Abstract
Ewing's sarcoma family tumors (ESFTs) are aggressive malignancies which frequently harbor characteristic EWS-FLI1 or EWS-ERG genomic fusions. Here we report that these fusion products interact with the DNA damage response protein and transcriptional co-regulator PARP-1. ESFT cells, primary tumor xenografts and tumor metastases were all highly sensitive to PARP1 inhibition. Addition of a PARP1 inhibitor to the second-line chemotherapeutic agent temozolamide resulted in complete responses of all treated tumors in an EWS-FLI1-driven mouse xenograft model of ESFT. Mechanistic investigations revealed that DNA damage induced by expression of EWS-FLI1 or EWS-ERG fusion genes was potentiated by PARP1 inhibition in ESFT cell lines. Notably, EWS-FLI1 fusion genes acted in a positive feedback loop to maintain the expression of PARP1, which was required for EWS-FLI-mediated transcription, thereby enforcing oncogene-dependent sensitivity to PARP-1 inhibition. Together, our findings offer a strong preclinical rationale to target the EWS-FLI1: PARP1 intersection as a therapeutic strategy to improve the treatment of Ewing's sarcoma family tumors.
Keywords: Ewing’s Sarcoma, Prostate cancer, Gene Fusion, FLI1, PARP1
Introduction
The Ewing’s sarcoma family of tumors (ESFTs) are malignant neoplasms of bone and soft tissue which frequently harbor reciprocal chromosomal translocations that result in the production of chimeric proteins containing the transcriptional activation domain from the Ewing’s sarcoma breakpoint region 1 gene (EWS) fused to the DNA binding domain of an ETS transcription factor (FLI1 or ERG) (1, 2). Dysregulation of these chimeric proteins have previously been implicated in abnormal proliferation, invasion, and tumorigenesis (3, 4). While the EWS-FLI or EWS-ERG fusion proteins represent potential disease-specific molecular targets, transcription factors such as these have been notoriously difficult to inhibit therapeutically. However, given the poor outcomes of patients with metastatic EFST (5), there is a clear need to improve therapy for these patients.
We have recently shown that an alternative strategy to inhibit transcription factor activity is to target critical interacting enzymes (6). Similar to ESFTs, 50% of prostate cancers harbor genomic rearrangements of ETS transcription factors (7). However, unlike the EWS-FLI or EWS-ERG fusions which produce a multifunctional chimeric protein, prostate cancer rearrangements usually place an androgen-regulated promoter upstream of an ETS gene (ERG or ETV1), resulting in increased ETS transcription factor activity by overexpression (3). We have previously demonstrated that PARP1 and DNA-dependent protein kinase (DNA-PKcs) are key ETS cofactors in prostate cancer, and therapeutic inhibition of PARP1 disrupts the growth of ETS positive, but not ETS negative, prostate cancer xenografts (6). Importantly, we demonstrated that this ETS protein interaction axis is mediated by the ETS DNA binding domain (6). While this interaction site is present in the EWS-FLI1 and EWS-ERG chimeras, these fusion proteins activate oncogenic pathways independent of their DNA binding domain (8). As such, despite the presence of the ETS DNA binding domain, the effectiveness of PARP inhibitors against these multifunctional gene fusion proteins is unclear.
Mechanistically, PARP1 is a functionally complex enzyme that has been shown to both drive transcription and to accelerate base excision repair (9–11). PARP1 inhibitors have demonstrated promising activity in early clinical trials (14), particularly in BRCA-mutant cancers defective in homologous repair (HR), in which they may cause replication fork stalling and subsequent synthetic lethal cell death (12, 13). We now hypothesize that the EWS-FLI and EWS-ERG gene fusions in ESFTs depend on the activity of PARP1 and may be targeted with PARP1 inhibition.
Materials and Methods
Cell Lines and inhibitors
PC3 , RD-ES, SAOS-2, A-204 (ATCC), CADO-ES1 (DSMZ), COG10 and COG258 cell lines were grown in RPMI 1640 (Invitrogen, Carlsbad, CA) supplemented with 10% FBS (Invitrogen). The genetic identity of each cell line was confirmed by genotyping samples. Olaparib was purchased from Axon Biochem.
Small RNA interference
Gene specific knockdown was accomplished using commercially available siRNA duplexes for EWS, ERG, FLI1, DNA-PKcs or PARP-1 (Dharmacon, Lafayette, CO). Two independent siRNA sequences were used for each gene for knockdown experiments.
