maintained for the duration of the procedure (0.8-1.5%). A dental acrylic implant was attached to the skull of the animal using stainless steel screws. A stainless steel post (used to restrain the monkey's head) and a stainless steel recording chamber (positioned to provide access to the medial vestibular nucleus) were attached to the implant. A 16-17 mm eye coil, consisting of 3 loops of Teflon coated stainless steel wire, was placed behind the conjunctiva of the right eye. The animals were allowed 2 weeks to recover from surgery before any experiments were performed.
Data acquisition
During the recording sessions, the monkey was comfortably seated in a primate chair, which was set upon a vestibular turntable. Head restrained recordings were performed initially while the room was dimly lit. An enamel-insulated tungsten microelectrode (7–10 MΩ impedance; Frederick Haer Co., Bowdoinham, ME) was used to record single unit activity as previously described (Sylvestre and Cullen 1999). Turntable velocity was measured using an angular velocity sensor (Watson Inc.). Unit activity, horizontal gaze and head positions, target position, and table velocity were recorded onto a digital audio tape for playback at a later time. Each unit recorded was analyzed off-line to ensure proper isolation. During playback, action potentials were distinguished using a windowing circuit (BAK Electronics Inc., Germantown, MD). This was operated manually such that a pulse was associated with the rising phase of each action potential. Gaze position, head position, target position, gaze velocity and head velocity signals were low-pass filtered at 250 Hz (eight-pole Bessel filter) and sampled at 1000 Hz.
Behavioral Paradigms
Neurons were initially recorded during standard head-restrained paradigms with the head in the stereotaxic position in order to characterize their sensitivities to eye movements and/or head velocity. Monkeys were trained to follow a laser target, which was projected onto a screen (60 cm in front of the monkey) in exchange for a juice reward. Saccadic eye movements were elicited by stepping the target between horizontal positions (±5, 10, 15, 20, 25, and 30°). Smooth pursuit eye movements were evoked by rotating a target sinusoidally in the horizontal plane with a frequency of 0.5 Hz, reaching a maximal velocity of 40°/sec. Neuronal sensitivities to head velocity were quantified by passively rotating monkeys about an earth-vertical axis (with a frequency of 0.5 Hz, ±40°/sec) in the dark [passive whole-body rotation (pWBR)] and while they cancelled their vestibulo-ocular reflex [passive whole-body rotation cancellation (pWBRc)]. The latter was achieved by having the monkey follow a laser that moved together with the turntable (i.e. at the same speed and frequency). Neuronal responses were also recorded during pWBR at frequencies of 1 and 2 Hz (±40°/sec).
Using the above mentioned paradigms, we characterized neurons (see below) that showed vestibular sensitivity during pWBRc as PVP (i.e., eye position sensitivity, pause during saccades) or VO (no eye position sensitivity) neurons (Roy and Cullen 2001b; 2002; Scudder and Fuchs 1992). In order to measure neuronal response to neck proprioceptive input we next characterized neuronal responses during a paradigm in which the neck proprioceptors were stretched, but there was no activation of vestibular receptors. To do this, the head was held stationary relative to space while the body was rotated underneath (body-under-head (BUH) paradigm, 1 Hz, ±40°/sec). We then recorded the responses of the same neurons during combined activation of vestibular and neck proprioceptive receptors; by manually rotating the head above a stationary body (passive head-on-body (PHB) paradigm) at a frequency of 1 Hz.
Once a neuron had been fully characterized during head-restrained conditions, the monkey's head was slowly and carefully released such that the monkey was able to make natural head movements only in the horizontal plane. This was obtained by means of a specially designed head-holder (Huterer and Cullen 2002) that enabled us to either completely immobilize the animal's head or allowed the monkey to freely rotate its head around the yaw axis (Sadeghi et al. 2007a; Sadeghi et al. 2007b). The response of the same neuron was then recorded during voluntary (i.e., active) horizontal eye–head gaze shifts made to orient targets as described previously (Roy and Cullen 2002). For the present study, we chose gaze shifts that had amplitudes of 35-45°. At the beginning of the gaze shift, as well as, at the end of the head movement, the eye-in-head position was close to the center (i.e., <2.5°). Velocities of head movements that were produced during such gaze shifts reached peak values of ∼140 °/sec.
Finally, to investigate the frequency response of PVP and VO neurons, we recorded from a subpopulation of neurons during BUH and PHB paradigms at rotation frequencies of 0.5 and 2 Hz.
Analysis of neuronal discharges
Gaze, head, target, and table signals were digitally filtered at 125 Hz. Eye position was calculated from the difference between gaze and head position signals. Gaze, eye, and head position signals were digitally differentiated to produce velocity signals. Neuronal firing rates were estimated using convolution of the spike train with an optimal digital filter (non-eye movement neurons; Cherif et al. 2008) and a Gaussian window (eye-movement neurons; Roy and Cullen 1998; 2002). Subsequent analysis was performed using custom algorithms (Matlab, Mathworks).
In this study, we present data from two classes of neurons in the medial vestibular nuclei that were sensitive to yaw rotations: (i) neurons that are sensitive to eye movements (i.e. position-vestibular-pause (PVP) neurons, which encode eye position during fixation, respond to smooth pursuit eye movement, and pause during saccades), and (ii) neurons that are not sensitive to any class of eye movement (vestibular-only (VO) neurons). In order to distinguish between these two types of neurons, periods of steady fixation and saccade-free smooth pursuit were analyzed using a multiple regression analysis and correlations between firing rate and eye position/velocity were assessed. A least squares regression analysis was then used to analyze the responses of both classes of neurons during passive and active head rotations. To quantify response modulation during pWBR and pWBRc, we optimized the coefficients of equations 1 and 2 (see Results) for VO and PVP neurons, respectively. Specifically, the values of the resting discharge (bias, spikes/second), and head velocity and acceleration coefficients [gpWBR in (spikes/sec)/(°/sec) and apWBR in (spikes/sec)/(°/sec2)] were estimated (Roy and Cullen 2002). Neuronal sensitivities (S in (spikes/sec)/(°/sec)) and phases (φ in degrees) relative to head velocity were then computed using the following equations:
where f is the frequency of the sinusoidal rotation. A comparable approach was used to calculate the sensitivity and phase relative to the body and head velocity during body-under-head and passive head-on-body rotations, respectively. In the following sections, we report the value of S and the phase value, rather than comparing the velocity and acceleration terms, in order to be able to compare our results with those of previous studies.
Values are expressed as mean ± SEM and a Student's t-test was used to determine whether the average of two measured parameters differed significantly from each other. A linear mixed-effect regression model (Littell et al. 2006) which accounts for the correlation of responses for each neuron was used to characterize frequency-dependent trends. Analyses were conducted using the mixed model procedure (PROC MIXED) of SAS, version 9.1.3 (SAS Institute, Inc., 2002-2003). A log-base 10 transformation was used to normalize response distributions as required. Because measurements on some frequencies were not available in some neurons, a Kenward-Roger correction was applied to the degrees of freedom for better control of Type I error (Kowalchuk et al. 2004).
Results
We recorded from 60 neurons in the vestibular nuclei (VN) in two cynomolgus monkeys. The neurons were categorized based on their eye and head sensitivities during head restrained paradigms as position-vestibular-pause (PVP, n=22) or vestibular-only (VO, n=38). Neurons in each group showed excitatory responses during either ipsilateral (type I cells) or contralateral (type II cells) rotations. For the purposes of this study, we combined type I and II neurons in the data analysis, since they responded similarly during each behavioral task. Below, we will first describe the criteria used for characterizing the cells. We will then show their responses to vestibular and/ or neck proprioceptor stimulation and consider integration of these two signals. Finally, we compare the response of each class of neurons during active head-on-body movements to their responses obtained during passive rotations of head and body.
