Abstract
Bacterial lipopolysaccharide endotoxins (LPS) elicit inflammatory responses reflective of acute bacterial infection. We determine if feeding ewes high (15.5 %) or low (8.5 %) CP diets for 10 d altered inflammatory responses to an i.v. bolus of 0 (control), 0.75 (L75), or 1.50 (L150) μg LPS/kg BW in a 2 × 3 factorial (n = 5/treatment). Rectal temperatures, heart and respiratory rates, blood leukocyte concentrations, and serum cortisol, insulin, and glucose concentrations were measured for 24 h after an LPS bolus (bolus = 0 h). In general, rectal temperatures were greater (P ≤ 0.05) in control ewes fed high CP, but LPS increased (P ≤ 0.05) rectal temperature in a dose-dependent manner at most times between 2 and 24 h after bolus. Peak rectal temperature in L75 and L150 occurred 4 h after bolus. A monophasic, dose-independent rise (P ≤ 0.023) in serum cortisol occurred from 0.5 to 24 h after bolus, with peak cortisol at 4 h. Serum insulin was increased (P ≤ 0.016) by LPS in a dose-dependent manner from 4 to 24 h after bolus. Insulin did not differ between control ewes fed high and low CP diets but was greater (P < 0.001) in L75 ewes fed low compared with high CP and in L150 ewes fed high compared with low CP. Elevated insulin was not preceded by elevated serum glucose. Total white blood cell concentrations were not affected (P ≥ 0.135) by LPS, but neutrophil and monocyte fractions of white blood cells were increased (P ≤ 0.047) by LPS at 12 and 24 h and at 24 h after bolus, respectively, and lymphocyte fraction was increased (P = 0.037) at 2 h and decreased (P ≤ 0.006) at 12 and 24 h after bolus. Red blood cell and hemoglobin concentrations and hematocrit (%) were elevated (P ≤ 0.022) by LPS at 2 and 4 h after bolus. Rectal temperatures and serum glucose were greater (P ≤ 0.033) in ewes fed a high CP diet before LPS injection, but these effects were lost at and within 2.5 h of bolus, respectively. Feeding high CP diets for 10 d did not reduce inflammation in ewes during the first 24 h after LPS exposure but may benefit livestock by preventing acute insulin resistance when endotoxin exposure is mild.
Keywords: Cortisol, crude protein, insulin, lipopolysaccharides, white blood cells
INTRODUCTION
Bacterial and viral infections reduce livestock performance, increase morbidity and death loss, and are leading sources of profit loss in livestock (Duff and Galyean, 2007). Exposure to bacterial lipopolysaccharides (LPS) replicates infection in livestock (Steiger et al., 1999; Thorgersen et al., 2010) and allows the study of inflammatory responses without the risk of using a live pathogen. In previous studies, LPS exposure depleted plasma AA and glucose concentrations (Briard et al., 2000; Waggoner et al., 2009) and elevated serum cortisol (You et al., 2008; Karrow et al., 2010). These findings indicate that catabolism increases during systemic inflammation (Soto et al., 1998) and may increase CP requirements (Waggoner et al., 2009). Effects of feeding CP above NRC requirements (NRC, 2007) on immune function and inflammation in livestock are not clear (Duff and Galyean, 2007), but CP deficiency in mice suppressed viral defenses, which in turn predisposed the mice to bacterial infections (Jakab et al., 1981). In this study, we sought to determine whether feeding diets with CP concentrations above NRC requirements over a relatively short period of time reduced inflammation in ewes exposed to bacterial endotoxins.
