Abstract
Melanoma is a common and deadly tumor that upon metastasis to the central nervous system (CNS) has median survival duration of less than 5 months. Activation of the signal transducer and activator of transcription 3 (STAT3) has been identified as a key mediator that drives the fundamental components of melanoma. We hypothesized that WP1066, a novel inhibitor of STAT3 signaling, would enhance the antitumor activity of cyclophosphamide (CTX) against melanoma, including disease within the CNS. The mechanisms of efficacy were investigated by tumor- and immune-mediated cytotoxic assays, in vivo evaluation of the reduction of regulatory T cells (Tregs) and by determining intratumoral p-STAT3 expression by immunohistochemistry. Combinational therapy of WP1066, with both metronomic and cytotoxic dosing of CTX, was investigated in a model system of systemic and intracerebral melanoma in syngeneic mice. Inhibition of p-STAT3 by WP1066 was enhanced with CTX in a dose-dependent manner. However, in mice with intracerebral melanoma, the greatest therapeutic benefit was seen in animals treated with cytotoxic CTX dosing and WP1066, whose median survival time was 120 days, an increase of 375%, with 57% long-term survivors. This treatment efficacy correlated with p-STAT3 expression levels within the tumor microenvironment. The efficacy of the combination of cytotoxic dosing of CTX with WP1066 is attributed to the direct tumor cytotoxic effects of the agents and has the greatest therapeutic potential for the treatment of CNS melanoma.
Keywords: Melanoma, cyclophosphamide, STAT3
INTRODUCTION
Metastasis to the central nervous system (CNS) in patients with melanoma is associated with a dismal prognosis, with a median survival interval of less than 5 months1, 2. Cyclophosphamide (CTX) has been used in the treatment of patients with stage IV melanoma3, 4 and to enhance various immunotherapeutic approaches5–10, presumably by decreasing regulatory T cells (Tregs)7, 11, 12, increasing proinflammatory cytokines8, 13 or inhibiting immunosuppressive cytokines14. The immunomodulatory, metronomic (20 mg/kg/day) dosing of CTX is less toxic than cytotoxic (150 mg/kg/day) dosing and has been shown to exert potent and long lasting antitumor effects in a number of animal tumor model systems15, 16; including murine metastatic melanoma models17, 18. The superiority of using metronomic CTX dosing over monotherapies has been demonstrated in murine melanoma model systems17–19; however evaluating this type of treatment strategy for patients with end-stage melanoma with CNS metastasis has been neglected.
The signal transducer and activator of transcription 3 (STAT3) is a key regulator of tumor growth, metastases, and tumor-associated immunosuppression in patients with malignancies, including melanoma20–23. Upon phosphorylation, p-STAT3 becomes activated, translocates to the nucleus and up regulates key genes in tumorigenesis and tumor immune evasion23, 24. Constitutively active p-STAT3 is frequently seen in melanoma and is found at higher levels in metastatic melanoma cell lines25, 26 and in melanoma brain metastases27. The inhibition of p-STAT3 suppresses melanoma cell invasiveness, inhibits proliferation, and prevents metastasis in murine models26. We have previously shown that blocking p-STAT3 with WP1066, a novel small molecule inhibitor of p-STAT3, has cytotoxic effects on melanoma cell lines20, enhances T-cell cytotoxicity against melanoma by inhibition of Tregs21, 28, and inhibits melanoma metastasis in a syngeneic murine model20, 21. The efficacy of combinational treatment of CTX with p-STAT3 inhibition in a murine model with CNS metastasis has not been studied. We therefore hypothesized that combination therapy of CTX with WP1066, an inhibitor of p-STAT3, would act additively against melanoma (including disease within the CNS) secondary to their combined effect on inhibiting Tregs to enhance cytotoxicity of effector T cells.
Materials and Methods
Agents
WP1066 was synthesized and supplied by Dr. Priebe (The University of Texas M. D. Anderson Cancer Center, Houston, TX). CTX (Sigma, St. Louis, MO) was dissolved in PBS immediately before use.
Murine models of melanoma
The B16/F10 murine melanoma has been previously described20. The in vivo experiments utilized 4- to 6-week-old female C57BL/6J mice (10/group) in accordance with Laboratory Animal Resources Commission standards and conducted according to an approved protocol, 08-06-11831. Induction of intracerebral B16 and to assess for the generation of immunological memory by tumor rechallenge has been previously described20, 29, 30. The intracerebral tumorigenic dose for the B16 cells was 5 × 102 in a total volume of 5 µl. In this murine model system, median survival is typically 15 days and grossly evident tumor is not usually seen until day 10–12 when the mice begin to manifest neurological symptoms. To induce pulmonary melanoma, 1 × 105 cells in a total volume of 100 µl were injected into the tail vein of the mouse. Treatment was initiated on day 3. For mice with intracerebral tumors, an animal was euthanized when they were unable to reach food or water, lost greater than 20% of its body weight, or was suffering from neurological deficits, which historically occurs within 24 hours of death. The etiology of death was confirmed to be tumor progression by autopsy of the CNS. For the mice with pulmonary lesions, the experiment was terminated after two weeks, and the number of pulmonary melanoma lesions were counted by two observers blinded to the treatment conditions (independently of each other) and tabulated.
Metronomic dosing of CTX, delivered o.g., was at a dose of 20 mg/kg every day (weekends off) until euthanasia/death or for a maximum of 3 weeks (whichever came first) in the intracerebral model and for 2 weeks in the pulmonary model. Cytotoxic CTX treatment, delivered i.p., was administered as two weekly cycles separated by a 1-week interval for the intracerebral model and for one cycle in the pulmonary model. Each cycle consisted of a total of three doses of CTX (150 mg/kg/per dose) administered every other day (total dose of 450 mg/kg, maximal tolerated dose)16. Since greater than 80% of mice with intracerebral B16 melanoma treated with WP1066 at 40 mg/kg survive long-term20, a subtherapeutic dose of 30 mg/kg of WP1066 was used so an additive/synergistic effect with CTX could be ascertained. WP1066 was administered via o.g. in a vehicle of DMSO/polyethylene glycol (PEG) 300 (1:4 ratio) on a once every other day schedule for 9 treatments (on Mondays, Wednesdays, and Fridays). DMSO/PEG 300 vehicle alone was used for the negative control group.
Cell survival assay
B16 cells were seeded at a density of 2,000 cells per well in 96-well culture plates and were treated with WP1066 at increasing concentrations of 0, 0.156, 0.313, 0.625, 1.25, 2.5, and 5.0 µM or at CTX concentrations of 0, 0.156 mg/ml (0.559 mM), 0.313 mg/ml (1.121 mM), 0.625mg/ml (2.239 mM), and 1.25 mg/ml (4.478 mM). The WP1066 diluent DMSO was used at a final concentration of 0.05% including as a control with the CTX. After 72 h of treatment, 25 µl of 5 mg/ml dimethyl thiazolyl diphenyl tetrazolium salt (MTT, Sigma-Aldrich, St. Louis, MO) solution were added to each well, and the cells were cultured for 3 h at 37° C in a humidified atmosphere of 5% CO2 and 95% air. The cells were lysed with 100 µl/well of lysing buffer (50% dimethylformamide, 20% sodium dodecyl sulfate [SDS], pH 5.6) and incubated at room temperature overnight. Cell viability was evaluated by reading the O.D. at 570 nm, and the IC50 was calculated.
