Abstract
Selective breeding for divergence in locomotion in a novel environment (bHR, bred High-Responder; bLR, bred Low-Responder) correlates with stress-reactivity, spontaneous anxiety-like behaviors and predicts vulnerability in a rodent model of depression. Identifying genetic factors that may account for such vulnerability are key determinants not only for the illness outcome but also for the development of better-tailored treatment options. Melanin-concentrating hormone (MCH) is a neuropeptide that exhibits some of the hallmarks of a regulator of affective states. The aim of this study was to ascertain the role of the MCH system in depression-like behaviors in bHR vs. bLR rats. bLR rats showed a 44% increase in hypothalamic pMCH mRNA and a 14% decrease in hippocampal CA1 MCH1R mRNA when compared to bHR rats. Interestingly, the amount of time that rats spent immobile in the FST (depressive-like behavior) correlated positively with the amount of hypothalamic pMCH mRNA and negatively with that of hippocampal CA1 MCH1R. The results indicate that the bLR-bHR is a useful rat model to investigate individual basal genetic differences that participate in the monitoring of emotional responsiveness (i.e., depression- and anxiety-like behaviors). They also point to the MCH system (i.e., chronically higher pMCH expression and consequently receptor down-regulation) as a candidate biomarker for the severity of depressive-like behavior. The data indicate that MCH1R participates in the modulation of depression-like behavior through a process that involves the CA1 region of the hippocampus, supporting the possible use of MCH1R antagonists in the treatment of depression.
1. Introduction
Reliable animal models of depression (Nestler and Hyman, 2010) are crucial to understanding the molecular mechanisms underlying depressive disorder and improving treatment options. Selective breeding for divergence in locomotion upon exposure to a novel environment (bHR, bred High-Responder; bLR, bred Low-Responder) has been used to generate an animal model which correlates strongly with stress-reactivity, spontaneous anxiety-like behaviors and other measures of “emotionality” (Stead et al., 2006). Moreover, novelty-seeking behavior has been recently shown to predict vulnerability in a rodent model of depression (Stedenfeld et al., 2011). Therefore, this animal model may be useful in identifying genetic factors that may account for vulnerability to depression-like behavior which are key determinants not only for illness outcome but also may be useful for developing better-tailored treatment options.
In addition to the well-known neurotransmitter monoamine hypothesis, many parallel processes exist that play a role in depression. These systems have a modulatory role in neural function by shaping complex intracellular processes, such as gene expression, receptor sensitivity and neurogenesis, among others. Melanin-concentrating hormone (MCH) is a 19 amino acid cyclic neuropeptide that exhibits some of the hallmarks of a regulator of affective states. MCH is expressed in the lateral hypothalamus and the zona incerta (Bittencourt et al., 1992) and in rodents, acts on one G protein-coupled receptor, MCH1R, which densely populates limbic brain regions such as the nucleus accumbens (NAc), prefrontal cortex (PFC), hippocampus and amygdala (Saito et al., 2001). MCH injection into the NAc has been shown to induce depressive-like behavior (Georgescu et al., 2005), and pharmacological (Borowsky et al., 2002) or genetic inactivation (Roy et al., 2006) of the MCH system exerts strong antidepressant and anxiolytic effects in acute assays. Moreover, MCH has been shown to modulate dopamine induced neuronal activity in the NAc (Chung et al., 2009). The MCH system has also been implicated in mood regulation through other brain regions, as it has been reported that hippocampal MCH1R expression is increased in the chronic mild stress depression model (Roy et al., 2007), and that injection of MCH into the dorsal raphe nucleus (DRN) induces depression-like behavior (Lagos et al., 2011). The role of the MCH system in regulating the propensity to manifest mood disorders, as measured by basal differences in the forced swim test — increase in the immobility time and decrease in the activity time — has not been investigated yet. This study takes advantage of the inborn differences in depression-like behavior in the bHR vs. bLR rat model to approach this question.
2. Experimental Procedures
bHR vs. bLR Rats
Male Sprague-Dawley rats from generation nine of the bHR/bLR breeding colony (University of Michigan, Ann Arbor, MI) were housed under a 12 h light/dark cycle with food and water available ad libitum. Thirty-six bHR and thirty-six bLR rats were used for this study. All rats were maintained at the University of Michigan animal facilities in accordance with the University Committee Use and Care of Animals. The experiments were conducted according to the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23, revised 1996). All efforts were made to minimize the number of animals used for this study.