In vitro assays
Soft agar colony formation, chemosensitivity, and basement membrane matrix invasion assays were performed per standard protocols (see SOM). Approaches for immunofluorescence, immunoprecipitation, western blot, COMET assays, luciferase reporter assays, and Oncomine analysis are also detailed in the SOM. Primer sequences available in Supplementary Table 1.
Xenografts SCID mice were purchased from Charles River, Inc. (Charles River Laboratory, Wilmington, MA) and implanted subcutaneously with RD-ES, PC3-luciferase-LACZ , or PC3-luciferase-EWS-FLI (1 × 106 cells/injection). After two weeks, mice with tumors were treated with Olaparib and/or Temozolamide. All procedures involving mice were approved by the University of Michigan UCUCA and conform to their relevant regulatory standards.
Additional experimental details are available in the SOM.
Results and Discussion
To test the hypothesis that EWS fusions interact with PARP1, we performed IP-immunoblot analysis on cell lines naturally harboring the rearrangements. Using an ERG or FLI1 antibody that recognizes the EWS-ERG and EWS-FLI1 fusion proteins, respectively, we pulled down both PARP1 and DNA-PKcs in a DNA-independent manner from two cell lines, CADO-ES1 (EWS-ERG) or RD-ES (EWS-FLI1) (Fig. 1A). Reverse IP from RD-ES cells performed using a PARP1 antibody enriched for EWS-FLI1 and DNA-PKcs in a DNA-independent manner (Fig. 1B), confirming the interaction between EWS fusions and PARP1.
Figure 1. Ewing’s sarcoma gene fusion products (EWS-FLI1 and EWS-ERG) interact with PARP1 and DNA-PKcs, and confer sensitivity to PARP1 inhibition.
A, Immunoprecipitation (IP)-immunoblot analysis performed on CADO-ES1 (EWS-ERG) cells using an ERG antibody and RD-ES (EWS-FLI1) cells using a FLI1 antibody. IPs were treated +/-100μg/mL ethidium bromide.
B, As in A, except using a PARP1 antibody on RD-ES cells.
C, Soft agar colony formation assays were performed using PC3 cells, overexpressing ERG, EWS-FLI1, EWS-ERG, or LACZ. Colonies counted after 3 weeks in culture. Representative photomicrographs shown. Scale bar= 50μM.
D, As in C, except in ETS-rearranged cells (VCaP, RD-ES, CADO-ES1, COG10 and COG258) and ETS-negative sarcoma controls (SAOS-2 and A-204).
Error bars indicate S.E.M. of three replicates. *p<0.05.
We then tested the sensitivity of ESFT cells to the PARP1 inhibitor Olaparib. To analyze the sensitivity of ESFT cells overexpressing the EWS-FLI1 and EWS-ERG fusions, compared to the prostate cancer harboring the TMPRSS2-ERG fusion, we performed soft agar colony formation assays on PC3 cells in which we had stably overexpressed these fusion products. Overexpression of any of these three ETS fusion genes caused sensitivity to Olaparib (p<0.05 at 3 and 10μM), and the EWS-FLI1 and EWS-ERG fusions were more sensitive than the TMPRSS2-ERG fusion (p<0.05 at 1μM) (Fig. 1C). Soft agar assays performed using CADO-ES1, RD-ES or VCaP (TMPRSS2-ERG rearranged prostate cancer) cells confirmed that while all ETS positive cell lines were more susceptible to Olaparib than the ETS negative cell line PC3, ESFT cell lines were even more sensitive than VCaP cells (p<0.05) (Fig. 1D). Importantly, Olaparib did not have an effect on an osteosarcoma cell line, SAOS-2, or a rhabdomyosarcoma cell line, A-204, which were used as sarcoma model controls without ETS rearrangement (Fig. 1D). We also found that two Ewing’s cell lines (COG10 and COG258 (both EWS-FLI1)) derived from front-line chemotherapy patient relapses (and thus heavily pretreated) were also extremely sensitive to PARP1 inhibition (Fig. 1D).
