Abstract
The 19p13.1 breast cancer susceptibility locus is a modifier of breast cancer risk in BRCA1 mutation carriers and is also associated with risk of ovarian cancer. Here we investigated 19p13.1 variation and risk of breast cancer subtypes, defined by estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER2) status, using 48,869 breast cancer cases and 49,787 controls from the Breast Cancer Association Consortium (BCAC). Variants from 19p13.1 were not associated with breast cancer overall or with ER-positive breast cancer but were significantly associated with ER-negative breast cancer risk [rs8170 Odds Ratio (OR)=1.10, 95% Confidence Interval (CI) 1.05 – 1.15, p=3.49 × 10-5] and triple negative (TN) (ER, PR and HER2 negative) breast cancer [rs8170 OR=1.22, 95% CI 1.13 – 1.31, p=2.22 × 10-7]. However, rs8170 was no longer associated with ER-negative breast cancer risk when TN cases were excluded [OR=0.98, 95% CI 0.89 – 1.07, p=0.62]. In addition, a combined analysis of TN cases from BCAC and the Triple Negative Breast Cancer Consortium (TNBCC) (n=3,566) identified a genome-wide significant association between rs8170 and TN breast cancer risk [OR=1.25, 95% CI 1.18 – 1.33, p=3.31 × 10-13]. Thus, 19p13.1 is the first triple negative-specific breast cancer risk locus and the first locus specific to a histological subtype defined by ER, PR, and HER2 to be identified. These findings provide convincing evidence that genetic susceptibility to breast cancer varies by tumor subtype and that triple negative tumors and other subtypes likely arise through distinct etiologic pathways.
Keywords: genetic susceptibility, association study, subtype, neoplasms, common variant
Introduction
It is becoming increasingly apparent that genetic susceptibility to breast cancer varies by expression levels of estrogen receptor (ER) in breast tumors. Studies of genetic loci identified in genome-wide association studies (GWAS) have shown that variants in 5p12, 8q24, 1p11.2, 9p21.3, 10q21.2, and 11q13 are associated with ER-positive breast cancer (1–8) but not ER-negative breast cancer, whereas variants in FGFR2, 2q35, TOX3, LSP1, MAP3K1, TGFB1, RAD51L1 and ESR1 are associated with both ER-positive and ER-negative disease (8–10). In addition, only a subset of these genetic risk factors for overall breast cancer (TOX3, 2q35, 5q11, LSP1, RAD51L1 and ESR1) have been associated with triple negative (TN) breast cancer, defined by ER, progesterone receptor (PR) and human epidermal growth factor receptor-2 (HER2) expression levels (10–12). To date no variants have been specifically associated with ER-negative or TN disease.
The 19p13.1 breast cancer susceptibility locus was first identified in a GWAS of BRCA1 carriers as a modifier of breast cancer risk (9). Single nucleotide polymorphisms (SNPs), rs8170 and either rs8100241 or rs2363956 (r2=1), from 19p13.1 were associated with risk of breast cancer (rs8170 Hazard Ratio (HR)=1.27, p=1.5 × 10−10; rs8100241 HR=0.84, p=1.6 × 10−10; rs2363956 HR=0.84, p=2.4 × 10−10). The same variants have also been associated with risk of ovarian cancer in the general population (13). In addition, replication studies have suggested associations between these SNPs and ER-negative and ER-positive breast cancer (9, 12), and also with triple negative disease (9, 12). The 19p13.1 locus contains the genes c19orf62 (MERIT40), ANKLE1, and ABHD8, but the causal variants underlying these associations with breast and ovarian cancer risk have yet to be identified.
Here we present a study of the 19p13.1 locus and breast cancer risk in the Breast Cancer Association Consortium (BCAC), an international consortium that has identified or confirmed genome-wide significant associations between commonly inherited variants in several loci and breast cancer risk. We investigated associations between rs8170 from the 19p13.1 locus and breast cancer risk using 48,869 breast cancer cases and 49,787 controls, and associations between rs8100241 and rs2363956 from 19p13.1 and breast cancer in a subset of the BCAC cohort. We also directly assessed differences in breast cancer risk by tumor subtype, defined by ER, PR, and HER2 status, and show that 19p13.1 variants are associated specifically with risk of TN breast cancer.
Materials and Methods
Ethics Statement
Study subjects were recruited on protocols approved by the Institutional Review Boards at each participating institution, and all subjects provided written informed consent.
BCAC studies
Thirty-nine studies from the Breast Cancer Association Consortium (BCAC) contributed genotype data (rs8170, rs8100241 and/or rs2363956) to this study (Supplementary Tables 1, 2). Women of white European ancestry were included from 37 BCAC studies based in Europe, North America, and Australia (49,897 cases, 48,306 controls). Asian women were included from two BCAC studies based in Thailand and Taiwan (1,198 cases, 1,481 controls). BCAC studies are described in detail in Supplementary Table 2. Study participants were recruited under protocols approved by the Institutional Review Board at each institution and all subjects provided written informed consent.
TNBCC studies
Thirteen studies from the Triple Negative Breast Cancer Consortium (TNBCC) were included in the triple negative-specific analysis of rs8170 (Supplementary Tables 1, 2). These studies included 1,350 TN breast cancer cases and 3,852 controls of women of white European ancestry. Samples included from the five TNBCC studies that are also involved in BCAC (BBCC, KARBAC, MCCS, SBCS, POSH) are unique to the TNBCC analysis and were not included in the BCAC analyses presented in this paper. TNBCC studies are described in detail in Supplementary Table 2. Study participants were recruited under protocols approved by the Institutional Review Board at each institution and all subjects provided written informed consent.