General characterization of response of neurons to passive sinusoidal rotation: Vestibular-only neurons
Vestibular only (VO) neurons were identified based on their lack of responses to eye movements during head restrained paradigms. As has been previously demonstrated (e.g., see Roy and Cullen 2001b), VO neurons are insensitive to eye position and velocity during ocular fixation, smooth pursuit, and saccadic eye movements, but respond to head-in-space motion during passive whole-body rotation (pWBR). Based on this criterion, 38 of the recorded neurons were identified as VO cells (20 in monkey G and 18 in monkey D). To quantify the response of neurons during pWBR we used the following equation:
| (1) |
where fr is the firing rate, bias is a constant equal to the resting neural discharge, k is the cell's sensitivity to eye position, and gpWBR and apWBR are constant coefficients. Coefficients in equation (2) were estimated using least squared optimization, as has been previously described by Roy and Cullen (2002). As for VO neurons, estimates of gpWBR and apWBR were then used to calculate the neuron's sensitivity and phase lead with respect to head velocity (see Methods). Next, to further dissociate each PVP neuron's vestibular and eye movement sensitivities, responses were also characterized during cancellation of the VOR (pWBRc). In contrast to VO neurons, and consistent with previous studies of PVP neurons in other primate species (squirrel monkey: Cullen and McCrea 1993; rhesus monkey: Roy and Cullen 2002), vestibular sensitivities during pWBRc were significantly reduced (18%) compared to those measured during rotation in the dark (paired t-test, p = 0.02).
Figure 1A1 shows the response of an example neuron during sinusoidal rotations with a frequency of 1Hz (peak velocity of 40 °/sec). The neuron was strongly modulated, with excitatory responses during ipsilateral head movements produced by rotations about an earth-vertical axis. Using equation (1) the estimation of the response of the example neuron provided a resting discharge of 83 spikes/sec, sensitivity of 0.59 (spikes/sec)/(°/sec) with a VAF of 0.95 and a response phase lead of 33°. For the population of neurons we measured a resting discharge of 56 ± 4 spikes/sec, and estimated a sensitivity of 0.55 ± 0.06 (spikes/sec)/(°/sec) and phase lead of 32 ± 3° relative to head velocity (average population VAF = 0.69 ± 0.03). The responses of a subset of neurons were also recorded at frequencies of 0.5 (n=37) and 2 (n=35) Hz. In agreement with a recent report (Dickman and Angelaki 2004), vestibular sensitivity significantly increased (from 0.46 ± 0.06 to 0.74 ± 0.09 (spikes/sec)/(°/sec)) as a function of rotation frequency (mixed model regression on transformed data, p<0.0001). In contrast, we found that response phase was constant over the same frequency range (29 ± 3° to 37 ± 3°, mixed model regression, p = 0.06).
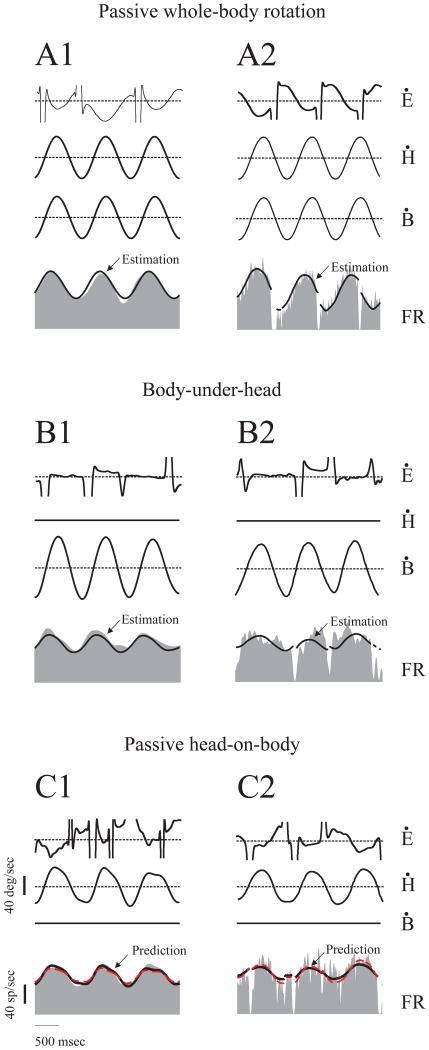
Figure 1.
Activity of an example VO and PVP neuron. (A) Responses to vestibular stimulation during passive whole-body rotation. Both neurons increased their discharges during ipsilaterally directed head movements. The VO neuron (A1) was insensitive to eye movements (not shown), whereas the PVP neuron (A2) increased its discharge for contralaterally directed eye movements (not shown) and paused for ipsilaterally directed saccades. Vestibular sensitivities were estimated using equations 1 and 2 for VO and PVP cells, respectively (solid black traces superimposed on neuronal responses). (B) Responses to neck proprioceptive stimulation during body-under-head rotations. The example VO (B1) and PVP (B2) neurons both responded to ipsilaterally directed rotations of body under a fixed head. Neuronal sensitivities to neck movement were estimated using equation 3 (superimposed solid black traces). (C) Responses to concurrent vestibular and neck proprioceptive stimulation during passive head-on-body rotation. The example VO (C1) and PVP (C2) neurons were excited by ipsilaterally directed rotations. Addition of the vestibular and neck sensitivities (see text, equation 4) accurately predicted (dashed red line) the firing rate and was similar to the estimated response (solid black line). Ė, eye velocity; Ḣ, head velocity; Ḃ, body velocity; FR, firing rate.
General characterization of response of neurons to passive sinusoidal rotation: Position-vestibular-pause neurons
Position-vestibular-pause (PVP) neurons were identified on the basis of their sensitivity to head velocity during pWBR and eye position sensitivity during ocular fixation. The neurons also paused during ipsilateral or contralateral saccades for type I and II cells, respectively. Sensitivity to eye position (k) for these neurons was calculated using a regression analysis on eye positions and firing rates during periods of steady fixation. Cells were then characterized in response to pWBR paradigm, where the following model was used to fit the firing rate during periods of slow phase eye movements:
| (2) |
where fr is the firing rate, bias is a constant equal to the resting neural discharge, k is the cell's sensitivity to eye position, and gpWBR and apWBR are constant coefficients. Coefficients in equation (2) were estimated using least squared optimization, as has been previously described by Roy and Cullen (2002). As for VO neurons, estimates of gpWBR and apWBR were then used to calculate the neuron's sensitivity and phase lead with respect to head velocity (see Methods). Next, to further dissociate each PVP neuron's vestibular and eye movement sensitivities, responses were also characterized during cancellation of the VOR (pWBRc). In contrast to VO neurons, and consistent with previous studies of PVP neurons in other primate species (squirrel monkey: Cullen and McCrea 1993; rhesus monkey: Roy and Cullen 2002), vestibular sensitivities during pWBRc were significantly reduced (18%) compared to those measured during rotation in the dark (paired t-test, p = 0.02).
Figure 1A2 shows an example PVP neuron which was excited by contralaterally-directed yaw rotations (1 Hz, 40 °/sec peak velocity). This example neuron had a resting discharge of 94 spikes/sec, sensitivity of 0.61 (spikes/sec)/(°/sec) and the response had a phase lead of 8° (VAF of estimation = 0.83). This neuron was typical of our population of PVP neurons (n = 22; 15 in monkey G and 7 in monkey D), for which the mean resting discharge was 111 ± 12 spikes/sec and the response sensitivity was 0.79 ± 0.13 with a phase lead of 21 ± 3° re. head velocity (VAF = 0.57 ± 0.05). A subpopulation of PVP neurons were also tested at 0.5 (n = 19) and 2 Hz (n = 21). Over this frequency range, the mean response sensitivity of the population increased slightly from 0.72 ± 0.1 to 0.87 ± 0.13 (spikes/sec)/(°/sec) (mixed model regression on transformed data, p=0.01), while there was no significant change in response phase (27 ± 3° to 20 ± 2 °, mixed model regression, p = 0.1). These trends are consistent with those reported in rhesus monkey (Dickman and Angelaki 2004), suggesting similarities in the rotational response dynamics of both VO and PVP neurons of cynomolgus and rhesus monkeys.
