Abstract
Purpose
To assess trends in the use of ancillary diagnostic tests in the evaluation of patients with open-angle glaucoma (OAG) and glaucoma suspects over the past decade.
Design
Retrospective longitudinal cohort analysis.
Participants
169,917 individuals with OAG and 395,721 with suspected glaucoma age ≥40 enrolled in a national United States managed care network between 2001–2009.
Methods
Claims data were analyzed to assess trends in visual field (VF) testing, fundus photography (FP), and other ocular imaging (OOI) testing for patients with OAG or suspected glaucoma in 2001–2009. Repeated measures logistic regression was performed to identify differences in the odds of undergoing these procedures in 2001, 2005, and 2009 and whether differences exist for patients under the exclusive care of optometrists versus ophthalmologists.
Main Outcome Measures
Odds and annual probabilities of undergoing VF testing, FP, and OOI for OAG from 2001–2009.
Results
For patients with OAG, the odds of undergoing VF testing decreased by 36% from 2001 to 2005, 12% from 2005 to 2009, and 44% from 2001 to 2009. By comparison, the odds of having OOI increased by 100% from 2001 to 2005, 24% from 2005 to 2009, and 147% from 2001 to 2009. Probabilities of undergoing FP were relatively low (13–25%) for both provider types and remained fairly steady over the decade. For patients cared for exclusively by optometrists, the probability of VF testing decreased from 66% in 2001 to 44% in 2009. Among those seen exclusively by ophthalmologists, the probability of VF testing decreased from 65% in 2001 to 51% in 2009. The probability of undergoing OOI increased from 26% in 2001 to 47% in 2009 for patients of optometrists and from 30% in 2001 to 46% in 2009 for patients of ophthalmologists. By 2008, patients with OAG receiving care exclusively by optometrists had a higher probability of undergoing OOI than VF testing.
Conclusion
During 2001–2009 OOI rose dramatically whereas VF testing declined considerably. Since OOI has not been shown to be as effective at detecting OAG or disease progression compared to VF testing, increased reliance upon OOI technology, in lieu of VF testing, may be detrimental to patient care.
Over the past decade, other ocular imaging (OOI) devices, beyond straightforward fundus photography (FP), have assisted eye-care providers in diagnosing and managing patients with open-angle glaucoma (OAG) and suspected glaucoma. Confocal scanning laser ophthalmoscopy, scanning laser polarimetry, and optical coherence tomography have been promoted as enabling clinicians to better detect structural damage to the optic nerve and retinal nerve fiber layer (NFL). These procedures can be performed quickly, are painless, relatively easy to perform on most patients, and require no subjective patient input. However, it is unclear how well these devices can detect glaucomatous progression1; moreover, they may yield unreliable results in some patients2,3 and can be quite costly.4 Studies assessing the diagnostic capability of OOI devices in discriminating eyes with early OAG from those without glaucoma have found moderate sensitivity, at best, (ranging from 68%–91% at specificities of 80–97%) for detecting OAG.5,6 Furthermore, OOI devices have been found to not perform as well as careful assessment of the optic disc at identifying patients with OAG.5
Little is known about patterns of utilization of these newer imaging procedures in patients with OAG. This study uses longitudinal data from a large managed-care network to study recent trends throughout the United States in the use of three common ancillary approaches for evaluating patients with OAG or suspected glaucoma: visual field (VF) testing, fundus photography (FP), and OOI.
Methods
Data Source
The i3 InVision Data Mart database (Ingenix, Eden Prairie, MN) contains detailed fully de-identified records of all beneficiaries in a large national managed-care network. We had access to data for all beneficiaries with any form of eye care from January 1, 2001, through December 31, 2009. This subset comprises beneficiaries who had one or more International Classification of Diseases (ICD-9-CM)7 codes for any eye-related diagnosis (360–379.9); Current Procedural Terminology (CPT-4)8 code for any eye-related visits, diagnostic or therapeutic procedures (65091–68899 or 92002–92499); or any other claim submitted by an ophthalmologist or optometrist during their time in the medical plan. We had access to all beneficiaries’ medical claims (inpatient, outpatient, skilled nursing facility) for ocular and nonocular conditions, and sociodemographic information (age, sex, race, education, financial wealth).
Patients
We identified all persons aged 40 or older who were in the database for at least 1 continuous year and had at least 1 eye-care-provider (ophthalmologist or optometrist) visit during their time in the plan. Persons who were in the plan for fewer than 365 days or who had noncontinuous enrollment were excluded. ICD-9-CM diagnostic codes were used to identify patients with OAG (365.1, 365.10, 365.11, 365.12, 365.15) and suspected glaucoma (365.0, 365.00, 365.01, 365.04). Beneficiaries with codes for suspected glaucoma and subsequently an OAG diagnosis were considered glaucoma suspects from the time of their suspect diagnosis until just before their first OAG diagnosis; thereafter, they were considered to have OAG.
Analyses
Statistical analyses were performed by using SAS, 9.2 (SAS Institute, Cary, NC), software. Participant characteristics were summarized for those with OAG and suspected glaucoma using means and standard deviations for continuous variables and frequencies and percentages for categorical variables.
Comparison of Ancillary Glaucoma Testing Among Newly-Diagnosed Glaucoma Patients in 2003 vs 2007
We identified two groups: patients with incident OAG diagnosed in 2003 and those diagnosed in 2007. To be considered to have incident OAG, a patient must not have received an OAG diagnosis during a look-back period of 2 or more years (2001–2002 for the 2003 cohort, 2001–2006 for 2007 cohort), had at least one OAG diagnosis during 2003 and 2007, respectively, and had at least 2 years continuous coverage in the plan after OAG diagnosis. These two cohorts were followed from their initial OAG diagnosis to determine the proportions undergoing each of the following ancillary procedures for glaucoma in the first 12, 15, 18 and 24 months following OAG diagnosis: VF testing (CPT-4 codes 92081, 92082, 92083), FP (code 92250), and OOI testing (code 92135).We calculated the proportions undergoing precisely one, multiple, and none of these three types of diagnostic tests (VF, OOI, FP) during the specified time intervals and the proportion undergoing two or more types of diagnostic tests on the same day. Similar analyses were done for incident cases of suspected glaucoma diagnosed in 2003 and 2007. There were no limits set by the managed care network on the number of VF, FP, or OOI tests that could be performed on a given patient (personal communication, United Health Care, December 28, 2010).