Genotyping
Genotyping of rs8170, rs8100241, and rs2363956 in BCAC was performed using a TaqMan allelic discrimination assay or the Sequenom iPLEX platform (Sequenom, San Diego, CA, USA) via standard protocols. Robust quality control criteria, established by BCAC, were applied as detailed in previous consortium studies (4). Briefly, the genotyping concordance was verified with internal duplicates and overall data quality was ensured using independent genotyping of 96 CEU samples by each genotyping center. We excluded all samples from any study with more than two discordant genotypes on the CEU plate. All studies met the specified criteria for call rate (>95%).
Rs8170 and rs8100241 genotyping in TNBCC samples was performed using a single multiplex on the iPLEX Mass Array platform (Sequenom) as part of a larger 22-SNP genotyping project. Samples were plated by study as random mixtures of cases and controls with no-template and CEPH controls in every plate. Genotyping quality for SNPs and samples was evaluated using an iterative quality control process. SNPs and samples were excluded based on the following criteria: SNP call rate <95%, Hardy-Weinberg equilibrium (HWE) p-value <0.01 among controls, and sample call rate <95%.
Pathology and tumor markers
Pathology analyses of BCAC data were conducted using studies of white European women only. All studies except CTS, GC-HBOC, and UKBGS provided data on ER and PR status of tumors, and 25 studies provided data on HER2 (Supplementary Table 3). The collection of pathology and tumor marker information for BCAC has been described previously (14). Briefly, studies provided information on histopathologic subtype, grade of differentiation, tumor size, nodal involvement, and stage at diagnosis of breast tumors. ER/PR status was most commonly defined using data from medical records. ER and PR negative status was defined as <10% of the tumor cells stained. HER2 negative status was typically defined as a score of 0 or 1+ on a HER2 immunohistochemistry (IHC) scale of 0–3+.
TNBCC cases were defined as individuals with an ER–negative, PR–negative and HER2–negative breast cancer. Definition of ER and PR negative status were <1% cells stained positive for DEMOKRITOS, DFCI, FCCC, and MCCS; <10% cells stained positive for BBCC, KARBAC; intensity score (0,1,2,3) percentage of cells stained (0–100%) <50 for SBCS; or an Allred score <3 for RPCI and POSH. Definition of HER2 negative status was score 0 or 1+ by IHC for BBCC, DEMOKRITOS, FCCC, MCCS, POSH, RCPI, SBCS; or IHC score 0, 1+ or 2+ and FISH negative for DFCI, KARBAC. CK5/6 and EGFR IHC data for identification of basal tumors were not available.
Statistical methods
Departure from Hardy-Weinberg equilibrium (HWE) was assessed in controls using a goodness of fit test. Evidence of departure was not observed in any of the participating studies (HWE p≥0.001). Single SNP analyses were conducted using unconditional logistic regression separately for white Europeans and Asians. Analyses utilizing only BCAC case-control studies were adjusted for study, and analyses utilizing all BCAC studies (case-control and case-only studies) were adjusted for country. SNP associations were tested in a log-additive model. To obtain additional information, we also used a 2 degree of freedom test, calculating odds ratios and 95% confidence intervals separately for heterozygotes and rare homozygotes. Consideration of age made no substantial difference to the results, assessed by both the exclusion of studies for which the age of controls was not known and the adjustment for age in 5-year categories and as a continuous covariate. Subtype-specific associations defined by ER, PR and HER2 status were estimated for white Europeans with invasive breast cancer using polytomous logistic regression with control status as the reference outcome, adjusting for country or study where appropriate. SNP associations were tested in a log-additive model. Heterogeneity in the OR by subtypes was tested by applying polytomous logistic regression to cases-only, treating the number of minor alleles as the outcome. Triple-negative specific analyses were conducted among cases with known ER, PR, and HER2 status using polytomous logistic regression with ER-negative (excluding triple negative) and triple negative cases compared to controls as the reference outcome, adjusting for country. BCAC and TNBCC analyses were performed in a combined data set using raw genotype data for rs8170 and rs8100241 from each consortium, and analyses were adjusted for country and consortium. Interaction and haplotype analyses were conducted using the combined BCAC and TNBCC data set adjusting for country. Haplotype analyses were conducted using the haplo.glm function from the haplo.stats package in R with default parameters.
Results
We first evaluated three SNPs in the 19p13.1 locus- rs8170, rs8100241, and rs2363956-for associations with overall risk of invasive breast cancer in BCAC studies of white European women. Rs8170 was genotyped in all 37 studies (47,671 cases and 48,306 controls), while only a subset of studies genotyped rs8100241 (21,645 cases and 21,521 controls) or rs2363956 (17,857 cases and 20,648 controls) (Supplementary Table 1). Neither rs8170 nor rs2363965 was associated with risk of overall invasive breast cancer. However, the A allele of rs8100241 was associated with a small increased risk of breast cancer [Odds Ratio (OR)=1.04, 95% Confidence Interval (CI) 1.01 – 1.08, p=2.88 × 10−3] (Table 1). Results were very similar when excluding four case-only studies (Supplementary Table 4). No associations were observed between rs8170, rs8100241, or rs2363956 and risk of ductal carcinoma in situ (DCIS). Similarly, no association was observed between rs8170 or rs8100241 and risk of invasive breast cancer in two BCAC studies of Asian women including 1,198 breast cancer cases and 1,481 controls, although power to detect an association with rs8170 was limited due to a very low minor allele frequency of 0.20% in this population (Supplementary Table 5). Adjustment for age did not change the magnitude or significance of our results.
