Summary
The transcription factor Dmp1 is a Ras/HER2-activated haplo-insufficient tumor suppressor that activates the Arf/p53 pathway of cell cycle arrest. Recent evidence suggests that Dmp1 may activate p53 independently of Arf in certain cell types. Here we report findings supporting this concept with the definition an Arf-independent function for Dmp1 in tumor suppression. We found that Dmp1 and p53 can interact directly in mammalian cells via the carboxyl-terminus of p53 and the DNA-binding domain of Dmp1. Expression of Dmp1 antagonized ubiquitination of p53 by Mdm2 and promoted nuclear localization of p53. Dmp1-p53 binding significantly increased the level of p53, independent of Dmp1’s DNA-binding activity. Mechanistically, p53 target genes were activated synergistically by co-expression of Dmp1 and p53 in p53−/−; Arf−/−cells and genotoxic responses of these genes were hampered more dramatically in Dmp1−/− and p53−/− cells than in Arf−/− cells. Together, our findings identify a robust new mechanism of p53 activation mediated through by direct physical interaction between Dmp1 and p53.
Keywords: Dmp1 (Dmtf1), p53, Mdm2, Arf, ubiquitination, nuclear localization
Introduction
Upon cellular stresses, such as DNA damage, oncogene activation, and hypoxia, the tumor suppressor p53 is activated and initiates a transcriptional program that induces DNA repair, cell cycle arrest, apoptosis, or autophagy (1–3). This stress signaling plays critical roles in the prevention of tumor formation and, indeed, p53-null mice or p53 knock-in mice that express mutant p53 are highly prone to tumor development (4). A central regulator of the p53 pathway is the Mdm2 protein (Hdm2 in humans) that inhibits transcriptional activity, nuclear localization, and protein stability of p53 (5–7). Homozygous deletion of Mdm2 results in embryonic lethality at the blastocyst stage due to unrestricted apoptosis. Deletion of p53 abrogates this effect, indicating the critical in vivo function of Mdm2 is the negative regulation of p53 activity (7). The Mdm2 (and also Hdm2) gene is regulated by p53 through direct binding of the protein to the p53-responsive elements located within the P2 promoter (7). Mutations in TP53 that disrupt p53 function occur in 50% of human cancers (8); the alteration of regulators for p53 is found in most of the remaining 50%. The Hdm2 gene is amplified in ~35% of human sarcomas and ~7% of all cancers without TP53 mutation, but the protein is overexpressed in 40–80% of late-stage metastatic cancers in the absence of gene amplification (7, 9).
The activity of Mdm2 is negatively regulated by p19Arf (p14ARF in humans) in response to oncogenic stress (10–13). p19Arf is an alternative reading frame gene product generated from the Ink4a/Arf locus which also encodes the cyclin-dependent kinase inhibitor p16Ink4a. p19Arf directly binds to Mdm2, thereby stabilizing and activating p53. Arf induction by potentially harmful growth-promoting signals forces early-stage cancer cells to undergo p53-dependent and -independent cell cycle arrest, apoptosis, or autophagy providing a powerful mode of tumor suppression (10–13). The Arf promoter monitors latent oncogenic signals in vivo, and thus Arf-null mice are also highly prone to spontaneous tumor development (14, 15). Arf is regulated at both transcriptional and protein levels (16–19). The Arf promoter is directly activated by E2Fs and Dmp1 while it is repressed by overexpression of nuclear proteins such as Bmi1, Twist, Tbx2/3, and Pokemon (16, 17).
Dmp1 (cyclin D binding myb-like protein 1; Dmtf1) is a tumor suppressor that is deleted in ~40% of human non-small cell lung carcinomas (20–24). Mitogenic signals from oncogenic Ras and HER2/neu have been shown to activate the Dmp1 promoter, while physiological mitogens as well as genotoxic stimuli mediated by NF-κB cause repression (25–28). It has been considered that the Dmp1 protein acts as a tumor suppressor by directly activating the Arf promoter, and thereby inducing Arf-, p53-dependent cell cycle arrest (16, 21, 22). Eμ-Myc, K-rasLA, and HER2/neu-driven tumor development was significantly accelerated in both Dmp1+/− and Dmp1−/−mice with no significant differences in the survival between the two cohorts, suggesting that Dmp1 is haplo-insufficient for tumor suppression (22, 23, 28). In Eμ-Myc lymphomas, the combined frequencies of p53 mutation and Arf deletion in mice of Dmp1+/− or Dmp1−/−background were much lower than that in Dmp1+/+ littermates, indicating that Dmp1 is a physiological regulator of the Arf-p53 pathway in vivo (22). Of note, the frequency of p53 mutation (~40%) was significantly decreased in both Dmp1+/− and Dmp1−/− backgrounds (< 10%) even in K-rasLA lung cancer model where the Ink4a/Arf involvement was rare (23). This suggests an Arf-independent mechanism of p53 regulation by Dmp1 in epithelial tissues.
In this study, we searched for binding partners for the Dmp1 protein to explain the Arf-independent function of Dmp1 in tumor suppression. We show that Dmp1 physically interacts with p53 to neutralize the activity of Hdm2 on p53’s ubiquitination, nuclear localization, and transcription. We also demonstrate that Dmp1 has Arf-independent mechanisms of p53 activation in vivo. We propose that the physical interaction between Dmp1 and p53 will synergize with Arf promoter activation by Dmp1 to enhance the activity of the p53 pathway.
Materials and Methods
Cell culture, retrovirus preparation, and infection
NIH 3T3, H1299, A549, and U2OS cells were cultured and transfected as described previously (16, 20). Passage 6 Arf; p53-double knockout MEFs were received from Drs. C. Sherr and M. Roussel. For preparation of retroviruses, human kidney 293T cells were transfected with a helper ecotropic retrovirus plasmid defective in psi-2 packaging sequences, together with pMSCV-IRES-puro vectors containing Dmp1, Dmp1 mutant, or p53 cDNA (16).
Detection of endogenous Dmp1-p53 interaction
Subconfluent U2OS cells were co-treated with 2 μM etoposide (Toposar®, Teva) and 25 μM MG-132 for 6 hrs, then harvested in EBC buffer. 1 mg of total protein was immunoprecipitated with 4 μg of antibodies to Dmp1 (RAD to the full-length His-Dmp1 [29], RAX [26] or RAZ [24]), antibodies to p53 (sc-126, sc-6243), or control immunoglobulins, followed by incubation with protein G-sepharose and extensive wash with lysis buffer (30). Sites of antibody bindings were visualized by sc-126 for p53 and RAD for Dmp1, followed by Trueblot™ secondary antibodies (eBioscience). Endogenous Dmp1-p53 interaction was also studied with 1 mg of mouse thymus lysates injected with 0.6 mg/30 g etoposide.