Behavioral Testing
All behavioral testing in bHR and bLR rats was performed at University of Michigan: (1) locomotion in a novel environment; (2) anxiety-like behavior (elevated-plus maze, EPM); and (3) depression-like behavior (forced-swim test, FST: Day 1, 15-min and Day 2, 5-min test). Each behavioral test was screened in consecutive weeks (see Figure 1A) as previously described elsewhere (Stead et al., 2006; Jama et al., 2008). In brief, locomotion in a novel environment was monitored by computer in 5-min intervals over 60 min by placing rats into clear acrylic 43 × 21.5 × 25.5 cm (high) cages equipped with infrared photocell emitters. For the EPM, which is constructed of black Plexiglas with four elevated arms (70 cm from the floor × 45 cm long × 12 cm wide), each rat is placed at the beginning of the 5-min test, in the central square facing a closed arm. The computerized tracking system records the latency to first enter the open arm, and the amount of time spent in the open arm over the course of the 5-min test. For the FST rats were placed in vertical Plexiglas cylinders containing water (25°C) at a depth of 30 cm. On the first day of the test, rats were placed in water for a 15-min period (pretest phase, Day 1). Twenty-four hours later, rats were exposed again to the above experimental conditions and percent time spend immobile vs. active (climbing, swimming) behaviors were videotaped and scored for a 5-min period (test phase, Day 2). Rats were killed by decapitation either 15-min or 24-h after the last test (Day 2, 5-min FST session), trunk-blood was collected for plasma corticosterone (CORT) measurements performed by a radioimmunoassay from MP Biomedicals (Orangeburg, NY), and their brains removed and fast frozen for further gene expression analysis.
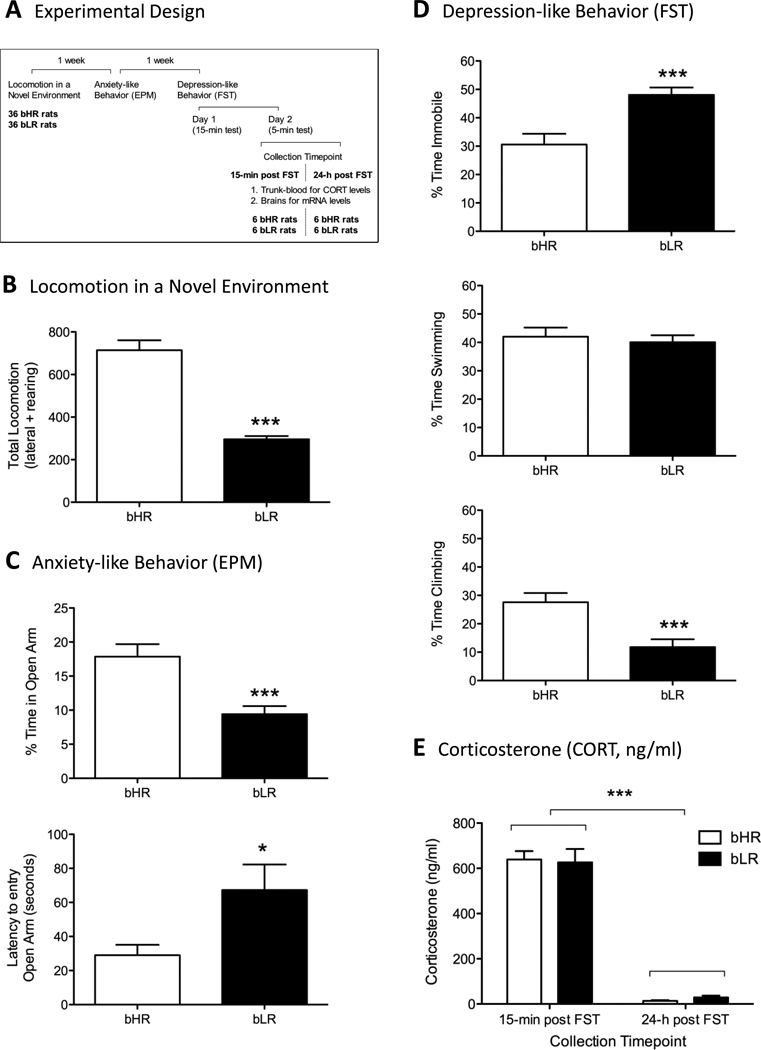
Figure 1.
A. Experimental Design. B. Locomotion in a Novel Environment. C. Anxiety-like Behavior (EPM). D. Depression-like Behavior (FST, 5-min period, test phase, Day 2). E. Corticosterone (CORT, ng/ml).