Table 1.
19p13.1 single SNP associations with breast cancer among white European women
| Cases | Controls | OR (95% CI) | Ptrend | |
|---|---|---|---|---|
| Invasive breast cancer | ||||
| rs8170 | ||||
| CC | 31,083 | 31,673 | 1.00 | |
| CT | 14,807 | 14,917 | 0.99 (0.96 – 1.02) | |
| TT | 1,781 | 1,716 | 0.95 (0.89 – 1.02) | |
| Log-additive | 0.98 (0.96 – 1.01) | 0.17 | ||
| rs8100241 | ||||
| GG | 5,128 | 4,968 | 1.00 | |
| GA | 10,848 | 10,711 | 1.05 (1.01 – 1.10) | |
| AA | 5,669 | 5,842 | 1.09 (1.03 – 1.15) | |
| Log-additive | 1.04 (1.01 – 1.08) | 2.88 × 10−3 | ||
| rs2363956 | ||||
| TT | 4,396 | 5,315 | 1.00 | |
| TG | 8,876 | 10,215 | 1.01 (0.96 – 1.06) | |
| GG | 4,585 | 5,298 | 1.02 (0.96 – 1.07) | |
| Log-additive | 1.01 (0.98 – 1.04) | 0.59 | ||
|
| ||||
| DCIS | ||||
| rs8170 | ||||
| CC | 1,523 | 28,349 | 1.00 | |
| CT | 699 | 13,412 | 1.02 (0.93 – 1.12) | |
| TT | 83 | 1,548 | 0.95 (0.75 – 1.19) | |
| Log-additive | 1.00 (0.93 – 1.09) | 0.90 | ||
| rs8100241 | ||||
| GG | 346 | 4,276 | 1.00 | |
| GA | 722 | 9,123 | 1.01 (0.88 – 1.15) | |
| AA | 390 | 4,900 | 1.03 (0.88 – 1.20) | |
| Log-additive | 1.01 (0.93 – 1.10) | 0.75 | ||
| rs2363956 | ||||
| TT | 141 | 5,066 | 1.00 | |
| TG | 317 | 10,039 | 0.99 (0.81 – 1.22) | |
| GG | 159 | 5,225 | 0.93 (0.74 – 1.19) | |
| Log-additive | 0.97 (0.85 – 1.09) | 0.60 | ||
Given that the 19p13.1 susceptibility locus was first identified as a modifier of breast cancer risk in BRCA1 mutation carriers (9), who predominantly develop tumors with an ER-negative or TN phenotype, we next evaluated associations between these three SNPs and risk of invasive breast cancer subtypes as defined by ER, PR, and HER2 status (Table 2). Since genotype data were available for rs8170 in the entire BCAC data set, we focused on this SNP in the analyses of breast cancer subtypes. When considering ER status alone, rs8170 was associated with risk of ER-negative breast cancer [OR=1.09, 95% CI 1.05 – 1.14, p=6.69 × 10−5], but not with ER-positive breast cancer [OR=0.99, 95% CI 0.96 – 1.02, p=0.38] [pHet=1.61 × 10−5] (Table 2). A similar pattern was observed for PR status [PR-negative OR=1.05, 95% CI 1.01 – 1.10, p=7.39 × 10−3] [pHet=6.52 × 10−3] (Table 2). When considering both ER and PR status, rs8170 was associated only with tumors negative for both markers [OR=1.10, 95% CI 1.05 – 1.16, p=4.10 × 10−5] (Table 2). Incorporation of HER2 status demonstrated that the 19p13.1 locus was associated with risk of TN breast cancer [OR=1.21, 95% CI 1.13 – 1.31, p=2.97 × 10−7], but not any other combination of ER, PR and HER2 status [pHet=1.32 × 10−5]. In particular, rs8170 was not associated with risk of developing HER2-negative tumors that were ER-positive or PR-positive [OR=1.00, 95% CI 0.97 – 1.04, p=0.80], indicating that rs8170 is associated with TN rather than HER2-negative disease. The estimate of effect for rs8170 was stronger among TN breast cancers (OR=1.21) than all ER-negative breast cancers (OR=1.09). Analysis of rs8170 among cases only was consistent with the case-control analyses (Supplementary Table 6). Similar patterns by subtype were observed for rs8100241 and rs2363956 (Supplementary Table 7). Exclusion of the four case-only BCAC studies did not substantially alter these findings (Supplementary Table 8).
Table 2.