Longitudinal Trends in Ancillary Glaucoma Testing
Repeated measures logistic regression was performed to compare the odds of undergoing each ancillary glaucoma procedure in each year from 2001 to 2009 for a patient with OAG. The regression models were adjusted for age, sex, race, education level, net worth, region of residence in the United States, insurance plan type, type of eye-care professional providing their care (ophthalmologist or optometrist only, or both), time in the plan (by year), hypertension, hyperlipidemia, diabetes mellitus, obesity, and comorbid ocular conditions that could warrant the use of these diagnostic tests—specifically, cystoid and diabetic macular edema, exudative age-related macular degeneration, nonproliferative and proliferative diabetic retinopathy, other retinal conditions, and other conditions that can affect the optic nerve (i.e., other glaucomas and optic neuropathies). (Table 1, available at http://aaojournal.org) Preliminary analysis showed evidence of a non-linear trend in the use of these procedures, hence, the effect of time was modeled as nonlinear (quadratic) in the regression models. Furthermore, since this quadratic effect of time could have been different in the presence of different comorbid ocular conditions, interactions were included between the effect of time and macular edema, exudative macular degeneration, other retinal diseases, and other diseases of the optic nerve for the OOI and FP models and interactions between time and other diseases of the optic nerve for the VF models. Comparisons of the odds of receiving VF testing, FP, and OOI were performed for those with OAG in the plan during 2001 versus 2005, 2005 versus 2009, and 2001 versus 2009 to capture changes in utilization over time. While performing these comparisons, all other ocular and nonocular conditions were assumed to be at their average levels as computed from the data. Similar analyses were performed for glaucoma suspects.
In this analysis, separate models were created for those receiving care provided by one or more ophthalmologists but no optometrist, one or more optometrists only, and both provider types. Each model was adjusted for the same covariates listed above. The estimated odds of receiving each procedure in each year were converted to probabilities. These probabilities were estimated assuming all other ocular and nonocular conditions to be at their average levels as computed from the data, and assuming white race, male sex, age 60, preferred provider organization insurance plan type, residence in a Northeastern state, high school education, and $75,000–150,000 household net worth.
For all analyses, p-values less than 0.05 were considered statistically significant. The University of Michigan Institutional Review Board (IRB) determined this study was exempt from requiring IRB approval.
RESULTS
Of the 2,854,417 patients who met the study inclusion criteria, 169,917 (6.0%) had ≥1 OAG diagnosis and 395,721 (13.9%) had ≥1 suspected glaucoma diagnosis. The mean ± standard deviation (SD) time in the plan for those with OAG was 4.1 ± 2.2 years and for those with suspected glaucoma 4.4 ± 2.3 years. The mean age ± (SD) of those who were eligible for the study was 55.1 ± 10.3 years. Patients with suspected glaucoma were younger than those with OAG (56.4 ±10.2 years vs. 61.8 ± 11.3 years). The sample with OAG included 115,378 whites (79.4%), 15,601 blacks (10.7%), 8,970 Latinos (6.2%), 4,064 Asian Americans (2.8%), and 91,087 women (53.6%). (Table 2).
Table 2.
Sociodemographic Characteristics of Individuals in the Sample with Open-angle Glaucoma or Suspected Glaucoma
| OAG (N=169,917) |
Glaucoma suspect (N=395,721) |
||||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Sex | Male | 78830 | 46.4 | 167663 | 42.4 |
| Female | 91087 | 53.6 | 228058 | 57.6 | |
| Race* | White | 115378 | 79.4 | 277286 | 81.8 |
| Black | 15601 | 10.7 | 24371 | 7.2 | |
| Latino | 8970 | 6.2 | 22507 | 6.6 | |
| Asian | 4064 | 2.8 | 11376 | 3.4 | |
| Other race | 1262 | 0.9 | 3319 | 1.0 | |
| Net Worth | < $25,000 | 12599 | 8.6 | 26236 | 7.6 |
| $25–75,000 | 10011 | 6.8 | 21627 | 6.3 | |
| $75–150,000 | 19292 | 13.2 | 42316 | 12.3 | |
| $150–500,000 | 65603 | 44.8 | 154312 | 44.9 | |
| $ < 500,000 | 38828 | 26.5 | 99244 | 28.9 | |
| Region of Residence | NE | 28959 | 17.1 | 75953 | 19.2 |
| SE | 72323 | 42.6 | 156979 | 39.7 | |
| MW | 48949 | 28.8 | 116770 | 29.5 | |
| West | 19476 | 11.5 | 45566 | 11.5 | |
| Other region | 181 | 0.1 | 406 | 0.1 | |
| Ocular comorbidities | Other nerve | 57026 | 33.6 | 72190 | 18.2 |
| Wet AMD | 3288 | 1.9 | 3823 | 1.0 | |
| Macular edema | 4308 | 2.5 | 6098 | 1.5 | |
| NPDR | 10778 | 6.3 | 18343 | 4.6 | |
| PDR | 3924 | 2.3 | 4504 | 1.1 | |
| Other retina | 36163 | 21.3 | 66866 | 16.9 | |
Race was unknown for 24642 individuals with OAG and 56862 glaucoma suspects
NE = Northeast; SE = Southeast; MW = Midwest; AMD = age-related macular degeneration; NPDR = non-proliferative diabetic retinopathy; PDR =proliferative diabetic retinopathy; “Other nerve” refers to other conditions that can affect the optic nerve such as other types of glaucoma or optic neuropathy; “Other retina” refers to other conditions that can affect the retina besides diabetic retinopathy and macular edema; OAG = open-angle glaucoma
Diagnostic Testing in First Year after Initial OAG Diagnosis: 2003 vs. 2007 Cohorts
The number of individuals with incident OAG with at least 12 months of follow-up in 2003 and 2007 was 5,610 and 5,147 persons, respectively. The proportions of patients in the 2003 and 2007 cohorts, respectively, undergoing ancillary glaucoma testing within 12 months of their OAG diagnosis were 63% and 65% for VF testing, 17% and 23% for FP, and 38% and 53% for OOI. (Tables 3 and 4) In the first year after diagnosis, the proportion undergoing multiple types of tests increased from the 2003 cohort to the 2007 cohort: 13% of the 2003 cohort, compared with 18% in the 2007 cohort, had VF testing plus FP; 7% and 14%, respectively, had FP plus OOI; 28% and 40% had VF plus OOI; and 6% and 12% had all three tests. (p<0.01 for all comparisons) The percentage of patients undergoing only VF testing (no OOI or FP) decreased from 27% in the 2003 cohort to 18% in the 2007 cohort (p<0.01), remained steady at 3% for FP only (no VF or OOI) (p>0.2), and increased from 9% to 11% for OOI only (no VF or FP) (p<0.01). From 2003–2004 to 2007–2008, the use of VF testing without any OOI decreased from 34% of patients to 25% of patients, whereas use of OOI without VF testing increased from 10% to 13%. (p<0.01 for both comparisons) Few patients in either cohort received OOI plus FP (0.3–2.3%), or all three procedures at a single visit (0.1–0.9%); the proportion undergoing VF testing with OOI on the same day increased from 13% in the 2003 cohort to 21% in the 2007 cohort.(p<0.01 for all comparisons) The proportion of patients undergoing none of the three ancillary glaucoma procedures decreased from 25% in 2003–2004 to 19% in 2007–2008.(p<0.01) (Tables 5 and 6 shows results for the 2003 and 2007 glaucoma-suspect cohorts and over different lengths of time since OAG diagnosis.)
Table 3.