In cell p53 ubiquitination assays
This assay was adapted from Li et al (31). H1299 cells were transfected with His-tagged ubiquitin, p53, Mdm2, and increasing amounts of Dmp1. Cells were treated with 25 μM proteasome inhibitor MG-132 (Calbiochem) for six hrs prior to harvesting to prevent degradation of ubiquitinated p53. Cells were lysed in EBC buffer without EDTA, sonicated, and cleared by centrifugation at 20,000g for 10 minutes. One milligram of cell lysate was incubated with 30 μL of 50% Ni-NTA agarose/EBC without EDTA slurry for 2 hrs at 4°C with rotation. The samples were then centrifuged at 10,000g for 2 minutes at 4°C and the supernatant (unbound) lysate was removed and saved. The Ni-NTA resin was washed four times with 800 μL EBC without EDTA. His-tagged proteins were eluted from the Ni-NTA agarose by incubating in EBC buffer at 100°C for 5 minutes. The samples were then centrifuged at 10,000g for 2 minutes at 4°C and the supernatant (immunoprecipitated protein) was analyzed by Western blotting using antibodies for p53, Dmp1, Hdm2, and Actin.
Induction of DNA damage response by doxorubicin injection in mice
Arf-null mice were obtained from NCI Mouse Repository. p53-null mice were purchased from the Jackson Laboratory (Bar Harbor, Maine). Six-week-old wild-type, Arf−/−, Dmp1−/−, and p53−/−mice (all C57BL/6) were intravenously injected with 0.6 mg doxorubicin/30 g mouse for 0, 2, and 4 hrs, after which they were sacrificed, and thymi and lungs were harvested. Apoptosis was evaluated in vivo using antibodies to cleaved caspase 3 (Cell Signaling). For p53 target genes, p21Cip1/Waf1 and bbc3 (puma) mRNA levels were quantitated by real-time PCR Taqman assay (ABI 7500) (26, 27). For quantification of p53 binding to p21Cip1 and bbc3 promoters by real-time PCR, Taqman assays were custom designed by ABI to detect target sequences using mouse β-actin genomic DNA as an internal control. All procedures involving mice were approved by IACUC.
In vitro protein binding assays, in vitro ubiquitination assay, immunofluorescence, immunohistochemistry, luciferase assays, and EMSA are explained in the Supplementary Materials and Methods.
Results
Dmp1 physically interacts with p53
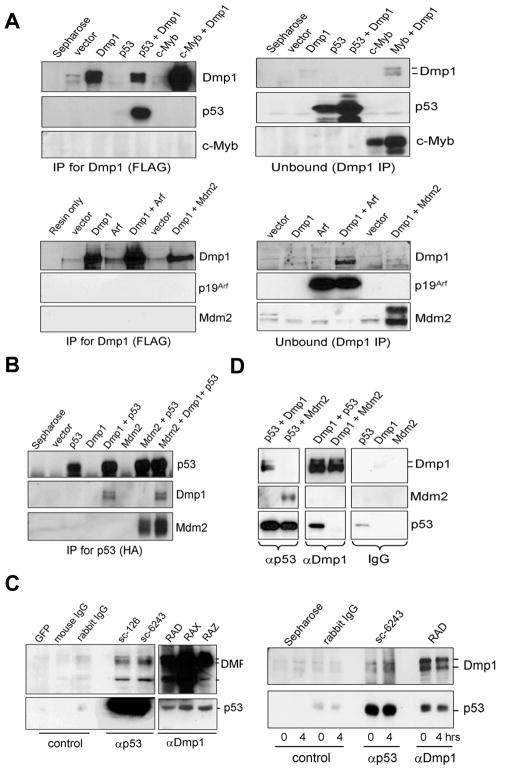
Published studies indicate that Dmp1 regulates p53 levels in vivo since Dmp1-null tumors have significantly decreased incidence of p53 mutation (22, 23). Thus, the half-life of p53 was studied by activating Dmp1:ER in MCF10A cells (ARF-null), and found that it was extended from 41 minutes to 3.5 hrs (Supplementary Fig. S1). To identify which key components of the Arf-Mdm2-p53 tumor suppressor pathway interact with Dmp1, the protein was transiently co-expressed with p53, Mdm2, p19Arf, or c-Myb in 3T3 cells (Fig. 1A, B; Supplementary Fig. S2A; the input protein levels are shown in Supplementary Fig. 3). Immunoprecipitation with FLAG antibody showed that only p53 interacted with Dmp1 among these four proteins. Reciprocal immunoprecipitation with HA antibody verified that Dmp1 was co-immunoprecipitated (co-IP) with p53 (Fig. 1B). Physical interaction of Mdm2-p53 was also confirmed by co-IP under the same experimental condition. Of note, the presence of Dmp1 did not interfere with Mdm2-p53 binding, suggesting that Dmp1 binds to p53 using a domain distinct from the Mdm2-binding region (Fig. 1B, last lane). Additionally, Dmp1 did not interact with c-Myc, E2F2, or E2F3, further demonstrating the specificity of Dmp1-p53 binding (Supplementary Fig. S2B, C).
Fig. 1. Dmp1 co-immunoprecipitates with p53.
(A), (B) NIH 3T3 cells were transfected with cDNA for FLAG-Dmp1, HA-p53, c-Myb, p19Arf, and/or Mdm2 expression vectors. The cleared lysates were immunoprecipitated with antibodies to (A) Dmp1 (FLAG) or (B) p53 (HA), and analyzed by Western blotting.
(C, left panels) Endogenous Dmp1-p53 interaction in U2OS cells treated with etoposide. Etoposide/MG-132 treated lysates were immunoprecipitated with control GFP antibody (sc-9996), control IgGs or antibodies to p53 (sc-126, sc-6243) or Dmp1 (RAD, RAX, RAZ), followed by blotting with Dmp1 or p53 antibodies.
(C, right panels) Endogenous Dmp1-p53 interaction in the mouse thymus injected with etoposide. Lysates were immunoprecipitated with control immunoglobulins, p53 antibody (sc-6243), or Dmp1 antibody (RAD) and analyzed by Western blotting.
(D) Recombinant FLAG-Dmp1, HA-p53, and FLAG-Mdm2 proteins were purified from baculovirus-infected Sf9 cells. In vitro binding assays were conducted by using 4 μg of proteins followed by immunoprecipitation with p53 (sc-6243), Dmp1 (RAX), or control IgGs, and analyzed by Western blottings.
Next, the interaction of endogenous human DMP1 (hDMP1) with p53 was examined using U2OS cell lysates treated with the topoisomerase inhibitor etoposide. The hDMP1 protein was co-immunoprecipitated with p53 using two different antibodies to p53 (Fig. 1C, left panels). In agreement, p53 was also co-immunoprecipitated with three different antibodies to Dmp1 (RAD, RAX, and RAZ) (24, 26, 29) (Fig. 1C, left panels). Endogenous Dmp1-p53 interaction was also demonstrated in the thymus from mice injected with etoposide (Fig. 1C, right panels). The occurrence of DNA-damage response by etoposide treatment was confirmed through detection of γH2AX (Supplementary Figure S4). To determine whether the Dmp1-p53 interaction was direct or indirect, purified proteins from baculovirus-infected Sf9 cell lysates were used (Fig. 1D). A significant amount (~5% of input) of Flag-Dmp1 was identified in complex with HA-p53 in vitro. Reciprocal immunoprecipitation showed that HA-p53 (~5% of input) was pulled down with an antibody to Dmp1 (Fig. 1D). We also confirmed direct interaction between Mdm2 and p53, but not Dmp1 and Mdm2 (Fig. 1D, middle panels). Together, our data show Dmp1 physically interacts with p53, but not to p19Arf or Mdm2.