Given the sensitivity of ESFTs to PARP inhibition, we sought to explore the mechanism underlyng this sensitivity. In prostate cancer, TMPRSS2-ERG overexpression causes DNA damage, which is then potentiated by PARP inhibition (6). Surprisingly, Ewing’s cells demonstrated significantly more γH2A.X foci (a marker of DNA double strand breaks) per cell than TMPRSS2-ERG positive VCaP cells (p<0.05 for all EFST cells) (Fig. 2A) and 1μM Olaparib caused massive induction of γH2A.X foci (Supplementary Fig. S1A). Neutral COMET assays confirmed that natural EFST cells have more DNA double strand breaks per cell than VCaP cells (p<0.05) (Fig. 2A). Importantly, overexpression of EWS-FLI1 in PC3 cells led to increased DNA breaks (p<0.05) (Supplementary Fig. S1B) and knockdown of either EWS-FLI1 or EWS-ERG fusions caused a reduction in COMET tail moment, and significantly decreased the induction of DNA damage after Olaparib treatment (p<0.05) (Fig. 2B and Supplementary Figs. S2A, S2B, S3A and S3B) suggesting that Olaparib-potentiated DNA damage is due to expression of the EWS-FLI1 and EWS-ERG fusion genes. Inhibition of non-homologous end-joining (NHEJ) can synergistically potentiate DNA damage in the context of deficient homologous recombination (HR). However, siRNA knockdown of XRCC4 (needed for NHEJ) had little effect (Supplementary Fig. S4), suggesting that EFST cells have functional HR.Although PARP1 inhibition dramatically accentuated DNA damage, it did not have an effect on short-term cell viability (Supplementary Fig. S5A and S5B).
Figure 2. Ewing sarcoma gene fusion products confer increased DNA damage and cell invasion, which can be modulated by genetic or pharmacologic approaches.
A, Staining and quantification of γH2A.X foci in prostate and EFST cells shown on left axis. On right axis, average tail moments from neutral COMET assays are shown. Representative COMET tails are shown, with calculated tail moments depicted numerically.
B, Neutral COMET assays performed on cells treated with siRNA and/or 1μM Olaparib as indicated.
C, Boyden chamber transwell migration assays were performed 48 hours after siRNA transfection or Olaparib treatment. For siRNA experiments, cells were plated in serum-free media and incubated for another 48 hours before fixing and staining. Representative photomicrographs are shown.
D, As in C, except sarcoma control cell lines that do not harbor EFST gene fusions were used.
Error bars indicate S.E.M. of three replicates. *p<0.05.
We next assessed the effect of PARP1 inhibition on short-term cell invasion. Intriguingly, siRNA against EWS, PARP1 or DNA-PKcs reduced CADO-ES1 and RD-ES cell invasion (p<0.05), while knockdown of key components of DNA repair pathways (XRCC1 for base excision repair, XRCC3 for HR and XRCC4 for NHEJ) had no effect (Fig. 2C and Supplementary Figs. S2A and S2B), supporting a DNA repair-independent role of the enzymes. Importantly, Olaparib disrupted ESFT cell invasion (p<0.05) at approximately one log-fold lower concentration than VCaP cell invasion (6) and did not affect ETS negative, control sarcoma cell invasion (Fig. 2D and Supplementary Figs. S2A and S2B).