Use of Glaucoma Diagnostic Procedures and Different Combinations of Procedures for Incident Glaucoma Diagnosed in 2003 and 2007
| Use of Diagnostic Testing for Incident Glaucoma in 2003 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Base* | ≥ 1 procedure during interval |
≥ 1 instance of same-day procedure during interval |
≥ 1 of each procedure during respective interval |
Only one type of procedure during interval |
VF yes /OOI no (%) |
OOI yes/ VF no (%) |
None | |||||||||||
| VF(%) | FP(%) | OOI (%) |
VF & FP (%) |
FP & OOI (%) |
VF & OOI (%) |
3 tests (%) |
VF & FP (%) |
FP & OOI (%) |
VF & OOI (%) |
3 tests (%) |
VF only (%) |
FP only (%) |
OOI only (%) |
|||||
| Within 12 months after first record of glaucoma | 5610 | 62.6 | 17.1 | 37.8 | 6.3 | 0.3 | 12.6 | 0.1 | 13.4 | 7.0 | 28.2 | 6.1 | 27.0 | 2.8 | 8.6 | 34.3 | 9.6 | 25.1 |
| Within 15 months after first record of glaucoma | 5308 | 67.0 | 19.1 | 41.6 | 7.2 | 0.3 | 14.2 | 0.2 | 15.8 | 8.5 | 32.7 | 7.5 | 26.0 | 2.3 | 7.9 | 34.3 | 8.9 | 21.8 |
| Within 18 months after first record of glaucoma | 5047 | 69.4 | 20.6 | 44.7 | 7.8 | 0.3 | 16.0 | 0.2 | 17.3 | 10.1 | 36.5 | 9.0 | 24.6 | 2.2 | 7.2 | 32.9 | 8.2 | 20.2 |
| Within 24 months after first record of glaucoma | 4557 | 73.3 | 23.5 | 49.8 | 9.3 | 0.5 | 18.5 | 0.2 | 20.5 | 13.0 | 42.3 | 12.0 | 22.4 | 2.0 | 6.5 | 31.0 | 7.5 | 17.3 |
| Use of Diagnostic Testing for Incident Glaucoma in 2007 | ||||||||||||||||||
| Within 12 months after first record of glaucoma | 5147 | 64.5 | 22.9 | 53.1 | 8.3 | 2.3 | 20.8 | 0.9 | 17.7 | 13.8 | 39.9 | 11.5 | 18.5 | 3.0 | 11.0 | 24.7 | 13.2 | 19.3 |
| Within 15 months after first record of glaucoma | 4853 | 69.3 | 25.6 | 57.3 | 9.7 | 2.5 | 23.5 | 1.0 | 20.8 | 16.7 | 45.9 | 14.5 | 17.2 | 2.7 | 9.3 | 23.4 | 11.4 | 16.6 |
| Within 18 months after first record of glaucoma | 4592 | 72.0 | 27.4 | 60.8 | 10.3 | 2.8 | 25.3 | 1.1 | 23.0 | 18.9 | 50.0 | 16.8 | 15.8 | 2.3 | 8.7 | 22.0 | 10.8 | 14.9 |
| Within 24 months after first record of glaucoma | 4102 | 75.3 | 30.2 | 64.8 | 11.6 | 3.3 | 28.1 | 1.3 | 26.2 | 22.8 | 55.3 | 20.9 | 14.7 | 2.1 | 7.6 | 20.1 | 9.5 | 13.1 |
Base included those who were in the plan for the specific interval after getting diagnosed
OOI = ocular imaging; FP = fundus photography; VF = visual field testing
Table 4.
Changes in Use of Glaucoma Diagnostic Procedures and Different Combinations of Procedures for Incident Glaucoma Diagnosed in 2003 and 2007
| ≥ 1 procedure during interval |
≥ 1 instance of same-day procedure during interval |
≥ 1 of each procedure during respective interval |
Only one type of procedure during interval |
VF yes / OOI no (%) |
OOI yes / VF no (%) |
None | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VF(%) | FP(%) | OOI (%) | VF & FP (%) |
FP & OOI (%) |
VF & OOI (%) |
3 tests (%) |
VF & FP (%) |
FP & OOI (%) |
VF & OOI (%) |
3 tests (%) |
VF only (%) |
FP only (%) |
OOI only (%) |
||||
| Change in proportions from 2003 to 2007 (12 months) | +2.0 | +5.8 | +15.3 | +2.0 | +2.0 | +8.2 | +0.8 | +4.4 | +6.8 | +11.6 | +5.5 | −8.5 | +0.2 | +2.4 | |||
| Change in proportions from 2003 to 2007 (15 months) | +2.3 | +6.5 | +15.7 | +2.5 | +2.3 | +9.3 | +0.9 | +5.0 | +8.2 | +13.2 | +7.0 | −8.8 | +0.4 | +1.4 | −10.9 | +2.5 | −5.2 |
| Change in proportions from 2003 to 2007 (18 months) | +2.6 | +6.8 | +16.1 | +2.5 | +2.5 | +9.3 | +0.9 | +5.6 | +8.8 | +13.5 | +7.7 | −8.8 | +0.1 | +1.6 | −10.9 | +2.6 | −5.3 |
| Change in proportions from 2003 to 2007 (24 months) | +2.1 | +6.7 | +15.0 | +2.3 | +2.8 | +9.5 | +1.1 | +5.7 | +9.8 | +13.0 | +8.9 | −7.7 | +0.1 | +1.1 | −10.9 | +2.0 | −4.2 |
Statistical comparison of changes in proportions of a given procedure or group of procedures from 2003 to 2007 using chi-square tests with Holm’s adjustment for multiple comparisons. Bold indicates p ≤ 0.01
OOI = ocular imaging; FP = fundus photography; VF = visual field testing
Table 5.