Risk of invasive breast cancer associated with rs8170 among white Europeans defined by ER, PR, and HER2 tumor status
| N | rs8170 | Ptrend | Case-only | |
|---|---|---|---|---|
| OR (95%CI) | Phet | |||
| ER Status | ||||
| Controls | 48,306 | 1.00 | --- | |
| ER+ | 25,649 | 0.99 (0.96 – 1.02) | 0.38 | 1.61 × 10−5 |
| ER− | 7,641 | 1.09 (1.05 – 1.14) | 6.69 × 10−5 | |
| PR Status | ||||
| Controls | 48,306 | 1.00 | --- | |
| PR+ | 19,996 | 0.99 (0.96 – 1.03) | 0.71 | 6.52 × 10−3 |
| PR− | 10,444 | 1.05 (1.01 – 1.10) | 7.39 × 10−3 | |
| ER/PR Status | ||||
| Controls | 48,306 | 1.00 | --- | |
| ER+/PR+ | 18,811 | 0.99 (0.96 – 1.02) | 0.60 | |
| ER+/PR− | 4,294 | 0.99 (0.93 – 1.05) | 0.66 | 3.68 × 10−4 |
| ER−/PR+ | 1,102 | 1.04 (0.93 – 1.16) | 0.47 | |
| ER−/PR− | 6,092 | 1.10 (1.05 – 1.16) | 4.10 × 10−5 | |
| ER, PR and HER2 Status | ||||
| Controls | 45,684 | 1.00 | --- | |
| (ER+ or PR+)/HER2− | 11,774 | 1.00 (0.97 – 1.04) | 0.80 | |
| (ER+ or PR+)/HER2+ | 1,918 | 1.02 (0.94 – 1.11) | 0.62 | 1.32 × 10−5 |
| ER−/PR−/HER2− | 2,216 | 1.21 (1.13 – 1.31) | 2.97 × 10−7 | |
| ER−/PR−/HER2+ | 1,109 | 0.94 (0.85 – 1.05) | 0.31 | |
OR, odds ratio; CI, confidence interval; ER, estrogen receptor; PR progesterone receptor; HER2, human epidermal growth factor receptor-2; +, positive; −, negative; Phet, Case-only heterogeneity p-value.
We next investigated whether variants in the 19p13.1 locus were associated specifically with risk of TN disease by comparing TN cases (ER−, PR−, HER2−) to non-TN, ER-negative cases (ER−, PR+ or HER2+) in an analysis of ER-negative breast cancers with known ER, PR, and HER2 status (Table 3). Rs8170 was not associated with risk of ER-negative breast cancer when excluding TN cases [OR=0.98, 95% CI 0.89 – 1.07, p=0.63], but remained strongly associated with risk of TN breast cancer [OR=1.21, 95% CI 1.13 – 1.31, p=2.94 × 10−7, pHet=9.07 × 10−5]. Given that basal-like tumors account for approximately 80% of TN tumors (15), we also evaluated the influence of cytokeratin 5/6 (CK5/6) and epidermal growth factor receptor (EGFR) basal-tumor marker status on the 19p13.1 association with breast cancer risk. Due to limited data for these markers (Supplementary Table 3), we focused on rs8170 to maximize power to detect differences by basal status. Rs8170 was significantly associated with risk of basal-like TN tumors [OR=1.27, 95% CI 1.07 – 1.50, p=0.0069], but was not associated with risk of non-basal TN tumors [OR=1.03, 95%CI 0.79 – 1.34, p=0.83] [pHet=0.026] (Supplementary Table 9). Furthermore, rs8170 was not associated with either ER-positive basal tumors (n=301) [OR=0.90, 95% CI 0.73 – 1.10, p=0.30] or ER-negative, non-TN basal tumors (n=122) [OR=0.89, 95% CI 0.64 – 1.23, p=0.48] [pHet=0.80]. This suggests that the 19p13.1 locus is exclusively associated with TN, basal-like tumors. However, because of the small sample size and potential misclassification of CK5/6 and EGFR, these results need to be confirmed in larger studies of breast cancer subtypes.
Table 3.
Triple negative-specific risk associated with rs8170
| N | OR (95% CI) | Ptrend | Case-only | |
|---|---|---|---|---|
| Phet | ||||
| All BCAC studies | ||||
| Controls | 45,684 | 1.00 | ||
| ER− (non-TN) | 1,584 | 0.98 (0.89 – 1.07) | 0.63 | |
| TN | 2,216 | 1.21 (1.13 – 1.31) | 2.94 × 10−7 | 1.77 × 10−4 |
| TNBCC studies | ||||
| Controls | 3,852 | 1.00 | ||
| TN | 1,350 | 1.26 (1.13 – 1.40) | 3.02 × 10−5 | NA |
| BCAC + TNBCC studies | ||||
| Controls | 52,158 | 1.00 | ||
| ER− (non-TN) | 1,584 | 0.98 (0.89 – 1.07) | 0.60 | |
| TN | 3,566 | 1.25 (1.18 – 1.33) | 4.24 × 10−13 | 2.51 × 10−6 |
ER, estrogen receptor; TN, triple negative; −, negative.