In Situ Hybridization Analysis
Rats were sorted based on immobility time in the FST (i.e., the classic measure of depression-like behavior) and a subset of 6 bHR and 6 bLR rats from each collection timepoint were selected for further molecular analysis. For mRNA in situ hybridization, tissue was cryostat-cut at −20°C in 10 µm sections and mounted on SuperFrost Plus slides (Fisher Scientific), and stored at −80°C until further processing. Briefly, as described elsewhere (Garcia-Fuster et al., 2009; Chung et al., 2009), tissue was fixed in 4% paraformaldehyde at room temperature, rinsed with aqueous buffers, and dehydrated with graded alcohols. After air-drying, the sections were hybridized with a 35S-labeled cRNA probe. The following rat probes were cloned from cDNA fragments with specific primers using standard in vitro transcription methodology: pMCH was a kind gift from Dr. Jean-Louis Nahon (Institute de Pharmacologie Moleculaire et Cellulaire, Valbonne, France), and MCH1R. The probes were labeled with incorporation of 35S-UTP and hybridized to tissue overnight at 60°C. The next day, sections were washed with increasing stringency, dehydrated with graded alcohols, air-dried, and exposed to film. Film exposure time was chosen to maximize signal. Digital images of the brain sections were quantified using a computer-based image analysis system (MCID, Image Research Inc., St Catharines, ON, Canada). Integrated optical density (IOD, µCi/g of probe × Area) in specific brain regions (pMCH, hypothalamus; MCH1R, PFC, NAc-Shell, amygadala, CA1) was measured and the corresponding values of radioactivity were determined by interpolation from a standard curve generated from 14C standards (American Radiolabeled Chemicals, St Louis, MO). The values obtained represent the average of measurements taken from 18 (hypothalamus) or 10–12 (PFC, NAc-Shell, amygdala, CA1 region of the hippocampus) sections per animal in the specified brain sites. The specificity of the hybridization signal was confirmed with sense probe controls for both probes (data not shown).
3. Results
Behavioral Phenotype: bHR vs. bLR Rats
Selective breeding has generated two lines of rats with marked differences in their locomotor responses to the mild stress of a novel environment. A student’s two-tail t-test was used for the statistical evaluation of bHR vs. bLRs rats for each behavioral test. As anticipated, bLR rats were markedly less active (249±15) in a novel environment when compared to bHR rats (713±45) (F1,70=77.1, p<0.0001, Figure 1B). Results in the EPM reflect a balance between natural exploratory drives in the rat and its fear of open and exposed spaces. Consistent with an increase in anxiety-like behavior (Figure 1C), bLR rats spent significantly less time in the open arm of the plus maze (10±1; F1,70=14.0, p<0.001) and a greater latency to enter the open arms of the maze (66±15; F1,70=5.6, p<0.05) compared with bHR rats (18±2 and 28±6 respectively). The FST is an assay commonly used in depression research and is based on the observation that rats, following initial escape oriented movements, develop an immobile posture (i.e., behavioral despair) when place in an inescapable cylinder of water. Consistent with an increase in depression-like behavior (Figure 1D), during the 5-min period (test phase, Day 2), bLRs rats spent significantly more time immobile (48±3; F1,62=14.0, p<0.001) and less time active (climbing behavior, 12±3; F1,62=14.8, p<0.001) than bHR rats (31±4 and 28±3 respectively).
Corticosterone Levels
CORT secretion 15-minutes post FST was 627±59 ng/ml for bLR and 639±37 ng/ml and bHR rats respectively (Figure 1E). When blood was collected 24-h post FST, CORT secretion was 29±8 ng/ml for bLR and 14±3 ng/ml and bHR rats respectively. A two-way ANOVA showed a significant effect of collection timepoint (15-min vs. 24-h; F1,56=450.9, p<0.0001), but no significant phenotype effect (bLR vs. bHR; F1,56=0.003, n.s.) or timepoint × phenotype interaction (F1,56=0.237, n.s.).