General characterization of response of neurons to passive neck rotation
We next addressed whether neurons were directly influenced by the stimulation of neck proprioceptors. The responses of VO and PVP neurons were recorded while the body was rotated beneath the earth-fixed head (i.e. body-under-head (BUH) rotations). Notably, in this paradigm neck proprioceptors were activated, but there was no stimulation of vestibular receptors. Figure 1B1 shows the response of the example VO neuron to BUH rotations at the frequency of 1 Hz. The sensitivity of VO neurons to neck proprioceptive stimulation was quantified using the following equation:
| (3) |
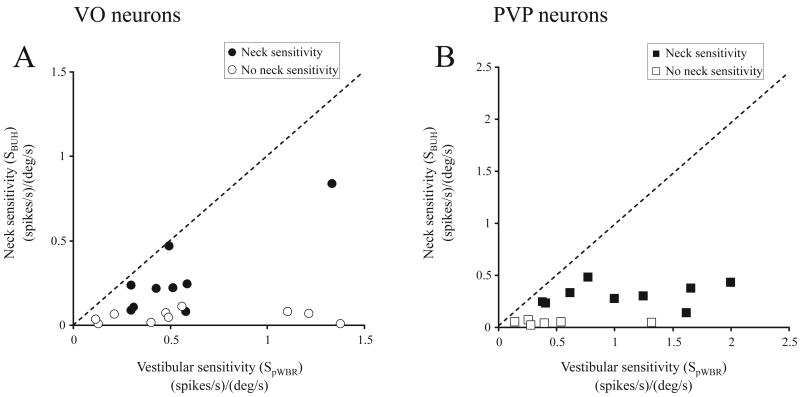
where fr is the firing rate, bias is a constant equal to the resting neural discharge, and gBUH and aBUH are constant coefficients. For the analysis of PVP neurons, we used a comparable approach that included an additional eye position term to account for a neuron's eye-position sensitivity. Approximately half of the populations of VO and PVP neurons - 55% (16/20 in monkey G and 5/18 in monkey D) of VO and 50% (7/15 in monkey G and 4/7 in monkey D) of PVP cells - was sensitive to neck proprioceptive stimulation (i.e., SBUH > 0.1 (spikes/sec)/(°/sec)). The example VO and PVP neurons shown in Figures 1B1 and 1B2 are typical in that their responses to neck proprioceptive stimulation (during BUH rotation) were ∼50% smaller than their responses during vestibular stimulation (pWBR in Figures 1A1 and 1A2). The example VO neuron had a neck sensitivity of 0.25 (spikes/sec)/(°/sec) and a phase lead of 51° relative to body velocity (VAF of 0.93), while the example PVP neuron had a neck sensitivity of 0.32 (spikes/sec)/(°/sec) and a phase lag of 23° relative to body velocity (VAF of 0.45). Figure 2 compares, on a cell by cell basis, the neck proprioceptive (SBUH) and vestibular (SpWBR) sensitivities of VO (n = 19; Figure 2A) and PVP (n = 15; Figure 2B) neurons recorded during both pWBR and BUH paradigms (1Hz, 40 °/sec). Notably, neck proprioceptive sensitivities (during BUH rotation) were on average ∼50% smaller than vestibular sensitivities (during pWBR) and as a result most of the data points fall close to the x-axis. This relationship was also true when neck and vestibular velocity or acceleration coefficients were compared individually (see Supplementary Figure 1).
Figure 2.
Comparison of vestibular and neck proprioceptive sensitivities of VO (A) and PVP (B) neurons. The plots in A (VO) and B (PVP) depict the relationship between the calculated neuronal sensitivities (see Methods) obtained during pWBR and BUH rotations. Neurons were considered as neck sensitive (filled symbols) if this calculated neck sensitivity was greater than 0.1 (spikes/s)/(°/s) (see Methods). Note that neck proprioceptive sensitivities were, on average, ∼50% smaller than vestibular sensitivities, even for neck sensitive neurons, and as a result most of the data points fall close to the x-axis.
Overall, there was no difference in the vestibular sensitivities (measured during whole body rotation) for VO and PVP neurons that were sensitive versus insensitive to neck proprioceptive inputs (VO neurons, p > 0.4; PVP neurons, p > 0.3). Moreover, while the average of the absolute value of neck sensitivities for neck-sensitive VO and PVP neurons were comparable (0.24 ± 0.04 and 0.23 ± 0.03 (spikes/secc)/(°/sec), respectively), neck-related responses led velocity more for VO than PVP neurons (14 ± 5° versus 1 ± 10°, respectively). Notably, of the neurons that were sensitive to neck rotation ∼78% (25/32) had vestibular and neck proprioceptive responses that were antagonistic. This finding is similar to those reported in previous studies (squirrel monkey: Gdowski and McCrea 2000; cat: Kasper et al. 1988). When the relative direction of the neck response was taken into account (i.e. if vestibular and neck sensitivities were oppositely directed, the sign of the neck sensitivity was considered negative) the average neck sensitivities of the population of VO and PVP neurons were relatively small (i.e., 0.096 ± 0.08 and 0.17 ± 0.07, respectively).
A subpopulation of neck-sensitive neurons were also tested at 0.5 (18 VO and 8 PVP neurons) and 2 Hz (11 VO and 10 PVP neurons). Although neck sensitivities of VO neurons showed a statistically significant increase as a function of frequency (0.5-2Hz) (mixed model regression on transformed data, p = 0.01), the change was quite small (0.19 ± 0.03 to 0.29 ± 0.04 (spikes/sec)/(°/sec)). For PVP neurons the neck sensitivities remained constant over the range of frequencies tested (0.24 ± 0.06 to 0.45 ± 0.13 (spikes/sec)/(°/sec); mixed model regression on transformed data, p = 0.13). Similarly, there was no significant change (mixed model regression, p > 0.5) in response phases over this frequency range (-2 ± 5 to 3 ± 4 and 13 ± 10 to 17 ± 12 for VO and PVP neurons, respectively).
Integration of vestibular and neck proprioceptive signals during passive head-on-body rotations
In order to address how vestibular and neck proprioceptive inputs are combined at the level of single neurons, neuronal responses were next characterized during passive head-on-body (PHB) rotations. Figure 1C1 shows the response of our example VO neuron to PHB rotation. Superimposed on the unit's response is our estimation of the response (solid black line) using equation (1), where the estimated coefficients (now gPHB and aPHB) quantify the response to combined vestibular and neck stimulation. (Note that it is not possible to dissociate these two inputs during PHB rotation since one is the inverse of the other).
We then assessed whether a given neuron's modulation during PHB could be predicted by its vestibular and neck related responses during pWBR and BUH, respectively, using the equation:
| (4) |
where gpWBR and apWBR are constant coefficients obtained during pWBR, and gBUH and aBUH are constant coefficients obtained during BUH rotations. In the analysis of PVP neurons, an additional eye position term was included to account for each neuron's eye-position sensitivity. Figure 1C shows the estimation (solid black line superimposed on firing rate) for our example VO (Figure 1C1) and PVP (Figure 1C2) neurons. Model fits provided a VAF of 0.89 and 0.45, respectively. For both VO and PVP neurons, the predicted responses closely matched neuronal responses (dashed red lines, VAF= 0.7 and 0.4, respectively). Thus, during PHB, the responses of both example neurons could be well predicted by the linear summation of vestibular and neck related responses during pWBR and BUH, respectively.