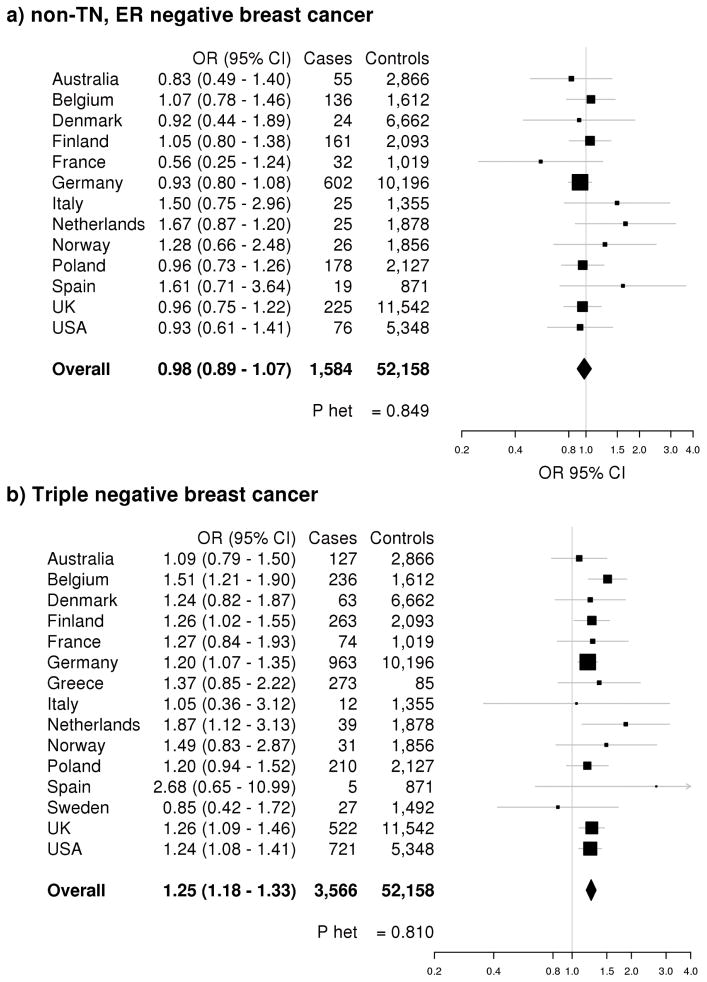
We next extended our evaluation of 19p13.1 variants to non-overlapping subjects (1,350 TN cases, 3,852 controls) from the Triple Negative Breast Cancer Consortium (TNBCC) (Supplementary Table 1) (12). Among the TNBCC studies alone, rs8170 was associated with an increased risk of TN breast cancer [OR=1.26, 95% CI 1.13 – 1.40, p=3.02 × 10−5] (Table 3). Importantly, the combined rs8170 genotype data from BCAC and TNBCC (n=3,566 TN cases), yielded a genome wide significant association with risk of TN breast cancer [OR=1.25, 95% CI 1.18 – 1.33, p=4.24 × 10−13] (Table 3). There was no evidence for heterogeneity of the ORs by country for either TN or non-TN, ER-negative breast cancer in the combined analysis (Figure 1). The difference in effect estimates between TN and non-TN, ER-negative breast cancer was highly significant [pHet=2.51 × 10−6], indicating that rs8170 is a TN-specific risk variant. A similar pattern was observed for rs8100241, which was inversely associated only with TN disease [OR=0.81, 95% CI 0.76 – 0.86, p=1.91 × 10−12] and not with non-TN, ER-negative disease [OR=0.94, 95% CI 0.86 – 1.03, p=0.19] in the combined data set [pHet=3.30 × 10−3] (Supplementary Table 10).
Figure 1. 19p13.1 (rs8170) association with risk of non-TN, ER-negative and TN breast cancer.
Forest plots for rs8170 and risk of (a) non-TN, ER-negative breast cancer and (b) TN breast cancer are shown by country. Country-specific odds ratios (95% CIs) are denoted by black boxes (black lines). Overall OR estimates are represented by black diamonds, where diamond width corresponds to 95% CI bounds. Box and diamond heights are inversely proportional to precision of the OR estimate. P-values for heterogeneity (pHet) of odds ratios by country are shown.
To better understand the influence of 19p13.1 variants on risk of TN breast cancer, we included both rs8170 and rs8100241 in a multivariate model in the combined BCAC and TNBCC data set. Both rs8170 [OR=1.16, 95% CI 1.07 – 1.26, p=6.14 × 10−4] and rs8100241 [OR=0.85, 95% CI 0.79 – 0.91, p=5.10 × 10−6] remained significantly associated with risk of TN breast cancer with only slight attenuation of the ORs. However, when considering the association of one SNP stratified by the genotype of the other, we found that the effect of rs8170 was restricted to individuals with the rs8100241 “GA” genotype [OR=1.29, 95%CI 1.14 – 1.45, p=3.13 × 10−5] and that the effect of rs8100241 was restricted to individuals with the rs8170 “CC” genotype [OR=0.82, 95%CI 0.76 – 0.89, p=9.90 × 10−7] (Supplementary Table 11a). This is reflected by a significant interaction between these SNP [Interaction OR=1.21, 95% CI 1.06 – 1.37, p=0.0036] (Supplementary Table 11b). A haplotype analysis for these two SNPs found that the C-G and T-G haplotypes (rs8170-rs8100241) were both associated with risk of TN breast cancer compared to the C-A haplotype [C-G OR=1.17, 95% CI 1.09 – 1.25, p=1.00 × 10−5; T-G OR=1.35, 95% CI 1.25 – 1.46, p=2.51 × 10−14], while the T-A haplotype was not observed at all (Supplementary Table 12), suggesting that both SNPs tag the causal variant. No interactions were observed between these SNPs among other subtypes defined by any combination of ER, PR, and HER2 status.
Due to overlap between the BCAC samples in this analysis and a subset of those in SEARCH and the TNBCC, which were previously examined in an initial generalization of 19p13.1 SNP associations with BRCA1-related tumors (9), we performed a sensitivity analysis removing these studies from the ER and ER/PR/HER2 subtype analyses. The effect estimates in this sensitivity analysis were very similar to those from the complete BCAC analysis, with only slight attenuation of significance (Supplementary Table 13).