Use of Glaucoma Diagnostic Procedures and Different Combinations of Procedures for Incident Suspected Glaucoma Diagnosed in 2003 and 2007
| Use of Diagnostic testing for Incident Suspected Glaucoma in 2003 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Base | ≥ 1 procedure during interval |
≥ 1 instance of same-day procedure during interval |
≥ 1 of each procedure during respective interval |
Only one type of procedure during interval |
VF yes / OI no (%) |
OI yes / VF no (%) |
None | |||||||||||
| VF(%) | FP(%) | OI(%) | VF & FP (%) |
FP & OI (%) |
VF & OI (%) |
3 tests (%) |
VF & FP (%) |
FP & OI (%) |
VF & OI (%) |
3 tests (%) |
VF only (%) |
FP only (%) |
OI only (%) |
|||||
| Within 12 months after first record of glaucoma suspect | 13510 | 51.1 | 17.0 | 31.5 | 5.4 | 0.5 | 10.7 | 0.3 | 11.2 | 4.8 | 19.6 | 3.9 | 24.2 | 4.9 | 11.0 | 31.5 | 11.9 | 32.1 |
| Within 15 months after first record of glaucoma suspect | 12757 | 53.0 | 18.1 | 33.7 | 5.8 | 0.5 | 11.7 | 0.3 | 12.3 | 5.9 | 22.0 | 4.9 | 23.6 | 4.8 | 10.7 | 31.0 | 11.7 | 30.5 |
| Within 18 months after first record of glaucoma suspect | 12050 | 54.2 | 18.7 | 34.9 | 6.1 | 0.5 | 12.3 | 0.3 | 13.0 | 6.5 | 23.3 | 5.4 | 23.3 | 4.6 | 10.4 | 30.8 | 11.6 | 29.7 |
| Within 24 months after first record of glaucoma suspect | 10698 | 56.0 | 20.6 | 37.0 | 6.8 | 0.6 | 13.5 | 0.4 | 14.6 | 8.1 | 25.8 | 6.8 | 22.3 | 4.6 | 9.9 | 30.2 | 11.3 | 28.2 |
| Use of Diagnostic testing for Incident Suspected Glaucoma in 2007 | ||||||||||||||||||
| Within 12 months after first record of glaucoma suspect | 16627 | 51.1 | 26.4 | 43.1 | 9.0 | 2.3 | 17.1 | 1.1 | 17.3 | 10.8 | 27.6 | 9.0 | 15.3 | 7.3 | 13.7 | 23.6 | 15.6 | 26.0 |
| Within 15 months after first record of glaucoma suspect | 15708 | 53.1 | 27.7 | 45.1 | 9.6 | 2.4 | 18.2 | 1.2 | 18.7 | 12.3 | 29.7 | 10.3 | 15.0 | 7.0 | 13.4 | 23.4 | 15.4 | 24.5 |
| Within 18 months after first record of glaucoma suspect | 14722 | 54.2 | 28.6 | 46.3 | 10.0 | 2.5 | 19.1 | 1.2 | 19.6 | 13.3 | 31.1 | 11.2 | 14.7 | 6.9 | 13.0 | 23.1 | 15.2 | 23.8 |
| Within 24 months after first record of glaucoma suspect | 13019 | 55.9 | 30.3 | 48.3 | 10.9 | 2.8 | 20.4 | 1.3 | 21.1 | 15.1 | 33.5 | 12.7 | 13.9 | 6.8 | 12.4 | 22.4 | 14.8 | 22.5 |
Base included those who were in the plan for the specific interval after getting diagnosed
OI = ocular imaging; FP = fundus photography; VF = visual field testing
Statistical comparison of changes in proportions of a given procedure or group of procedures from 2003 to 2007 using chi-square test with Holm’s adjustment for multiple comparisons. Bold indicates p ≤ 0.01
Table 6.
Changes in Use of Glaucoma Diagnostic Procedures and Different Combinations of Procedures for Incident Suspected Glaucoma Diagnosed in 2003 and 2007
| ≥ 1 procedure during interval | ≥ 1 instance of same-day procedure during interval |
≥ 1 of each procedure during respective interval |
Only one type of procedure during interval |
VF yes / OOI no (%) |
OOI yes / VF no (%) |
None | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VF(%) | FP(%) | OOI (%) | VF & FP (%) |
FP & OOI (%) |
VF & OOI (%) |
3 tests (%) |
VF & FP (%) |
FP & OOI (%) |
VF & OOI (%) |
3 tests (%) |
VF only (%) |
FP only (%) |
OOI only (%) |
||||
| Change in proportions from 2003 to 2007 (12 months) | 0.0 | +9.4 | +11.6 | +3.5 | +1.9 | +6.4 | +0.8 | +6.1 | +6.1 | +7.9 | +5.1 | −8.9 | +2.4 | +2.7 | −7.9 | +3.7 | −6.1 |
| Change in proportions from 2003 to 2007 (15 months) | +0.1 | +9.6 | +11.4 | +3.8 | +2.0 | +6.5 | +0.8 | +6.4 | +6.4 | +7.7 | +5.4 | −8.6 | +2.3 | +2.6 | −7.7 | +3.7 | −6.0 |
| Change in proportions from 2003 to 2007 (18 months) | 0.0 | +10.0 | +11.4 | +3.9 | +2.0 | +6.7 | +0.9 | +6.7 | +6.8 | +7.8 | +5.8 | −8.6 | +2.3 | +2.6 | −7.8 | +3.6 | −5.9 |
| Change in proportions from 2003 to 2007 (24 months) | −0.1 | +9.8 | +11.3 | +4.1 | +2.3 | +7.0 | +1.0 | +6.5 | +7.0 | +7.7 | +5.9 | −8.4 | +2.2 | +2.4 | −7.8 | +3.5 | −5.7 |
OOI = ocular imaging; FP = fundus photography; VF = visual field testing
Statistical comparison of changes in proportions of a given procedure or group of procedures from 2003 to 2007 using chi-square test with Holm’s adjustment for multiple comparisons. Bold indicates p ≤ 0.01
Odds of Diagnostic Testing in 2001 vs. 2005 vs. 2009
In multivariable analysis, patients with OAG had a 36%-reduced odds of undergoing VF examination in 2005, compared with 2001 (adjusted odds ratio [OR]=0.64 [95% confidence interval (CI) = 0.62–0.66]); a 12%-reduced odds of doing so in 2009, compared with 2005 (adjusted OR=0.88 [CI=0.86–0.90]); and a 44%-reduced odds in 2009 relative to 2001 (adjusted OR=0.56 [CI=0.55–0.58]). The odds of undergoing FP in 2005 were decreased 4% from 2001 (adjusted OR=0.96 [CI=0.93–1.00]), but by 2009 had increased by 13% relative to 2005 (adjusted OR=1.13 [CI=1.10–1.16]) and by 8% relative to 2001 (adjusted OR=1.08 [CI=1.05–1.12]). The odds of undergoing OOI were increased by 100% in 2005 relative to 2001 (adjusted OR=2.00 [CI=1.94–2.05]), by 24% in 2009 relative to 2005 (adjusted OR=1.24 [CI=1.22–1.26]), and by 147% in 2009 relative to 2001 (adjusted OR=2.47 [CI=2.40–2.54]).