Immunoblotting analysis
B16 cells were seeded at a density of 2 × 106 cells/well in 6-well culture plates and incubated overnight in RPMI medium at 37° C in an atmosphere containing 5% CO2. Afterwards, B16 cells were cultured in the absence or presence of WP1066 (2.5 µM, 5 µM), CTX [0.75 mg/ml (below the IC50), 1.5 mg/ml (above the IC50], or the combination of 2.5 µM of WP1066 with CTX (0.75 mg/ml, 1.5 mg/ml) for 2 hours. Afterwards, B16 cells were pelleted and rinsed with ice-cold PBS at 1500 rpm for 5 minutes then placed for 30 minutes in ice-cold lysis buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 1 mM EDTA) containing 1% Triton-X-100 plus phosphatase and protease inhibitors (Sigma-Aldrich). The lysates were centrifuged at 14,000 rpm for 10 minutes at 4° C. The supernatants were collected and their protein content was quantified. Equal amounts of protein (65 µg) were electrophoretically fractionated in 8% polyacrylamide gels containing SDS, transferred to nitrocellulose membranes, and subjected to immunoblot analysis with specific antibodies against p-STAT3 (Tyr705), STAT3 (Cell Signaling Technology, Inc., Danvers, MA), and β-actin (Sigma-Aldrich). Autoradiography of the membranes was performed using Amersham ECL Western blotting chemiluminescent detection reagents (Amersham Biosciences). The densities of the protein bands relative to that of the β-actin protein control were measured with the Image J program provided by the National Institutes of Health (http://rsb.info.nih.gov/ij/index.html).
Immune cytotoxicity assay
Spleens from 4- to 6-week-old female mice with intracerebral melanoma (positive control), without tumor or treatment (negative control), or with intracerebral tumors treated for two week were harvested and dissociated into a single cell suspension. After erythrocytes in the spleens were lysed with 1X RBC lysis buffer (eBioscience), splenocytes were washed once with RPMI 1640 medium and were used as effector cells to target B16 cells in standard cytotoxicity assays31. The ratio of splenocyte, effector cells to 4 µM CSFE-labeled B16 target cells was 100:1. Viability was assessed based on propidium iodide (PI; BD Biosciences) staining of CFSE-labeled B16 cells by a FACSCaliber flow cytometer (BD Biosciences), and data analysis was performed by using FlowJo software (TreeStar, Ashland, OR).
Immunohistochemistry
Formalin-fixed, paraffin-embedded 4µm sections of the CNS melanoma or lung melanoma were stained for p-STAT3 expression using a rabbit polyclonal anti-p-STAT3 (Tyr705) antibody (1:50; Cell Signaling Technology, Danvers, MA) as previously described32. To detect expression of the macrophage-restricted cell surface glycoprotein F4/80, a purified anti-mouse F4/80 (1:50; Biolegend, San Diego, CA) antibody was used. To detect CD8 expression, a rat monoclonal anti-CD8 antibody (1:50; Abcam, Cambridge, MA) was used. Two independent observers (L-YK, TW) blinded to treatment cohorts quantitatively evaluated intranuclear staining of p-STAT3 expression by analyzing the tumors using high-power fields (max: ×400 objective and ×100 eyepiece) of each specimen in the regions with the highest relative positive staining for that individual specimen in duplicate. Three sections of each tumor and four fields of view of each section were analyzed. Each observer recorded a total cell count and the number of cells with positive intranuclear p-STAT3 staining. The duplicate numbers were then averaged for the final number of cells and were expressed as the percentage of positive cells per specimen. The analysis was secondarily validated and reviewed by the neuropathologist (GNF).
Determination of inhibition of immune cells in vivo
To ascertain the inhibition of the immune populations within the spleen and peripheral blood compartments, tumor-bearing mice were treated with CTX, WP1066, or CTX in combination with WP1066, for 14 days as described above. Single-cell suspensions were prepared from spleens and the peripheral blood of mice and single cells were surface-stained with FITC-conjugated anti-CD4 (L3T4) or PE-conjugated anti-CD8 (53-6.7) and were intracellularly stained with APC-conjugated-FoxP3 (clone FJK-16s; eBioscience, San Diego, CA). The cell number of CD4+ and CD8+ T cells in the peripheral blood was counted based on positive surface staining of the respective markers relative to the total cell count of PBMCs. The percentage of FoxP3+ Tregs was calculated within the peripheral blood and within the CD4 compartment as previously described33.
Statistics
Kaplan-Meier product-limit survival probability estimates of overall survival were calculated34 and log-rank tests35 were performed to compare overall survival between treatment groups and the control arm. No multiple comparison adjustment was done. All the P values are unadjusted and a value below 0.05 was considered statistically significant. Computations were carried out in SAS version 9.2. Student’s t-test was performed for the ex vivo and in vitro data.
Results
Blockade of p-STAT3 with WP1066 enhances the cytotoxic effects of CTX on the tumor
To determine if CTX and WP1066 exert an additive effect on direct tumor cytotoxicity, the IC50 of the agents, both individually and in combination, were evaluated on the melanoma cell line B16. The IC50 doses of WP1066 and CTX for B16 cells were 2.43 µM (0.865 µg/ml) and 4.04 mM (1.128 mg/ml), respectively. Using the IC50 dose of WP1066 (2 µM), the addition of CTX enhanced tumor cytotoxicity (Fig. 1A).
Figure 1.

Cyclophosphamide (CTX) and WP1066 exert an additive cytotoxicity. A. The combination of CTX and WP1066 exerts direct cytotoxic effects on B16 cells. B. Western blot analysis reveals that p-STAT3 inhibition by WP1066 was enhanced with CTX in a dose-dependent manner in B16 cells.
B16 has both constitutive activation of p-STAT3 (Fig. 1B) and inducible activity36. To ascertain whether CTX exerts an additive effect with WP1066 on the inhibition of p-STAT3, B16 cells were cultured in the absence or presence of WP1066, CTX, or both, and the levels of p-STAT3, total STAT3, and β-actin evaluated by Western blot analysis. Inhibition of p-STAT3 by WP1066 was enhanced with CTX in a dose-dependent manner in the B16 cells. Specifically, the combination of either 0.75 or 2.5 mg/ml of CTX with 2.5 µM of WP1066 has a similar inhibitory effect on pSTAT3 as 5 µM of WP1066 alone. This would suggest that CTX can enhance the inhibition of p-STAT3 in conjunction with WP1066 but cannot exert direct inhibition of p-STAT3 at these doses (Fig. 1B).