In Situ Hybridization Analysis
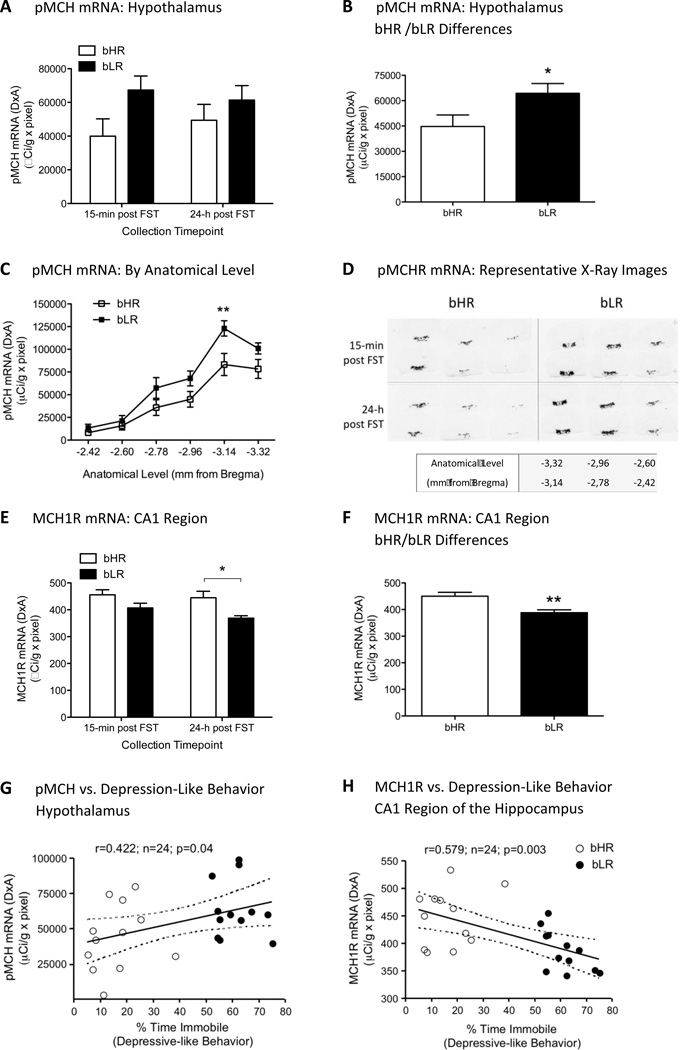
As mentioned earlier, six rats per group and timepoint of analysis sorted based on FST performance (i.e., immobility in the FST) were selected for molecular analysis (i.e., pMCH and MCH1R mRNA levels). When analyzing pMCH in hypothalamic regions, a two-way ANOVA showed no significant effect of collection timepoint (15-min vs. 24-h post FST; F1,20=0.036, n.s.), a significant effect of phenotype (bLR vs. bHR; F1,20=4.6, p<0.05) and no interaction collection timepoint × phenotype (F1,20=0.714, n.s., Figure 2A). In fact, when comparing bLR with bHR rats independently of the collection timepoint, bLR showed a 44% increase in hypothalamic pMCH mRNA when compared to bHR rats (p<0.05, Figure 2B). Moreover, if the analysis was split by anatomical level (see Figure 2C), a two-way ANOVA revealed an effect of phenotype (bLR vs. bHR; F1,129=16.89, p<0.001), an effect of anatomical level (Bregma −2.42 to −3.32 mm; F5,129=40.07, p<0.001), but no interaction between phenotype and anatomical level (F5,129=1.223, n.s.). Post-hoc analysis revealed that changes in pMCH mRNA mainly occurred at the posterior level of the hypothalamus (distance from Bregma: −3.14 mm, p<0.01).
Figure 2.
A. pMCH mRNA in hypothalamus of bHR/bLR rats by ISH analysis. Data represents mean ± SEM mRNA levels of bHR and bLR rats at the two timepoints of analysis (15-min and 24-h post FST). *p<0.05, bHR vs. bLR (effect of phenotype). B. pMCH mRNA in hypothalamus: bHR/bLR differences independently of the collection timepoint. *p<0.05. C. pMCH mRNA distribution in hypothalamus by anatomical level (−2.42 to −3.32 mm from Bregma). D. Representative pMCH mRNA in situ hybridization images in the hypothalamus (−2.42 to −3.32 mm from Bregma) of bHR vs. bLR rats 15-min or 24-h post FST. Bottom: representative slide showing anatomical level for each tissue section. E. MCH1R mRNA in CA1 region of the hippocampus. **p<0.01, bHR vs. bLR (effect of phenotype), *p<0.05, bHR vs. bLR at the 24-h collection timepoint. F. MCH1R mRNA in CA1: overall bHR/bLR differences. G. pMCH mRNA in hypothalamus correlates with depression-like behavior (% time immobile). H. MCH1R mRNA in CA1 inversely correlates with depressive-like behavior (% time immobile).