Discussion
Here we report on the identification of the first TN breast cancer specific susceptibility locus at 19p13.1. We found that rs8170 was strongly associated with risk of TN breast cancer [OR=1.25, p=4.24 × 10−13], but was not associated with ER-positive [OR=0.99, p=0.38] or non-TN, ER-negative [OR=0.98, p=0.63] breast cancer. Further analyses based on basal tumor markers suggested that the 19p13.1 variants are associated specifically with basal-like TN tumors [OR=1.27, p=0.0069]. Ongoing histopathology studies in BCAC involving characterization of the CK5/6 and EGFR status of tumors may increase the numbers of TN-basal cases and allow re-evaluation of this finding in the future. We were well powered to detect an association between 19p13.1 variants and these breast cancer subtypes in more than 32,000 cases and 48,000 controls. Importantly, our ability to evaluate risk of breast cancer across histological subtypes in a single, large consortium strengthens the validity of the findings. Heterogeneity in hormone receptor and basal marker status across studies may influence our ability to detect associations with breast tumor subtypes at 19p13.1. However, in a sensitivity analysis including only cases from studies with the most stringent criteria for defining hormone receptor status (<1% of cells stained positive for ER and PR, HER2 0 or 1+ on IHC), the effect estimates were very similar to those from the complete analysis of the ER-negative, non-TN and TN subtypes. These findings have important implications for understanding genetic susceptibility to breast cancer, because they suggest that additional susceptibility variants for specific subtypes of breast cancer remain to be identified.
TN breast cancer accounts for approximately 15% of all breast cancer among women of European descent and differs substantially from other subtypes of breast cancer by expression and genomic profiles and by epidemiologic characteristics (15). Women with TN breast cancer are more likely to be younger, have an earlier age at menarche, higher body mass index during premenopausal years, higher parity, and a lower lifetime duration of breast feeding and in the US are more likely to be African American or Latina (16–18), and TN tumors are associated with more aggressive disease and poorer survival (15, 19, 20). The biological and clinical distinctions between TN and other breast cancer subtypes are concordant with the identification of TN-specific genetic risk factors and provide additional evidence for a distinct TN tumor etiology. This highlights the importance of additional subtype-specific breast cancer studies, and studies of breast cancer in additional populations such as African Americans and Latinas, since it is not known whether similar associations with the SNPs described here exist in these populations.
The three 19p13.11 variants measured in this study are located in the genes C19orf62 and ANKLE1 and are approximately 13kb from the gene ABHD8. C19orf62, which encodes the MERIT40 protein, is currently hypothesized to be the most likely cancer susceptibility gene in this region due to the known interaction between MERIT40 and BRCA1. MERIT40 is integral to the localization of the BRCA1-A complex during DNA double-strand break repair, specifically through the recruitment and retention of the BRCA1-BARD1 ubiquitin ligase and the BRCC36 deubiquitination enzyme (21–24). However, both ANKLE1 (ankyrin repeat and LEM domain containing 1) and ABHD8 (abhydrolase domain containing 8) encode proteins of uncharacterized functions, making conjecture about the involvement of these proteins in cancer-related processes difficult.
It is unknown whether a single causal variant or multiple rare variants underlie the 19p13.1 association, affecting TN risk through dysregulation of these or other nearby genes. Conversely, the causal variant at 19p13.1 may lie in a regulatory element that confers risk to TN disease through long-range effects on distant genes. Although the biology underlying this association is unknown, it is likely that the functional consequences of variants at 19p13.1 are to modify genes or proteins that cooperate with other factors in signaling pathways critical to the development of the TN phenotype. One can speculate that the causal 19p13.1 variants directly initiate and promote TN tumor development, or alternatively that the 19p13.1 causal variants act to change the morphology of an existing malignant breast lesion to a TN phenotype early in tumorigenesis. Re-sequencing and fine-mapping efforts in TN breast cancer cases will be important for identification of the causal variants in the 19p13.1 locus and the mechanism by which these variants specifically influence risk of TN breast cancer.
In conclusion, our study provides convincing evidence that the 19p13.1 locus is specifically associated with risk of TN disease, confirming that some breast cancer susceptibility loci differ by histological breast tumor subtype defined by ER, PR and HER2 status. This report provides further evidence that TN tumors and other subtypes likely arise through distinct etiologic pathways. Genetic and functional studies of TN breast cancer will be necessary to identify the mechanism underlying the 19p13.1 association and to identify additional TN-specific susceptibility loci.