WP1066 does not further enhance the therapeutic effects of cyclophosphamide on pulmonary melanoma lesions
To determine whether CTX and WP1066 show additive efficacy against melanoma within the lung, metronomic CTX (o.g.), cytotoxic CTX (i.p.) or WP1066 (o.g.) were administered alone or in conjunction with one another in C57BL/6J mice who had pulmonary melanoma. In an attempt to observe an additive effect of STAT3 inhibition and CTX, a subtherapeutic dose (30 mg/kg) of WP1066 was used. The number of melanoma lesions in untreated tumor-bearing mice was 26.8 ± 11.3. Although there was no statistical difference in development of lung lesions between mice treated with the subtherapeutic WP1066 dose (33 ± 16.1) and the untreated mice (P> 0.05), administration of CTX by both metronomic and cytotoxic methods reduced the number of pulmonary lesions compared with the control (7 ± 3.5 P= 0.049 and 0.7 ± 0.6; P= 0.01, respectively). Reduction in the number of melanoma lesions was even more significant using cytotoxic CTX dosing than using metronomic CTX dosing (P= 0.03). However, no additive effect was observed in combinational therapy groups compared with groups treated alone with either metronomic (7.8 ± 3.6) or cytotoxic (0.5 ± 0.6) CTX dosing (Fig. 2).
Figure 2.

Cyclophosphamide (CTX) is efficacious against pulmonary melanoma. Subtherapeutic WP1066 did not inhibit B16 pulmonary melanoma; however, both metronomic and cytotoxic CTX dosing exerted a therapeutic effect compared with the control (P<0.05). The decrease in the number of melanoma lesions was more significant for cytotoxic compared with metronomic CTX dosing (P= 0.03). Regardless of metronomic or cytotoxic CTX dosing, subtherapeutic WP1066 did not exert additive effect.
WP1066 does enhance the therapeutic effects of cyclophosphamide against CNS melanoma
To determine whether combinational therapy of metronomic CTX dosing and WP1066 yields an additive benefit against established CNS tumors, C57BL/6J mice with intracerebral B16 were treated as previously described. The median overall survival time for tumor-bearing mice treated with the WP1066 vehicle control or PBS was 16 days (range, 13–21 days; n=10). No improvement in the median survival time was observed with the subtherapeutic WP1066 dose alone (15 days; range, 10–18 days; n= 7; P= 0.29) or metronomic (o.g.) dosing of CTX alone (18 days; range 17–36 days; n=10; P= 0.1) compared with the control. The median overall survival time of 21 days (range, 17–75 days; n=10) was slightly longer for mice with intracerebral melanoma treated with the subtherapeutic WP1066 dose in combination with metronomic CTX dosing, and this was statistically significant compared with vehicle control mice (P=0.004) and those treated with WP1066 (P<0.0001) alone but not with those treated with metronomic CTX dosing alone (P= 0.13) (Fig. 3A).
Figure 3.

Cyclophosphamide exerts an additive effect with WP1066 against intracerebral melanoma. A. Survival data from C57BL/6J mice treated with metronomic cyclophosphamide (CTX) dosing, cytotoxic CTX dosing, WP1066, or in combination after B16 cells were established in the brain. The median survival time was not significantly enhanced with subtherapeutic WP1066 or with metronomic CTX monotherapy; however the combination exerted therapeutic benefit in comparison to the control (P=0.004) and WP1066 monotherapy (P<0.0001) but not with metronomic CTX monotherapy (P= 0.13). B. Median survival was significantly enhanced by cytotoxic CTX monotherapy compared with the vehicle control (P< 0.0001) and was further enhanced with subtherapeutic WP1066 in comparison to cytotoxic CTX dosing alone (P= 0.03), WP1066 alone (P=0.0001), and the control (P=0.0002). In mice that survived long term, subsequent rechallenge by injection of B16 cells into the contra lateral hemisphere indicated that no immunological memory was induced. These experiments were repeated in their entirety with similar results.
We next determined the effects of cytotoxic CTX dosing in combination with WP1066. The median overall survival time for tumor-bearing mice treated with the WP1066 vehicle control or PBS was 17 days (range, 13–17 days; n=9). No improvement in the median survival time was observed with the subtherapeutic WP1066 dose alone (17.5 days; range, 14–21 days; n= 8; P= 0.10). Survival was significantly enhanced by cytotoxic CTX monotherapy relative to the vehicle control (32 days; range, 24–108 days; P< 0.0001). The median survival time of mice with intracerebral melanoma treated with a subtherapeutic WP1066 dose and cytotoxic CTX dosing was 120 days (range, 26–127 days; n=7), which was significantly longer than for mice treated using cytotoxic CTX dosing alone (P= 0.03), WP1066 (P=0.0001), and the controls (P=0.0002) (Fig. 3B). For the mice treated with the combined therapy of WP1066 and cytotoxic CTX dosing, 57% survived long term, and there was at least a 375% increase in median survival time compared with the cytotoxic CTX alone group when the experiment was terminated to perform the tumor rechallenge experiments. At the time of death, CNS autopsy demonstrated there were no significant differences in tumor burden between treatment groups. Death was usually secondary to leptomeningeal spread of B16 tumor and secondary hydrocephalus. This would preclude measuring tumor size and harvesting for Western Blot analysis.
To determine whether mice with intracerebral tumors treated with both WP1066 and cytotoxic or metronomic dosing of CTX were able to generate long-lasting protective immune memory, mice were rechallenged with B16 cells implanted in the contra lateral hemisphere. Upon rechallenge, mice treated with the combination of the subtherapeutic WP1066 dose in conjunction with either metronomic or cytotoxic CTX dosing, had median survival times of 17 and 21 days, respectively, which did not differ significantly from the median survival time of naïve, control mice. We did not see long lasting immunity after intracerebral rechallenge experiment in any treatment groups.
WP1066 does not further enhance immune-mediated cytotoxic effects of CTX
To determine if there was an enhancement of immunological tumor cytotoxicity correlating with the therapeutic in vivo efficacy of the combinational therapy, we evaluated immune cytotoxic responses directed toward B16 melanoma cells. Splenocytes from non-tumor-bearing mice, and tumor-bearing mice treated with WP1066, metronomic (o.g.) CTX dosing, cytotoxic (i.p.) CTX dosing, or a combination of WP1066 with CTX were isolated and co-cultured with CFSE-labeled B16 target cells for 48 h to assess immune cytotoxicity toward B16 cells. Immune-mediated cytotoxicity decreased by 37% in tumor-bearing mice compared with non-tumor-bearing controls (P =0.008). The immune cells including CD3+ T cells, natural killer (NK) cells and NKT cells (splenocytes) from the tumor-bearing mice treated with WP1066, metronomic CTX dosing, and cytotoxic CTX dosing increased cytotoxic clearance of the B16 target cells by 30%, 77% and 69%, respectively, relative to tumor-bearing control mice (P = 0.05, P = 0.001, and P= 0.006, respectively). However, combinational therapy of WP1066 with either metronomic or cytotoxic CTX dosing did not significantly enhance immune-mediated cytotoxic clearance of the B16 target cells in comparison with monotherapy (Fig. 4A).