MCH1R mRNA was measured in key relevant areas such as the PFC, NAc-shell, CA1 region of the hippocampus and the amygdala (see Table 1). A two-way ANOVA showed no significant effect of collection timepoint (15-min vs. 24-h post FST), no effect of phenotype (bLR vs. bHR) and no significant interaction for collection timepoint × phenotype for the PFC (F1,20=0.013, n.s., F1,20=0.706, n.s., F1,20=1.395, n.s., respectively), NAc-shell (F1,20=1.189, n.s., F1,20=2.429, n.s., F1,20=2.235, n.s., respectively) and the amygdala (F1,20=1.910 n.s., F1,20=0.072, n.s., F1,20=0.072, n.s., respectively) (Table 1). When analyzing MCH1R in the CA1 region of the hippocampus, a two-way ANOVA showed no significant effect of collection timepoint (15-min vs. 24-h post FST; F1,20=1.826, n.s.), a significant effect of phenotype (bLR vs. bHR; F1,20=11.75, p<0.01) and no interaction between collection timepoint × phenotype (F1,20=0.554, n.s., Figure 2E). Post-hoc analysis revealed a decrease in MCH1R mRNA in bLR rats when comparing to bHR rats at the 24-h collection timepoint (p<0.05). Independent of the collection timepoint, bLR rats showed a 14% decrease in hippocampal CA1 MCH1R mRNA when compared to bHR rats (p<0.01, Figure 2F).
Table 1.
MCH1R mRNA Levels in Relevant Areas of Analysis
| 15-min post-FST |
24-h post FST |
|||
|---|---|---|---|---|
| bHR | bLR | bHR | bLR | |
| PFC | 912 ± 34 | 930 ± 34 | 969 ± 90 | 862 ± 28 |
| NAc-Shell | 3239 ± 83 | 3230 ± 54 | 3298 ± 177 | 2859 ± 204 |
| CA1 | 456 ± 19 | 407 ± 17 | 445 ± 24 | 369 ± 9, * |
| Amygdala | 416 ± 11 | 425 ± 15 | 398 ± 23 | 398 ± 12 |
Data repesents mean IOD (µCi/g × pixel) ± SEM
p<0.05 when comparing bHR vs. bLR
Remarkably, the amount of time spent immobile in the FST (depressive-like behavior) positively correlated with the amount of pMCH mRNA present in the hypothalamus (r=0.422, n=24, p<0.05, Figure 2G) and negatively correlated with the amount of MCH1R mRNA in the CA1 region of the hippocampus (r=0.579, n=24, p<0.01, Figure 2H).
4. Discussion
Taken together the results suggest that the bLR-bHR model is a useful tool to investigate individual basal genetic differences that might lead to differences in emotional responsiveness (i.e., depression- and anxiety-like behaviors). A description of the breeding strategy and initial behavioral characterization of the bHR and bLR lines was previously published (Stead et al., 2006) and has been extended for several generations across multiple behavioral and neurobiological dimensions (see Clinton et al., 2007, 2008, Perez et al., 2009, Clinton et al., 2010, Stedenfeld et al., 2011, Turner et al., 2011). Remarkably, since novelty-induced locomotion is a behavior that is correlated with measures of anxiety-like behavior, Turner and colleagues (2011) recently showed that an FGF2 treatment early in life rescued the anxiety-like phenotype in vulnerable rats (bLRs) without altering locomotion activity and depression-like behavior in bLR rats, therefore dissociating the effect of locomotion from other behaviors. In order to assess for possible changes in behavior and to be able to correlate the behavioral phenotype with molecular changes it is really important to phenotype each generation of the breeding lines. For this particular generation, bLR rats showed increased anxiety- and increased depression-like behavior when compared to bHR rats. While bHR/bLR rats have differences in stress responses for mild stressors (i.e., given the option to explore) (Kabbaj et al., 2000), in a more severely stressful situation like the FST they show no differences in CORT levels (see Figure 1E) suggesting that their distinct molecular signature is not influenced by the behavioral testing. This behavioral phenotype was accompanied by differences in the MCH system gene expression, which likely represent basal differences in gene levels rather than a differential response to the stressor caused by the behavioral tests.