Comparisons by Type of Provider
Visual Field Testing
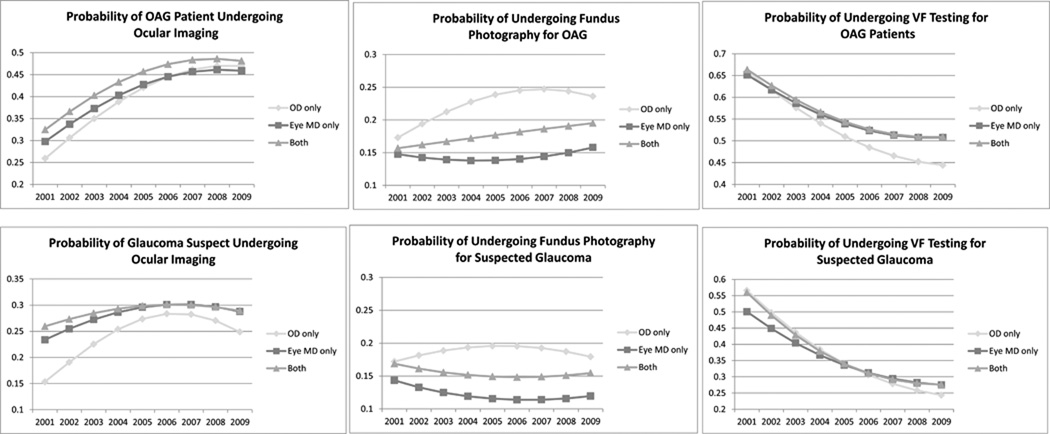
Among OAG patients and glaucoma suspects seen by optometrists, ophthalmologists, or both, the odds of VF testing were reduced in 2005 compared with 2001, and reduced in 2009 relative to 2005. (Table 7) The probability of an OAG patient undergoing VF testing decreased steadily from 2001 to 2009—over all, from 64% to 50%; under ophthalmologist-only care, from 65% to 51%; and under optometrist care, from 66% to 44%. Glaucoma suspects showed similar trends. The probability of undergoing VF testing was higher under optometrists’ care than under ophthalmologists’ care in 2001 (57% vs. 50%, respectively), but the reverse was true by the end of the decade (24% vs. 28%, respectively). (p<0.05 for both comparisons)(Figure 1)
Table 7.
Odds of Undergoing Different Diagnostic Tests for Open-Angle Glaucoma and Suspected Glaucoma in 2005 vs 2001, 2009 vs 2005, and 2009 vs 2001
| Ocular Imaging | |||||||
|---|---|---|---|---|---|---|---|
| Optometrist only | Ophthalmologist only | Both providers | |||||
| OR (CI) | p-value* | OR (CI) | p-value* | OR (CI) | p-value* | ||
| OAG | 2005 vs 2001 | 2.07 (1.82–2.36) | <0.0001 | 1.76 (1.70–1.82) | <0.0001 | 1.75 (1.64–1.87) | <0.0001 |
| 2009 vs 2005 | 1.22 (1.14–1.31) | <0.0001 | 1.14 (1.11–1.16) | <0.0001 | 1.10 (1.06–1.15) | 0.0001 | |
| 2009 vs 2001 | 2.53 (2.22–2.88) | <0.0001 | 2.00 (1.93–2.07) | <0.0001 | 1.93 (1.81–2.06) | <0.0001 | |
| Gl. Suspect | 2005 vs 2001 | 2.08 (1.92–2.24) | <0.0001 | 1.38 (1.34–1.42) | <0.0001 | 1.21 (1.15–1.28) | <0.0001 |
| 2009 vs 2005 | 0.88 (0.85–0.91) | <0.0001 | 0.96 (0.94–0.98) | <0.0001 | 0.96 (0.92–0.99) | 0.3841 | |
| 2009 vs 2001 | 1.82 (1.69–1.97) | <0.0001 | 1.32 (1.29–1.36) | <0.0001 | 1.16 (1.10–1.23) | <0.0001 | |
| Fundus Photography | |||||||
| Optometrist only | Ophthalmologist only | Both providers | |||||
| OR (CI) | p-value* | OR (CI) | p-value* | OR (CI) | p-value* | ||
| OAG | 2005 vs 2001 | 1.50 (1.31–1.71) | 0.0002 | 0.92 (0.89–0.97) | 0.0005 | 1.15 (1.06–1.24) | 0.1848 |
| 2009 vs 2005 | 0.98 (0.91–1.06) | 1.0000 | 1.17 (1.13–1.21) | < 0.0001 | 1.13 (1.07–1.19) | 0.0020 | |
| 2009 vs 2001 | 1.48 (1.29–1.68) | 0.0026 | 1.08 (1.04–1.13) | 0.4732 | 1.30 (1.20–1.40) | 0.0001 | |
| Gl. Suspect | 2005 vs 2001 | 1.17 (1.09–1.26) | 0.3876 | 0.78 (0.75–0.81) | <0.0001 | 0.86 (0.81–0.92) | <0.0001 |
| 2009 vs 2005 | 0.90 (0.86–0.93) | <0.0001 | 1.04 (1.01–1.06) | 0.4732 | 1.04 (1.00–1.09) | 0.8583 | |
| 2009 vs 2001 | 1.05 (0.98–1.13) | 1.0000 | 0.81 (0.78–0.84) | <0.0001 | 0.90 (0.84–0.96) | <0.0001 | |
| Visual Field Testing | |||||||
| Optometrist only | Ophthalmologist only | Both providers | |||||
| OR (CI) | p-value* | OR (CI) | p-value* | OR (CI) | p-value* | ||
| OAG | 2005 vs 2001 | 0.53 (0.48–0.59) | <0.0001 | 0.63 (0.61–0.64) | <0.0001 | 0.61 (0.57–0.64) | <0.0001 |
| 2009 vs 2005 | 0.76 (0.72–0.81) | <0.0001 | 0.88 (0.86–0.90) | <0.0001 | 0.87 (0.83–0.91) | <0.0001 | |
| 2009 vs 2001 | 0.41 (0.37–0.45) | <0.0001 | 0.55 (0.54–0.57) | <0.0001 | 0.53 (0.50–0.56) | <0.0001 | |
| Gl. Suspect | 2005 vs 2001 | 0.40 (0.38–0.42) | <0.0001 | 0.51 (0.49–0.52) | <0.0001 | 0.41 (0.39–0.43) | <0.0001 |
| 2009 vs 2005 | 0.62 (0.60–0.64) | <0.0001 | 0.75 (0.74–0.76) | <0.0001 | 0.74 (0.71–0.76) | <0.0001 | |
| 2009 vs 2001 | 0.25 (0.23–0.26) | <0.0001 | 0.38 (0.37–0.39) | <0.0001 | 0.30 (0.29–0.31) | <0.0001 | |
OR = odds ratio, CI = confidence interval; Gl. suspect = glaucoma suspect; OAG = open-angle glaucoma.
p-values with Holm’s adjustment to account for multiple comparisons
Regression models were adjusted for age, sex, race, education level, net worth, region of residence in the United States, insurance plan type, time in the plan (by year), hypertension, hyperlipidemia, diabetes mellitus, obesity, and comorbid ocular conditions that could warrant the use of these diagnostic tests—including cystoid and diabetic macular edema, exudative age-related macular degeneration, nonproliferative and proliferative diabetic retinopathy, other retinal conditions, and other conditions that can affect the optic nerve (i.e., other glaucomas and optic neuropathies).
Figure 1. Probability of Undergoing Different Glaucoma Diagnostic Tests From 2001–2009 for Individuals with Open-angle Glaucoma or Suspected Glaucoma.
OD only = Enrollees under optometrist-only care; Eye MD only = Enrollees under ophthalmologist-only care; Both = Enrollees receiving care by at least one of each provider type during their time in the plan; VF = Visual Field; OAG = open-angle glaucoma
Probabilities were estimated assuming all other ocular and nonocular conditions to be at their average levels as computed from the data, and assuming white race, male sex, age 60, preferred provider organization insurance plan type, residence in a Northeastern state, high school education, and $75,000–150,000 household net worth.