Figure 4.
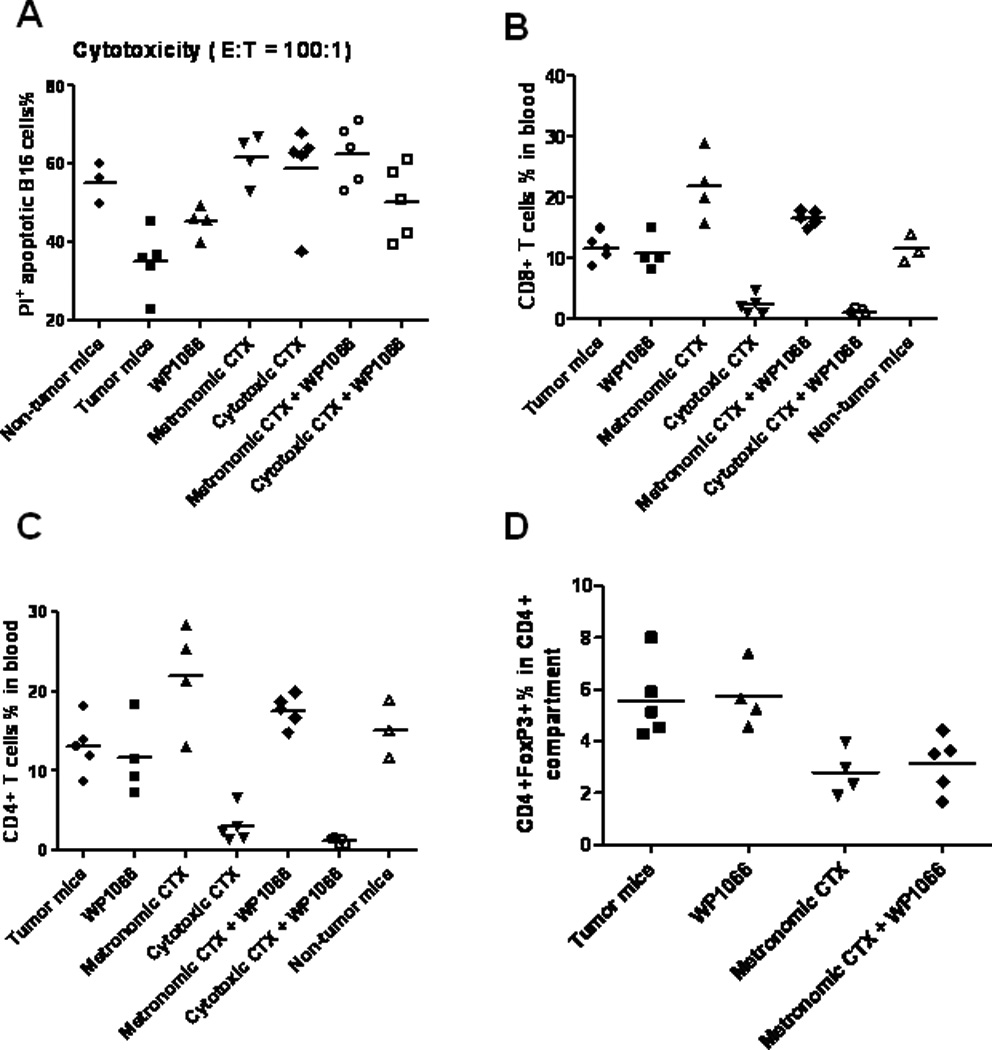
In vivo immune modulation does not correlate with combination treatment response. A. There was a decrease in immune-mediated cytotoxicity in tumor-bearing mice compared with non-tumor-bearing controls (P = 0.008). The effector cells from tumor-bearing mice that were treated with WP1066, metronomic CTX dosing, and cytotoxic CTX demonstrated increased cytotoxic clearance of the B16 target cells compared with tumor-bearing control mice (P = 0.05, P = 0.001, and P = 0.006, respectively). However, therapies combining WP1066 with either metronomic or cytotoxic CTX dosing did not exert an additive tumor cytotoxic effect. B. The number of CD8+ T cells was enhanced with metronomic CTX dosing (P= 0.007) and suppressed with cytotoxic CTX dosing (P<0.0001). However, the CD8+ effector cells were not further modulated with WP1066 in conjunction with CTX (P> 0.05). C. The number of CD4+ T cells was enhanced with metronomic CTX dosing (P= 0.04) and was suppressed by cytotoxic CTX dosing (P= 0.0005); however the number of CD4+ T cells was not further modulated with WP1066 in conjunction with CTX (P> 0.05). D. Within the CD4 compartment, metronomic CTX dosing inhibited Tregs (CD4+Foxp3+) (P< 0.05) compared with the tumor-bearing control; however WP1066 did not further inhibit the number of Tregs (P>0.05).
WP1066 does not further modulate the influence of CTX on the immune cell populations
To determine the in vivo effects of metronomic (o.g.) CTX dosing, cytotoxic (i.p.) CTX dosing, WP1066 administration, and combinational therapy on the number of CD8+, CD4+, and CD4+Foxp3+ Tregs, tumor-bearing mice were treated for 14 days as described above. Metronomic CTX dosing enhanced the number of CD8+ (P= 0.007) (Fig. 4B) and CD4+ T cells (P= 0.04) (Fig. 4C) and inhibited the number of Tregs within the CD4 compartment (P<0.05) (Fig. 4D) compared with the control tumor-bearing mice. The combination of metronomic CTX dosing and subtherapeutic WP1066 dosing did not further enhance the number of CD8+ (Fig. 4B) or CD4+ T cells (Fig. 4C) or further inhibit the number of CD4+FoxP3+ Tregs in the peripheral blood (P > 0.05) (Fig. 4D). In contrast, cytotoxic CTX dosing significantly suppressed CD8+ T cells (P< 0.0001) (Fig. 4B), CD4+ T cells (P= 0.0005) (Fig. 4C), and CD4+FoxP3+ Tregs (P=0.0004) (data not shown) compared with the tumor-bearing control mice and this was not further modulated with WP1066 (P> 0.05).
