Abstract
Background
Noninvasive methods are desirable for longitudinal studies examining drug efficacy and disease resolution defined as decreases in epidermal thickness in mouse models of psoriasiform skin disease. This would eliminate the need for either sacrificing animals or collecting serial skin biopsies to evaluate changes in disease progression during an individual study. Quantification of epidermal thickness using Optical Coherence Tomography (OCT) provides an alternative to traditional histology techniques.
Methods
Using the KC-Tie2 doxycycline-repressible psoriasiform skin disease mouse model, OCT imaging was completed on diseased back skin of adult KC-Tie2 (n=3-4) and control (n=3-4) mice, followed immediately by the surgical excision of the same region for histological analyses. Animals were then treated with doxycycline to suppress transgene expression and reverse the skin disease and additional OCT images and tissues were collected 2 and 4 weeks following. Epidermal thickness was measured using OCT and histology.
Results
OCT and histology both demonstrated KC-Tie2 mice had significantly thicker epidermis (~4-fold; p<0.0001) than control animals. By two weeks following gene repression, decreases in epidermal thickness were observed using both OCT and histology, and were sustained through 4 weeks. Correlation analyses between histology and OCT values at all time points and in all animals revealed high significance (R2=0.78); with correlation being highest in KC-Tie2 mice (R2=0.92) compared to control animals (R2=0.16).
Conclusion
Noninvasive OCT imaging provided similar values as those collected using standard histological measures in thick skin of KC-Tie2 mice but became less reliable in thinner control mouse skin, possibly reflecting limitations in resolution of OCT. Future advances in resolution of OCT may improve and allow greater accuracy of epidermal thickness measurements.
Introduction
Psoriasis is a complex, multifaceted skin disease affecting ~2% of the population and whose pathogenesis has not been fully elucidated. The generation and study of murine models of psoriasis have provided preclinical insight into disease mechanisms (1) and in vivo avenues for testing new therapeutics (2-4). However, evaluation of skin disease severity and its longitudinal resolution during and after treatment is almost always determined using histological evaluation of acanthosis. This requires either multiple skin tissue biopsies throughout the duration of the treatment regime or animal sacrifice at various time points and the use of additional animals with a non-longitudinal experimental design. A reproducible, non-invasive method for measuring epidermal thickness would reduce the number of sacrificed animals and provide a method of quantifying disease severity through real-time images of affected skin.
Non-invasive techniques have gained significant interest for dermatological uses in recent years (5-9). Optical coherence tomography (OCT) is one such technique capable of generating 2D images of tissue microstructure similar to cross-sectional histological images (10). The mechanism of OCT is similar in principle to ultrasound but infrared light is used instead of sound waves, and echo time is measured using interferometry (11). In-depth measurements are recorded while a light beam is scanned along a transverse distance, and this two-dimensional array of data represents a cross section of the sample, which can be processed and displayed as a 2D gray-scale image.
OCT provides exceptional resolution over other conventional ultrasound imaging, magnetic resonance imaging or computed tomography and can be done in real time to a depth of 1-2 mm typically in opaque tissues (12). Originally pioneered for use as a retinal scanning technique, OCT has been studied in human dermatological applications, including high resolution images of skin structure and collagen content in tissues and blood flow (7, 9). The epidermal and dermal layers, as well as hair follicles and sweat ducts, are distinguishable by OCT, and the demarcation between the epidermis and dermis is evident in most images (13, 14). It has also been documented that inflammatory skin conditions in humans, such as psoriasis and dermatitis, can be analyzed for morphological changes including blistering, inflammation and edema using OCT and these outcomes may be correlated with histology (15).
We have previously described a psoriasiform murine model in which Tie2 is overexpressed in keratinocytes (KCs), resulting in a chronic inflammatory skin disease phenotype containing an abundance of infiltrating macrophages, dendritic cells, and T cells and containing a significantly thicker epidermis (16) This model demonstrated resolution of the skin phenotype within a four week period following gene repression or systemic CsA treatment (16), TNFα inhibition or antigen cell depletion (17) and following cutaneous nerve injury (18). In these experiments, drug treatment efficacy and/or skin disease improvement was evaluated either by longitudinal pre- vs post-treatment tissue collection and analyses (18) or was examined in a non-longitudinal manner (16, 17), requiring the invasive collection of skin biopsies. We now report a reproducible, longitudinal, non-invasive real-time method for measuring epidermal thickness using OCT in mice across a 4 week treatment period and demonstrate high correlation with histological measures.