In particular the data shows that in bLR rats, which manifest a more depressive-like behavior than bHR rats, MCH is produced at higher levels in the hypothalamus, suggesting that MCH release may be chronically higher. Changes in pMCH mRNA occurred at the posterior level of the hypothalamus (distance from Bregma: −3.14 mm), which has been described to show more clustering of MCH-positive neurons (Kerman et al., 2007). Additionally, the receptor is down regulated in the CA1 region of the hippocampus, indicating that this brain region may be most affected by higher basal MCH release, and thus important in manifesting the increased depression-like behavior. Interestingly, the more posterior part of the lateral hypothalamus projects to the hippocampus (Villalobos and Ferssiwi, 1987), supporting that caudal alterations in MCH expression might be driving the reduction of MCH1R in the CA1 region of the hippocampus. Moreover, independently of the phenotype, the amount of time a rat spent immobile while in the FST (i.e., measure of depression-like behavior) positively correlated with the amount of pMCH mRNA expression in the hypothalamus and negatively correlated with the amount of MCH1R mRNA in the CA1 region, suggesting the MCH system may be useful as a biomarker for the severity of depressive-like behavior.
It has previously been proposed that the hyperactivity of the MCH-ergic system could be involved in the physiopathology of depression. For instance, MCH microinjections into the NAc or the DRN induced depressive-like behavior when evaluated in the forced swim test — increase in the immobility time and decrease in the time spent in climbing or swimming behaviors (Georgescu et al., 2005, Lagos et al., 2011). The current data suggests the CA1 region of the hippocampus, in addition to the previously described NAc and the DRN (Georgescu et al., 2005, Lagos et al., 2011), may be involved in emotional behaviors induced by MCH. Interestingly, MCHR1 mRNA expression has also been reported to be elevated in the chronic mild stress depression model in mice (Roy et al., 2007). In fact, MCH1R facilitates behavioral performance in hippocampal dependent learning tasks by acting on CA1 glutamatergic synaptic transmission and long-term synaptic plasticity (Pachoud et al., 2010). The MCH system has been described to enhance memory retention in the hippocampus (Varas et al., 2002), further indicating that the MCH system is involved in hippocampal function.
These data suggest that MCH1R is involved in regulating depression-like behavior possibly through a process involving the hippocampus, and therefore support the use of MCH1R antagonists in the treatment of depression (see Chung et al., 2011). Thus far the reported data have studied the link between the MCH system and mood disorders in assays that relied on central administrations of the MCH peptide (Georgescu et al., 2005, Lagos et al., 2011), on selective MCH1R antagonists into relevant brain areas (e.g., Chaki et al., 2005, David et al., 2007, Gehlert et al., 2009) or on knockout genetics (Roy et al., 2007). Moreover, unpublished data from the Civelli laboratory showed that acute intracerebroventricular injections of an MCH1R antagonist (TPI 136117) induced an antidepressant-like effect based on FST performance in adult rats, while had no effect on locomotion. The present data adds a novel dimension by suggesting an association between basal levels of the MCH system gene expression and the manifestation of depression-like behavior when evaluated by immobility time spent in the forced swim test. In conclusion, dysregulation of the MCH system gene expression (i.e., chronically higher MCH release and receptor down-regulation) could be a predisposing factor for developing affective disorders. Future experiments will ascertain whether the administration of the MCH1R antagonist into specific brain regions such as the CA1 region of the hippocampus could protect the predisposition or ameliorate the behavioral phenotype manifested in bLR rats.
Acknowledgments
Role of Funding Source
This study was supported by NIH DA024746, an Established Investigator Award from the National Alliance for Research on Schizophrenia and Depression (NARSAD), and a grant from the Tourette Syndrome Association to OC, and by NIDA 5P01DA021633-02, NIMH Conte Center Grant #L99MH6039, Office of Naval Research (ONR) N00014-09-1-0598, and The Pritzker Neuropsychiatric Research Foundation to HA & SJW. MJGF is a ‘Ramón y Cajal’ Researcher (MICINN-UIB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
MJGF, GSP and OC designed the experiments and wrote the manuscript. SMC, SJW and HA performed the behavioral testing on bLR and bHR rats and provided their brains. MJGF conducted the experiments and analyzed the data. All authors contributed to and have approved the final manuscript.
Conflict of Interest
The authors declare no competing financial interests in relation to the work described.