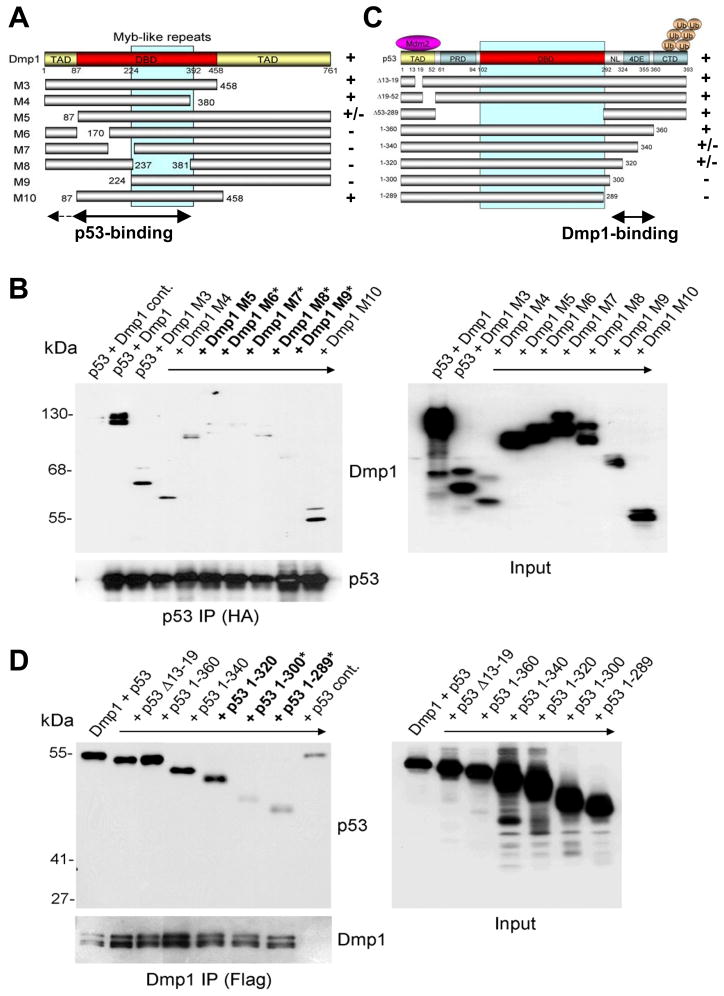
Mapping of p53-bindig domain on Dmp1 and Dmp1-binding domain on p53
Mapping the p53-bindig domain on Dmp1 was accomplished by co-expressing Dmp1 mutants with wild-type p53 in Sf9 and 3T3 cells. We found the Dmp1 mutants M5, M6, M7 M8, and M9 (20) were unable to interact with p53, suggesting that amino acid residues 1–380 are vital to Dmp1’s interaction with p53 (Fig. 2A, B). Specifically, the DNA-binding domain of Dmp1 (amino acids 87–380) is considered to play a major role in the Dmp1-p53 interaction since the M10 mutant bound to p53 at the same level as wild-type. The p53-interaction domain on Dmp1 was mapped to amino acids 87–380 in NIH 3T3 cells (Supplementary Fig. S5A, C). Conversely, the Dmp1-binding domain was mapped to amino acid residues 290–360 of p53 (nuclear localization signal and tetramerization domain (Fig. 2C, D; Supplementary Fig. S5B, C), consistent with the finding that Dmp1-binding to p53 did not interfere with Mdm2-p53 interaction (Fig. 1B). For verification, the His-tagged minimal-binding region of Dmp1 for p53 (M14: amino acids 87–224) and His-tagged, full-length or carboxyl-terminal p53 (amino acids 290–393:Δ1–289) were purified from bacteria and protein-protein binding assays were conducted by IP-Western blotting (Supplementary Fig. S6). Our results show Dmp1 M14 bound directly to wild-type p53 and p53Δ1–289.
Fig. 2. Mapping the protein interaction domains in Dmp1 and p53.
(A) Schematic presentation of wild-type Dmp1 and its deletion mutants.
(C) Schematic presentation of wild-type p53 and its deletion mutants. Sf9 cells were co-infected with baculoviruses encoding either wild-type or deletion mutants of Flag-Dmp1 and HA-p53. Lysates were immunoprecipitated with (B) anti-HA agarose or (D) anti-Flag M2 agarose and analyzed by Western blotting with Dmp1 and p53 antibodies. Bold Dmp1 or p53 mutants showed partial interaction with p53 or Dmp1, respectively. Bold plus asterisk mutants showed no interaction. TAD: transactivation domain; DBD: DNA-binding domain; PRD: proline-rich domain; NL: nuclear localization signal; 4DE: tetramerization domain; CTD: carboxyl-terminal regulatory domain.