WP1066 exerts an additive effect to CTX inhibition of the p-STAT3 pathway within the tumor microenvironment
To clarify why there was an additive effect of CTX with WP1066 in the efficacy experiments against melanoma within the CNS but to a much lesser degree than in the lung, we investigated the levels of pSTAT3 within the local tumor microenvironments and their modulation with combination therapy. The intranuclear p-STAT3 expression within the melanomas in each group was measured using immunohistochemistry and expressed as the percentage of p-STAT3-positive cells. The presence of cytoplasmic melanin was not included in the analysis. In the CNS melanoma of control mice, p-STAT3 expression was 12.7 ± 2.3% (range 2.8–21.8%; n = 8). This was diminished to 9.2 ± 2.8% (range 2.3–18.7%; n = 9; P=0.170) in mice treated with sub-therapeutic WP1066. Metronomic CTX did not affect p-STAT3 expression at 12.7 ± 3.7% (range 4.6–37.2%; n = 8) p-STAT3 expression. In mice with CNS tumors treated with cytotoxic CTX, the p-STAT3 level was 4.5 ± 1.2% (range 1.9–10.7%; n = 7; P=0.011) and this was further reduced in combination with WP1066 to 2.3 ± 0.8% (range 0–6.5%; n = 7; P=0.001) (Fig. 5A). In marked contrast, in the control, established lung melanoma, the p-STAT3 expression was low at 2.4 ± 0.6% (range 0–3.7%; n = 5). Mice treated with sub-therapeutic WP1066 and metronomic CTX had 2.0 ± 0.4% (range 1.4–2.7%; n = 4) and 1.7 ± 0.7 % (range 0.8–3%; n =5) p-STAT3 expression, respectively. Secondary to the already low levels of p-STAT3, the addition of WP1066 did not provide any further additive effect to CTX in modulating p-STAT3 inhibition in lung tumor model (Fig. 5B). Although a significant difference could be found in the p-STAT3 expression in tumors between the brain and the lung in animals treated with the metronomic CTX, a determination of p-STAT3 levels in the cytotoxic CTX group could not be used to provide secondary validation due to the low incidence of lung tumors (i.e. marked efficacy of cytotoxic CTX in the lung). The numbers of tumor infiltrating macrophage/microglia and CD8 T cells in the tumor microenvironment between treatment groups was not significant (data not shown).
Figure 5.
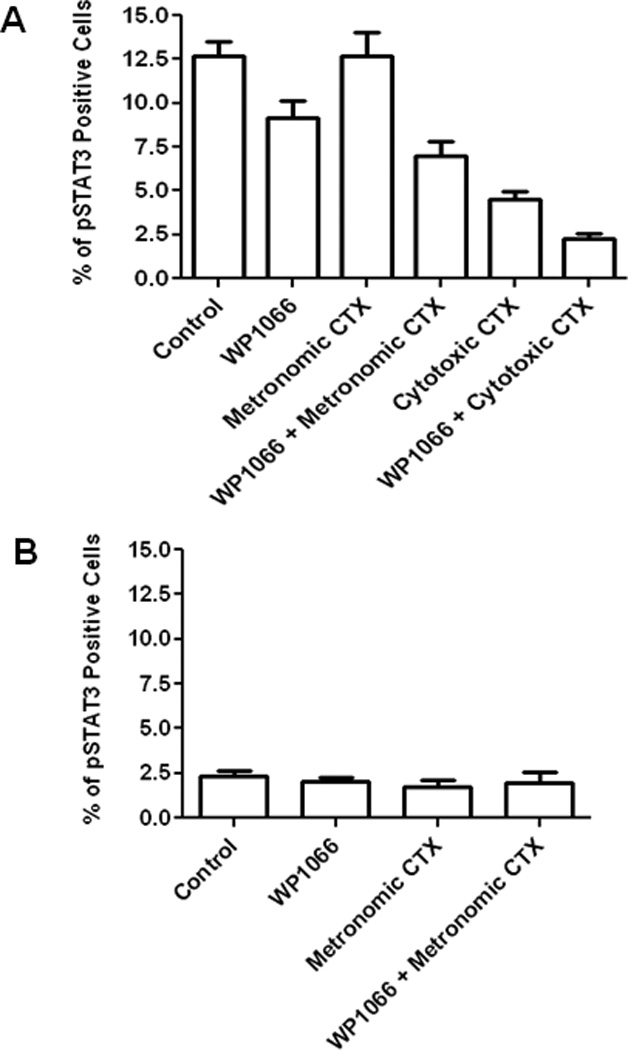
Intratumoral p-STAT3 expression correlates with treatment response. (A) The intratumoral levels of p-STAT3 in melanoma within the CNS were significantly lower in mice treated with cytotoxic CTX dosing than those in the control mice (P = 0.011). The combination of metronomic CTX dosing or cytotoxic CTX dosing with WP1066 also significantly inhibited p-STAT3 expression in tumors in comparison with the control mice (P = 0.052 and P = 0.001, respectively). Error bars represent standard error of the mean. (B) The intratumoral levels of p-STAT3 in melanoma within the lung were lower than those in melanoma within the CNS. There was no significantly difference among various treatment groups in the lung.
Discussion
The refractory nature of melanoma and intracerebral melanoma in particular has been problematic in the clinical setting, with typical response rates of less than 10% with single chemotherapeutic agents37, 38. Previous reports have shown superior effects of various combinations of metronomic CTX dosing with other therapeutics over monotherapies in murine melanoma models17, 39. Indeed, Shiraga et al. showed increased survival time and reduced pulmonary metastasis with a metronomic CTX schedule compared with cytotoxic CTX dosing19. Moreover, studies comparing the effectiveness of combinational therapies of metronomic and cytotoxic CTX dosing have shown that tumor control was better in combinational treatment groups with a metronomic CTX schedule than in those with cytotoxic CTX dosing15, 18. In contrast, our results indicate that the survival benefit and number of long term survivors were greater with cytotoxic CTX dosing plus WP1066, especially for treatment of intracerebral melanoma when compared with monotherapies or the combination of metronomic CTX dosing and WP1066. Our findings of the superior efficacy of cytotoxic CTX dosing may be, in part, due to our murine model not being particularly sensitive to CTX toxicity except for slight weight loss at the end of the first cycle of the treatment, which resolved during the 1 week break for the treatment, and did not reoccur during the second cycle of the treatment. Unlike our report, serious toxicity of the cytotoxic CTX schedule was reported in the CB-17 SCID mouse tumor model15 probably attributable to the already immunocompromised stage of this latter model. Our study is also the first to demonstrate that CTX is partially exerting its therapeutic activity of Treg suppression28 and inhibition of melanoma tumorigenesis25–27 by blocking the p-STAT3 pathway. However, given the therapeutic response of the lung melanoma to CTX despite the relatively low levels of p-STAT3, it is likely that CTX exerts direct anti-tumor effects independent of the p-STAT3 pathway.