MATERIALS AND METHODS
Animals and Experimental Procedure
All procedures involving animals were approved by the New Mexico State University Institutional Animal Care and Use Committee. Thirty mature (4 to 6 yr of age) Rambouillet ewes in moderate body condition (61.8 ± 1.4 kg BW) were housed in adjacent individual 1.5 × 4-m pens and given free access to shade and water for the duration of the experiment. Ewes were randomly assigned to treatment combinations in a 2 × 3 factorial arrangement. Each ewe was fed either above (high CP) or just below (low CP) NRC requirements for dietary CP (NRC, 2007; Table 1) at an amount equal to 3 % of initial BW (as fed basis). Ewes were fed once daily at 1800 for 10 d. Beginning at 0900 on d 11, each ewe was injected intravenously with a bolus of bacterial LPS (Escherichia coli O55:B55, Sigma Chemical Co., St. Louis, MO) at 0 (control), 0.75 (L75), or 1.50 (L150) μg/kg BW. A bulk LPS solution was prepared by dissolving 2,000 μg LPS in 100 mL of sterile physiological saline. Thus, injected volumes ranged from 1.8 to 5.6 mL of LPS solution, depending on individual BW and prescribed LPS treatment. Each control ewe was intravenously injected with 2 mL sterile physiological saline. Blood samples, heart rate, respiratory rate, and rectal temperature were collected from each ewe 0.5 h before (−0.5 h) and immediately before (0 h) LPS injection, and at 0.5, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 12, and 24 h after LPS injection. Feeding on this day occurred immediately after sample collection at 12 h.
Table 1.
Ingredient and nutrient composition of diets fed to mature ewes once daily for 10 d before administration of bacterial LPS.
| Item | Low CP | High CP |
|---|---|---|
| Ingredient, % of DM | ||
| Sudan hay | 60.0 | 60.0 |
| Corn grain, cracked | 33.9 | 13.9 |
| Soybean meal | - | 20.0 |
| Molasses | 5.0 | 5.0 |
| Limestone | 0.75 | 0.75 |
| Salt | 0.20 | 0.20 |
| Dicalcium phosphate | 0.15 | 0.15 |
| Nutrient, % of DM1 | ||
| ADF | 28.4 | 29.5 |
| CP | 8.5 | 15.5 |
| Ca | 0.93 | 0.94 |
| P | 0.34 | 0.41 |
Analyzed by SDK Laboratories (Hutchinson, KS).
Blood Sample Analyses
Serum Cortisol, Insulin, and Glucose
Blood samples were collected via jugular venipuncture into 10-mL vacuum tubes (Corvac serum-separator, Kendall Health Care, St. Louis, MO). Blood samples were kept at room temperature for 30 to 60 min and then centrifuged (1,500 × g at 4°C for 15 min). After centrifugation, serum was stored in plastic vials at −80°C until thawed for assay. Serum cortisol concentrations were quantified by solid-phase RIA using components of a commercial kit (Coat-A-Count, Siemens Medical Solutions Diagnostics, Los Angeles, CA) with modifications described by Kiyma et al. (2004). Within-assay and between-assay CV for cortisol determinations were less than 10%. Serum insulin concentrations were determined from duplicate 50-μL aliquots with a commercial ELISA kit (Ovine Insulin; ALPCO Diagnostics, Windham, NH) for samples collected at 0, 0.5, 2, 4, 12, and 24 h after bolus. Serum glucose concentrations were quantified colorimetrically with a commercially available hexokinase reagent (Infinity TR15241, Thermo Scienticic, Waltham, MA), as previously described (Waggoner et al., 2009). Within-assay and between-assay CV for insulin and glucose determinations were less than 15%.
Complete Blood Counts
Whole blood samples were collected by jugular venipuncture into EDTA-containing vacuum tubes at 0, 2, 4, 12, and 24 h after LPS bolus. Samples were packaged on ice immediately after collection and were shipped overnight to the Veterinary Diagnostics Services, Albuquerque, NM, for complete blood count analysis, which included quantification of total white blood cells, total red blood cells, hemoglobin, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin concentration, platelets, and neutrophil, lymphocyte, monocyte, eosinophil, and basophil fractions of total white blood cells.
Statistical Analysis
Data for respiratory rate, heart rate, rectal temperature, serum cortisol, serum glucose, serum insulin, white blood cell fractions, and hematological parameters were analyzed as a split plot design with ewe as the experimental unit. The main plot contained the 2 × 3 factorial arrangement of dietary CP (high CP or low CP), LPS (0, 0.75, or 1.50 μg/kg BW), and diet x LPS interaction. The sub plot contained time from LPS bolus and all associated interactions. Data were analyzed using the MIXED procedure of SAS (SAS Instit., Inc., Cary, NC) with repeated measures (time) and compound symmetry covariance structure. When F-tests were significant (P ≤ 0.05), linear (dose-dependent) and quadratic (dose-independent) relationships between LPS treatment means were examined with orthogonal polynomial contrasts. Area under curve for rectal temperature over the first 6 h after bolus was calculated by trapezoidal summation. Because of pretreatment differences between LPS-injected and control ewes for red blood cells, hemoglobin, and hematocrit, these data were reanalyzed with value at 0 h included in the model. Data are presented as least squares means ± SE.