Dmp1 antagonizes ubiquitination of p53 by Mdm2
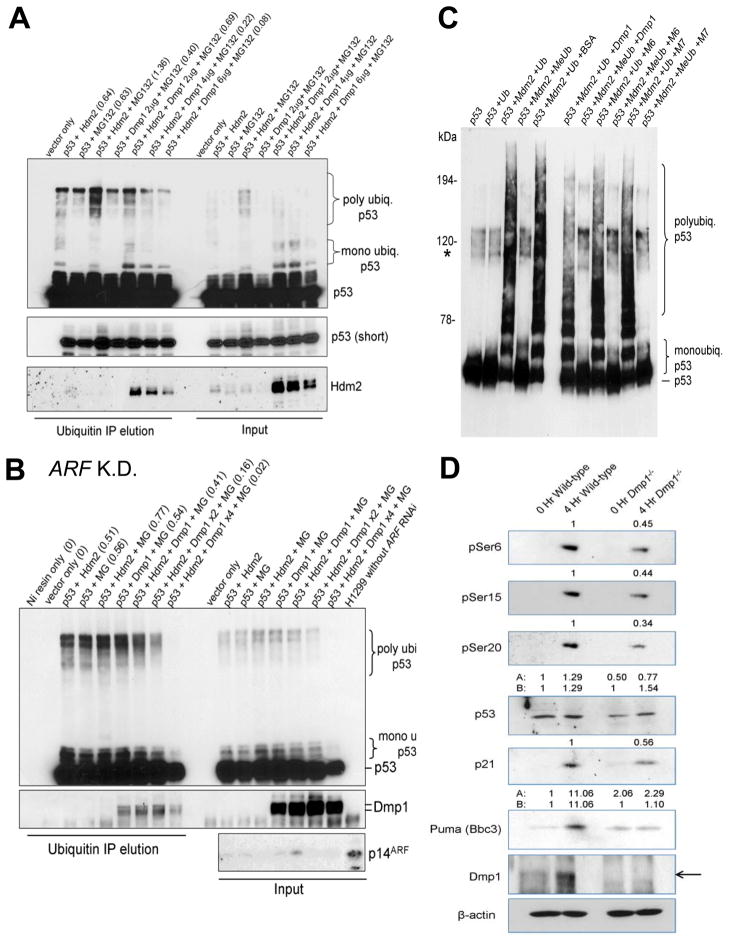
Mdm2 (or Hdm2) has been reported to inactivate p53 by 1) accelerating ubiquitin-mediated degradation of p53, 2) inhibiting nuclear import of p53 to the nucleus, and/or by 3) inhibiting transcriptional activity of p53 (5–7, 13). To assess the effect of Dmp1 expression on p53 ubiquitination by Hdm2, H1299 cells were co-expressed with His-tagged ubiquitin, p53, Hdm2, and increasing amounts of Dmp1. Ubiquitinated proteins were brought down with Ni-NTA resin, and samples were analyzed by Western blotting. Results show that Dmp1 overexpression decreased p53 polyubiquitination by Hdm2 in the presence or absence of proteasome inhibitor (Fig. 3A; Supplementary Fig. S7). H1299 cells transfected with shRNA to p14ARF also showed significant inhibition of p53 polyubiquitination by Dmp1 indicating that the effect was ARF-independent (Fig. 3B). To study the effects of endogenous Dmp1 on p53 stability, hDMP1 was knocked down by specific shRNA (23). Cells depleted for DMP1 exhibited over a 60% reduction in endogenous p53 protein levels compared to vector (Supplementary Fig. S8A) while p53 transcript levels remained unchanged (Supplementary Fig. S8B). Therefore, modulation of p53 levels by Dmp1 does not take place at the transcriptional level. Furthermore, endogenous p53 ubiquitination was drastically increased (Supplementary Fig. S8C) upon DMP1 knockdown. In vitro p53 ubiquitination reactions indicate that Hdm2 accelerated poly-ubiquitination of p53, while co-expression of Hdm2 and Dmp1 significantly inhibited Hdm2-mediated ubiquitination of p53 in dose-dependent fashion (Fig. 3C, Supplementary Fig. S10). Conversely, auto-ubiquitination of Hdm2 was not inhibited by Dmp1 (Supplementary Fig. S11) suggesting that Dmp1’s mode of action involves its direct binding with p53 and does not function as a general/non-specific inhibitor of Hdm2’s ubiquitin ligase activity. The M6 and M7 Dmp1 mutants that do not bind to p53 had little effect in p53 ubiquitination in vitro, indicating that direct physical interaction between Dmp1 and p53 is essential for Dmp1 to interfere with Hdm2 activity (Fig. 3C). To observe the effect of Dmp1-loss on p53 levels in vivo, 5-week-old Dmp1−/− and pure wild-type mice were treated by intravenous injection of doxorubicin for four hours. The animals were sacrificed, the thymus resected, and total protein prepared for analysis by PAGE. Steady - state p53 levels in untreated animals were 50% lower in mice of Dmp1-null background compared to wild-type. A 30% reduction in total p53 was observed in the thymus from Dmp1−/− mice treated with doxorubicin (Fig. 3D, the 4th lane). Notably, the amount of phosphorylated p53 (pSer6, 15, 20) was further (60–70%) reduced in Dmp1−/− thymus than wild-type (Figure 3D, 1st – 3rd lanes), and the levels of p53 target gene products were proportionate to the levels of phosphorylated p53 (Figure 3D, 5th – 6th lanes). These data indicated that Dmp1 not only plays critical roles in setting basal p53 levels in tissues, but also is involved in the activation of p53 in response to DNA-damage.
Fig. 3. Dmp1 modulates p53 ubiquitination in vivo and in vitro.
(A) Dmp1 modulates p53 ubiquitination. H1299 cells were transfected with His-ubiquitin, p53, Hdm2, and increasing amounts of Dmp1. Cells were treated with the proteasome inhibitor MG-132 and ubiquitinated p53 was isolated with Ni-NTA resin and analyzed by Western blotting with the indicated antibodies. The numbers in the parentheses show densitometric values of polyubiquitinated p53.
(B) In cell p53 ubiquitination assay using H1299 cells transfected with p14ARF shRNA. Polyubiquitination of p53 by Hdm2 was significantly inhibited by Dmp1 (47% by Dmp1 x1, 79% by Dmp1 x2, and 98% by 4x Dmp1) expression in H1299 cells with 90% downregulation of p14ARF by specific shRNA, indicating that the inhibitory effect of Dmp1 on p53 ubiquitination was independent of ARF. K.D.: knockdown; MG: MG132.
(C) Dmp1 blocks p53 ubiquitination by Mdm2 in vitro. In vitro ubiquitination assays were performed in the presence of purified p53, Mdm2 (E3), E2 (ubch5a), E1, and ATP using either ubiquitin (poly- and mono-ubiquitination) or methyl-ubiquitin (mono-ubiquitination only). Dmp1 M6 and M7 mutants that do not interact with p53 did not block poly- or mono-ubiquitination. Asterisk indicates endogenous Sf9 ubiquitination of p53.
(D) Both total and phosphorylated p53 levels are reduced in Dmp1−/− thymus compared to wild-type. Total protein was isolated from the thymus of Dmp1-null and wild-type mice after intravenous injection with doxorubicin for 4 hours and resolved by SDS-PAGE. The numbers indicate relative expression levels of each protein determined by densitometric analyses of Western blotting data.
Dmp1 antagonizes nuclear export of p53 by Hdm2
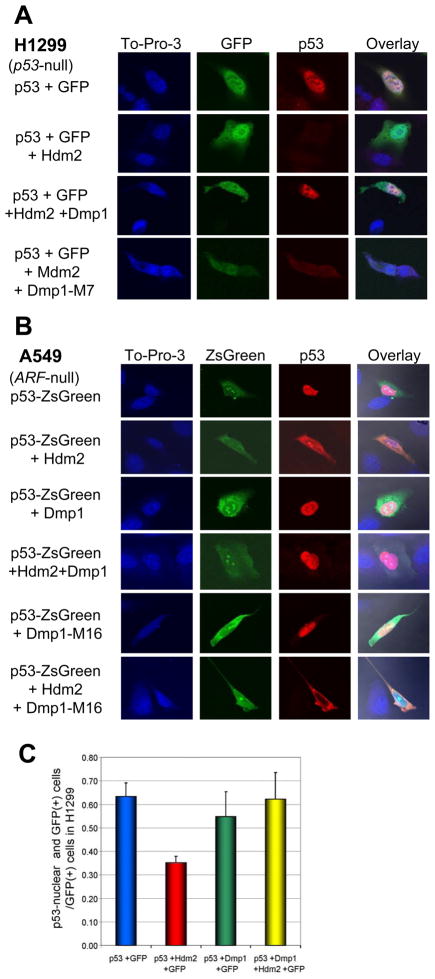
Next, we investigated whether Dmp1 altered the localization of p53. H1299 cells were co-transfected with GFP, p53, Hdm2, and/or Dmp1, probed for p53, and analyzed by confocal microscopy (Fig. 4A). As anticipated, Hdm2 expression resulted in an absolute reduction in p53 levels (Fig. 4A, 2nd panels). This effect was rescued by concurrent expression of Dmp1 (Fig. 4A, the 3rd panels) while the M7 mutant was unable to reverse the negative effects of Hdm2 (Fig. 4A, the 4th panels). Increased nuclear localization of p53 by Dmp1 was verified in endogenous proteins since MCF7 cells (ARF-null) depleted of hDMP1 by shRNA resulted in nuclear exclusion of p53, while, in control cells, p53 was retained in the nucleus (Supplementary Fig. S9). Subcellular localization of p53 was also studied in A549 cells (ARF-null) co-expressed with p53-ZsGreen, Dmp1, and Hdm2 (Fig. 4B). Expression of Hdm2 caused accumulation of p53 in the cytoplasm (the 2nd panels) while addition of Dmp1 restored nuclear localization of p53 (the 4th panels), indicating that the effect of Dmp1 is independent of p14ARF. The M16 Dmp1 mutant (Δ87–237, deletion for both M6 and M7) failed to restore nuclear accumulation of p53 when co-expressed with Hdm2 (Fig. 4B, the 6th panels), further supporting our hypothesis that physical interaction between Dmp1 and p53 is essential to antagonize the activity of Hdm2.