To clarify the in vivo mechanism of activity, we evaluated the immune-mediated cytotoxic responses and the inhibition of various immune cell populations. The absolute number of CD8+ and CD4+ cells was the highest and number of CD4+FoxP3+ Tregs the lowest in the CD4 peripheral blood compartment in the metronomic CTX group. These findings are consistent with earlier reports that have shown that the single dose of CTX or metronomic CTX schedule enhances CD8+, CD4+ cells and inhibits CD4+ FoxP3+ Tregs9, 10. WP1066 did not further potentiate the inhibition of Tregs likely secondary to our use of subtherapeutic doses to evaluate therapeutic synergy. In contrast, although there was a decreased in the number of CD4+FoxP3+ Tregs in the CD4 peripheral blood compartment in the cytotoxic CTX group, there was also a concordant decrease of CD4 and CD8+ effector T cells. We therefore predicted that the use of metronomic CTX dosing and WP1066 would exert the greatest therapeutic benefit in vivo because there was a decrease in Treg levels but not in levels of antitumor effector cells. However, this was not the case, and the animals that underwent cytotoxic CTX dosing and WP1066, which had the greatest therapeutic benefit, showed the lowest absolute numbers of effector T cells such as CD4+ and CD8+ T cells and Tregs in the peripheral blood. Furthermore, no additive immune-cell mediated cytotoxicity was observed with the combination of WP1066 with either metronomic or cytotoxic dosing of CTX.
Although additive efficacy was shown with cytotoxic CTX dosing and WP1066 in the intracerebral melanoma model, an additive effect with either the metronomic or cytotoxic CTX dosing in combination with WP1066 was not seen in the pulmonary melanoma model. The observed effect of cytotoxic CTX could be due to increased blood-brain barrier and tumor vascular permeability to WP1066. The active metabolite of CTX has limited permeability across the blood-brain barrier40, but cytotoxic CTX has been shown to induce apoptosis in endothelial cells of the tumor vasculature41 and increase vascular permeability through endothelial cell damage42. Another possible explanation is that because maximal efficacy was already obtained in the lung melanoma group treated with cytotoxic CTX dosing, that the addition of WP1066 did not have an additive effect in this model system; however, this would not provide an explanation for the lung melanoma group receiving metronomic CTX dosing because there was still modest development of lung melanoma. The discrepancy between in vivo efficacy and the lack of correlative synergistic immune-mediated modulation could be due to the suboptimal selection of the immune monitoring time points relative to tumor development. The temporal relationship of the immune responses relative to tumor progression and metastasis could be assessed in in vivo models that fluorescently labeling the melanoma and the immune subpopulations43. The incongruity did prompt a direct evaluation of the mechanism of activity of the combination directly within the tumor microenvironment. A marked dose-response effect of CTX with WP1066 was observed on the inhibition of the STAT3 pathway within the microenvironment of the CNS melanoma but not within the lung melanomas. This may be due to a greater induction of the STAT3 pathway within the CNS secondary to the presence of reactive astrocytes44 elaborating IL-645 that are not present in the lung indicating that the intrinsic properties of the tumor microenvironment, including inducible pathways of tumorigenesis may render an enhanced sensitivity to STAT3 inhibitory agents.
A limitation of the intracerebral melanoma model system used in this study is the rapidity of tumor progression. We would have liked to have evaluated the combinational therapy in a model system of grossly evident tumor; however, in mice with intracerebral B16, grossly evident tumor is not apparent until days 10–12, when they are already becoming neurologically compromised. Since median survival is 15 days in untreated mice, the therapeutic window is restricted. Furthermore, it is unclear if CTX can achieve sufficient therapeutic effects in patients with melanoma metastasis within the brain parenchyma although CTX has been shown to achieve sufficient levels in the cerebrospinal fluid of primates to mediate tumor cytotoxicity46. Chemotherapeutic regimens that include CTX have demonstrated responses in patients with CNS medulloblastoma and germinoma47 and CNS breast metastasis48 suggesting that CTX may exert effects against tumors within the CNS. Determination of CNS intratumoral levels of CTX could be elucidated in dogs diagnosed with CNS melanoma or alternatively, within the context of a clinical trial involving a small cohort of patients prior to initiation of a larger Phase II study, by preoperative administration of CTX, surgical resection of the CNS melanoma metastasis and the direct determination of the intratumoral concentrations of CTX49. In conclusion, we have shown a significant additive antitumor effect of WP1066 with cytotoxic dosing of CTX in an established murine intracerebral melanoma model, which suggests that further clinical testing of the combination of a STAT-3 inhibitor with CTX appears warranted.
Acknowledgments
This work was supported by the Anthony Bullock III Foundation (ABH), the Mitchell Foundation (ABH), and the U. S. National Institutes of Health grants CA120813-03, A177225-01, P50 CA093459 (ABH), and P50 CA093459 (WP). The funding organizations had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. We thank Audria Patrick and David M. Wildrick, Ph.D., for editorial assistance.
Abbreviations used
- CNS
Central nervous system
- CTX
cyclophosphamide
- STAT3
signal transducer and activator of transcription 3
Footnotes
Waldemar Priebe and Amy B. Heimberger hold patents and have a financial interest in the development of WP1066.