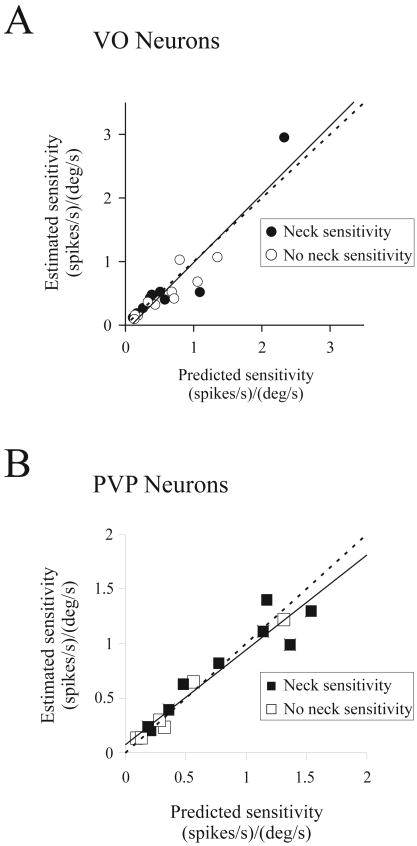
Similar results were obtained for our entire population of VO and PVP neurons. Figure 3 compares the estimated and predicted magnitudes of response sensitivities (S, see Methods) of VO (n = 19; Figure 3A) and PVP (n = 15; Figure 3B) neurons recorded during all three passive paradigms (i.e., pWBR, BUH, and PHB; 1Hz, 40 °/sec). Overall, the data points fall close to the unity line, consistent with the proposal that predictions and estimates were comparable (VO neurons: 0.72 ± 0.2 vs. 0.61 ± 0.2 (spikes/sec)/(°/sec), p = 0.45 and PVP neurons: 0.62 ± 0.1 vs. 0.57 ± 0.1 (spikes/sec)/(°/sec), p = 0.26). This was true both for neurons that were sensitive (9 VOs and 9 PVPs) or insensitive (10 VOs and 6 PVPs) to neck proprioceptive inputs. This relationship was also true when estimated and predicted neck velocity or acceleration coefficients were compared individually (see Supplementary Figure 2). Moreover, response sensitivities and phases to PHB rotations remained relatively constant over the range of frequencies tested (0.5-2Hz) for both neck sensitive and insensitive VO neurons (mixed model regression, p > 0.1) and PVP neurons (mixed model regression, p > 0.1).
Figure 3.
Comparison of estimated and predicted sensitivities of VO (A) and PVP (B) neurons to passive head-on-body rotations. The linear addition of vestibular and neck proprioceptive sensitivities (obtained during pWBR and BUH rotations, respectively) provided a good prediction of each neurons modulation during passive head-on-body rotations (VAFs for estimates and predictions: 0.67 ± 0.04 and 0.51 ± 0.05 for VO neurons; 0.50 ± 0.06 and 0.33 ± 0.09 for PVP neurons). The slope of the line fit to the data was not significantly different from unity (dashed line), indicating that predictions were accurate whether neurons were sensitive (filled symbols) or insensitive (open symbols) to neck proprioceptive inputs.
Attenuated responses during active head-on-body movements
In our last experiment, the responses of VO and PVP neurons were recorded during active head-on-body movements (i.e. gaze shifts). In this condition, not only are the neck proprioceptors activated by the rotation of the head on the body, but the monkey also generates a command to move the head. To address whether we could predict a neuron's response based solely on its sensitivity to vestibular and neck inputs (as we could during passive head-on-body movements (i.e., Figure 3)), we recorded from the same neurons during active eye head gaze shifts and compared their responses to those obtained during pWBR and PHB paradigms. Responses were characterized both for the period during which gaze was redirected (Figure 4A: gs period), as well as during the 10-80 msec period following the end of the gaze shift (Figure 4A: post-gs), where the gaze was stable but the head continued to move.
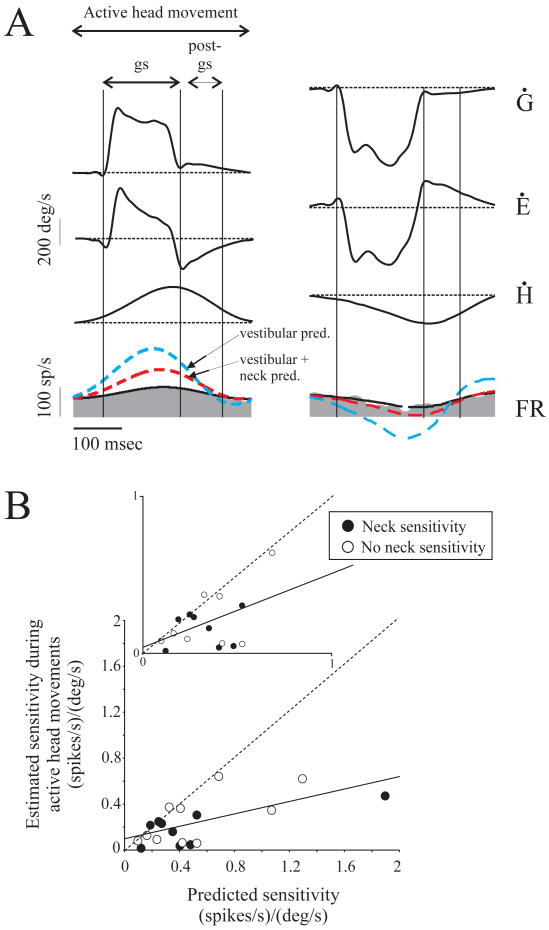
Figure 4.
Neuronal responses to active head-on-body movements. (A) Example of a neck-sensitive VO neuron during ipsilateral (left panel) and contralateral (right panel) gaze shifts. Predictions made using either the vestibular sensitivity obtained during pWBR (blue dashed line) or the sum of vestibular and neck sensitivities obtained during pWBR and BUH, respectively, (red dashed line) both overpredicted neuronal responses during gaze shifts as well as during the post-gaze shift period. For comparison, the response estimated using equation 1 is also shown (black solid trace). (B) Cell by cell comparison of VO neuron sensitivities to passive versus active head-on-body movements. The slope of the line fit to the data was significantly less than unity (dotted line). Similar results were obtained when neurons sensitive (filled circles) or insensitive (open circles) to neck proprioceptive inputs were considered separately. Moreover, when we considered only neurons with sensitivities < 1 (i.e. the majority of neurons), estimated sensitivities were consistently less then those predicted based on passive stimulation of the vestibular and neck proprioceptive receptors (inset). Ġ, gaze velocity; Ė, eye velocity; Ḣ, head velocity; FR, firing rate.
Vestibular-only neurons
The shaded gray area in Figure 4A shows the response of an example VO neuron during gaze shifts. For each neuron, we first used equation (4) to attempt to predict responses based on vestibular and neck-related sensitivities measured during pWBR and BUH paradigms. As is shown for the example neuron, this prediction (red dashed line; sensitivity = 0.35 (spikes/sec)/(°/sec); VAF = 0.20) provided a poor fit, as compared to an estimate in which coefficients were independently optimized (black solid line; sensitivity = 0.2 (spikes/sec)/(°/sec); VAF = 0.73). For comparison, the response predicted based solely on the neuron's vestibular sensitivity (i.e. modulation during pWBR) is also shown (blue dashed line). As can be seen in Figure 4A, neither prediction approaches the attenuation that occurs during either the gaze shift or the 10-80 msec period that followed; both predictions substantially overestimated the neuron's response. This was true for the population of VO neurons that was recorded during all passive rotations, as well as, during active gaze shifts (n = 19). Overall, responses during active head movements were significantly less than those predicted based on linear summation of vestibular and neck sensitivities obtained during passive rotations (0.25 ± 0.04 vs. 0.53 ± 0.1 (spikes/sec)/(°/sec), paired t-test, p = 0.0009).