References
- Bittencourt JC, Presse F, Arias C, Peto C, Vaughan J, Nahon JL, Vale W, Sawchenko PE. The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization. J. Comp. Neurol. 1992;319:218–245. doi: 10.1002/cne.903190204. [DOI] [PubMed] [Google Scholar]
- Borowsky B, Durkin MM, Ogozalek K, Marzabadi MR, DeLeon J, Lagu B, Heurich R, Lichtblau H, Shaposhnik Z, Daniewska I, Blackburn TP, Branchek TA, Gerald C, Vaysse PJ, Forray C. Antidepressant, anxiolytic and anorectic effects of a melanin-concentrating hormone-1 receptor antagonist. Nat. Med. 2002;8:825–830. doi: 10.1038/nm741. [DOI] [PubMed] [Google Scholar]
- Chaki S, Funakoshi T, Hirota-Okuno S, Nishiguchi M, Shimazaki T, Iijima M, Grottick AJ, Kanuma K, Omodera K, Sekiguchi Y, Okuyama S, Tran TA, Semple G, Thomsen W. Anxiolytic- and antidepressant-like profile of ATC0065 and ATC0175: nonpeptidic and orally active melanin-concentrating hormone receptor 1 antagonists. J. Pharmacol. Exp. Ther. 2005;313:831–839. doi: 10.1124/jpet.104.081711. [DOI] [PubMed] [Google Scholar]
- Chung S, Hopf FW, Nagasaki H, Li CY, Belluzzi JD, Bonci A, Civelli O. The melanin-concentrating hormone system modulates cocaine reward. Proc. Natl. Acad. Sci. U.S.A. 2009;106:6772–6777. doi: 10.1073/pnas.0811331106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S, Parks GS, Lee C, Civelli O. Recent updates on the melanin-concentrating hormone (MCH) and its receptor system: lessons from MCH1R antagonists. J. Mol. Neurosci. 2011;43:115–121. doi: 10.1007/s12031-010-9411-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton SM, Vazquez DM, Kabbaj M, Kabbaj MH, Watson SJ, Akil H. Individual differences in novelty-seeking and emotional reactivity correlate with variation in maternal behavior. Horm. Behav. 2007;51:655–664. doi: 10.1016/j.yhbeh.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton S, Miller S, Watson SJ, Akil H. Prenatal stress does not alter innate novelty-seeking behavioral traits, but differentially affects individual differences in neuroendocrine stress responsivity. Psychoneuroendocrinology. 2008;33:162–177. doi: 10.1016/j.psyneuen.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton SM, Bedrosain TA, Abraham AD, Watson SJ, Akil H. Neural and environmental factors implicating maternal behavior differences in high- versus low-novelty-seeking rats. Horm. Behav. 2010;54:463–473. doi: 10.1016/j.yhbeh.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David DJ, Klemenhagen KC, Holick KA, Saxe MD, Mendez I, Santarelli L, Craig DA, Zhong H, Swanson CJ, Hegde LG, Ping XI, Dong D, Marzabadi MR, Gerald CP, Hen R. Efficacy of the MCHR1 antagonist N-[3-(1-{[4-(3,4-difluorophenoxy)phenyl]methyl}(4-piperidyl))-4-methylphenyl]-2-methylpropanamide (SNAP 94847) in mouse models of anxiety and depression following acute and chronic administration is independent of hippocampal neurogenesis. J. Pharmacol. Exp. Ther. 2007;321:237–248. doi: 10.1124/jpet.106.109678. [DOI] [PubMed] [Google Scholar]
- Garcia-Fuster MJ, Clinton SM, Watson SJ, Akil H. Effect of cocaine on Fas-associated protein with death domain in the rat brain: individual differences in a model of differential vulnerability to drug abuse. Neuropsychopharmacology. 2009;34:1123–1134. doi: 10.1038/npp.2008.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehlert DR, Rasmussen K, Shaw J, Li X, Ardayflo P, Craft L, Coskun T, Zhang HY, Chen Y, Witkin JM. Preclinical evaluation of melanin-concentrating hormone receptor 1 antagonism for the treatment of obesity and depression. J. Pharmacol. Exp. Ther. 2009;329:429–438. doi: 10.1124/jpet.108.143362. [DOI] [PubMed] [Google Scholar]