Experimental Design and Methods
Animals
The KC-specific (K5-tTA) driver line and the TetosTek/Tie2 responder line have been described previously (16, 19, 20). Animal husbandry was performed between the K5tTA line and the TetosTek/Tie2 line and offspring were genotyped using PCR and DNA extracted from ear biopsies as previously described (16). We have previously published that animals inheriting a single copy of each gene (K5tTA and TetosTek/Tie2 ; called double- or bi-transgenic KC-Tie2 mice) have ~50-fold increase in Tie2 mRNA and spontaneously develop a psoriasiform skin phenotype by 8 weeks of age (16). Littermates inheriting one or no transgenes do not express protein derived from either transgene and therefore serve as experimental controls.
Adult control and KC-Tie2 mice (n=3-4) were anesthetized using Avertin® (250mg/kg); prior to imaging animals were shaved and remaining hair removed with a depilatory (Nair, Church & Dwight Co. Inc., Princeton, New Jersey). Pre-treatment OCT images were captured from the midline dorsal surface of each mouse; following which a 4mm punch biopsy of the same region was harvested. Animals were then placed on doxycycline food (200mg/kg rodent chow; BioServ, NJ, USA) to repress gene expression and two and four weeks following, additional OCT images of skin adjacent to the original site were captured and punch biopsies of the same skin collected.
All animal protocols were approved by the Case Western Reserve University institutional animal care and use committee (IACUC) and conformed to the American Association for Accreditation of Laboratory Animal Care guidelines.
OCT Image Capture and Epidermal Thickness Measurement
Pre and post-doxycycline OCT images were collected for each mouse over the four week period. At each image capture location a small amount of glycerin was applied to the skin, and the area was covered with a glass coverslip to minimize the strong scattering from the skin surface. All images were acquired at 10 frames per second using a bench-top spectral domain OCT system in the Biomedical Engineering Department at Case Western Reserve University, which provides an axial resolution of ~ 6 μm and a lateral resolution of ~ 5 μm using a broad-band light source centered at 0.84 μm. The light intensity on the tissue was approximately 1 mW.
Epidermal thickness was measured on the captured images using Image Pro Plus Interactive Software (MediaCybernetics, Bethesda, MD). The spatial calibration tool was used to calibrate the measurement tool according to the following picture dimensions: Lateral = 4μm/pixel in X direction (4.0mm/1000 pixels) and Axial = 1.367μm/pixel in Y direction (2.8mm/2048 pixels, corrected for index of refraction (value=1.38)(21)). Epidermal thickness on OCT images was calculated by drawing a line from the skin surface reflection (entrance signal/echo) to the first well-demarcated change of reflectivity with clear signal-poor zones, as previously described (7, 22). Four line measurements were collected per image and the average thickness of each image was calculated.
Histological Processing and Epidermal Thickness Measurement
Skin biopsies were placed in 10% buffered formalin (Surgipath Medical Industries, Richmond, IL) overnight at 4°C prior to dehydration and embedding (Sakura Finetech, Torrance, CA). H&E staining was completed on 5μm thick paraffin sections using standard protocols. For each mouse, epidermal thickness was quantified using approaches similar to those described above and as described previously (16-18, 23).
Statistics
All data are represented as mean ± standard error of the mean (SEM). Between group comparisons were analyzed using a Student’s T test and Pearson Correlation Coefficients (R) and the coefficient of determination (R2) are reported.
Results
OCT and histological measures of the epidermis of untreated adult mouse skin revealed significant increases in the thickness in KC-Tie2 mouse skin (70.92 ± 3.2μm OCT; 61.32 ± 2.2μm histology) compared to littermate controls (33.09 ± 9.6μm OCT; 15.97 ± 1.1μm; p=0.00097 for OCT and p<0.0001 for histology; Figures 1 and 2E) confirming previous reports (16-18).
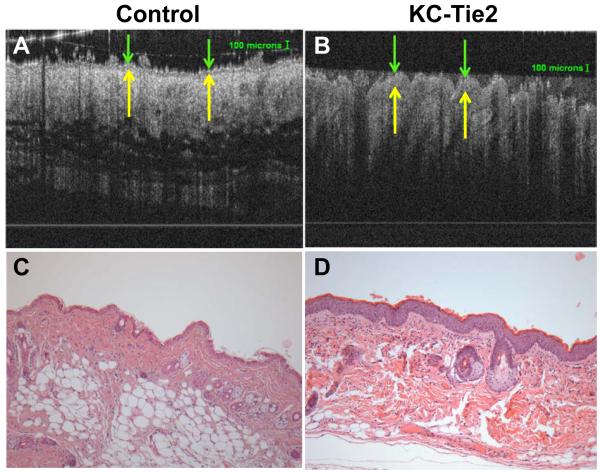
Figure 1. Noninvasive OCT imaging and standard histological methods can be used to measure epidermal thickness in control and KC-Tie2 mouse skin.