Figure 4B compares estimated and predicted sensitivities during the gaze shift period on a cell by cell basis for neck-sensitive (closed symbols) and neck-insensitive (open symbols) neurons. Almost all the data points fall below the unity line (dashed), indicating that estimated sensitivities were less than expected based on the linear summation of vestibular and neck sensitivities. The slope of the line fit to the data points was significantly different from unity (p < 0.0001). This was also true when only neurons with smaller head velocity sensitivities (inset) were considered (p < 0.001). Notably, vestibular sensitivities were attenuated regardless of whether the cells were sensitive to neck proprioceptive stimulation (0.18 ± 0.03 vs. 0.45 ± 0.1 (spikes/sec)/(°/sec), n = 9, paired t-test, p < 0.02) or showed no neck sensitivity (0.35 ± 0.09 vs. 0.50 ± 0.1 (spikes/sec)/(°/sec), n = 10, paired t-test, p < 0.03). Thus, unlike the passive condition, the response of VO neurons during active head-on-body movements cannot be predicted by the simple addition of vestibular and neck signals
Position-vestibular-pause neurons
A comparable approach was used to investigate the information encoded by PVP neurons during the active head movements made during gaze shifts. Similar to VO neurons, the responses of PVPs were attenuated during the gs period. Indeed, we were unable to quantify the responses during the gaze shift period because of the few spikes present. However, in contrast to VO cells, the responses of PVP neurons were not attenuated during the post-gs period when compared to values obtained during PHB rotations at 1 Hz (n = 15, paired t-test, p = 0.73); neuronal activities were accurately predicted by linear addition of the vestibular and neck sensitivities (equation 4). This observation was true for neurons that were sensitive to neck proprioceptive stimulation (0.66 ± 0.21 vs. 0.62 ± 0.18 (spikes/sec)/(°/sec), n = 9, paired t-test, p = 0.83) and those with no neck sensitivity (0.80 ± 0.33 vs. 0.73 ± 0.27 (spikes/sec)/(°/sec), n = 6, paired t-test, p = 0.78). In addition, the average VAFs for the population of neurons for estimations and predictions were comparable (0.49 ± 0.06 and 0.32 ± 0.04, respectively). It is also noteworthy, that in the present study the PVP neurons of our cynomolgus monkeys were largely silent (i.e., paused) during the gaze shift component of the movement. This contrasts with previous studies of PVPs in rhesus monkeys (Roy and Cullen 2002), where we have reported attenuation but not complete inhibition. One likely explanation for this difference is that cynomolgus monkeys generally produced lower peak head velocities (143 ± 4 °/s) in our studies compared to rhesus monkeys (317 ± 22 °/s). As a result, neurons were less likely to be driven out of the saccadic pathway mediated inhibition during gaze shifts.
Discussion
During everyday activities, both vestibular and neck afferents are typically simultaneously activated. The results of recent studies have suggested important differences in the way in which these signals are combined at the level of the vestibular nucleus in squirrel (saimiri sciureus) versus rhesus (macaca mulatta) monkeys (examples of new and old world monkeys, respectively). Notably, neurons in squirrel monkeys, but not rhesus monkeys are sensitive to passive stimulation of neck proprioceptors (reviewed in Cullen and Roy 2004). Here, to establish whether two species within a single genus could also use different strategies for multimodal integration, recordings were made in another species of the macaca genus. We found that more than 50% of both PVP and VO neurons of the macaca fascicularis (cynomolgus) monkey are modulated by neck proprioceptive stimulation. Neck and vestibular sensitivities were antagonistic such that responses were reduced during passive head-on-body rotation, in a manner predicted by the linear summation of neck and vestibular inputs. Interestingly, during active head-on-body movements responses showed even greater attenuation – suggesting further integration with the head motor command. Taken together, our findings show differences in multimodal integration strategies even within the macaca genus. In the following sections we discuss the neck and vestibular signals carried by the vestibular nuclei, and address the differential neural substrate for multimodal integration between different species and animals.
Vestibular signals carried by VN neurons
Our data from the medial vestibular nuclei of cynomolgus monkey are consistent with those of previous studies in rhesus monkey. We found that vestibular sensitivity increased as a function of frequency of rotation (pWBR) over the range of 0.5 – 2.0 Hz for both VO and PVP neurons. Dickman and Angelaki (2004) have previously reported a similar increase in the sensitivity of both non-eye movement-related neurons (i.e., VO neurons) and eye movement-related neurons (i.e., PVP neurons) over the same range. Most recently, Ramachandran and Lisberger (2008) tested the responses of rhesus PVP neurons to stimuli over an extended frequency range (0.5-20 Hz) and reported a similar increase in sensitivity for the range of frequencies corresponding to those tested here, and an increase <3 fold overall. In the present study, we also found that response phase was reasonably constant across this same frequency range for both groups of neurons. Similar findings have been reported in rhesus monkeys (Dickman and Angelaki 2004) where the phase of the response was relatively constant over the same frequency range.
Neck proprioceptive signals are carried by VN neurons
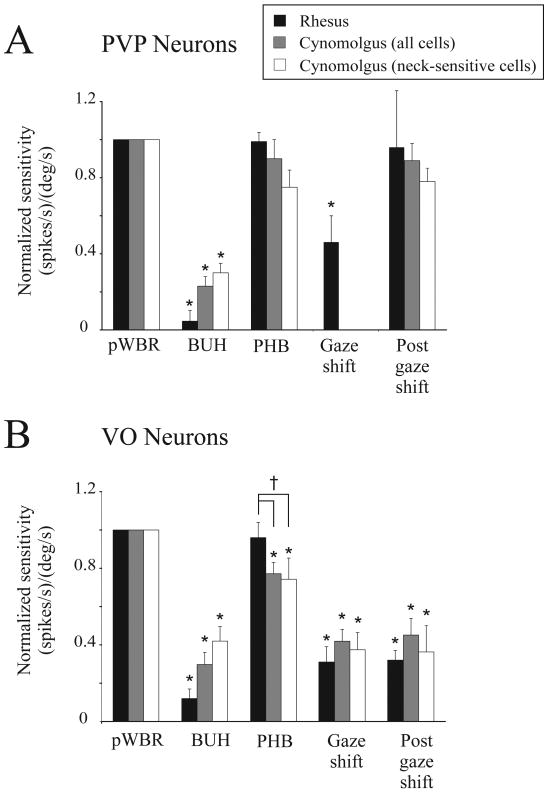
Information from neck proprioceptors is sent to the vestibular nuclei (VN) via monosynaptic excitatory connections between the central cervical nucleus (CCN) and contralateral VN (Sato et al. 1997) as well as by means of direct projections from the cerebellum (Akaike 1983; Eccles et al. 1974; Furuya et al. 1975; Noda et al. 1990; Robinson et al. 1994). Previous studies in decerebrate cats (Kasper et al. 1988; lateral and descending vestibular nuclei) as well as in alert squirrel monkeys (Gdowski and McCrea 2000) have shown that a significant number of neurons in the vestibular nuclei are modulated in response to neck (BUH) stimulation (50 vs. 78%, respectively). Here, we found that ∼50% of both VO and PVP neurons in the medial vestibular nucleus of cynomolgus monkeys are sensitive to neck proprioceptive stimulation. Even when neurons with and without neck sensitivity are pooled together, both VO and PVP neurons showed significant neck sensitivity (Figure 5, BUH paradigm, grey bars). Interestingly we also observed a noticeable difference in the proportion of neck sensitive cells between the two animals, particularly for VO neurons (80% in monkey G and 28% monkey D). However, it is important to emphasize that the results in both animals were markedly different from those obtained in our recent studies in rhesus monkeys (macaca mulatta) where neither VO nor PVP neurons are responsive to passive stimulation of the neck proprioceptors (Roy and Cullen 2001b; 2002) (Figure 5A and 5B, BUH paradigm, black bars).