Fundus Photography
The overall probability of undergoing FP among OAG patients changed little throughout the decade, ranging from 15.3% in 2004 to 17.0% in 2009. During each year examined, the probability of FP among patients with OAG and glaucoma suspects was higher with optometrist care than with ophthalmologist care. (Figure 1)
Other Ocular Imaging
Among patients with OAG, the odds of undergoing OOI were elevated in 2005 and 2009 relative to 2001, irrespective of provider type. Among glaucoma suspects, the likelihood was elevated in 2005 compared with 2001 but was reduced in 2009 relative to 2005 for each provider category. The odds of receiving OOI in 2009 relative to 2001 were significantly elevated among OAG patients—by 153% with optometrist-only care, 100% with ophthalmologist-only care, and 93% under joint ophthalmologist-optometrist care (p<0.05 for OAG patients under optometrist-only care versus those under ophthalmologist-only care). Trends were similar, although less pronounced, with glaucoma suspects. (p<0.05) (Table 7) The annual probability of undergoing OOI increased from 26% in 2001 to 47% in 2009 for patients of optometrists and from 30% in 2001 to 46% in 2009 for patients of ophthalmologists. Patients with OAG under the exclusive care of optometrists had lower probabilities of undergoing OOI relative to patients under the exclusive care of ophthalmologists from 2001–2005. After 2005, the probabilities were similar for both groups. From 2001–2009, the probability of undergoing OOI was higher for glaucoma suspects under the exclusive care of ophthalmologists as compared to those under the exclusive care of optometrists. For those under the exclusive care of ophthalmologists, from 2001 through 2007, the probability of undergoing OOI for suspected glaucoma has gradually risen each year and reached a plateau of approximately 30% of patients from 2007 through 2009. Glaucoma suspects under the care of optometrists experienced an even more dramatic rise in the probability of undergoing OOI from 2001 through 2006. However, from 2006 through 2009, the probability of undergoing OOI decreased from 28% to 25% for this group. (Figure 1)
Overall Trends by Provider Type
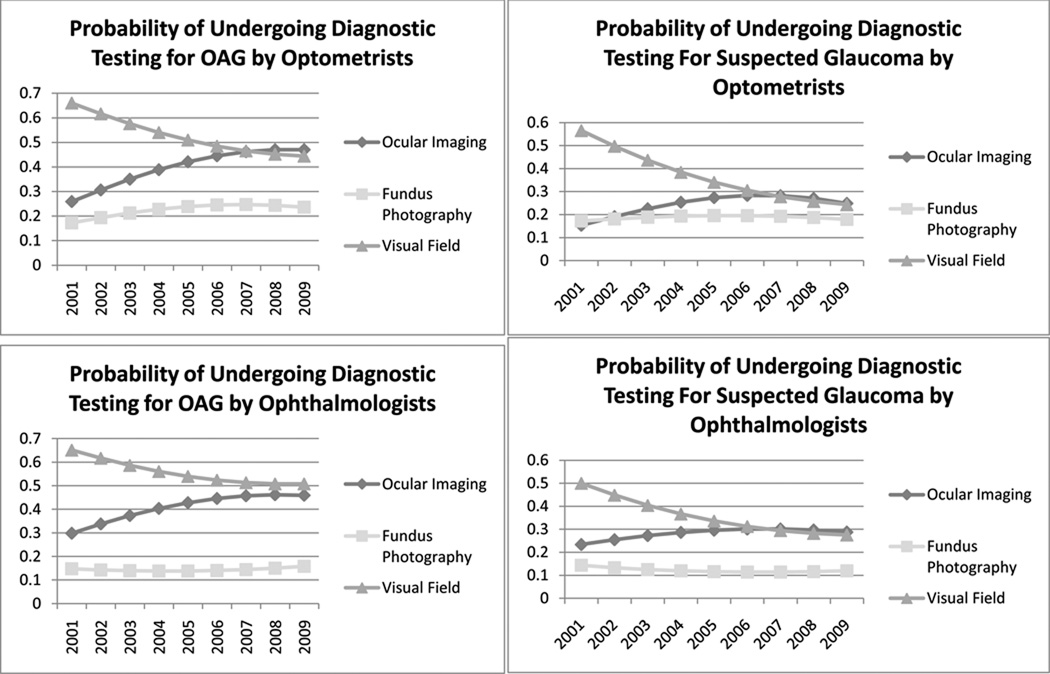
From 2001–2009, patients with OAG under exclusive care by optometrists were observed to have increasing probabilities of OOI coupled with decreasing probabilities of VF testing over the course of the decade. By 2008, the probability of undergoing OOI exceeded the probability of undergoing VF testing for this group. Similar trends were seen for patients under exclusive care by ophthalmologists or under the care of both provider types, though the probability of undergoing VF testing continued to be slightly higher than OOI for these groups through 2009. (Figure 2)
Figure 2. Trends in the Use of Different Glaucoma Diagnostic Tests from 2001–2009 for Enrollees Receiving Exclusive Care by Optometrists or Ophthalmologists.
Probabilities were estimated assuming all other ocular and nonocular conditions to be at their average levels as computed from the data, and assuming white race, male sex, age 60, preferred provider organization insurance plan type, residence in a Northeastern state, high school education, and $75,000–150,000 household net worth.
OAG = open-angle glaucoma
Details of all of the models can be found as Supplemental Tables 8–22, available at http://aaojournal.org.
DISCUSSION
Over the past decade, we find substantially increased use of OOI and dramatically decreased use of VF testing in the management of patients with OAG and suspected glaucoma by ophthalmologists and optometrists. By 2008, in fact, OAG patients under the exclusive care of an ophthalmologist had nearly as high a probability of undergoing OOI as VF testing while and those under the exclusive care of an optometrist actually had a higher probability of undergoing OOI than VF testing. Probabilities of undergoing FP for OAG patients were relatively low throughout the decade, ranging from 13% to 25%.
Little is known in the literature about utilization rates for different ancillary glaucoma procedures. Using 1995–1999 data on Medicare patients in the year before they had glaucoma surgery, Coleman and colleagues found that VF testing rates were suboptimal relative to the recommended standard of care—especially among racial minorities, persons ≥85 years old, and patients with ocular comorbidities.9 Although direct comparison of that study with ours is challenging due to differences in the average age of the study populations and study timing (OOI was not commercially available in the mid-1990s), both studies demonstrate that many OAG patients are not undergoing routine VF testing. Considering the importance of VF testing in glaucoma management, it is disconcerting that >25% of individuals with newly-diagnosed OAG in 2003 and 2007 have no record of undergoing VF testing within 2 years of diagnosis and that the odds of undergoing VF testing in 2009 relative to 2001 decreased 45% for those under exclusive care by ophthalmologists and 59% for those under the care of optometrists. These findings suggest that greater effort needs to be made to better educate ophthalmologic and optometric trainees of the importance of VF testing in glaucoma management.