RESULTS
Respiratory Rate and Heart Rate
There was a dose of LPS × time interaction (P ≤ 0.005) for respiratory rate and heart rate. Respiratory rate was greater (P ≤ 0.056) in control ewes than in LPS-treated ewes at 1 and 2.5 h after bolus, and heart rate was elevated (P ≤ 0.031) in L150 ewes at 5 and 12 h compared with controls. Respiratory rates in L75 and L150 ewes increased (P ≤ 0.001) from 3 to 4 h after bolus compared with rates at 0 h. In control and L75 ewes, heart rates were constant across the 24-h sampling period, whereas rate transiently increased (P = 0.051) in L150 ewes at 1.5 h compared with rate at 0 h (data not shown).
Rectal Temperature
There was a LPS × dietary CP concentration × time interaction (P = 0.020) for rectal temperature (Figure 1). In general, rectal temperatures were greater (P ≤ 0.05) in control ewes fed high CP compared with low CP, but LPS increased (P ≤ 0.05) rectal temperature in a dose-dependent manner, regardless of diet. Peak rectal temperature occurred 4 h after bolus in both L75 and L150. Area under curve for rectal temperature during the first 6 h after bolus did not differ (P = 0.580) between ewes fed high and low CP (108545 ± 286 and 108772 ± 286 units, respectively), but was increased (P < 0.001) by LPS independent of dose (106,039 ± 350, 110,061 ± 350, and 109,876 ± 350 units for controls, L75, and L150, respectively).
Figure 1.
Rectal temperatures of ewes fed high or low CP diets and administered i.v. boluses of LPS or saline (n = 5 per LPS x CP). Means with different superscripts at each time point differ (P ≤ 0.05). Where necessary, overlapping means share superscripts. Effects on rectal temperature were: LPS × CP × time (P = 0.020).
Serum Cortisol Concentration
There was an LPS × time interaction (P ≤ 0.001) for serum cortisol concentrations. In control ewes, serum cortisol concentrations (Figure 2) were constant (P ≥ 0.05) across the 24-h sampling period, but LPS injection increased (P ≤ 0.023) cortisol in a dose-independent manner at 0.5 h after bolus and all subsequent times. Peak serum cortisol in L75 and L150 ewes occurred 4 h after bolus. Serum cortisol concentrations did not differ (P = 0.293) between ewes fed high or low CP diets.
Figure 2.
Serum cortisol concentrations of ewes fed high or low CP diets and administered i.v. boluses of LPS or saline (n = 5 per LPS x CP). “*” indicates times at which a dose-independent effect of LPS was observed (P ≤ 0.047). Effects on serum cortisol concentration were: LPS x dietary CP x time (P = 0.466), CP x time (P = 0.642), LPS x time (P ≤ 0.001), LPS x CP (P = 0.327), time (P ≤ 0.001), and CP (P = 0.293).