Figure 5.
Comparison of VO and PVP neuronal responses in rhesus and cynomolgus monkeys during different paradigms. (A) Average vestibular and neck sensitivity of PVP neurons measured during pWBR and BUH rotations, respectively. Responses were normalized relative to vestibular sensitivity obtained during pWBR. PVP neurons in rhesus monkey exhibit negligible neck proprioceptive sensitivity (BUH, black bar), while those in cynomolgus monkey are significantly modulated (BUH, white bar). Although, sensitivities were slightly attenuated during PHB rotations compared to pWBR, the difference was not significant for cynomolgus PVP neurons. During active eye-head gaze shifts, the modulation of rhesus PVP neurons (black bars) was reduced when compared to passive movements (rhesus normalized sensitivity = 0.46 ± 0.14). However, during the post gaze shift interval, neuronal sensitivities were similar to those obtained during PHB rotation (rhesus normalized sensitivity = 0.96 ± 0.34 vs. 0.99 ± 0.05, respectively). Data for rhesus PVP neurons was taken from Roy and Cullen (2002; n = 24 for pWBR, gaze shifts and post gaze shifts, and n = 12 for BUH and PHB). (B) Average vestibular and neck sensitivity of VO neurons measured during pWBR and BUH rotations, respectively. Responses were normalized relative to vestibular sensitivity obtained during pWBR. VO neurons in rhesus monkey exhibit negligible neck proprioceptive sensitivity (BUH, black bar), while those in cynomolgus monkey are significantly modulated (BUH, white bar). Responses of VO neurons in the cynomolgus monkey during PHB rotations were significantly decreased compared to those obtained during pWBR (PHB, white bar). In contrast, rhesus monkey VO neurons did not show a change in their responses (PHB, black bar, normalized sensitivity = 0.96 ± 0.08). During gaze shifts and post-gaze shift intervals, neuronal responses were attenuated in both species (rhesus monkey normalized sensitivity = 0.31 ± 0.08 and 0.32 ± 0.05, respectively). Data for rhesus VO neurons was taken from Roy and Cullen (2001; n = 40 for pWBR, gaze shifts and post gaze shifts, n = 15 for BUH, and n = 23 for PHB). See text for details of cynomolgus monkey data. * shows significant difference with pWBR, † shows significant difference between rhesus and cynomolgus monkeys.
The apparent differences across species in relation to the strategy used to integrate neck information with vestibular processing (at the level of the vestibular nucleus) could potentially reflect differences in behavioral strategies used in daily life. For example, the cervico-ocular reflex (COR) is activated by rotation of the neck and works in conjunction with the vestibulo-ocular reflex (VOR) to stabilize visual gaze. Prior studies have evaluated the performance of this reflex by passively rotating the body under a stationary head (i.e. the BUH paradigm used in the present study) in order to evaluate the effect of neck proprioceptor activation on eye movements. Notably, over the same frequency range used in the study, squirrel monkeys have a robust COR with gains as large as 0.4 (Gdowski et al. 2001; Gdowski and McCrea 2000). In contrast, comparable stimulation in rhesus monkeys demonstrates that the COR response is negligible in this species (Roy and Cullen 2002). Thus, when only these two species are considered – the neck sensitivity of neurons in the vestibular nuclei is predictive of the strength of the COR. Based on this observation, one might predict that the COR response produced by cynomolgus monkeys would also be robust. Interestingly, however, when we tested our monkeys, no consistent COR response was observed: reflex gains were virtually non-existent with mean values of 0.03, 0.02 and 0.02 for 0.5, 1 and 2 Hz, respectively. Similarly, no significant COR in cats is present during yaw rotations in this frequency range (Baker et al. 1982). Thus, it seems that although neck proprioceptive afferent projections to VN are present in cat (non-primate mammal), squirrel monkey (New World monkey), rhesus monkey (Old World monkey), and cynomolgus monkey (Old World monkey), there are differences in the way the neck signal is utilized by VN neurons in these animals. Putative functional roles for these neck signals are further discussed below.
One possibility is that the difference between the two species of macaques is the result of a sampling bias. However, this seems unlikely. In the present study we found that ∼50% of neurons (in both animals) were sensitive to the activation of neck proprioceptors. In our previous studies in rhesus, we directly tested the neck sensitivity of a comparable number of neurons (total = 27). Assuming a 50% chance of neck sensitivity, the probability that these previous studies have only recorded from neurons that have no neck sensitivity would be 0.527 (0.000000007). In addition, differences in the training history of monkeys are not likely to underlie the observed differences. Notably, the training, experimental setup and experimental procedures that rhesus and cynomolgus monkeys underwent in our lab were identical. As such, we believe that the observed difference between the two species is not an artifact of the experiments and is the result of differences between the neural processing by the two species (see below).
Multimodal integration: convergence of vestibular and neck signals
In the present study, during passive head-on-body rotations, the responses of all neurons could be predicted based on the sum of their vestibular sensitivity during passive whole body rotation and neck sensitivity during passive rotation of the body under head. This finding is consistent with previous studies in anesthetized/decerebrate cats (Kasper et al. 1988) and alert squirrel monkeys (Gdowski et al. 2001). Similar to these previous studies, we also found that for both PVP and VO neurons, neck and vestibular sensitivities were typically antagonistic such that their summation during passive head-on-body movements resulted in attenuation of the vestibular response, where the effect was more pronounced for VO neurons (Figure 5A and 5B, compare pWBR and PHB paradigms). This result differs from that found in rhesus monkeys (Roy and Cullen 2001b) where all neurons were insensitive to neck motion, and modulation was comparable during passive whole body and passive head on body rotations (Figure 5, compare black bars for PHB and pWBR).
During active head-on-body rotations, however, the summation of vestibular and neck sensitivities (estimated in passive rotation paradigms) did not reliably predict neuronal responses. First, during active eye-head gaze shifts, the modulation of PVP neurons was greatly attenuated relative to the summation prediction. Neuronal firing often completely paused during the early part of the gaze shift, gradually resuming activity at gaze shift end. This observed attenuation of the PVP modulation during gaze shifts effectively reduces the efficacy of the VOR. This is important since the VOR is counterproductive during gaze shifts; it would produce an eye movement command in the direction opposite to that of the intended gaze shift (Cullen et al. 2004). Taken together with similar findings in other species (rhesus monkey: reviewed in Angelaki and Cullen 2008; reviewed in Cullen and Roy 2004; Roy and Cullen 2002; squirrel monkey: Gdowski and McCrea 2000), our results are consistent with the proposal that during gaze shifts saccadic burst neurons in the brain stem send a strong inhibitory input to PVP neurons to reduce the efficacy of the VOR (Cullen and Roy 2004).
In contrast, during active head movements where gaze is stable (such as the interval immediately following a gaze shift where the eye is aligned with the target, but the head is still moving), PVP neurons in the cynomolgus monkey were substantially modulated. Moreover, responses could be predicted by the linear summation of neck and vestibular signals (estimated during pWBR and BUH rotation, respectively) as was the case during passive head on body movements. Accordingly, as shown in Figure 5A, sensitivities were comparable during PHB and Post gaze shift interval. That PVP neurons continue to respond during active head movements when gaze is stable has important functional implications since, to hold gaze (i.e., eye-in-space) stable, it is beneficial for the oculomotor system to generate a VOR. This finding is also consistent with the results of previous studies in rhesus monkeys (Figure 5A, black bars; Roy and Cullen 2002).