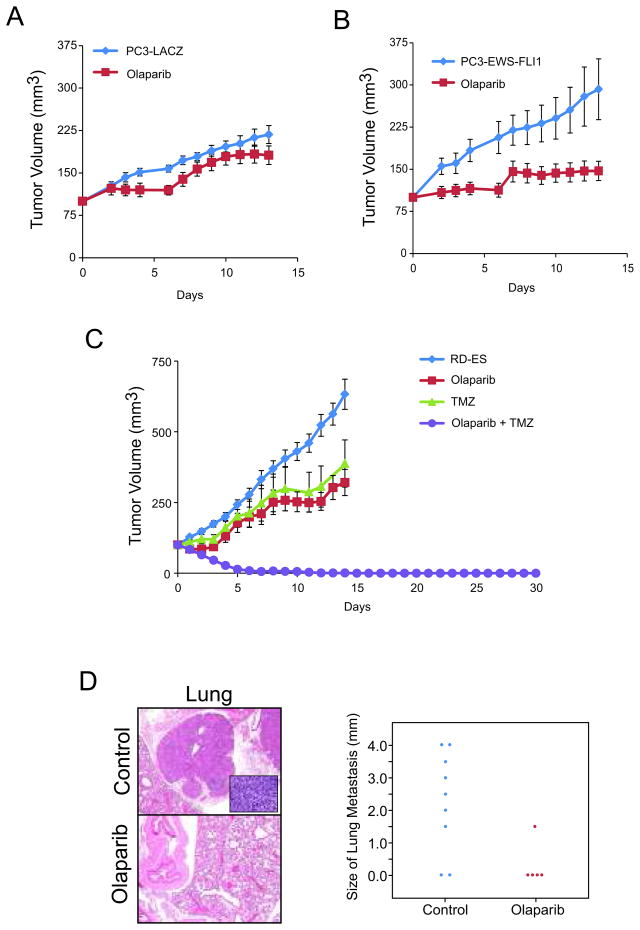
Because PARP inhibition disrupts the long term growth and invasion of ESFT cells, we assessed the ability of EWS-FLI1 to sensitize xenografts to Olaparib. Surprisingly, Olaparib significantly impeded PC3-EWS-FLI1 (P<0.001), but not PC3-LACZ, xenograft growth (Fig. 3A and 3B), demonstrating that EWS-FLI1 overexpression is alone sufficient to sensitize PC3 xenografts to Olaparib. Because PARP1 inhibitors are unlikely to be used clinically as a monotherapy, we also combined PARP1 inhibition with the DNA alkylating agent temozolomide (TMZ) to treat a highly aggressive ESFT xenograft model (RD-ES) harboring an endogenous EWS-FLI1 rearrangement. TMZ potentiates the effects of PARP1 inhibitors (16) and is currently being assessed in Ewing’s sarcoma patients as second-line therapy (17). Surprisingly, TMZ alone only delayed RD-ES xenograft growth (p<0.001) (Fig. 3C). However, Olaparib also caused significant delay in RD-ES xenograft growth (p<0.001), and, when administered in combination with TMZ, led to an immediate tumor regression and a highly significant sustained complete response. Mice were maintained without therapy for an additional 30 days without any observable recurrence (p<0.001). Furthermore, we harvested lungs from RD-ES xenografted mice, assessed metastasis and found only 1/5 mice treated with Olaparib developed lung metastasis as compared to 6/8 untreated mice (p=0.058) (Fig. 3D). This is the first mouse model demonstrating that PARP1 activity is required for metastasis.
Figure 3. PARP inhibition prevents EWS-FLI1 positive xenograft growth and metastasis.
A, Two weeks after engraftment, PC3-LACZ xenografts were treated with 100mg/kg Olaparib BID. Tumor volume measurements were recorded daily.
B, As in A, except with PC3-EWS-FLI1 xenografts.
C, As in A, except RD-ES were treated with Olaparib (100mg/kg BID) alone, TMZ (50mg/kg QD) alone or in combination.
D, At the RD-ES xenograft assay endpoint, lungs were analyzed for metastases. Representative images were taken with a 4x objective (inset at 40x). In the plot, each dot represents an individual lung metastasis for different treatments as indicated along the x-axis. Metastasis diameter was measured and depicted along the y-axis.
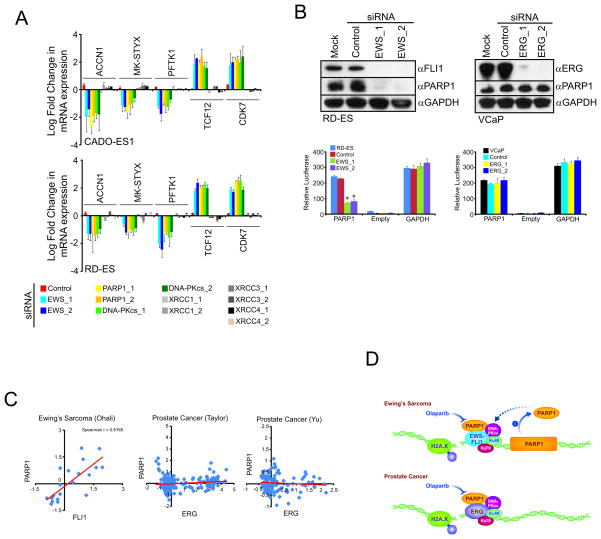
Given that PARP1 has roles in both DNA repair and transcriptional regulation (9, 10), we hypothesized that the sensitivity of ESFT xenograft growth and metastasis depends on both PARP1-mediated roles. Consistent with this, knockdown of PARP1 or DNA-PKcs, but not other DNA repair proteins, disrupted transcriptional activity to a similar extent as fusion targeting EWS siRNA as evidenced by disruption of consensus EWS-FLI1 target genes (18) (Fig. 4A and Supplementary Figs. S2A, S2B).