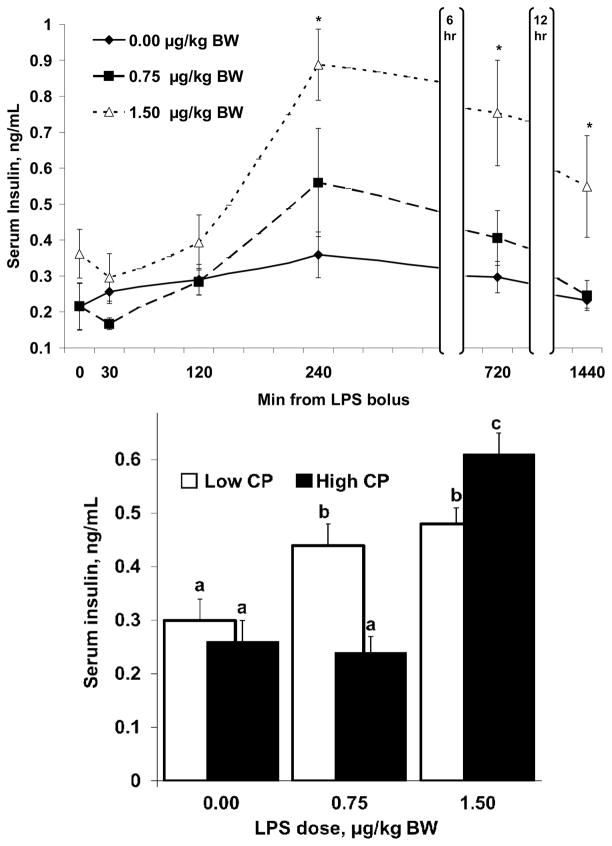
Serum Glucose and Insulin Concentrations
A dietary CP concentration × time interaction was observed (P = 0.002) for serum glucose concentrations. Serum glucose (Figure 3) was greater (P ≤ 0.033) in ewes fed high CP diets compared with those fed low CP diets at 1 and 2 h, and tended (P ≤ 0.092) to be greater at 0, 0.5, and 2.5 h after bolus. Serum glucose was reduced (P ≤ 0.059) in a dose-independent manner by LPS at 1.5 h and from 2.5 to 24 h after bolus. An LPS x time interaction was observed (P = 0.006) for serum insulin concentrations. Additionally, a tendency was observed (P = 0.083) for an LPS x CP interaction. Insulin (Figure 4) was constant (P ≥ 0.05) across the sampling period in control ewes but was increased (P ≤ 0.016) in a dose-dependent manner by LPS at 2 h and remained elevated in L150 ewes at 12 and 24 h after bolus. Serum insulin did not differ (P = 0.101) between high and low CP diets in control ewes but was greater (P < 0.001) in L75 ewes fed low CP and in L150 ewes fed high CP.
Figure 3.
Serum glucose concentrations of ewes administered i.v. boluses of LPS or saline (top; n = 5 per LPS x CP) after being fed high or low CP diets for 10 d (bottom). “*” indicates times at which a dose-independent effect of LPS was observed (top; P ≤ 0.059) or times at which means for ewes fed high or low dietary CP differed (bottom; P ≤ 0.033). “#” indicates times at which means for ewes fed high or low CP tended to differ (P ≤ 0.092). Effects on serum glucose concentration were: LPS x CP x time (P = 0.841), LPS x time (P = 0.197), CP x time (P = 0.002), CP x LPS (P = 0.806), time within CP (P ≤ 0.001), and LPS (P = 0.022).
Figure 4.
Serum insulin concentrations of ewes administered i.v. boluses of LPS or saline (top; n = 5 per LPS x CP) after being fed high or low CP diets for 10 d (bottom). ”*” indicates times at which a dose-dependent effect (4 and 12 h) or dose-independent effect (24 h) of LPS was observed (P ≤ 0.016). Means with different superscripts differ (P < 0.001). Effects on serum insulin concentration were: LPS x dietary CP x time (P = 0.350), LPS x time (P = 0.006), CP x time (P = 0.677), CP x LPS (P = 0.083), and time within LPS (P ≤ 0.001).
White Blood Cells
There was an LPS x time interaction (P ≤ 0.049) for neutrophil, lymphocyte, monocyte, and eosinophil fractions of total white blood cells, and a dietary CP concentration × time interaction (P = 0.050) for eosinophil fraction. Number of total white blood cells per microliter of blood was not affected (P ≥ 0.431) by LPS dose or CP concentration, but was less (P ≤ 0.014) at 2 h (7.7 ± 1.1 cells × 103) and 4 h (8.6 ± 1.2 cells × 103) than at 0 h (13.7 ± 1.0 cells × 103), 12 h (14.0 ± 1.4 cells × 103), and 24 h (16.5 ± 1.5 cells × 103) in all ewes, independent of diet or LPS dose. In control ewes, neutrophil, lymphocyte, and monocyte fractions of total white blood cells (Figure 5) were constant (P ≥ 0.05) across the 24-h sampling period. Neutrophil fractions were increased (P ≤ 0.010) by LPS in a dose-independent manner at 12 and 24 h. Lymphocyte fractions were increased (P ≤ 0.051) by LPS in a dose-independent manner at 2 h, not changed (P = 0.558) at 4 h, and decreased (P ≤ 0.006) in a dose-independent manner at 14 and 24 h after bolus. Monocyte fractions were increased by LPS (P = 0.026) in a dose-independent manner 24 h after bolus but were not different (P ≥ 0.05) before 24 h. Eosinophil fractions did not differ (P = 0.158) among LPS treatments but were less (P = 0.043) in ewes fed high CP at 2 and 24 h after bolus. We did not observe any effects (P ≥ 0.05) due to dietary CP, LPS, or time from bolus on basophil fraction of total white blood cells (data not shown).