Supplementary Material
Acknowledgments
Grant Support
This work was supported by the National Institutes of Health grant CA122340, a Specialized Program of Research Excellence (SPORE) in Breast Cancer (CA116201), the Komen Foundation for the Cure and the Breast Cancer Research Foundation (BCRF); BCAC is funded by CR-UK [C1287/A10118, C1287/A12014] and by the European Community’s Seventh Framework Programme under grant agreement n° 223175 (HEALTH-F2-2009-223175) (COGS), and by the European Union COST programme [BM0606]. D.F.E. is a Principal Research Fellow of Cancer Research UK; SBCS: Breast Cancer Campaign (2004Nov49 to AC) and Yorkshire Cancer Research Core Funding (Institute for Cancer Studies); ABCS: Dutch Cancer Society grant [NKI 2009-4363 and NKI 2007-3839 to MKS] and the Dutch National Genomics Initiative; ACP: Breast Cancer Research Trust, UK. ES is funded by National Institute for Health Research (NIHR) Comprehensive Biomedical Research Centre, Guy’s & St. Thomas’ NHS Foundation Trust in partnership with King’s College London. IT is funded by the Oxford Biomedical Research Centre; ABCFS, NC-BCFR and OFBCR: National Cancer Institute, National Institutes of Health (NIH) under RFA-CA-06-503 and through cooperative agreements with members of the Breast Cancer Family Registry (BCFR) and Principal Investigators, including Cancer Care Ontario (U01 CA69467), Northern California Cancer Center (U01 CA69417), University of Melbourne (U01 CA69638); ABCFS: National Health and Medical Research Council of Australia, the New South Wales Cancer Council, the Victorian Health Promotion Foundation (Australia) and the Victorian Breast Cancer Research Consortium. J.L.H. is a National Health and Medical Research Council (NHMRC) Australia Fellow and a Victorian Breast Cancer Research Consortium Group Leader. M.C.S. is a NHMRC Senior Research Fellow and a Victorian Breast Cancer Research Consortium Group Leader; CNIO-BCS: Genome Spain Foundation, the Red Temática de Investigación Cooperativa en Cáncer and grants from the Asociación Española Contra el Cáncer and the Fondo de Investigación Sanitario (PI081583 and PI081120); The California Teachers Study: California Breast Cancer Act of 1993, National Institutes of Health (R01 CA77398), the Lon V Smith Foundation [LVS39420]), and the California Breast Cancer Research Fund (contract 97-10500); UCIBCS: NIH [CA58860, CA92044] and the Lon V Smith Foundation [LVS39420]; ESTHER: Baden Württemberg Ministry of Science, Research and Arts, and the VERDI study supported by a grant from the German Cancer Aid (Deutsche Krebshilfe); GENICA: Federal Ministry of Education and Research (BMBF) Germany (01KW9975/5, 01KW9976/8, 01KW9977/0 and 01KW0114), the Robert Bosch Foundation, Stuttgart, Deutsches Krebsforschungszentrum (DKFZ) Heidelberg, Institute for Prevention and Occupational Medicine of the German Social Accident Insurance (IPA), Bochum, and the Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany: KBCP: Finnish Cancer Society, the Academy of Finland (grant number 127220), the special Government Funding (EVO) of Kuopio University Hospital (grant number 5654113 and 5501) and by the strategic funding of the University of Eastern Finland; OBCS: Finnish Cancer Foundation, the Academy of Finland, the University of Oulu, the Oulu University Hospital and Biocenter Oulu; RPCI: P30 grant to RPCI (CA016056-32); The Breakthrough Generations Study: Breakthrough Breast Cancer and the Institute of Cancer Research (ICR). ICR acknowledges NHS funding to the NIHR Biomedical Research Centre; PBCS: Intramural Research Funds of the National Cancer Institute, USA; HEBCS: Helsinki University Central Hospital Research Fund, Academy of Finland (132473), the Finnish Cancer Society, and the Sigrid Juselius Foundation; MARIE: Deutsche Krebshilfe e.V., grant number 70-2892-BR I, the Hamburg Cancer Society, the German Cancer Research Center (DKFZ) and the Federal Ministry of Education and Research (BMBF) Germany grant 01KH0402; GESBC: Deutsche Krebshilfe e. V. [70492] and the state of Baden-Württemberg through the Medical Faculty of the University of Ulm [P.685]; BSUCH: Dietmar Hopp Foundation, the German Cancer Research Center, DKFZ and the Helmholtz association; GC-HBOC: Deutsche Krebshilfe [107054], the Center of MolecularMedicine, Cologne, the German Cancer Research Center, DKFZ and the Helmholtz society; BBCS: Cancer Research UK, Breakthrough Breast Cancer and the National Cancer Research Network (NCRN); kConFab: National Breast Cancer Foundation, the National Health and Medical Research Council (NHMRC) and by the Queensland Cancer Fund, the Cancer Councils of New South Wales, Victoria, Tasmania and South Australia, the Cancer Foundation of Western Australia, and Cancer Australia #628333; LMBC: ‘Stichting tegen Kanker’ (232-2008 and 196-2010);MCCS: Australian National Health and Medical Research Council [grants #209057, 251533, 396414, 504711], Cancer Council Victoria, and VicHealth; ORIGO: Dutch Cancer Society; SEARCH: Cancer Research UK [C8197/A10123, C8197/A10123 and C490/A10124]. AMD was supported by Cancer Research UK grant [C8197/A10865] and by the Joseph Mitchell Fund; FCCC: National Institutes of Health (U01 CA69631, 5U01 CA113916 to A.K.G.), the Eileen Stein Jacoby Fund, The University of Kansas Cancer Center, and the Kansas Bioscience Authority Eminent Scholar Program. A.K.G. is the Chancellors Distinguished Chair in Biomedical Sciences endowed Professor.