Representative OCT images (A-B) and H&E stained skin (C-D) taken from the same dorsal skin region of control (A, C) and KC-Tie2 (B,D) mice. Green arrows indicate the apical surface of the skin and yellow arrows demarcate the dermo-epidermal junction.
Figure 2. OCT image analyses and histology validation identify significant increases in the thickness of the epidermis in KC-Tie2 mice which decreases two weeks following doxycycline treatment.
Representative images taken with histology (A-B) and OCT (C-D) of KC-Tie2 mouse skin prior to beginning treatment (A, C) and 4 weeks following doxycycline treatment (B, D). Epidermal thickness measures using OCT and histology confirm untreated KC-Tie2 mice have significantly thicker epidermis than control mice (E). Following two weeks of doxycycline treatment, the increase in epidermal thickness is reversed as measured using OCT and is significantly decreased as measured histologically. Four weeks of doxycycline returns all epidermal thickness measures to similar levels in control and KC-Tie2 mice regardless of imaging modality. * p<0.05 compared to control animals.
We have previously reported the successful reversal of skin disease, as measured by epidermal thickness, 4 weeks following gene repression using dietary doxycycline supplementation (16). We now demonstrate that two weeks following doxycycline exposure, KC-Tie2 mouse skin was significantly thinner than pre-treatmentlevels using OCT (23.24 ± 2.1μm, p<0.0001) and standard histological approaches (17.84 ± 1.3μm, p<0.0001), and returned to control mouse levels as demonstrated using OCT image analyses of epidermal thickness (p=0.22). However, histological examination demonstrated that the epidermal thickness remained modestly increased in KC-Tie2 mice compared to control animals following two weeks of doxycycline treatment (17.84 ± 1.3μm KC-Tie2 vs 12.78 ± 0.9μm control; p=0.048) (Figure 2E). Four weeks of doxycycline supplementation returned all epidermal thickness measures to similar levels for both KC-Tie2 and control mice using both OCT (19.58 ± 0.7μm KC-Tie2 vs 17.54 ± 0.9μm controls; p=0.19) and histology (18.8 ± 0.5μm KC-Tie2 vs 17.8 ± 0.5μm controls; p=0.28) approaches (Figure 2A-E).
Correlation analyses using all histology and OCT measures obtained for control and KC-Tie2 animals across the longitudinal study revealed significant correlation amongst OCT and histological measures (R = 0.8827, p< 0.0001 (t=7.968, DF=18); Figure 3A). Additional analyses based upon mouse strain revealed highly significant correlations in KC-Tie2 animals across all time points (Figure 3B; R = 0.9593, p < 0.0003 (t = 9.61, DF = 8)), however, OCT measures of epidermal thickness in control mice were more variable (evidenced by larger error bar; Figure 2E) and this finding was also reflected in the correlation analyses (Figure 3C; R = 0.4205, p = 0.2276 (t = 1.311, DF = 8)).
Figure 3. Correlation analyses reveal strong correlation between OCT and histology measures.
Epidermal thickness measured using OCT and histology across (A) all groups of mice and all treatment groups demonstrates strong correlation. (B) KC-Tie2 animal data alone shows stronger correlation between the measures, however (C) control mouse levels show significantly more variability decreasing correlative strength perhaps reflecting limitations in resolution for measuring thin skin. R2 values as indicated.
Conclusions
The ability to successfully measure epidermal thickness in a longitudinal, non-invasive real-time manner in murine skin would provide a novel innovative tool for examining the efficacy of drug therapies in mouse models of inflammatory skin disease. Here, we demonstrate using KC-Tie2 animals and doxycycline gene repression that OCT can be used successfully to monitor improvements in skin disease, as measured by epidermal thickness in the same animals across a 4 week period, and that the OCT values correlate with those obtained using standard histological analyses.