Figure 5.
Neutrophil (top), lymphocyte (middle), and monocyte (bottom) fractions of total white blood cells (WBC) of ewes fed high or low CP diets and administered i.v. boluses of 0 (◆), 0.75 (■), or 1.50 (△) μg/kg BW LPS. (n = 5 per LPS x CP). “*” (dose-dependent) and “#” (dose-dependent) indicate times at which effects of LPS were observed (P ≤ 0.016). Effects on these fractions were: LPS x dietary CP x time (P ≥ 0.217), LPS x time (P ≤ 0.004), CP x time (P ≥ 0.075), CP x LPS (P ≥ 0.300), time (P ≤ 0.004), and CP (P ≥ 0.224).
Hematological Parameters
There was an LPS × time interaction (P ≤ 0.039) for red blood cells, hemoglobin, and hematocrit. Red blood cells, hemoglobin, and hematocrit (Figure 6) did not differ (P ≥ 0.05) between dietary CP concentrations, but were increased (P ≤ 0.022) in a dose-independent manner by LPS at 2 and 4 h after bolus. We did not observe any effects (P ≥ 0.05) due to dietary CP, LPS, or time from bolus on mean corpuscular volumes, mean corpuscular hemoglobin concentrations, or platelets (data not shown).
Figure 6.
Red blood cells (top), hemoglobin (middle), and hematocrit (bottom) of ewes fed high or low CP diets and administered i.v. boluses of 0 (◆), 0.75 (■), or 1.50 (△) μg/kg BW LPS. (n = 5 per LPS x CP). “*” indicates times at which a dose-independent effect of LPS was observed (P ≤ 0.022). Effects on these blood components were: LPS x dietary CP x time (P ≥ 0.388), LPS x time (P ≤ 0.034), CP x time (P ≥ 0.181), CP x LPS (P ≥ 0.217), time (P ≤ 0.002), CP (P ≥ 0.316), and time 0 (P ≤ 0.001).
DISCUSSION
Our results show that feeding CP above NRC requirements for 10 d did not reduce inflammation during the first 24 h after exposure to bacterial endotoxin. Rectal temperatures were greater in control ewes fed high CP compared with low CP for most of the first 6 h after saline bolus. Furthermore, rectal temperatures in all ewes fed high CP were generally greater than those fed low CP before LPS bolus, indicating greater CP concentration in the diet increased metabolic heat production as previously shown in rats (Yamaoka et al., 2009). Ahmed and Abdellatif (1995) found similar effects of dietary CP on body temperature in unchallenged desert sheep. However, CP effects were not observed in ewes injected with LPS, as rectal temperatures were elevated in a dose-dependent manner to magnitudes that were similar between high CP and low CP-fed ewes. This finding indicates that increased metabolic heat production due to greater CP is covered up by the febrile response to LPS or that metabolic disregulation occurs with endotoxicity.
Eosinophils were the only class of leukocytes affected by CP concentration and, along with basophils, were not affected by LPS injection. All other leukocytes responded to LPS at some point within 24 h of exposure, and all of these responses were independent of dietary CP. Dietary CP concentration previously had no effect on leukocyte profiles in healthy, unchallenged ewes (Gentry et al., 1999) and neutrophil and monocyte responses to endotoxin exposure were consistent with observations in pigs (Williams et al., 2009). Though leukocytes are known to produce pyrogenic cytokines, LPS-induced febrile responses in the present study preceded observed changes in blood leukocyte profiles and were likely initiated by a source other than white blood cells. Furthermore, we observed this rise in rectal temperature to be monophasic, while Whyte et al. (1989) observed a biphasic febrile response that bracketed changes in leukocyte profiles and attributed only the second peak in rectal temperature to leukocyte-derived cytokines. Morimoto et al. (1987) further explained that in rabbits, the initial rise in temperature is due to non-leukocyte derived pyrogenic compounds and that any leukocyte-induced pyrogenesis may be delayed by several hours. Regardless, of their influence on rectal temperature, leukocyte responses to LPS exposure were not affected by dietary CP concentration. Red blood cells, hemoglobin, and hematocrit all increased after LPS injection. Thorgersen et al. (2010) reported a similar increase in hemoglobin in pigs, though the mechanism by which this occurs is unclear.