Unlike PVP neurons, the responses of VO neurons were consistently attenuated during active head-on-body movements regardless of the ongoing gaze behavior. The summation of vestibular and neck sensitivities (estimated during passive rotation paradigms) overestimated neuronal responses during and following gaze shifts. Accordingly, as is shown in Fig. 4B (gray and white bars) neuronal sensitivities were reduced during gaze shifts as well as the post gaze shift interval when compared to passive whole-body rotations. Moreover, unlike PHB the suppression in neck-sensitive neurons was greater than could be accounted for based on the summation of vestibular and neck sensitivities during PHB rotations (Figure 5B, compare white bars for PHB). Comparable levels of attenuation during active head movements have been reported for VO neurons in rhesus monkey (black bars; Roy and Cullen 2001), as well as for many VO neurons in squirrel monkeys (Gdowski and McCrea 2000). Notably, while direct inputs from neck proprioceptors have not been observed in alert rhesus monkeys during body-under-head rotations, neck signals are used to gate in an inhibitory signal during active head rotations in conditions where there is a match between the motor command to the neck and the resultant proprioceptive feedback (e.g., Roy and Cullen 2004). We propose that a similar mechanism underlies the augmented suppression modulation during active head movements in cynomolgus monkeys.
Multimodal Integration in the vestibular nuclei: functional role
What is the functional role of the multimodal integration observed in the vestibular nuclei of cynomolgus monkeys? Information from the vestibular system describes the motion of the head relative to space. However, during self-motion the maintenance of posture and balance requires knowledge of body movement. The integration of vestibular and neck proprioceptive signals - the latter of which provides information concerning the orientation of the head relative to the body - could underlie a coordinate transformation of head-centered information (i.e. head movement) into a body-centered reference frame (reviewed in Angelaki and Cullen 2008).
In rhesus monkeys, neurons that receive both vestibular and neck inputs have been reported in the fastigial nucleus of the cerebellum (Brooks and Cullen 2007). In turn, neurons in the fastigial nucleus have been shown to encode self-motion in a body-centered reference frame (Kleine et al. 2004; Shaikh et al. 2005; Shaikh et al. 2004). The fastigial nucleus has strong reciprocal connections with the vestibular nuclei, which in the case of cynomolgus monkeys appears to result in similar firing behaviors. The similarity is most notable, for vestibular-only (VO) neurons which have been implicated in vestibular spinal reflexes and also most likely send projections to higher-order structures within the cerebellum and cortex.
Implications for the observed integration of signals
There are more than 19 different recognized species within the macaque genus, which is considered the most widespread primate genus over the planet (Morales and Melnick 1998). While it is estimated that one species of macaque will have no more than about 1% difference in its genomic sequence from another (Page and Goodman 2001; Stewart and Disotell 1998), there are variations across species in habitat, diet, and social behavior. For example, cynomolgus monkeys can spend up to 97% of their time in trees whereas rhesus monkeys are more terrestrial (Wheatley 1980). The need to adopt a more arboreal posture might enhance the efficacy of mechanisms that support the stabilization of the head and/or torso. In contrast, inherently a terrestrial posture is more biomechanically secure. It is possible that the differences in strategies used for the integration of vestibular and proprioceptive sensory information in cynomolgus versus rhesus monkeys reflect, at least in part, this difference in life style.
Ultimately, which of these species of monkeys provides a better model for multimodal integration in the human brain remains to be determined. Humans and Old World monkeys diverged at about 25 million years ago (Goodman et al. 1998; Osada et al. 2001). The average human-macaque sequence identity is ∼93% (International HapMap Consortium 2007). Prior studies have shown that small genetic variations can result in marked phenotypic changes (Goodman et al. 1990). For example, mutations in the phenylalanine hydroxylase gene in human that cause phenylketonuria is actually part of the normal genome of the macaque and yet have no phenotypic effect in this animal (International HapMap Consortium 2007). As such, it is evident that, even with only small variants in the genome different strategies can evolve to solve functional problems.
Supplementary Material
Acknowledgments
We thank Dr. José A. Correa, McGill University Statistical Consulting Service, for help in statistical analysis of data with SAS software. We thank Marion Van Horn, Jessica Brooks, and Mohsen Jamali for critically reading the manuscript. This work was supported by the Canadian Institutes of Health Research (CIHR) and NIH R01 DC02390.
References
- Ackermann R, Cheverud J. Morphological Integration in Primate Evolution. In: Pigliucci M, Preston K, editors. Phenotypic Integration: Studying the Ecology and Evolution the Ecology and Evolution of Complex Phenotypes. New York: Oxford University Press; 2004. pp. 302–317. [Google Scholar]
- Akaike T. Electrophysiological analysis of cerebellar corticovestibular and fastigiovestibular projections to the lateral vestibular nucleus in the cat. Brain Res. 1983;272:223–235. doi: 10.1016/0006-8993(83)90568-1. [DOI] [PubMed] [Google Scholar]
- Angelaki DE, Cullen KE. Vestibular system: the many facets of a multimodal sense. Annu Rev Neurosci. 2008;31:125–150. doi: 10.1146/annurev.neuro.31.060407.125555. [DOI] [PubMed] [Google Scholar]
- Baker J, Goldberg J, Peterson B, Schor R. Oculomotor reflexes after semicircular canal plugging in cats. Brain Res. 1982;252:151–155. doi: 10.1016/0006-8993(82)90989-1. [DOI] [PubMed] [Google Scholar]
- Brooks J, Cullen KE. Society for Neuroscience. San Diego, CA: 2007. Reference frames and reafference in the rostral fastigial nucleus. [Google Scholar]
- Cherif S, Cullen KE, Galiana HL. An improved method for the estimation of firing rate dynamics using an optimal digital filter. J Neurosci Methods. 2008;173:165–181. doi: 10.1016/j.jneumeth.2008.05.021. [DOI] [PubMed] [Google Scholar]
- Cullen KE, Huterer M, Braidwood DA, Sylvestre PA. Time course of vestibuloocular reflex suppression during gaze shifts. J Neurophysiol. 2004;92:3408–3422. doi: 10.1152/jn.01156.2003. [DOI] [PubMed] [Google Scholar]
- Cullen KE, McCrea RA. Firing behavior of brain stem neurons during voluntary cancellation of the horizontal vestibuloocular reflex. I. Secondary vestibular neurons. J Neurophysiol. 1993;70:828–843. doi: 10.1152/jn.1993.70.2.828. [DOI] [PubMed] [Google Scholar]
- Cullen KE, Roy JE. Signal processing in the vestibular system during active versus passive head movements. J Neurophysiol. 2004;91:1919–1933. doi: 10.1152/jn.00988.2003. [DOI] [PubMed] [Google Scholar]
- Dickman JD, Angelaki DE. Dynamics of vestibular neurons during rotational motion in alert rhesus monkeys. Exp Brain Res. 2004;155:91–101. doi: 10.1007/s00221-003-1692-1. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Rantucci T, Sabah NH, Taborikova H. Somatotopic studies on cerebellar fastigial cells. Exp Brain Res. 1974;19:100–118. doi: 10.1007/BF00233397. [DOI] [PubMed] [Google Scholar]