Fig. 4. Dmp1 stabilizes p53 nuclear localization in the presence of Hdm2.
(A) Subcellular localization of p53 in H1299 cells transfected with GFP, p53, Hdm2, Dmp1, and/or Dmp1-M7. The M7 Dmp1 mutant lacks amino acids 170–237 of Dmp1 (Fig. 2A) and does not interact with p53. p53 was stained using p53 mouse monoclonal antibody (sc-126) and Alexa 594 conjugated goat anti-mouse antibody. To-pro-3 was used as a nucleic acid stain.
(B) Subcellular localization of p53 in A549 cells (ARF-null) transfected with p53-ZsGreen, Hdm2, Dmp1, and Dmp1-M16. Expression of Hdm2 caused accumulation of p53 in the cytoplasm while expression of Dmp1 restored nuclear localization of p53, indicating that the effect of Dmp1 is independent of p14ARF. The M16 Dmp1 mutant (lacking amino acids 87–237 of Dmp1 [Fig. 2A] does not interact with p53) cannot restore localization of p53 to the nucleus when co-expressed with Hdm2, suggesting that physical interaction between Dmp1 and p53 is essential to antagonize the activity of Hdm2.
(C) Quantification of cells positive for nuclear p53 and GFP vs. GFP only cells from panel A.
Synergism between Dmp1 and p53 on p53 target gene transcription in Arf-deficient cells
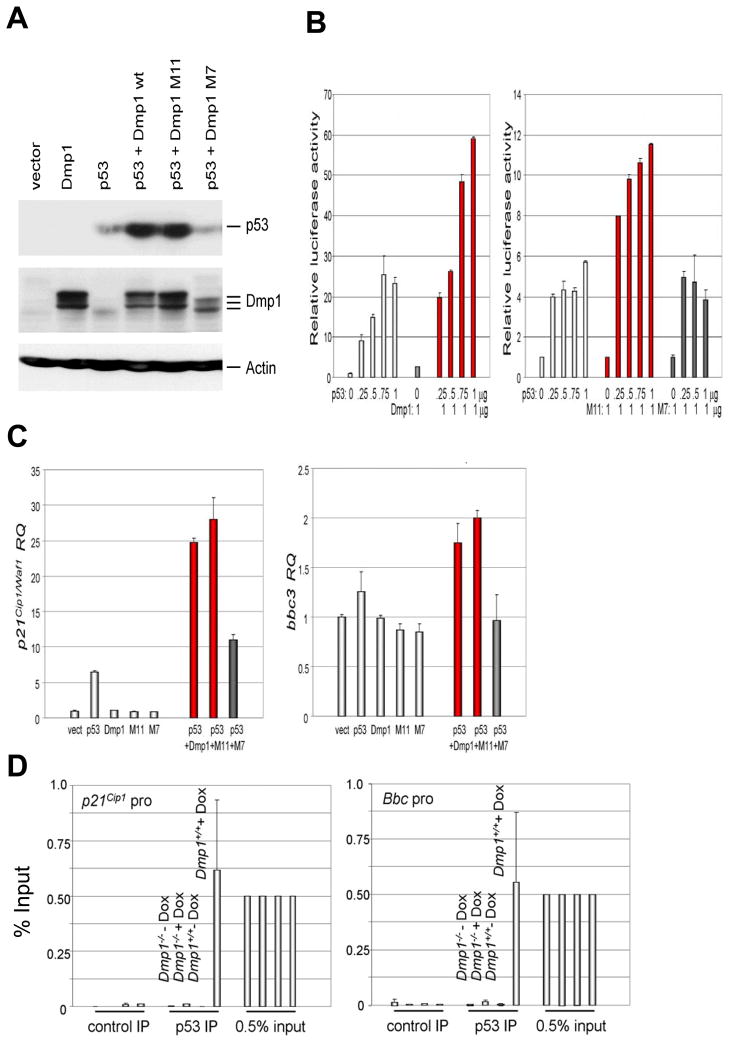
Next Arf; p53 double knockout (DKO) cells were transiently co-expressed with wild-type p53 and Dmp1 (and its mutants) to study the consequences on p53 function. We saw significant accumulation of p53 in cells co-expressing p53 and Dmp1 wild-type or the M11 mutant that does not bind to DNA, but not in those expressing p53 plus the M7 mutant that does not bind to p53 (Fig. 5A) (20). The increase in p53 levels upon co-expression of Dmp1 correlated with synergistic activation of the p21Cip1/Waf1 promoter luciferase construct in Arf; p53 double-knockout MEFs, (Fig. 5B, left panel). The Dmp1 M11 mutant that does not bind to DNA (20) also synergized with p53 on the p21 promoter activation (Fig. 5B, right panel), suggesting that this synergistic effect between p53 and Dmp1 was independent of DNA-binding of the latter. Since the M7 mutant did not contribute to the stability of p53, it had no effect on p53-mediated transactivation of the p21 promoter (Fig. 5B, right panel). The results of the p21Cip1/Waf1 reporter assays were further verified by stably co-expressing p53 and Dmp1 in Arf−/−; p53−/− MEFs and quantitating endogenous p21Cip1/Waf1 mRNA by real-time PCR (Fig. 5C). Synergism between p53 and Dmp1 was also demonstrated by real-time PCR for bbc3 (puma) in Arf−/−; p53−/− MEFs, albeit to a lesser extent (Fig. 5C, right). Thus, synergistic activation of the p21 and bbc3 promoters by Dmp1 (wild-type and M11 mutant) and p53 was a consequence of increased p53 levels.
Fig. 5. Dmp1 stabilizes the p53 protein and synergizes with p53 to activate its targets in Arf-null cells.