Unlike other components of inflammation, insulin response to LPS was affected by dietary CP concentrations. In ewes fed low CP, insulin concentrations after injection of either LPS dose increased similarly when compared with controls. However, in ewes fed high CP, the smaller LPS dose did not affect insulin, while the larger dose increased it to an even greater extent than in ewes fed low CP. Additionally, elevated insulin concentrations were not preceded by hyperglycemia but instead coincided with elevated cortisol concentrations, indicating that serum insulin was elevated as a result of insulin insensitivity. Andrews and Walker (1999) reported that increased glucocorticoid action can oppose insulin signaling and reduce sensitivity in tissues, while cytokines may also contribute to transient insulin resistance (Kushibiki et al., 2000). Our data indicate that a larger dose of LPS was needed to elicit elevated insulin in ewes fed high CP diets but, when elicited, the elevation was more robust than in ewes fed low CP. Elevated insulin concentrations may have also resulted from stimulation by nonglucose substrates such as propionate, butyrate, or AA (Mcatee and Trenkle, 1971; Brockman, 1982), which may increase during inflammation.
In summary, feeding CP above NRC requirements for 10 d did little to alter the inflammatory response to injection of bacterial endotoxins but did appear to increase the dose needed to elicit acute insulin resistance. Furthermore, effects of high CP on serum glucose and rectal temperatures that were observed in the absence of LPS were lost after LPS exposure. These findings indicate that increasing CP concentration in the diet for a short period of time does not reduce inflammation during the first 24 h after endotoxin exposure but may benefit livestock by tempering insulin resistance when endotoxin exposure is mild.
Footnotes
Research was supported in part by the New Mexico Agric. Exp. Stn., Las Cruces, NM and by NIH award number R01DK084842 (S.W.L.) and by the Interdisciplinary Training in Cardiovascular Research (T32) institutional training grant (HL07249).
LITERATURE CITED
- Ahmed MMM, Abdellatif AM. Effect of dietary protein level on thermoregulation, digestion and water economy in desert sheep. Small Ruminant Research. 1995;18:51–56. [Google Scholar]
- Andrews RC, Walker BR. Glucocorticoids and insulin resistance: old hormones, new targets. Clin Sci. 1999;96:513–523. doi: 10.1042/cs0960513. [DOI] [PubMed] [Google Scholar]
- Briard N, Dadoun F, Pommier G, Sauze N, Lebouc Y, Oliver C, Dutour A. IGF-I/IGFBPs system response to endotoxin challenge in sheep. J Endocrinol. 2000;164:361–369. doi: 10.1677/joe.0.1640361. [DOI] [PubMed] [Google Scholar]
- Brockman RP. Insulin and glucagon responses in plasma to intraportal infusions of propionate and butyrate in sheep (Ovis aries) Comp Biochem Physiol A Comp Physiol. 1982;73:237–238. doi: 10.1016/0300-9629(82)90062-7. [DOI] [PubMed] [Google Scholar]
- Duff GC, Galyean ML. BOARD-INVITED REVIEW: Recent advances in management of highly stressed, newly received feedlot cattle. J Anim Sci. 2007;85:823–840. doi: 10.2527/jas.2006-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry LR, Fernandez JM, Ward TL, White TW, Southern LL, Bidner TD, Thompson DL, Jr, Horohov DW, Chapa AM, Sahlu T. Dietary protein and chromium tripicolinate in Suffolk wether lambs: effects on production characteristics, metabolic and hormonal responses, and immune status. J Anim Sci. 1999;77:1284–1294. doi: 10.2527/1999.7751284x. [DOI] [PubMed] [Google Scholar]