- Fleagle J. Size distribution of living and fossil primate faunas. Paleobiology. 1978;4:67–76. [Google Scholar]
- Fukushima K. Corticovestibular interactions: anatomy, electrophysiology, and functional considerations. Exp Brain Res. 1997;117:1–16. doi: 10.1007/pl00005786. [DOI] [PubMed] [Google Scholar]
- Furuya N, Kawano K, Shimazu H. Functional organization of vestibulofastigial projection in the horizontal semicircular canal system in the cat. Exp Brain Res. 1975;24:75–87. doi: 10.1007/BF00236018. [DOI] [PubMed] [Google Scholar]
- Gdowski GT, Belton T, McCrea RA. The neurophysiological substrate for the cervico-ocular reflex in the squirrel monkey. Exp Brain Res. 2001;140:253–264. doi: 10.1007/s002210100776. [DOI] [PubMed] [Google Scholar]
- Gdowski GT, McCrea RA. Neck proprioceptive inputs to primate vestibular nucleus neurons. Exp Brain Res. 2000;135:511–526. doi: 10.1007/s002210000542. [DOI] [PubMed] [Google Scholar]
- Goodman M, Porter CA, Czelusniak J, Page SL, Schneider H, Shoshani J, Gunnell G, Groves CP. Toward a Phylogenetic Classification of Primates Based on DNA Evidence Complemented by Fossil Evidence. MOLECULAR PHYLOGENETICS AND EVOLUTION. 1998;9:585–598. doi: 10.1006/mpev.1998.0495. [DOI] [PubMed] [Google Scholar]
- Goodman M, Tagle DA, Fitch DHA, Wendy Bailey JC, Koop BF, Benson P, Slightom JL. Primate evolution at the DNA level and a classification of hominoids. Journal of Molecular Evolution. 1990;30:260–266. doi: 10.1007/BF02099995. [DOI] [PubMed] [Google Scholar]
- Huterer M, Cullen KE. Vestibuloocular reflex dynamics during high-frequency and high-acceleration rotations of the head on body in rhesus monkey. J Neurophysiol. 2002;88:13–28. doi: 10.1152/jn.2002.88.1.13. [DOI] [PubMed] [Google Scholar]
- International HapMap Consortium. A second generation human haplotype map of over 3.1 million SNPs. Nature. 2007;449:851–861. doi: 10.1038/nature06258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper J, Schor RH, Wilson VJ. Response of vestibular neurons to head rotations in vertical planes. II. Response to neck stimulation and vestibular-neck interaction. J Neurophysiol. 1988;60:1765–1778. doi: 10.1152/jn.1988.60.5.1765. [DOI] [PubMed] [Google Scholar]
- Kleine JF, Guan Y, Kipiani E, Glonti L, Hoshi M, Buttner U. Trunk position influences vestibular responses of fastigial nucleus neurons in the alert monkey. J Neurophysiol. 2004;91:2090–2100. doi: 10.1152/jn.00849.2003. [DOI] [PubMed] [Google Scholar]
- Kowalchuk RK, Keselman HJ, Algina J, Wolfinger RD. The Analysis of Repeated Measurements with Mixed-Model Adjusted F Tests. Educational and Psychological Measurement. 2004;64:224–242. [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. SAS for Mixed Models. Cary, NC: SAS Institute; 2006. [Google Scholar]
- Morales JC, Melnick DJ. Phylogenetic relationships of the macaques (Cercopithecidae: Macaca), as revealed by high resolution restriction site mapping of mitochondrial ribosomal genes. J Hum Evol. 1998;34:1–23. doi: 10.1006/jhev.1997.0171. [DOI] [PubMed] [Google Scholar]
- Noda H, Sugita S, Ikeda Y. Afferent and efferent connections of the oculomotor region of the fastigial nucleus in the macaque monkey. J Comp Neurol. 1990;302:330–348. doi: 10.1002/cne.903020211. [DOI] [PubMed] [Google Scholar]
- Osada N, Hida M, Kusuda J, Tanuma R, Iseki K, Hirata M, Suto Y, Hirai M, Terao K, Suzuki Y, Sugano S, Hashimoto K. Assignment of 118 novel cDNAs of cynomolgus monkey brain to human chromosomes. Gene. 2001;275:31–37. doi: 10.1016/s0378-1119(01)00665-5. [DOI] [PubMed] [Google Scholar]
- Page SL, Goodman M. Catarrhine phylogeny: noncoding DNA evidence for a diphyletic origin of the mangabeys and for a human-chimpanzee clade. Mol Phylogenet Evol. 2001;18:14–25. doi: 10.1006/mpev.2000.0895. [DOI] [PubMed] [Google Scholar]
- Ramachandran R, Lisberger SG. Neural substrate of modified and unmodified pathways for learning in monkey vestibulo-ocular reflex. J Neurophysiol. 2008 doi: 10.1152/jn.90498.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson FR, Phillips JO, Fuchs AF. Coordination of gaze shifts in primates: brainstem inputs to neck and extraocular motoneuron pools. J Comp Neurol. 1994;346:43–62. doi: 10.1002/cne.903460104. [DOI] [PubMed] [Google Scholar]
- Roy JE, Cullen KE. Dissociating self-generated from passively applied head motion: neural mechanisms in the vestibular nuclei. J Neurosci. 2004;24:2102–2111. doi: 10.1523/JNEUROSCI.3988-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy JE, Cullen KE. A neural correlate for vestibulo-ocular reflex suppression during voluntary eye-head gaze shifts. Nat Neurosci. 1998;1:404–410. doi: 10.1038/1619. [DOI] [PubMed] [Google Scholar]
- Roy JE, Cullen KE. Passive activation of neck proprioceptive inputs does not influence the discharge patterns of vestibular nuclei neurons. Ann N Y Acad Sci. 2001a;942:486–489. doi: 10.1111/j.1749-6632.2001.tb03776.x. [DOI] [PubMed] [Google Scholar]
- Roy JE, Cullen KE. Selective processing of vestibular reafference during self-generated head motion. J Neurosci. 2001b;21:2131–2142. doi: 10.1523/JNEUROSCI.21-06-02131.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy JE, Cullen KE. Vestibuloocular reflex signal modulation during voluntary and passive head movements. J Neurophysiol. 2002;87:2337–2357. doi: 10.1152/jn.2002.87.5.2337. [DOI] [PubMed] [Google Scholar]
- Sadeghi SG, Chacron MJ, Taylor MC, Cullen KE. Neural variability, detection thresholds, and information transmission in the vestibular system. J Neurosci. 2007a;27:771–781. doi: 10.1523/JNEUROSCI.4690-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi SG, Minor LB, Cullen KE. Response of vestibular-nerve afferents to active and passive rotations under normal conditions and after unilateral labyrinthectomy. J Neurophysiol. 2007b;97:1503–1514. doi: 10.1152/jn.00829.2006. [DOI] [PubMed] [Google Scholar]
- Sato H, Ohkawa T, Uchino Y, Wilson VJ. Excitatory connections between neurons of the central cervical nucleus and vestibular neurons in the cat. Exp Brain Res. 1997;115:381–386. doi: 10.1007/pl00005708. [DOI] [PubMed] [Google Scholar]
- Scudder CA, Fuchs AF. Physiological and behavioral identification of vestibular nucleus neurons mediating the horizontal vestibuloocular reflex in trained rhesus monkeys. J Neurophysiol. 1992;68:244–264. doi: 10.1152/jn.1992.68.1.244. [DOI] [PubMed] [Google Scholar]
- Shaikh AG, Ghasia FF, Dickman JD, Angelaki DE. Properties of cerebellar fastigial neurons during translation, rotation, and eye movements. J Neurophysiol. 2005;93:853–863. doi: 10.1152/jn.00879.2004. [DOI] [PubMed] [Google Scholar]
- Shaikh AG, Meng H, Angelaki DE. Multiple reference frames for motion in the primate cerebellum. J Neurosci. 2004;24:4491–4497. doi: 10.1523/JNEUROSCI.0109-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CB, Disotell TR. Primate evolution - in and out of Africa. Curr Biol. 1998;8:R582–588. doi: 10.1016/s0960-9822(07)00367-3. [DOI] [PubMed] [Google Scholar]
- Sylvestre PA, Cullen KE. Quantitative analysis of abducens neuron discharge dynamics during saccadic and slow eye movements. J Neurophysiol. 1999;82:2612–2632. doi: 10.1152/jn.1999.82.5.2612. [DOI] [PubMed] [Google Scholar]
- Wheatley B. In: Feeding and ranging of east Bornean Macaca fascicularis. Lindburg D, editor. New York: Princeton; 1980. pp. 215–246. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.