(A) Dmp1 stabilizes p53 in Arf; p53 double knockout (DKO) mouse embryonic fibroblasts. Accumulation of p53 was observed upon co-expression of wild-type Dmp1 or M11, but not M7 indicating stability of p53 is conferred through direct binding with Dmp1 and is not a result of Dmp1’s DNA binding.
(B) Synergistic increase in p53 transcriptional target activity. Left panel: Arf; p53 DKO MEFs were co-transfected with mouse p21Cip1/Waf1 luciferase reporter and increasing doses of p53, wild-type Dmp1 only, or Dmp1 plus p53. Right panel: p21 reporter assay was conducted with M11 (DNA binding deficient) or M7 (p53 binding deficient) Dmp1 mutant plus p53. Error bars indicate SEM.
(C) Real-time PCR Taqman assay of p21Cip1/Waf1 (left) and bbc3 (right) mRNA in Arf; p53 DKO MEFs infected with retrovirus expressing p53, Dmp1, and/or Dmp1 mutants. Expression of p53 increased the p21 mRNA 7 fold, while co-expression of p53 and Dmp1 or M11 mutant induced the mRNA 25–28 fold (red bars). Synergistic induction of bbc3 mRNA by p53 and Dmp1 or M11 was also observed albeit less efficiently than p21 mRNA. The M7 Dmp1 mutant defective in p53 interaction had little effect on p21 or bbc3 mRNA induction by p53 (black bars). Error bars indicate SEM.
(D) Dmp1-dependent binding of p53 to the bbc3 and p21Cip1 promoters in mouse thymus in response to doxorubicin injection. Wild-type and Dmp1-null mice (6-week-old) were tail-injected with 0.6 mg doxorubicin/30 g mouse and thymi were harvested at 0 and 4 hrs. The binding of p53 to target gene (p21Cip1 and bbc3) promoters was studied by chromatin immunoprecipitation, followed by real-time PCR. Significant binding of p53 to the p21Cip1 and bbc3 promoters were found in wild-type, but not in Dmp1-null thymus, suggesting that Dmp1 is indispensable for p53 to bind to these promoters. Control IP: IP with protein G sepharose only.
We then tail-injected doxorubicin in wild-type and Dmp1-null mice, harvested the thymus at 0 and 4 hrs post-treatment, and binding of p53 to target gene (p21Cip1 and bbc3) promoters was studied by chromatin immunoprecipitation followed by real-time PCR (Fig. 5D). Significant binding of p53 (0.5% of input levels) to the p21Cip1 and bbc3 promoters was found in wild-type, but not in Dmp1-null thymus, suggesting that Dmp1 is indispensable for p53 binding to these promoters in vivo. Together, our data indicate that 1) expression of Dmp1 interferes with the known activities of Hdm2 on p53’s ubiquitination and nuclear localization in an Arf-independent fashion, and that 2) p53-binding to target genes in Dmp1-dependent.
Arf-independent function of Dmp1 on p53 in vivo
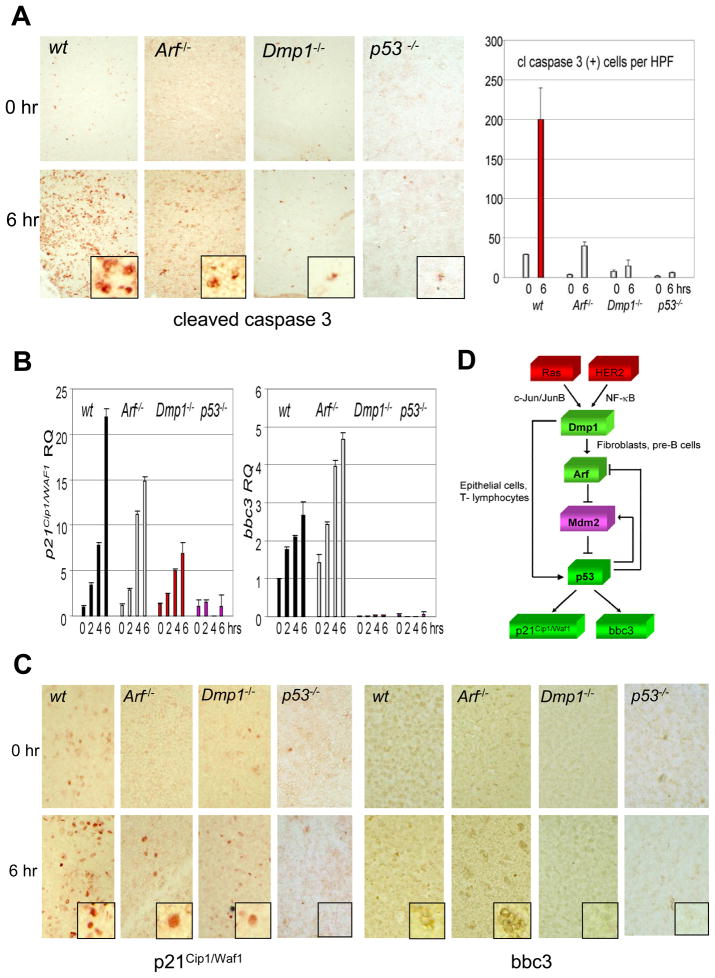
To demonstrate the Arf-independent function of Dmp1 on p53 in vivo, we injected doxorubicin into wild-type, Arf-null, Dmp1-null, and p53-null mice, harvested the thymus at 2 hr intervals, and studied the expression of the p53 targets p21Cip1/Waf1 and bbc3 by real-time PCR and immunohistochemistry (Fig. 6). Thymus was chosen as a target tissue since Dmp1 was highly expressed in the medulla in our previous histochemical studies (26). Cleaved caspase 3 was detectable in thymic medulla of wild-type mice 6 hrs after injection of doxorubicin, indicating apoptotic cell death (Fig. 6A, wt). Activation of p53 was confirmed by pSer6 and pSer15-specific antibodies (data not shown). Cleaved caspase 3 staining was significantly decreased in the thymus from Arf−/− mice and was barely detectable in that from Dmp1−/− or p53−/− mice (Fig. 6A). Consistent with these findings, p21 mRNA induction (~23-fold in wild-type) was more significantly compromised in Dmp1−/− mice (~7-fold at 6 hrs) than in Arf−/− mice (~15-fold), and bbc3 was barely detectable in Dmp1−/− thymus even 4–6 hrs after injection of doxorubicin (Fig. 6B). These data were confirmed by immunohistochemical staining of thymic tissues with p21 and bbc3-specific antibodies (Fig. 6C). Likewise, p21 and bbc3 induction by doxorubicin was more seriously compromised in Dmp1−/− lungs than in Arf−/− lungs (Supplementary Fig. S12A, B), eliminating the possibility this effect is cell type specific. Together, our data demonstrate Arf-independent activation of p53 by Dmp1 in response to genotoxic drug in vivo. The current model for the mechanism of regulation of p53 by Dmp1 is summarized in Fig. 6D.
Fig. 6. p53 target gene expression in mouse thymus injected with doxorubicin and current model for the mechanism of regulation of p53 by Dmp1.