Figure 4. EWS-FLI1 maintains a feed forward loop that drives PARP1 expression.
A, CADO-ES1 or RD-ES cells were treated with siRNA for 48 hours. qPCR was then performed for several EWS-ETS target genes.
B, Immunoblot analysis of cells treated with control or EWS siRNA. Bar graphs are PARP1 promoter reporter luciferase assays were performed on cells treated with siRNA for 24 hours and then transfected with the promoter reporter for an additional 24 hours.
C, Oncomine scatter plots of publically available gene expression data sets. Each point represents gene expression values from an individual patient. First author of each published study is indicated and referenced in the S.O.M..
D, Model depicting proposed mechanism of increased PARP sensitivity of ESFTs as compared to ETS positive prostate cancers.
Error bars indicate S.E.M. of three replicates. *p<0.05.
In modulating EWS-FLI1 and EWS-ERG expression, we observed that both EWS fusions maintain PARP1 mRNA expression levels (p<0.05) (Supplementary Figs. S2A, S3A, S6A and S6B). This was unexpected because the ETS gene fusions found in prostate cancer do not regulate PARP1 mRNA expression (6), but consistent with reports demonstrating that ETS transcription factors drive PARP1 expression in ESFTs (19, 20). Likewise, in EFST cells, knockdown of the EWS-FLI1 fusion led to a decrease in PARP1 protein expression and promoter activity (p<0.05), while ERG siRNA did not have these effects in VCaP cells (Fig. 4B). Conversely, EWS-FLI1 expression was not altered by Olaparib in RD-ES cells (Supplementary Figs. S6C and S6D). Finally, Oncomine tumor microarray compendium analysis demonstrated that PARP1 mRNA expression correlates with FLI1 expression in EFST patient samples (p<0.01, Spearman correlation shown in figure), but did not correlate with ERG expression in prostate cancer (Fig. 4C). Taken together, our data suggest that PARP1 is critical to a positive-feedback loop in EFST, in which the EWS-FLI fusion product drives expression of PARP1, which then further promotes transcriptional activation by EWS-FLI.
In conclusion, our data demonstrate that ESFT cells and xenografts are sensitive to PARP1 inhibition, to a significantly greater degree than their ETS-fusion-positive prostate cancer counterparts. This increased sensitivity may stem from both greater potentiation of DNA damage by PARP1 inhibition, as well as a EWS-FLI-PARP1 positive feedback loop in transcriptional activation (Fig. 4D). Our findings suggest that PARP1 inhibition should be explored as a strategy for targeted therapy in Ewing’s sarcoma, which may represent a tumor type that may be as profoundly affected by PARP inhibitors as BRCA1/2 mutated carcinomas (12–14).
Supplementary Material
Acknowledgments
We thank Xuhong Cao and Steven Kronenberg for technical support.
Grant Support: This work was funded in part by the DOD National Functional Genomics Center (W81XWH-11-1-0520). A.M.C. is a Howard Hughes Medical Institute Investigator and is supported by the Doris Duke Foundation, the American Cancer Society and is a University of Michigan Taubman Scholar. A.M.C., J.C.B., F.Y.F, and S.A.T. are supported by the Prostate Cancer Foundation.
Footnotes
The authors declare no competing financial interests.