Our findings are consistent with previous work (7, 24-26) demonstrating a correlation between epidermal thickness measurements by OCT and histology in human skin although conclusions from several studies suggested that OCT and histology could not be used interchangeably (24, 25), perhaps reflecting differences between in vivo tissue evaluated using OCT and ex vivo tissue evaluated by histology, which is invariably altered somewhat upon formalin fixation and paraffin embedding. Our results suggest that this is not necessarily recapitulated in mouse skin, perhaps reflective of human skin being significantly thicker than murine skin, such that processing and dehydration issues in mouse skin (which is significantly thinner) have less effect on histological outcomes. Work by the same group has recently revealed more similar and interchangeable outcomes between OCT and histology when epidermal thickness histology values are measured using fresh frozen skin sectioned on a cryostat, suggesting this method provides a more similar preservation of relative and absolute dimensions of skin layers than that obtained following routine paraffin embedding (26).
The ability of OCT to resolve the structures within the skin and allow measurement of epidermal thickness makes it an attractive non-invasive technique for measurement of epidermal thickness (13). Although the thinness of the murine epidermis may have led to better correlation with OCT for formalin fixed paraffin embedded skin compared to similar human skin, the thinness of the epidermis in control animals (only 1-2 cell layers thick, and very near the resolution limit of the OCT system) most likely also contributes to the lack of strong correlation between the histological and OCT measurements in control animals. This could be reflective of “noise” resulting from being near the resolution limit of the OCT system in addition to potential difficulty in identifying the dermo-epidermal junction when only two cell layers separate the two skin layers. Even in thicker human psoriasis skin, definitive identification of the dermo-epidermal junction can be challenging because neither the skin nor the dermo-epidermal junction are flat and there is marked papillomatosis, particularly in psoriasis (15, 27, 28). Moreover, manual image capture and quantitation of the thickness (as performed in our studies) can be time consuming and vulnerable to inter- and intra-observer variability (28) Therefore, measurements of epidermal thickness are difficult, not withstanding limitations based on the technology itself. For this study, we used a fixed station OCT system designed by the Department of Biomedical Engineering at Case Western Reserve University. The necessity to manipulate the animal under the scanner made image capture challenging and potentially increased variability in our data, especially in the thin-skinned control mice and may explain the weakness of the correlation with histological thickness in the these animals.
An additional means for analyses outside of those used in the current study could also increase sensitivity and standardization. Such that, multiple methods for measuring epidermal thickness using OCT have been utilized including averaged A-scan analysis, peak-to-peak measurements, manual B-scan analysis, and more recent reports utilizing the shapelet-based image processing technique which requires no manual measurements (7, 24, 28). However, it has been argued that the second intensity peak seen in the A-scan method does not correspond to the dermo-epidermal junction but rather to fibrous structures in the upper dermis, which could potentially account for lack of agreement between histology and OCT measurements (22, 24, 27). Additional studies using these algorithms may offer a more standardized approach to the measurement of epidermal thickness as perhaps would utilizing an automated, or semi-automated computer-aided image analysis that would provide the advantage of reducing variability caused by the human observer.
Work by Zulfakar et al. (29) recently demonstrated a correlation between epidermal thickness measurements using OCT and histology in the GsdmA3Dfl/+ mouse, whose skin phenotype is characterized by inflammation mediated hair loss, keratinocyte hyperproliferation and epidermal thickening. In this study, changes in epidermal thickness were reported using both OCT (operating at 1325 nm) and histology following 10 days of treatment with multiple topical treatments, including betamethasone dipropionate, salicylic acid and fish oil. Similar and overlapping values were presented, although correlation analyses were not. Our findings are consistent with these, and provide additional correlative evidence demonstrating the efficacy of OCT for measuring both longitudinally and non-invasively the changes in skin disease severity, using epidermal thickness as the primary outcome, in murine models of inflammatory hyperplastic skin disease.
In conclusion, although histology remains the gold standard for measuring epidermal thickness, and as yet no proposal to replace such a technique with OCT has been made (13), our data provide additional evidence that noninvasive OCT image analyses can provide a reliable method for measuring epidermal thickness without the need for invasive and skin damaging biopsies or using multiple groups of animals for non-longitudinal experiments or analyses of multiple individual time points. Although several limitations of the current project were identified, improved technology should lead to the development of better resolution, greater discernability of skin structures, and decreased measurement variability, ultimately providing more sensitive and reliable methods for use in the future.
Acknowledgements
This work was supported in part by grants from the National Institutes of Health (P30AR39750 and P50AR05508 to NLW and R01CA114276 to AMR) and the Murdough Family Center for Psoriasis.