(A) Left: Cleaved caspase 3 staining of thymus from wild-type, Arf-null, Dmp1-null, and p53-null mice. Doxorubicin was injected into 6-week-old mice, and thymi were isolated before and 6 hrs after drug injection. Right: The number of cleaved caspase 3-positive cells per high power field has been counted and are shown in bar graph.
(B) Real-time PCR analysis of p21Cip1/Waf1 and bbc3 in the mouse thymus. p21Cip1/Waf1 mRNA induction was more seriously compromised Dmp1−/− or p53−/− mice than in Arf−/− mice. Bbc3 was barely detectable in Dmp1−/− or p53−/− thymi while the induction was not affected in Arf−/− mice. Error bars indicate SEM.
(C) Immunohistochemical staining of thymic tissues with p21Cip1/Waf1 and bbc3-specific antibodies showing significantly decreased induction of p53 target gene products in Dmp1−/− thymus than in Arf−/− thymus.
(D) Current model for the mechanism of regulation of p53 by Dmp1. Published studies showed that Dmp1 receives oncogenic signals from mutant Ras or overexpressed HER2 and directly transactivates the Arf promoter. This p19Arf induction, in turn, neutralizes all the activities of Mdm2 and activates p53. Our current study demonstrates that the Dmp1 protein directly interacts with p53 and blocks activities of Mdm2, especially in epithelial cells and T lymphocytes. These two mechanisms will act synergistically to boost the p53 activity to prevent tumorigenesis.
Discussion
We have now shown that Dmp1 physically interacts with p53 and antagonizes all the known activities of Mdm2 (or Hdm2), i.e., p53 ubiquitination, nuclear-cytoplasmic shuttling, and transactivation of target genes. Dmp1 is a critical transcriptional activator for Arf, and p19Arf (or p14ARF) can, in turn, stabilize nucleoplasmic p53 by binding to Mdm2 and sequestering it in the nucleolus or by directly inhibiting the ubiquitin ligase activity of Mdm2(6, 13). Moreover, Arf has also been shown to modulate the activity of other E3 ligases for Mdm2, such as ARF-BP1 (18). However, inhibition of ubiquitination and nuclear export of p53 by Dmp1 are considered to be Arf-independent since they were found in cells where p14ARF was knocked down or deleted. Synergistic activation of the p21Cip1/Waf1 promoter by p53 and Dmp1 was also Arf-independent since it was found p53; Arf-null cells. These Arf-independent mechanisms of p53 modulation by Dmp1 substantiate the decrease in frequency of p53 mutations in both Dmp1+/− and Dmp1−/−backgrounds we previously observed in the K-rasLA lung cancer model where p19Arf involvement is rare (23).
We have mapped the Dmp1-binding domain at the carboxyl-terminus of p53, suggesting that Dmp1-p53 binding does not interfere with p53 binding to target genes or physical interaction between Mdm2 and p53. Conversely, the p53-binding domain was mapped to the DNA-binding domain of Dmp1, therefore binding of Dmp1 to p53 and DNA are considered to be mutually exclusive. Indeed, the Dmp1 mutant that does not bind to DNA retained the activity to stimulate the p21Cip1/Waf1 promoter in a p53-dependent fashion. Among p53-binding partners reported so far, Dmp1 is unique in that it directly binds and activates the Arf promoter and at the same time physically interacts with p53 (32). Furthermore, most p53-interacting proteins that bind to the C-terminus of p53 (e.g. YB-1, YY1, or RelA) negatively regulate its function whereas Dmp1 regulates p53 positively in terms of stabilization and nuclear localization (30, 32). Thus, Dmp1 is a critical antagonist of Mdm2 in regulating the activity of p53.
The current study showed an unexpected role of Dmp1 in apoptosis: although it was induced to wild-type levels in Arf−/− tissues, the bbc3 gene was barely induced by doxorubicin in the thymi or lungs from Dmp1−/− or p53−/− mice. This indicates that the induction of bbc3 was dependent on Dmp1 and p53, but not Arf. Since both mouse and human bbc3 promoters lack Dmp1-binding motifs, it is speculated that Dmp1 physically interacts with p53 and regulates the bbc3 promoter. This model was supported by ChIP that showed binding of p53 to the bbc3 (and p21) promoter in wild-type thymus, but not in Dmp1−/− tissues (Fig. 5D).
Li et al demonstrated that low levels of Mdm2 activity induce monoubiquitination and nuclear export of p53, whereas high levels promote p53’s polyubiquitination and nuclear degradation (31). Our results demonstrate that Dmp1 inhibits both monoubiquitination and polyubiquitination of p53, suggesting that Dmp1 can overcome Hdm2 antagonization at both high and low levels. Interestingly, in our localization studies, we found that p53 is degraded when it is co-expressed with Hdm2 in H1299 cells (p53-null, ARF wt), but is exported from the nucleus in A549 cells (p53 wt, ARF-null). This is likely due to the fact that A549 cells contain higher amounts of p53, reflecting higher endogenous expression, likely inducing Hdm2-mediated monoubiquitination and nuclear export of the protein. In both cases, Dmp1 stabilizes nuclear p53 providing clear evidence that Dmp1 can inhibit both p53 nuclear degradation and p53 nuclear export induced by Hdm2.
Augmentation of Dmp1’s activity may be a useful target for future drug discovery for several reasons. First, Dmp1 initiates two independent mechanisms that act synergistically to neutralize the activity of Mdm2: one directly through the regulation of p53 stabilization, localization, and transactivation capabilities and the second indirectly through the regulation of the Arf promoter. In addition, Dmp1 is haplo-insufficient for tumor suppression; hence tumors often retain one allele of Dmp1, making modulation of this intact gene more feasible.
Supplementary Material
Acknowledgments
We are grateful to C. Sherr and M. Roussel for Arf-null mice, Arf; p53-double null MEFs; C. Prives, B. Vogelstein for plasmid DNAs; and S. Grossman, D. Livingston for FLAG-Mdm2 baculovirus. We thank M. Willingham, P. Taneja, and S. Zhu for their advice in immunohistochemistry, and K. Klein for editing. K. Inoue is supported by NIH/NCI 2R01CA106314, ACS RSG-07-207-01-MGO, and KG080179. D. Frazier was supported by the NIH training grant (5T32CA079448, F Torti). D. Maglic has been supported by DOD pre-doctoral fellowship BC100907.