References
- 1.Burchill SA. Ewing's sarcoma: diagnostic, prognostic, and therapeutic implications of molecular abnormalities. J Clin Pathol. 2003;56:96–102. doi: 10.1136/jcp.56.2.96. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12560386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Alava E, Kawai A, Healey JH, et al. EWS-FLI1 fusion transcript structure is an independent determinant of prognosis in Ewing's sarcoma. J Clin Oncol. 1998;16:1248–55. doi: 10.1200/JCO.1998.16.4.1248. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9552022. [DOI] [PubMed] [Google Scholar]
- 3.Brenner JC, Chinnaiyan AM. Translocations in epithelial cancers. Biochim Biophys Acta. 2009;1796:201–15. doi: 10.1016/j.bbcan.2009.04.005. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2752494/?tool=pubmed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tomlins SA, Laxman B, Dhanasekaran SM, et al. Distinct classes of chromosomal rearrangements create oncogenic ETS gene fusions in prostate cancer. Nature. 2007;448:595–9. doi: 10.1038/nature06024. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17671502. [DOI] [PubMed] [Google Scholar]
- 5.Pinkerton CR, Bataillard A, Guillo S, Oberlin O, Fervers B, Philip T. Treatment strategies for metastatic Ewing's sarcoma. Eur J Cancer. 2001;37:1338–44. doi: 10.1016/s0959-8049(01)00131-9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11435062. [DOI] [PubMed] [Google Scholar]
- 6.Brenner JC, Ateeq B, Li Y, et al. Mechanistic rationale for inhibition of poly(ADP-ribose) polymerase in ETS gene fusion-positive prostate cancer. Cancer Cell. 2011;19:664–78. doi: 10.1016/j.ccr.2011.04.010. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21575865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomlins SA, Rhodes DR, Perner S, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–8. doi: 10.1126/science.1117679. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16254181. [DOI] [PubMed] [Google Scholar]
- 8.Welford SM, Hebert SP, Deneen B, Arvand A, Denny CT. DNA binding domain-independent pathways are involved in EWS/FLI1-mediated oncogenesis. J Biol Chem. 2001;276:41977–84. doi: 10.1074/jbc.M106757200. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11553628. [DOI] [PubMed] [Google Scholar]
- 9.Kim MY, Mauro S, Gevry N, Lis JT, Kraus WL. NAD+-dependent modulation of chromatin structure and transcription by nucleosome binding properties of PARP-1. Cell. 2004;119:803–14. doi: 10.1016/j.cell.2004.11.002. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15607977. [DOI] [PubMed] [Google Scholar]
- 10.Krishnakumar R, Gamble MJ, Frizzell KM, Berrocal JG, Kininis M, Kraus WL. Reciprocal binding of PARP-1 and histone H1 at promoters specifies transcriptional outcomes. Science. 2008;319:819–21. doi: 10.1126/science.1149250. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18258916. [DOI] [PubMed] [Google Scholar]
- 11.Rouleau M, Patel A, Hendzel MJ, Kaufmann SH, Poirier GG. PARP inhibition: PARP1 and beyond. Nature Rev Cancer. 2010;10:293–301. doi: 10.1038/nrc2812. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2910902/?tool=pubmed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bryant HE, Schultz N, Thomas HD, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–7. doi: 10.1038/nature03443. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15829966. [DOI] [PubMed] [Google Scholar]
- 13.Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–21. doi: 10.1038/nature03445. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15829967. [DOI] [PubMed] [Google Scholar]
- 14.Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123–34. doi: 10.1056/NEJMoa0900212. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19553641. [DOI] [PubMed] [Google Scholar]
- 15.Lin PP, Wang Y, Lozano G. Mesenchymal Stem Cells and the Origin of Ewing's Sarcoma. Sarcoma. 2011 doi: 10.1155/2011/276463. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20953407. [DOI] [PMC free article] [PubMed]
- 16.Donawho CK, Luo Y, Luo Y, et al. ABT-888, an orally active poly(ADP-ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models. Clin Cancer Res. 2007;13:2728–37. doi: 10.1158/1078-0432.CCR-06-3039. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17473206. [DOI] [PubMed] [Google Scholar]
- 17.Wagner LM, McAllister N, Goldsby RE, et al. Temozolomide and intravenous irinotecan for treatment of advanced Ewing sarcoma. Pediatr Blood Cancer. 2007;48:132–9. doi: 10.1002/pbc.20697. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16317751. [DOI] [PubMed] [Google Scholar]
- 18.Siligan C, Ban J, Bachmaier R, et al. EWS-FLI1 target genes recovered from Ewing's sarcoma chromatin. Oncogene. 2005;24:2512–24. doi: 10.1038/sj.onc.1208455. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15735734. [DOI] [PubMed] [Google Scholar]
- 19.Soldatenkov VA, Albor A, Patel BK, Dreszer R, Dritschilo A, Notario V. Regulation of the human poly(ADP-ribose) polymerase promoter by the ETS transcription factor. Oncogene. 1999;18:3954–62. doi: 10.1038/sj.onc.1202778. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10435618. [DOI] [PubMed] [Google Scholar]
- 20.Newman RE, Soldatenkov VA, Dritschilo A, Notario V. Poly(ADP-ribose) polymerase turnover alterations do not contribute to PARP overexpression in Ewing's sarcoma cells. Oncol Rep. 2002;9:529–32. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11956622. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.