References Cited
- 1.Gudjonsson JE, Johnston A, Dyson M, Valdimarsson H, Elder JT. Mouse models of psoriasis. J Invest Dermatol. 2007;127(6):1292–308. doi: 10.1038/sj.jid.5700807. [DOI] [PubMed] [Google Scholar]
- 2.Gilhar A, Bergman R, Assay B, Ullmann Y, Etzioni A. The Beneficial Effect of Blocking Kv1.3 in the Psoriasiform SCID Mouse Model. J Invest Dermatol. 131(1):118–24. doi: 10.1038/jid.2010.245. [DOI] [PubMed] [Google Scholar]
- 3.Miyoshi K, Takaishi M, Nakajima K, Ikeda M, Kanda T, Tarutani M, et al. Stat3 as a Therapeutic Target for the Treatment of Psoriasis: A Clinical Feasibility Study with STA-21, a Stat3 Inhibitor. J Invest Dermatol. 131(1):108–17. doi: 10.1038/jid.2010.255. [DOI] [PubMed] [Google Scholar]
- 4.Abe R, Yamagishi S-i, Fujita Y, Hoshina D, Sasaki M, Nakamura K, et al. Topical application of anti-angiogenic peptides based on pigment epithelium-derived factor can improve psoriasis. Journal of Dermatological Science. 2010;57(3):183–91. doi: 10.1016/j.jdermsci.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 5.Patel JK, Konda S, Perez OA, Amini S, Elgart G, Berman B. Newer technologies/techniques and tools in the diagnosis of melanoma. Eur J Dermatol. 2008;18(6):617–31. doi: 10.1684/ejd.2008.0508. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez S, Gilaberte-Calzada Y. In vivo reflectance-mode confocal microscopy in clinical dermatology and cosmetology. Int J Cosmet Sci. 2008;30(1):1–17. doi: 10.1111/j.1468-2494.2008.00406.x. [DOI] [PubMed] [Google Scholar]
- 7.Gambichler T, Moussa G, Sand M, Sand D, Altmeyer P, Hoffmann K. Applications of optical coherence tomography in dermatology. J Dermatol Sci. 2005;40(2):85–94. doi: 10.1016/j.jdermsci.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Rallan D, Harland CC. Ultrasound in dermatology--basic principles and applications. Clin Exp Dermatol. 2003;28(6):632–8. doi: 10.1046/j.1365-2230.2003.01405.x. [DOI] [PubMed] [Google Scholar]
- 9.Kollias N, Stamatas GN. Optical non-invasive approaches to diagnosis of skin diseases. J Investig Dermatol Symp Proc. 2002;7(1):64–75. doi: 10.1046/j.1523-1747.2002.19635.x. [DOI] [PubMed] [Google Scholar]
- 10.Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, et al. Optical coherence tomography. Science. 1991;254(5035):1178–81. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zysk AM, Nguyen FT, Oldenburg AL, Marks DL, Boppart SA. Optical coherence tomography: a review of clinical development from bench to bedside. J Biomed Opt. 2007;12(5):051403. doi: 10.1117/1.2793736. [DOI] [PubMed] [Google Scholar]
- 12.Fujimoto JG. Optical coherence tomography for ultrahigh resolution in vivo imaging. Nat Biotechnol. 2003;21(11):1361–7. doi: 10.1038/nbt892. [DOI] [PubMed] [Google Scholar]
- 13.Pierce MC, Strasswimmer J, Park BH, Cense B, de Boer JF. Advances in optical coherence tomography imaging for dermatology. J Invest Dermatol. 2004;123(3):458–63. doi: 10.1111/j.0022-202X.2004.23404.x. [DOI] [PubMed] [Google Scholar]
- 14.Morsy H, Kamp S, Thrane L, Behrendt N, Saunder B, Zayan H, et al. Optical coherence tomography imaging of psoriasis vulgaris: correlation with histology and disease severity. Arch Dermatol Res. 2010;302(2):105–11. doi: 10.1007/s00403-009-1000-4. [DOI] [PubMed] [Google Scholar]
- 15.Welzel J, Bruhns M, Wolff HH. Optical coherence tomography in contact dermatitis and psoriasis. Arch Dermatol Res. 2003;295(2):50–5. doi: 10.1007/s00403-003-0390-y. [DOI] [PubMed] [Google Scholar]