Several factors likely contribute to the considerable rise in the use of OOI in OAG management. Ancillary imaging procedures can be performed quickly—often by technicians with little experience in operating the equipment; in addition, they are painless, require little patient cooperation and effort, do not rely on subjective patient input, and often can be obtained without pupil dilation. A survey found that OAG patients prefer undergoing OOI to having FP or VF testing.10 Devices such as confocal scanning laser ophthalmoscopy, optical coherence tomography, and scanning laser polarimetry have been shown to detect—or purport to detect—subtle evidence of damage to the optic nerve and retinal nerve fiber layer long before functional damage can be identified on standard perimetry11, although studies have shown that they have moderate sensitivity, at best, of discriminating between eyes with and without OAG.5,6 Some eye care providers may prefer "answers" provided by a quick glance at the numbers provided by OOI rather than the more time consuming analyses of VF parameters to determine the presence of glaucomatous damage and disease progression. Financial incentive may also drive use of this technology. Since OOI equipment is quite expensive to purchase, the more tests an eye-care provider orders, the quicker one will recoup equipment costs and eventually generate revenue. Finally, motivation to purchase and use OOI may be driven partly by the desire for providers to market that they are practicing cutting-edge medicine.12
For those enrollees who received only one of these three ancillary glaucoma tests in the year following their initial OAG diagnosis, VF testing continued to dominate over the other two tests, although the proportion of enrollees who received VF testing and no OOI dropped by 10% whereas the proportion who underwent OOI but no VF testing increased by 2%–3% from 2003 to 2007. This suggests that some eye-care providers are choosing to perform OOI not as an adjunct to the more traditional tests for glaucoma assessment but instead as a replacement. Since VF testing usually takes longer to perform, requires more patient effort and time to administer, is subjective, and at the time had a lower reimbursement rate than OOI, there are several incentives for eye-care providers to change how they evaluate OAG patients.
In our analyses, the bulk of the shifting utilization of ancillary glaucoma tests occurred between the years 2001 and 2005. We suspect that early in the decade few providers had access to OOI equipment to perform this testing yet many had access to equipment to perform perimetric testing. As providers became more familiar with OOI and began purchasing these devices, there was a rise in the use of this technology. By the middle of the decade, OOI had been integrated into many practices and there was a plateau in its use in the management of OAG. While the decline in VF testing was rather steep during the beginning of the decade, this trend, too, plateaued by the middle of the decade indicating that clinicians continue to find value in its use in glaucoma management.
According to the 2005 American Academy of Ophthalmology (AAO) Preferred Practice Patterns (PPP) for OAG and suspected glaucoma, depending on whether the patient’s glaucoma is adequately controlled, the length of time it has been controlled, and the extent of damage, individuals with OAG should undergo VF and optic nerve assessment every 1 to 12 months, and those with suspected glaucoma, every 3 to 24 months.13,14 Although the PPP guidelines refer to OOI as an effective method for identifying structural damage to the optic nerve and retinal nerve fiber layer, they offer little clinical guidance on the optimal timing intervals with which these tests should be administered and they do not recommend that OOI should replace VF testing or detailed recordings of the optic nerve appearance. In 2007, an Ophthalmic Technology Assessment Committee Panel commissioned by the AAO concluded: “The information obtained from imaging devices is useful in clinical practice when analyzed in conjunction with other relevant parameters that define glaucoma diagnosis and progression.”15
If the trends we observe continue, with providers’ relying more on OOI and less on more traditional measures to evaluate OAG patients, researchers will need to address limitations with current imaging technology—including inadequate representation of different races and ethnicities in the normative databases of some devices and challenges with generating reliable output with patients who have tilted discs16, moderate-to-high myopia17, or larger-or-smaller-than-average sized optic nerves.18 Some studies suggest that compared with careful evaluation of the optic nerve head or VF testing, OOI less effectively differentiates eyes with OAG from healthy eyes5,19, although other studies suggest otherwise.6 In addition, if eye-care providers are using OOI in lieu of VF testing, it is imperative for imaging devices to detect and quantify progression over time. Although some currently available software products enable these devices to identify disease progression20, the performance of these products has been inadequately researched. Furthermore, since the technology to conduct OOI is rapidly evolving, with newer devices replacing older models, if the newer equipment is incapable of assimilating information captured from the older equipment, it will continue to be difficult to use this technology to quantify disease progression. Finally, if OOI is to play an expanded role in glaucoma evaluation, studies should assess whether providers caring predominantly for racial minorities and other such high-risk groups have access to OOI equipment, given its relatively high cost.
It is disconcerting that 22–28% of patients with newly diagnosed suspected glaucoma and 13–17% of those with newly diagnosed OAG did not undergo VF, OOI, or FP procedures within 24 months after diagnosis. These findings echo those of a recent report by Friedman and colleagues.21 Although the proportion of OAG patients in our study who received none of these ancillary glaucoma tests decreased from 2003 to 2007, future efforts should identify why so many patients are still are not receiving the standard of care recommended by the AAO PPP. Without access to patients’ medical records, we cannot discern the reasons why so many insured patients in this sample were inadequately evaluated. Possible explanations include patient-related factors (opting not to follow up and undergo testing), provider-related factors (lacking knowledge of appropriate evaluation methods), or lack of access to the test equipment.
Although eye-care providers, in general, have been relying more on OOI and less on VF testing and FP in their management of patients with OAG and glaucoma suspects, we find that the actual rates of use of these tests differ by type of eye-care provider. For example, the likelihood of using OOI for a patient with OAG in 2009 relative to 2001 increased significantly among ophthalmologists but even more dramatically among optometrists (by 100% vs. 153%, respectively). Likewise, the odds of performing VF testing in 2009 relative to 2001 declined for both provider types, but more so for optometrists (by 59%) than for ophthalmologists (by 45%). Possible explanations for the differing rates between provider types include differences in education and training, in patient case-mix, in access to diagnostic equipment, or in attitudes towards revenue generation.
A strength of using a large health-care claims database for our study is the ample numbers of individual patients with OAG and suspected glaucoma to perform a robust analysis of utilization patterns over time, and to stratify those analyses by provider type and adequately adjust for key potential confounders. Additionally, the data come from different types of clinical practices and settings (e.g., academic centers vs. private practices; rural vs. urban vs. suburban) throughout the country. Moreover, because all the patients had insurance, insurance status was not a barrier to the receipt of these services.
Our study is limited by our lack of access to patient medical records, which would contain information on clinical parameters; thus, we cannot determine whether patient care in specific instances was inadequate, appropriate, or excessive. Additional studies using other data sources should examine this important question and seek to further explore the factors driving some of the differences we note. Second, we had no information on the types of diagnostic equipment that eye-care providers had access to, which would certainly influence the types of tests ordered. Our results may be nongeneralizable to patient populations without any or adequate insurance coverage, in which we would expect to find even lower levels of utilization. Other important questions pertaining to utilization of ancillary glaucoma tests which cannot be adequately explored with claims data alone include how eye care providers opt to follow patients who have abnormal tests to identify disease progression and whether providers are more apt to follow OAG patients with one of these types of tests versus another depending on glaucoma severity.