References
- 1.Sampson JH, Carter JH, Jr., Friedman AH, Seigler HF. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurg. 1998;88:11–20. doi: 10.3171/jns.1998.88.1.0011. [DOI] [PubMed] [Google Scholar]
- 2.Staudt M, Lasithiotakis K, Leiter U, Meier F, Eigentler T, Bamberg M, Tatagiba M, Brossart P, Garbe C. Determinants of survival in patients with brain metastases from cutaneous melanoma. Br J Cancer. 2010;102:1213–1218. doi: 10.1038/sj.bjc.6605622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borne E, Desmedt, Duhamel A, Mirabel X, Dziwniel V, Maire C, Florin V, Martinot V, Penel N, Vercambre-Darras S, Mortier L. Oral Metronomic cyclophosphamide in elderly with metastatic melanoma. Invest New Drugs. 2009 doi: 10.1007/s10637-009-9298-5. [DOI] [PubMed] [Google Scholar]
- 4.Gunturu KS, Meehan KR, Mackenzie TA, Crocenzi TS, McDermott D, Usherwood EJ, Margolin KA, Crosby NA, Atkins MB, Turk MJ, Ahonen C, Fuse S, et al. Cytokine working group study of lymphodepleting chemotherapy, interleukin-2, and granulocyte-macrophage colony-stimulating factor in patients with metastatic melanoma: clinical outcomes and peripheral-blood cell recovery. J Clin Oncol. 28:1196–1202. doi: 10.1200/JCO.2009.24.8153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D, Garrido C, Chauffert B, Solary E, Bernard Bonnotte B, Martin F. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34:336–344. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- 6.Mihalyo MA, Doody AD, McAleer JP, Nowak EC, Long M, Yang Y, Adler AJ. In vivo cyclophosphamide and IL-2 treatment impedes self-antigen-induced effector CD4 cell tolerization: implications for adoptive immunotherapy. J Immunol. 2004;172:5338–5345. doi: 10.4049/jimmunol.172.9.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.North RJ. Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J Exp Med. 1982;155:1063–1074. doi: 10.1084/jem.155.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Proietti E, Greco G, Garrone B, Baccarini S, Mauri C, Venditti M, Carlei D, Belardelli F. Importance of cyclophosphamide-induced bystander effect on T cells for a successful tumor eradication in response to adoptive immunotherapy in mice. J Clin Invest. 1998;101:429–441. doi: 10.1172/JCI1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu JY, Wu Y, Zhang XS, Yang JL, Li HL, Mao YQ, Wang Y, Cheng X, Li YQ, Xia JC, Masucci M, Zeng YX. Single administration of low dose cyclophosphamide augments the antitumor effect of dendritic cell vaccine. Cancer Immunol Immunother. 2007;56:1597–1604. doi: 10.1007/s00262-007-0305-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taieb J, Chaput N, Schartz N, Roux S, Novault S, Menard C, Ghiringhelli F, Terme M, Carpentier AF, Darrasse-Jese G, Lemonnier F, Zitvogel L. Chemoimmunotherapy of tumors: cyclophosphamide synergizes with exosome based vaccines. J Immunol. 2006;176:2722–2729. doi: 10.4049/jimmunol.176.5.2722. [DOI] [PubMed] [Google Scholar]
- 11.Berd D, Mastrangelo MJ. Effect of low dose cyclophosphamide on the immune system of cancer patients: reduction of T-suppressor function without depletion of the CD8+ subset. Cancer Res. 1987;47:3317–3321. [PubMed] [Google Scholar]
- 12.Polak L, Turk JL. Reversal of immunological tolerance by cyclophosphamide through inhibition of suppressor cell activity. Nature. 1974;249:654–656. doi: 10.1038/249654a0. [DOI] [PubMed] [Google Scholar]
- 13.Schiavoni G, Mattei F, Di Pucchio T, Santini SM, Bracci L, Belardelli F, Proietti E. Cyclophosphamide induces type I interferon and augments the number of CD44(hi) T lymphocytes in mice: implications for strategies of chemoimmunotherapy of cancer. Blood. 2000;95:2024–2030. [PubMed] [Google Scholar]
- 14.Matar P, Rozados VR, Gonzalez AD, Dlugovitzky DG, Bonfil RD, Scharovsky OG. Mechanism of antimetastatic immunopotentiation by low-dose cyclophosphamide. Eur J Cancer. 2000;36:1060–1066. doi: 10.1016/s0959-8049(00)00044-7. [DOI] [PubMed] [Google Scholar]
- 15.Man S, Bocci G, Francia G, Green SK, Jothy S, Hanahan D, Bohlen P, Hicklin DJ, Bergers G, Kerbel RS. Antitumor effects in mice of low-dose (metronomic) cyclophosphamide administered continuously through the drinking water. Cancer Res. 2002;62:2731–2735. [PubMed] [Google Scholar]
- 16.Shaked Y, Emmenegger U, Francia G, Chen L, Lee CR, Man S, Paraghamian A, Ben-David Y, Kerbel RS. Low-dose metronomic combined with intermittent bolus-dose cyclophosphamide is an effective long-term chemotherapy treatment strategy. Cancer Res. 2005;65:7045–7051. doi: 10.1158/0008-5472.CAN-05-0765. [DOI] [PubMed] [Google Scholar]
- 17.Cruz-Munoz W, Man S, Kerbel RS. Effective treatment of advanced human melanoma metastasis in immunodeficient mice using combination metronomic chemotherapy regimens. Clin Cancer Res. 2009;15:4867–4874. doi: 10.1158/1078-0432.CCR-08-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hermans IF, Chong TW, Palmowski MJ, Harris AL, Cerundolo V. Synergistic effect of metronomic dosing of cyclophosphamide combined with specific antitumor immunotherapy in a murine melanoma model. Cancer Res. 2003;63:8408–8413. [PubMed] [Google Scholar]
- 19.Shiraga E, Barichello JM, Ishida T, Kiwada H. A metronomic schedule of cyclophosphamide combined with PEGylated liposomal doxorubicin has a highly antitumor effect in an experimental pulmonary metastatic mouse model. Int J Pharm. 2008;353:65–73. doi: 10.1016/j.ijpharm.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 20.Kong LY, Abou-Ghazal MK, Wei J, Chakraborty A, Sun W, Qiao W, Fuller GN, Fokt I, Grimm EA, Schmittling RJ, Archer GE, Jr., Sampson JH, et al. A novel inhibitor of signal transducers and activators of transcription 3 activation is efficacious against established central nervous system melanoma and inhibits regulatory T cells. Clin Cancer Res. 2008;14:5759–5768. doi: 10.1158/1078-0432.CCR-08-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kong LY, Wei J, Sharma AK, Barr J, Abou-Ghazal MK, Fokt I, Weinberg J, Rao G, Grimm E, Priebe W, Heimberger AB. A novel phosphorylated STAT3 inhibitor enhances T cell cytotoxicity against melanoma through inhibition of regulatory T cells. Cancer Immunol Immunother. 2009;58:1023–1032. doi: 10.1007/s00262-008-0618-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kortylewski M, Yu H. Stat3 as a potential target for cancer immunotherapy. J Immunother. 2007;30:131–139. doi: 10.1097/01.cji.0000211327.76266.65. [DOI] [PubMed] [Google Scholar]
- 23.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 24.Wang T, Niu G, Kortylewski M, Burdelya L, Shain K, Zhang S, Bhattacharya R, Gabrilovich D, Heller R, Coppola D, Dalton W, Jove R, et al. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med. 2004;10:48–54. doi: 10.1038/nm976. [DOI] [PubMed] [Google Scholar]