References
- 1.Toledo F, Wahl GM. Regulating the p53 pathway: in vitro hypotheses, in vivo veritas. Nat Rev Cancer. 2006;6:909–23. doi: 10.1038/nrc2012. [DOI] [PubMed] [Google Scholar]
- 2.Menendez D, Inga A, Resnick MA. The expanding universe of p53 targets. Nat Rev Cancer. 2009;9:724–37. doi: 10.1038/nrc2730. [DOI] [PubMed] [Google Scholar]
- 3.Vousden KH, Prives C. Blinded by the light: the growing complexity of p53. Cell. 2009;137:413–31. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 4.Donehower LA, Lozano G. 20 years studying p53 functions in genetically engineered mice. Nat Rev Cancer. 2009;9:831–41. doi: 10.1038/nrc2731. [DOI] [PubMed] [Google Scholar]
- 5.Brooks CL, Gu W. p53 ubiquitination: Mdm2 and beyond. Mol Cell. 2006;21:307–15. doi: 10.1016/j.molcel.2006.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kruse J-P, Gu W. Modes of p53 regulation. Cell. 2009;137:609–22. doi: 10.1016/j.cell.2009.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manfredi JJ. The Mdm2-p53 relationship evolves: Mdm2 swings both ways as an oncogene and a tumor suppressor. Genes Dev. 2010;24:1580–9. doi: 10.1101/gad.1941710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brosh R, Rotter V. When mutants gain new powers: news from the mutant p53 field. Nat Rev Cancer. 2009;9:701–13. doi: 10.1038/nrc2693. [DOI] [PubMed] [Google Scholar]
- 9.Araki S, Eitel JA, Batuello CN, Bijangi-Vishehsaraei K, Xie XJ, Danielpour D, et al. TGF-beta1-induced expression of human Mdm2 correlates with late-stage metastatic breast cancer. J Clin Invest. 2010;120:290–302. doi: 10.1172/JCI39194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balaburski GM, Hontz RD, Murphy ME. p53 and ARF: unexpected players in autophagy. Trends Cell Biol. 2010;20:363–9. doi: 10.1016/j.tcb.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim WY, Sharpless NE. The regulation of INK4/ARF in cancer and aging. Cell. 2006;127:265–75. doi: 10.1016/j.cell.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Sherr CJ. Divorcing ARF and p53: an unsettled case. Nat Rev Cancer. 2006;6:663–73. doi: 10.1038/nrc1954. [DOI] [PubMed] [Google Scholar]
- 13.Sherr CJ, Weber JD. The ARF/p53 pathway. Curr Opin Genet Dev. 2000;10:94–9. doi: 10.1016/s0959-437x(99)00038-6. [DOI] [PubMed] [Google Scholar]
- 14.Kamijo T, Zindy F, Roussel MF, Quelle DE, Downing JR, Ashmun RA, et al. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell. 1997;91:649–59. doi: 10.1016/s0092-8674(00)80452-3. [DOI] [PubMed] [Google Scholar]
- 15.Kamijo T, Bodner S, van de Kamp E, Randle DH, Sherr CJ. Tumor spectrum in ARF-deficient mice. Cancer Res. 1999;59:2217–22. [PubMed] [Google Scholar]
- 16.Inoue K, Roussel MF, Sherr CJ. Induction of ARF tumor suppressor gene expression and cell cycle arrest by transcription factor DMP1. Proc Natl Acad Sci USA. 1999;96:3993–8. doi: 10.1073/pnas.96.7.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inoue K, Mallakin A, Frazier DP. Dmp1 and tumor suppression. Oncogene. 2007;26:4329–35. doi: 10.1038/sj.onc.1210226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen D, Kon N, Li M, Zhang W, Qin J, Gu W. ARF-BP1/Mule is a critical mediator of the ARF tumor suppressor. Cell. 2005;121:1071–83. doi: 10.1016/j.cell.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 19.Chen D, Shan J, Zhu WG, Qin J, Gu W. Transcription-independent ARF regulation in oncogenic stress-mediated p53 responses. Nature. 2010;464:624–7. doi: 10.1038/nature08820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inoue K, Sherr CJ. Gene expression and cell cycle arrest mediated by transcription factor DMP1 is antagonized by D-type cyclins through a cyclin-dependent-kinase-independent mechanism. Mol Cell Biol. 1998;18:1590–600. doi: 10.1128/mcb.18.3.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inoue K, Wen R, Rehg JE, Adachi M, Cleveland JL, Roussel MF, et al. Disruption of the ARF transcriptional activator DMP1 facilitates cell immortalization, Ras transformation, and tumorigenesis. Genes Dev. 2000;14:1797–809. [PMC free article] [PubMed] [Google Scholar]
- 22.Inoue K, Zindy F, Randle DH, Rehg JE, Sherr CJ. Dmp1 is haplo-insufficient for tumor suppression and modifies the frequencies of Arf and p53 mutations in Myc-induced lymphomas. Genes Dev. 2001;15:2934–9. doi: 10.1101/gad.929901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mallakin A, Sugiyama T, Taneja P, Matise LA, Frazier DP, Choudhary M, et al. Mutually exclusive inactivation of DMP1 and ARF/p53 in lung cancer. Cancer Cell. 2007;12:381–94. doi: 10.1016/j.ccr.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sugiyama T, Frazier DP, Taneja P, Morgan RL, Willingham MC, Inoue K. Role of DMP1 and its future in lung cancer diagnostics. Expert Rev Mol Diagn. 2008;8:435–47. doi: 10.1586/14737159.8.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sreeramaneni R, Chaudhry A, McMahon M, Sherr CJ, Inoue K. Ras-Raf-Arf signaling critically depends on the Dmp1 transcription factor. Mol Cell Biol. 2005;25:220–32. doi: 10.1128/MCB.25.1.220-232.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mallakin A, Taneja P, Matise LA, Willingham MC, Inoue K. Expression of Dmp1 in specific differentiated, nonproliferating cells and its repression by E2Fs. Oncogene. 2006;25:7703–13. doi: 10.1038/sj.onc.1209750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taneja P, Mallakin A, Matise LA, Frazier DP, Choudhary M, Inoue K. Repression of Dmp1 and Arf transcription by anthracyclins: critical roles of the NF-κB subunit p65. Oncogene. 2007;26:7457–66. doi: 10.1038/sj.onc.1210568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taneja P, Maglic D, Kai F, Sugiyama T, Kendig RD, Frazier DP, et al. Critical role of Dmp1 in HER2/neu-p53 signaling and breast carcinogenesis. Cancer Res. 2010;70:9084–94. doi: 10.1158/0008-5472.CAN-10-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mallakin A, Sugiyama T, Kai F, Taneja P, Kendig RD, Frazier DP, et al. The Arf-inducing Transcription Factor Dmp1 Encodes Transcriptional Activator of Amphiregulin, Thrombospondin-1, JunB and Egr1. Int J Cancer. 2010;126:1403–16. doi: 10.1002/ijc.24938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sui G, Affar el B, Shi Y, Brignone C, Wall NR, Yin P, et al. Yin Yang 1 is a negative regulator of p53. Cell. 2004;117:859–72. doi: 10.1016/j.cell.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 31.Li M, Brooks CL, Wu-Baer F, Chen D, Baer R, Gu W. Mono- versus polyubiquitination: differential control of p53 fate by Mdm2. Science. 2003;302:1972–5. doi: 10.1126/science.1091362. [DOI] [PubMed] [Google Scholar]
- 32.Braithwaite AW, Del Sal G, Lu X. Some p53-binding proteins that can function as arbiters of life and death. Cell Death Differ. 2006;13:984–93. doi: 10.1038/sj.cdd.4401924. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.