- 16.Wolfram JA, Diaconu D, Hatala DA, Rastegar J, Knutsen DA, Lowther A, et al. Keratinocyte but not endothelial cell specific overexpression of Tie2 leads to the development of psoriasis. Am J Pathol. 2009;174:1443–58. doi: 10.2353/ajpath.2009.080858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ward NL, Loyd CM, Wolfram JA, Diaconu D, Michaels CM, McCormick TS. Depletion of antigen presenting cells by clodronate liposomes reverses the psoriatic skin phenotype in KC-Tie2 mice. British Journal of Dermatology. 2010 doi: 10.1111/j.1365-2133.2010.10129.x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ostrowski SM, Belkadi A, Loyd CM, Diaconu D, Ward NL. Cutaneous denervation of psoriasiform mouse skin improves acanthosis and inflammation in a substance P and CGRP dependent manner. Journal of Investigative Dermatology. 2011 doi: 10.1038/jid.2011.60. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones N, Voskas D, Master Z, Sarao R, Jones J, Dumont DJ. Rescue of the early vascular defects in Tek/Tie2 null mice reveals an essential survival function. EMBO Rep. 2001;2(5):438–45. doi: 10.1093/embo-reports/kve093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diamond I, Owolabi T, Marco M, Lam C, Glick A. Conditional gene expression in the epidermis of transgenic mice using the tetracycline-regulated transactivators tTA and rTA linked to the keratin 5 promoter. J Invest Dermatol. 2000;115(5):788–94. doi: 10.1046/j.1523-1747.2000.00144.x. [DOI] [PubMed] [Google Scholar]
- 21.Tearney GJ, Brezinski ME, Southern JF, Bouma BE, Hee MR, Fujimoto JG. Determination of the refractive index of highly scattering human tissue by optical coherence tomography. Opt Lett. 1995;20(21):2258. doi: 10.1364/ol.20.002258. [DOI] [PubMed] [Google Scholar]
- 22.Neerken S, Lucassen GW, Bisschop MA, Lenderink E, Nuijs TA. Characterization of age-related effects in human skin: A comparative study that applies confocal laser scanning microscopy and optical coherence tomography. J Biomed Opt. 2004;9(2):274–81. doi: 10.1117/1.1645795. [DOI] [PubMed] [Google Scholar]
- 23.Ward NL, Hatala DA, Wolfram JA, Knutsen DA, Loyd CM. Cutaneous manipulation of vascular growth factors leads to alterations in immunocytes, blood vessels and nerves: Evidence for a cutaneous neurovascular unit. Journal of Dermatological Science. 2011;61(1):14–22. doi: 10.1016/j.jdermsci.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gambichler T, Boms S, Stucker M, Kreuter A, Moussa G, Sand M, et al. Epidermal thickness assessed by optical coherence tomography and routine histology: preliminary results of method comparison. J Eur Acad Dermatol Venereol. 2006;20(7):791–5. doi: 10.1111/j.1468-3083.2006.01629.x. [DOI] [PubMed] [Google Scholar]
- 25.Gambichler T, Boms S, Stucker M, Kreuter A, Sand M, Moussa G, et al. Comparison of histometric data obtained by optical coherence tomography and routine histology. J Biomed Opt. 2005;10(4):44008. doi: 10.1117/1.2039086. [DOI] [PubMed] [Google Scholar]
- 26.Gambichler T, Moussa G, Regeniter P, Kasseck C, Hofmann MR, Bechara FG, et al. Validation of optical coherence tomography in vivo using cryostat histology. Phys Med Biol. 2007;52(5):N75–85. doi: 10.1088/0031-9155/52/5/N01. [DOI] [PubMed] [Google Scholar]
- 27.Welzel J. Optical coherence tomography in dermatology: a review. Skin Res Technol. 2001;7(1):1–9. doi: 10.1034/j.1600-0846.2001.007001001.x. [DOI] [PubMed] [Google Scholar]
- 28.Weissman J, Hancewicz T, Kaplan P. Optical coherence tomography of skin for measurement of epidermal thickness by shapelet-based image analysis. Opt Express. 2004;12(23):5760–9. doi: 10.1364/opex.12.005760. [DOI] [PubMed] [Google Scholar]
- 29.Zulfakar MH, Alex A, Povazay B, Drexler W, Thomas CP, Porter RM, et al. In vivo response of GsdmA3Dfl/+ mice to topically applied anti-psoriatic agents: effects on epidermal thickness, as determined by optical coherence tomography and H&E staining. Exp Dermatol. 2011;20(3):269–72. doi: 10.1111/j.1600-0625.2010.01233.x. [DOI] [PubMed] [Google Scholar]