References
- 1.Stacey SN, Manolescu A, Sulem P, Rafnar T, Gudmundsson J, Gudjonsson SA, et al. Common variants on chromosomes 2q35 and 16q12 confer susceptibility to estrogen receptor-positive breast cancer. Nat Genet. 2007;39:865–9. doi: 10.1038/ng2064. [DOI] [PubMed] [Google Scholar]
- 2.Stacey SN, Manolescu A, Sulem P, Thorlacius S, Gudjonsson SA, Jonsson GF, et al. Common variants on chromosome 5p12 confer susceptibility to estrogen receptor-positive breast cancer. Nat Genet. 2008;40:703–6. doi: 10.1038/ng.131. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Closas M, Chanock S. Genetic susceptibility loci for breast cancer by estrogen receptor status. Clin Cancer Res. 2008;14:8000–9. doi: 10.1158/1078-0432.CCR-08-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia-Closas M, Hall P, Nevanlinna H, Pooley K, Morrison J, Richesson DA, et al. Heterogeneity of breast cancer associations with five susceptibility loci by clinical and pathological characteristics. PLoS Genet. 2008;4:e1000054. doi: 10.1371/journal.pgen.1000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas G, Jacobs KB, Kraft P, Yeager M, Wacholder S, Cox DG, et al. A multistage genome-wide association study in breast cancer identifies two new risk alleles at 1p11.2 and 14q24.1 (RAD51L1) Nat Genet. 2009;41:579–84. doi: 10.1038/ng.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turnbull C, Ahmed S, Morrison J, Pernet D, Renwick A, Maranian M, et al. Genome-wide association study identifies five new breast cancer susceptibility loci. Nat Genet. 2010;42:504–7. doi: 10.1038/ng.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmed S, Thomas G, Ghoussaini M, Healey CS, Humphreys MK, Platte R, et al. Newly discovered breast cancer susceptibility loci on 3p24 and 17q23.2. Nat Genet. 2009;41:585–90. doi: 10.1038/ng.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Figueroa JD, Garcia-Closas M, Humphreys M, Platte R, Hopper JL, Southey MC, et al. Associations of common variants at 1p11.2 and 14q24.1 (RAD51L1) with breast cancer risk and heterogeneity by tumor subtype: findings from the Breast Cancer Association Consortium. Hum Mol Genet. 2011;20:4693–706. doi: 10.1093/hmg/ddr368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antoniou AC, Wang X, Fredericksen ZS, McGuffog L, Tarrell R, Sinilnikova OM, et al. A locus on 19p13 modifies risk of breast cancer in BRCA1 mutation carriers and is associated with hormone receptor-negative breast cancer in the general population. Nat Genet. 2010;42:885–92. doi: 10.1038/ng.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broeks A, Schmidt MK, Sherman ME, Couch FJ, Hopper JL, Dite GS, et al. Low penetrance breast cancer susceptibility loci are associated with specific breast tumor subtypes: findings from the Breast Cancer Association Consortium. Hum Mol Genet. 2011;20:3289–303. doi: 10.1093/hmg/ddr228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunning AM, Healey CS, Baynes C, Maia AT, Scollen S, Vega A, et al. Association of ESR1 gene tagging SNPs with breast cancer risk. Hum Mol Genet. 2009;18:1131–9. doi: 10.1093/hmg/ddn429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stevens KN, Vachon CM, Lee AM, Slager S, Lesnick T, Olswold C, et al. Common breast cancer susceptibility loci are associated with triple-negative breast cancer. Cancer Res. 2011;71:6240–9. doi: 10.1158/0008-5472.CAN-11-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolton KL, Tyrer J, Song H, Ramus SJ, Notaridou M, Jones C, et al. Common variants at 19p13 are associated with susceptibility to ovarian cancer. Nat Genet. 2010;42:880–4. doi: 10.1038/ng.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang XR, Chang-Claude J, Goode EL, Couch FJ, Nevanlinna H, Milne RL, et al. Associations of breast cancer risk factors with tumor subtypes: a pooled analysis from the Breast Cancer Association Consortium studies. J Natl Cancer Inst. 2011;103:250–63. doi: 10.1093/jnci/djq526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–48. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 16.Yang XR, Sherman ME, Rimm DL, Lissowska J, Brinton LA, Peplonska B, et al. Differences in risk factors for breast cancer molecular subtypes in a population-based study. Cancer Epidemiol Biomarkers Prev. 2007;16:439–43. doi: 10.1158/1055-9965.EPI-06-0806. [DOI] [PubMed] [Google Scholar]
- 17.Schneider BP, Winer EP, Foulkes WD, Garber J, Perou CM, Richardson A, et al. Triple-negative breast cancer: risk factors to potential targets. Clin Cancer Res. 2008;14:8010–8. doi: 10.1158/1078-0432.CCR-08-1208. [DOI] [PubMed] [Google Scholar]
- 18.Millikan RC, Newman B, Tse CK, Moorman PG, Conway K, Dressler LG, et al. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat. 2008;109:123–39. doi: 10.1007/s10549-007-9632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109:1721–8. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 20.Irvin WJ, Jr, Carey LA. What is triple-negative breast cancer? Eur J Cancer. 2008;44:2799–805. doi: 10.1016/j.ejca.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 21.Feng L, Huang J, Chen J. MERIT40 facilitates BRCA1 localization and DNA damage repair. Genes Dev. 2009;23:719–28. doi: 10.1101/gad.1770609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shao G, Patterson-Fortin J, Messick TE, Feng D, Shanbhag N, Wang Y, et al. MERIT40 controls BRCA1-Rap80 complex integrity and recruitment to DNA double-strand breaks. Genes Dev. 2009;23:740–54. doi: 10.1101/gad.1739609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang B, Hurov K, Hofmann K, Elledge SJ. NBA1, a new player in the Brca1 A complex, is required for DNA damage resistance and checkpoint control. Genes Dev. 2009;23:729–39. doi: 10.1101/gad.1770309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong Y, Hakimi MA, Chen X, Kumaraswamy E, Cooch NS, Godwin AK, et al. Regulation of BRCC, a holoenzyme complex containing BRCA1 and BRCA2, by a signalosome-like subunit and its role in DNA repair. Mol Cell. 2003;12:1087–99. doi: 10.1016/s1097-2765(03)00424-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.