- 25.Kortylewski M, Jove R, Yu H. Targeting STAT3 affects melanoma on multiple fronts. Cancer Metastasis Rev. 2005;24:315–327. doi: 10.1007/s10555-005-1580-1. [DOI] [PubMed] [Google Scholar]
- 26.Xie TX, Wei D, Liu M, Gao AC, Ali-Osman F, Sawaya R, Huang S. Stat3 activation regulates the expression of matrix metalloproteinase-2 and tumor invasion and metastasis. Oncogene. 2004;23:3550–3560. doi: 10.1038/sj.onc.1207383. [DOI] [PubMed] [Google Scholar]
- 27.Xie TX, Huang FJ, Aldape KD, Kang SH, Liu M, Gershenwald JE, Xie K, Sawaya R, Huang S. Activation of stat3 in human melanoma promotes brain metastasis. Cancer Res. 2006;66:3188–3196. doi: 10.1158/0008-5472.CAN-05-2674. [DOI] [PubMed] [Google Scholar]
- 28.Zorn E, Nelson EA, Mohseni M, Porcheray F, Kim H, Litsa D, Bellucci R, Raderschall E, Canning C, Soiffer RJ, Frank DA, Ritz J. IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood. 2006;108:1571–1579. doi: 10.1182/blood-2006-02-004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heimberger AB, Crotty LE, Archer GE, Hess KR, Wikstrand CJ, Friedman AH, Friedman HS, Bigner DD, Sampson JH. Epidermal growth factor receptor VIII peptide vaccination is efficacious against established intracerebral tumors. Clin Cancer Res. 2003;9:4247–4254. [PubMed] [Google Scholar]
- 30.Sampson JH, Archer GE, Ashley DM, Fuchs HE, Hale LP, Dranoff G, Bigner DD. Subcutaneous vaccination with irradiated, cytokine-producing tumor cells stimulates CD8+ cell-mediated immunity against tumors located in the "immunologically privileged" central nervous system. Proc Natl Acad Sci U S A. 1996;93:10399–10404. doi: 10.1073/pnas.93.19.10399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009;206:1457–1464. doi: 10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kong LY, Wu A, Doucette T, Wei J, Priebe W, Fuller GN, Qiao W, Sawaya R, Rao G, Heimberger AB. Intratumoral mediated immunosuppression is prognostic in genetically engineered murine models of glioma and correlates to immune therapeutic response. Clin Cancer Res. doi: 10.1158/1078-0432.CCR-10-1693. ed. 2010/10/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fecci PE, Mitchell DA, Whitesides JF, Xie W, Friedman AH, Archer GE, Herndon JE, 2nd, Bigner DD, Dranoff G, Sampson JH. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006;66:3294–3302. doi: 10.1158/0008-5472.CAN-05-3773. [DOI] [PubMed] [Google Scholar]
- 34.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 35.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 36.Kong LY, Gelbard A, Wei J, Reina-Ortiz C, Wang Y, Yang EC, Hailemichael Y, Fokt I, Jayakumar A, Qiao W, Fuller GN, Overwijk WW, et al. Inhibition of p-STAT3 enhances IFN-alpha efficacy against metastatic melanoma in a murine model. Clin Cancer Res. 16:2550–2561. doi: 10.1158/1078-0432.CCR-10-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Camacho LH, Antonia S, Sosman J, Kirkwood JM, Gajewski TF, Redman B, Pavlov D, Bulanhagui C, Bozon VA, Gomez-Navarro J, Ribas A. Phase I/II trial of tremelimumab in patients with metastatic melanoma. J Clin Oncol. 2009;27:1075–1081. doi: 10.1200/JCO.2008.19.2435. [DOI] [PubMed] [Google Scholar]
- 38.Markovic SN, Geyer SM, Dawkins F, Sharfman W, Albertini M, Maples W, Fracasso PM, Fitch T, Lorusso P, Adjei AA, Erlichman C. A phase II study of bortezomib in the treatment of metastatic malignant melanoma. Cancer. 2005;103:2584–2589. doi: 10.1002/cncr.21108. [DOI] [PubMed] [Google Scholar]
- 39.Cruz-Munoz W, Man S, Xu P, Kerbel RS. Development of a preclinical model of spontaneous human melanoma central nervous system metastasis. Cancer Res. 2008;68:4500–4505. doi: 10.1158/0008-5472.CAN-08-0041. [DOI] [PubMed] [Google Scholar]
- 40.Genka S, Deutsch J, Stahle PL, Shetty UH, John V, Robinson C, Rapoport SI, Greig NH. Brain and plasma pharmacokinetics and anticancer activities of cyclophosphamide and phosphoramide mustard in the rat. Cancer Chemother Pharmacol. 1990;27:1–7. doi: 10.1007/BF00689268. [DOI] [PubMed] [Google Scholar]
- 41.Browder T, Butterfield CE, Kraling BM, Shi B, Marshall B, O'Reilly MS, Folkman J. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000;60:1878–1886. [PubMed] [Google Scholar]
- 42.Iwamoto Y, Fujita Y, Sugioka Y. YIGSR, a synthetic laminin peptide, inhibits the enhancement by cyclophosphamide of experimental lung metastasis of human fibrosarcoma cells. Clin Exp Metastasis. 1992;10:183–189. doi: 10.1007/BF00132750. [DOI] [PubMed] [Google Scholar]
- 43.Yang M, Jiang P, An Z, Baranov E, Li L, Hasegawa S, Al-Tuwaijri M, Chishima T, Shimada H, Moossa AR, Hoffman RM. Genetically fluorescent melanoma bone and organ metastasis models. Clin Cancer Res. 1999;5:3549–3559. [PubMed] [Google Scholar]
- 44.Shahani N, Gourie-Devi M, Nalini A, Raju TR. Cyclophosphamide attenuates the degenerative changes induced by CSF from patients with amyotrophic lateral sclerosis in the neonatal rat spinal cord. J Neurol Sci. 2001;185:109–118. doi: 10.1016/s0022-510x(01)00479-8. [DOI] [PubMed] [Google Scholar]
- 45.Aloisi F, Care A, Borsellino G, Gallo P, Rosa S, Bassani A, Cabibbo A, Testa U, Levi G, Peschle C. Production of hemolymphopoietic cytokines (IL-6, IL-8, colony-stimulating factors) by normal human astrocytes in response to IL-1 beta and tumor necrosis factor-alpha. J Immunol. 1992;149:2358–2366. [PubMed] [Google Scholar]
- 46.Arndt CA, Balis FM, McCully CL, Colvin OM, Poplack DG. Cerebrospinal fluid penetration of active metabolites of cyclophosphamide and ifosfamide in rhesus monkeys. Cancer Res. 1988;48:2113–2115. [PubMed] [Google Scholar]
- 47.Mahoney DH, Jr., Strother D, Camitta B, Bowen T, Ghim T, Pick T, Wall D, Yu L, Shuster JJ, Friedman H. High-dose melphalan and cyclophosphamide with autologous bone marrow rescue for recurrent/progressive malignant brain tumors in children: a pilot pediatric oncology group study. J Clin Oncol. 1996;14:382–388. doi: 10.1200/JCO.1996.14.2.382. [DOI] [PubMed] [Google Scholar]
- 48.Rosner D, Nemoto T, Lane WW. Chemotherapy induces regression of brain metastases in breast carcinoma. Cancer. 1986;58:832–839. doi: 10.1002/1097-0142(19860815)58:4<832::aid-cncr2820580404>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 49.Lang FF, Gilbert MR, Puduvalli VK, Weinberg J, Levin VA, Yung WK, Sawaya R, Fuller GN, Conrad CA. Toward better early-phase brain tumor clinical trials: a reappraisal of current methods and proposals for future strategies. Neuro Oncol. 2002;4:268–277. doi: 10.1093/neuonc/4.4.268. [DOI] [PMC free article] [PubMed] [Google Scholar]