- Georgescu D, Sears RM, Hommel JD, Barrot M, Bolaños CA, Marsh DJ, Bednarek MA, Bibb JA, Maratos-Flier E, Nestler EJ, DiLeone RJ. The hypothalamic neuropeptide melanin-concentrating hormone acts in the nucleus accumbens to modulate feeding behavior and forced-swim performance. J. Neurosci. 2005;25:2933–2940. doi: 10.1523/JNEUROSCI.1714-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jama A, Cecchi M, Calvo N, Watson SJ, Akil H. Inter-individual differences in novelty-seeking behavior in rats predict differential responses to desipramine in the forced swim test. Psychopharmacology. 2008;198:333–340. doi: 10.1007/s00213-008-1126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabbaj M, Devine DP, Savage VR, Akil H. Neurobiological correlates of individual differences in novelty-seeking behavior in the rat: differential expression of stress-related molecules. J. Neurosci. 2000;20:6983–6988. doi: 10.1523/JNEUROSCI.20-18-06983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerman IA, Bernard R, Rosenthal D, Beals J, Akil H, Watson SJ. Distinct populations of presympathetic-premotor neurons express orexin or melanin-concentrating hormone in the rat lateral hypothalamus. J. Comp. Neurol. 2007;505:586–601. doi: 10.1002/cne.21511. [DOI] [PubMed] [Google Scholar]
- Lagos P, Urbanavicius J, Scorza MC, Miraballes R, Torterolo P. Depressive-like profile induced by MCH microinjections into the dorsal raphe nucleus evaluated in the forced swim test. Behav. Brain Res. 2011;218:259–266. doi: 10.1016/j.bbr.2010.10.035. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat. Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachoud B, Adamantidis A, Ravassard P, Luppi PH, Grisar T, Lakaye B, Salin PA. Major impairments of glutamatergic transmission and long-term synaptic plasticity in the hippocampus of mice lacking the melanin-concentrating hormone receptor-1. J. Neurophysiol. 2010;104:1417–1425. doi: 10.1152/jn.01052.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez JA, Clinton SM, Turner CA, Watson SJ, Akil H. A new role for FGF2 as an endogenous inhibitor of anxiety. J. Neurosci. 2009;29 doi: 10.1523/JNEUROSCI.4829-08.2009. 6379-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M, David NK, Danao JV, Baribault H, Tian H, Giorgetti M. Genetic inactivation of melanin-concentrating hormone receptor subtype 1 (MCHR1) in mice exerts anxiolytic-like behavioral effects. Neuropsychopharmacology. 2006;31:112–120. doi: 10.1038/sj.npp.1300805. [DOI] [PubMed] [Google Scholar]
- Roy M, David N, Cueva M, Giorgetti M. A study of the involvement of melanin-concentrating hormone receptor 1 (MCHR1) in murine models of depression. Biol. Psychiatry. 2007;61:174–180. doi: 10.1016/j.biopsych.2006.03.076. [DOI] [PubMed] [Google Scholar]
- Saito Y, Cheng M, Leslie FM, Civelli O. Expression of the melanin-concentrating hormone (MCH) receptor mRNA in the rat brain. J. Comp. Neurol. 2001;435:26–40. doi: 10.1002/cne.1191. [DOI] [PubMed] [Google Scholar]
- Stead JD, Clinton S, Neal C, Schneider J, Jama A, Miller S, Vazquez DM, Watson SJ, Akil H. Selective breeding for divergence in novelty-seeking traits: heritability and enrichment in spontaneous anxiety-related behaviors. Behav. Genet. 2006;36:697–712. doi: 10.1007/s10519-006-9058-7. [DOI] [PubMed] [Google Scholar]
- Stedenfeld KA, Clinton SM, Kerman IA, Akil H, Watson SJ, Sved AF. Novelty-seeking behavior predicts vulnerability in a rodent model of depression. Physiol. Behav. 2011;103:210–216. doi: 10.1016/j.physbeh.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CA, Clinton SM, Thompson RC, Watson SJ, Jr, Akil H. Fibroblast growth factor-2 (FGF2) augmentation early in life alters hippocampal development and rescues the anxiety phenotype in vulnerable animals. Proc. Natl. Acad. Sci. U.S.A. 2011;108:8021–8025. doi: 10.1073/pnas.1103732108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varas M, Perez M, Monzon ME, de Barioglio SR. Melanin-concentrating hormone, hippocampal nitric oxide levels and memory retention. Peptides. 2002;23:2213–2221. doi: 10.1016/s0196-9781(02)00252-8. [DOI] [PubMed] [Google Scholar]
- Villalobos J, Ferssiwi A. The differential ascending projections from the anterior, central and posterior regions of the lateral hypothalamic area: an autoradiographic study. Neurosci. Lett. 1987;81:89–94. doi: 10.1016/0304-3940(87)90345-4. [DOI] [PubMed] [Google Scholar]