- Jakab GJ, Warr GA, Astry CL. Alterations of pulmonary defense mechanisms by protein depletion diet. Infect Immun. 1981;34:610–622. doi: 10.1128/iai.34.2.610-622.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karrow NA, You Q, McNicoll C, Hay J. Activation of the ovine hypothalamic-pituitary-adrenal axis and febrile response by interleukin-6: a comparative study with bacterial lipopolysaccharide endotoxin. Can J Vet Res. 2010;74:30–33. [PMC free article] [PubMed] [Google Scholar]
- Kiyma Z, Alexander BM, Van Kirk EA, Murdoch WJ, Hallford DM, Moss GE. Effects of feed restriction on reproductive and metabolic hormones in ewes. J Anim Sci. 2004;82:2548–2557. doi: 10.2527/2004.8292548x. [DOI] [PubMed] [Google Scholar]
- Kushibiki S, Hodate K, Ueda Y, Shingu H, Mori Y, Itoh T, Yokomizo Y. Administration of recombinant bovine tumor necrosis factor-alpha affects intermediary metabolism and insulin and growth hormone secretion in dairy heifers. J Anim Sci. 2000;78:2164–2171. doi: 10.2527/2000.7882164x. [DOI] [PubMed] [Google Scholar]
- McAtee JW, Trenkle A. Metabolic regulation of plasma insulin levels in cattle. J Anim Sci. 1971;33:438–442. doi: 10.2527/jas1971.332438x. [DOI] [PubMed] [Google Scholar]
- Morimoto A, Murakami N, Myogin T, Takada M, Teshirogi S, Watanabe T. Separate mechanisms inside and outside the blood—brain barrier inducing metabolic changes in febrile rabbits. The Journal of Physiology. 1987;392:637–649. doi: 10.1113/jphysiol.1987.sp016801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC. Nutrient requirements of small ruminants: Sheep, goats, cervids, and new world camelids. 1. National Academies Press; Washington, D.C: 2007. [Google Scholar]
- Soto L, Martin AI, Millan S, Vara E, Lopez-Calderon A. Effects of endotoxin lipopolysaccharide administration on the somatotropic axis. J Endocrinol. 1998;159:239–246. doi: 10.1677/joe.0.1590239. [DOI] [PubMed] [Google Scholar]
- Steiger M, Senn M, Altreuther G, Werling D, Sutter F, Kreuzer M, Langhans W. Effect of a prolonged low-dose lipopolysaccharide infusion on feed intake and metabolism in heifers. J Anim Sci. 1999;77:2523–2532. doi: 10.2527/1999.7792523x. [DOI] [PubMed] [Google Scholar]
- Thorgersen EB, Hellerud BC, Nielsen EW, Barratt-Due A, Fure H, Lindstad JK, Pharo A, Fosse E, Tonnessen TI, Johansen HT, Castellheim A, Mollnes TE. CD14 inhibition efficiently attenuates early inflammatory and hemostatic responses in Escherichia coli sepsis in pigs. FASEB J. 2010;24:712–722. doi: 10.1096/fj.09-140798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner JW, Loest CA, Turner JL, Mathis CP, Hallford DM. Effects of dietary protein and bacterial lipopolysaccharide infusion on nitrogen metabolism and hormonal responses of growing beef steers. J Anim Sci. 2009;87:3656–3668. doi: 10.2527/jas.2009-2011. [DOI] [PubMed] [Google Scholar]
- Whyte RI, Warren HS, Greene E, Glennon ML, Robinson DR, Zapol WM. Tolerance to low-dose endotoxin in awake sheep. J Appl Physiol. 1989;66:2546–2552. doi: 10.1152/jappl.1989.66.6.2546. [DOI] [PubMed] [Google Scholar]
- Williams PN, Collier CT, Carroll JA, Welsh TH, Jr, Laurenz JC. Temporal pattern and effect of sex on lipopolysaccharide-induced stress hormone and cytokine response in pigs. Domest Anim Endocrin. 2009;37:139–147. doi: 10.1016/j.domaniend.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Yamaoka I, Hagi M, Doi M. Circadian Changes in Core Body Temperature, Metabolic Rate and Locomotor Activity in Rats on a High-Protein, Carbohydrate-Free Diet. Journal of Nutritional Science and Vitaminology. 2009;55:511–517. doi: 10.3177/jnsv.55.511. [DOI] [PubMed] [Google Scholar]
- You Q, Karrow NA, Cao H, Rodriguez A, Mallard BA, Boermans HJ. Variation in the ovine cortisol response to systemic bacterial endotoxin challenge is predominantly determined by signalling within the hypothalamic-pituitary-adrenal axis. Toxicol Appl Pharmacol. 2008;230:1–8. doi: 10.1016/j.taap.2008.01.033. [DOI] [PubMed] [Google Scholar]