If future studies confirm our specific findings regarding the recent shift in eye-care providers’ methods for following OAG and suspected glaucoma, greater attention will need to be paid to more fully understanding and overcoming some of the known limitations associated with current OOI devices. It is essential that some of the newer software products aimed at detecting disease progression with these devices undergo adequate internal and external validation, and that manufacturers address issues of noncompatibility between results generated from older and newer models, so that patients’ disease progression can be carefully followed over time. Finally, until imaging devices can be demonstrated to identify the presence of glaucoma and more fully capture disease progression relative to more traditional equipment, such as VF testing and FP can, providers should use these newer devices, if at all, as an adjunct to, not a replacement for, perimetry and FP.
Supplementary Material
Acknowledgments
Grant support: National Eye Institute K23 Mentored Clinician Scientist Award (1K23EY019511-01); American Glaucoma Society Clinician Scientist Grant, Blue Cross Blue Shield of Michigan Foundation, and an unrestricted grant from Research to Prevent Blindness
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented in part at the 2011 American Glaucoma Society meeting, March 5, 2011
The authors have no proprietary or commercial interest in any material discussed in this manuscript
References
- 1.Leung CK, Cheung CY, Weinreb RN, et al. Evaluation of retinal nerve fiber layer progression in glaucoma: a comparison between the fast and the regular retinal nerve fiber layer scans. Ophthalmology. 2011;118:763–767. doi: 10.1016/j.ophtha.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 2.Rauscher FM, Sekhon N, Feuer WJ, Budenz DL. Myopia affects retinal nerve fiber layer measurements as determined by optical coherence tomography. J Glaucoma. 2009;18:501–505. doi: 10.1097/IJG.0b013e318193c2be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asrani S, Edghill B, Gupta Y, Meerhoff G. Optical coherence tomography errors in glaucoma. J Glaucoma. 2010;19:237–242. doi: 10.1097/IJG.0b013e3181b21f99. [DOI] [PubMed] [Google Scholar]
- 4.Jaffe GJ, Caprioli J. Optical coherence tomography to detect and manage retinal disease and glaucoma. Am J Ophthalmol. 2004;137:156–169. doi: 10.1016/s0002-9394(03)00792-x. [DOI] [PubMed] [Google Scholar]
- 5.Deleón-Ortega JE, Arthur SN, McGwin G, Jr, et al. Discrimination between glaucomatous and nonglaucomatous eyes using quantitative imaging devices and subjective optic nerve head assessment. Invest Ophthalmol Vis Sci. 2006;47:3374–3380. doi: 10.1167/iovs.05-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badalà F, Nouri-Mahdavi K, Raoof DA, et al. Optic disk and nerve fiber layer imaging to detect glaucoma. Am J Ophthalmol. 2007;144:724–732. doi: 10.1016/j.ajo.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Physician ICD-9-CM 2006. 9th revision, Clinical Modification. Chicago, IL: American Medical Association; 2006. [Google Scholar]
- 8.CPT 2006. Current Procedural Terminology Professional Edition. Chicago, IL: American Medical Association; 2006. [Google Scholar]
- 9.Coleman AL, Yu F, Rowe S. Visual field testing in glaucoma Medicare beneficiaries before surgery. Ophthalmology. 2005;112:401–406. doi: 10.1016/j.ophtha.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 10.Gardiner SK, Demirel S. Assessment of patient opinions of different clinical tests used in the management of glaucoma. Ophthalmology. 2008;115:2127–2131. doi: 10.1016/j.ophtha.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schuman JS. Spectral domain optical coherence tomography for glaucoma (an AOS thesis) Trans Am Ophthalmol Soc. 2008;106:426–458. [PMC free article] [PubMed] [Google Scholar]
- 12.Hillman BJ. Physicians’ acquisition and use of new technology in an era of economic constraints. In: Gelijins AC, editor. Technology and Health Care in an Era of Limits. vol 3. Washington, DC: National Academy Press; 1992. pp. 133–149. Medical Innovation at the Crossroads. [Google Scholar]
- 13.American Academy of Ophthalmology Glaucoma Panel. Preferred Practice Pattern. Primary Open-Angle Glaucoma. San Francisco, CA: American Academy of Ophthalmology; 2010. [Accessed July 24, 2011]. p. 32. Available at: http://one.aao.org/CE/PracticeGuidelines/PPP.aspx. [Google Scholar]
- 14.American Academy of Ophthalmology Glaucoma Panel. Preferred Practice Pattern. Primary Open-Angle Glaucoma Suspect. San Francisco, CA: American Academy of Ophthalmology; 2010. [Accessed July 24, 2011]. p. 12. Available at: http://one.aao.org/CE/PracticeGuidelines/PPP.aspx. [Google Scholar]
- 15.Lin SC, Singh K, Jampel HD, et al. Ophthalmic Technology Assessment Committee Glaucoma Panel. Optic nerve head and retinal nerve fiber layer analysis: a report by the American Academy of Ophthalmology. Ophthalmology. 2007;114:1937–1949. doi: 10.1016/j.ophtha.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park CY, Kim YT, Kee C. Evaluation of the influence of tilt of optic disc on the measurement of optic disc variables obtained by optical coherence tomography and confocal scanning laser ophthalmoscopy. J Glaucoma. 2005;14:210–214. doi: 10.1097/01.ijg.0000159129.93085.96. [DOI] [PubMed] [Google Scholar]
- 17.Leung CK, Mohamed S, Leung KS, et al. Retinal nerve fiber layer measurements in myopia: an optical coherence tomography study. Invest Ophthalmol Vis Sci. 2006;47:5171–5176. doi: 10.1167/iovs.06-0545. [DOI] [PubMed] [Google Scholar]
- 18.Ortega JL, Kakati B, Girkin CA. Artifacts on the optic nerve head analysis of the optical coherence tomography in glaucomatous and nonglaucomatous eyes. J Glaucoma. 2009;18:186–191. doi: 10.1097/IJG.0b013e31818159cb. [DOI] [PubMed] [Google Scholar]
- 19.Siam GA, Gheith ME, de Barros DS, et al. Limitations of the Heidelberg Retina Tomograph. Ophthalmic Surg Lasers Imaging. 2008;39:262–264. doi: 10.3928/15428877-20080501-16. [DOI] [PubMed] [Google Scholar]
- 20.Medeiros FA, Zangwill LM, Alencar LM, et al. Detection of glaucoma progression with Stratus OCT retinal nerve fiber layer, optic nerve head, and macular thickness measurements. Invest Ophthalmol Vis Sci. 2009;50:5741–5748. doi: 10.1167/iovs.09-3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedman DS, Nordstrom B, Mozaffari E, Quigley HA. Glaucoma management among individuals enrolled in a single comprehensive insurance plan. Ophthalmology. 2005;112:1500–1504. doi: 10.1016/j.ophtha.2005.02.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.