Abstract
Interferon-α2b (IFN-α2b) is used to treat melanoma but there is a need to improve its efficacy. IFN-α2b signaling requires STAT1/STAT2 tyrosine phosphorylation and is subject to negative regulation by phosphatases. In this study, we determined whether inhibition of the protein tyrosine phosphatase Shp2 could enhance IFN-α2b responses in human melanoma cells. Shp2 knockdown increased IFN-α2b-stimulated STAT1 Tyr-701 phosphorylation and ISRE-luciferase activity even though it did not affect STAT2 Tyr-690 phosphorylation in A375 cells. In A375 tumor xenografts, Shp2 knockdown enhanced the anti-melanoma effect of IFN-α2b. Furthermore, the Shp2 inhibitor SPI-112Me increased the IFN-α2b-induced STAT1 activation and anti-proliferative response in A375 and SK-MEL-2 cells. These results demonstrate that inhibition of Shp2 can enhance the anti-melanoma activity of IFN-α2b.
Keywords: Shp2, STAT1, Melanoma, Interferon
1. Introduction
Most patients with metastatic melanoma do not respond to therapy [1]. These patients have a median survival of 6–9 months. For patients with resectable melanoma but that are at high risk of relapse after surgery, preventing recurrence of the disease is a strategy to increase the survival of these patients. Interferon α (IFN-α) is used in adjuvant therapy for high risk melanoma patients after surgery. Clinical benefits of IFN-α therapy includes increase in the disease-free survival by ~9 months and the 5-year survival by 8–9% [2,3]. Therefore, the effects of IFN-α therapy are modest, and much is needed to improve the efficacy of IFN-α.
IFN-α has a direct anti-proliferative effect on melanoma cells and also modulates the immune response. Compared to immune cells, melanoma cells are less responsive to IFN-α [4]. IFN-α exerts its effects mainly through the Tyk2/Jak1-STAT1/STAT2 pathway to activate transcription from the IFN-stimulated response element (ISRE) [5].
Shp2 is a protein tyrosine phosphatase (PTP) [6] that positively regulates the Ras- Erk1/Erk2 (Erk1/2) pathway [7,8]. Gain-of-function Shp2 mutants have been linked to several types of human cancer, including leukemia [6,9,10] and melanoma [11], whereas the wildtype Shp2 is activated by protein tyrosine kinases and could play a crucial role in tumor growth of some cancer cells [12]. These findings have stimulated interest in developing Shp2 PTP inhibitors as novel anti-cancer drugs [13,14].
Shp2 was found to dephosphorylate STAT1 in EGF- and IFN-γ-stimulated A431 cells, but had no effect on STAT3 and STAT5 [15]. Signaling by IFN-α in melanoma involves not only STAT1 but also STAT2. It remains to be analyzed if Shp2 affects STAT2 tyrosine phosphorylation and if Shp2 inhibition is sufficient to augment the IFN-α responses in melanoma. In a previous study [16], it was shown that sodium stibogluconate synergizes with IFN-α in inhibiting proliferation of several cultured cancer cell lines and has anti-tumor activity against WM9 melanoma cells in a xenograft assay. Sodium stibogluconate was reported as a Shp1 PTP inhibitor that also inhibits Shp2 and PTP1B with ~10-fold less potency [17]. It is predicted that sodium stibogluconate may also cross inhibit other PTPs. Thus, it is not clear if Shp2 inhibition contributes or is sufficient to exert an enhanced anti-melanoma effect in combination with IFN-α. In this study, we assessed the effects of Shp2 inhibition on IFN-α2b responses in human melanoma cells. Our data show that suppression of Shp2 enhances the anti-tumor activity of IFN-α2b in A375 tumor xenografts grown in immune deficient mice.
2. Materials and Methods
2.1. Cells and reagents
A375 cells were cultured in DMEM/5% fetal bovine serum (FBS) at 37 °C and 5% CO2. Cells were infected with lentiviruses containing doxycycline (dox)-inducible Shp2 shRNAs R0946 and R1049 [12]. Puromycin-resistant cell colonies were isolated and screened by Shp2 immunoblotting to select A375/R0946 and A375/R1049 cells that displayed dox-inducible Shp2 knockdown. Authentication of A375 human melanoma cells by short tandem repeat (STR) profiling using the AmpF/STR Identifier method was performed through a commercial service (Biosynthesis, Lewisville, TX). The STR DNA profile of A375 cells used in our study matches that of the A375 cell line in the American Type Culture Collection (Manassas, VA). SK-MEL-2 cells were obtained from the laboratory of Dr. Srikumar Chellappan [18] and cultured in the same conditions as A375 cells.
IFN-α2b (INTRON A) was from Schering-Plough (Kenilworth, NJ). SPI-112Me was synthesized as reported [19]. Antibodies to Shp2, STAT1, STAT2, Erk1 and Erk2 (Erk1/2), phospho-Erk1/2 (pErk1/2), and β-actin were from Santa Cruz Biotechnology (Santa Cruz, CA). STAT1-pY701 (pSTAT1) and pSTAT2 antibodies were from Cell Signaling Technology (Danvers, MA).
2.2. Immunoblotting
Cells and tumor tissues were lysed with lysis buffer (50 mM Tis-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 25 mM NaF, 5 mM Na4P2O7, 1 mM dithiothreitol, 1 mM Na3VO4, 100 µg/ml of phenylmethylsulfonyl fluoride, 2 µg/ml leupeptin, 2 µg/ml aprotinin, and 1% Triton X-100). Cleared cell lysates were obtained by centrifugation at 4 °C with a microfuge. Equal amounts of cell lysate protein were analyzed by immunoblotting similar to that described previously [8,12,19].
2.3. Luciferase reporter assay
Cells were plated in 12-well plates (2 × 105 cells/well) for 24 h and transfected with 1.5 µg/ml pISRE-Luc (Clontech, Mountain View, CA) and 0.2 µg/ml β-gal plasmids using the LipofectAMINE 2000 reagent (Invitrogen). After incubation for 6 h, the medium was replaced with fresh DMEM/5%FBS and cultured overnight. For Shp2 knockdown, cells were used 4 days after dox-induction. For SPI-112Me treatment, cells were pretreated with SPI-112Me (12 µM) for 2 h. IFN-α2b treatment was at 1,000 IU/ml for 5 h. Luciferase and β-galactosidase activities were determined as described previously [19]. The luciferase activity was normalized with β-galactosidase activity as an internal control for transfection efficiency.
2.4. Cell proliferation assay
Cell proliferation were measured similar to that described previously [19,20]. Essentially, cells (1,000 cells/well) were plated in clear-bottom, black 96-well plates and incubated with dox, IFN-α2b, and/or SPI-112Me as specified in the figure legends. Relative viable cell number was determined using the Celltiter-Glo reagent (Promega, Madison, WI).
2.5. Tumor growth assay
NCr nu/nu mice (female, ~5 weeks old) were obtained from Charles River (Wilmington, MA). Each mouse received subcutaneous (s.c.) injections of 1 × 106 cells in 0.1 ml, one on each flank. Tumor sizes were measured with a caliper. Tumor volume was calculated using the formula: 0.5 × length × width2. Mice bearing tumors of similar sizes were selected for experiments (6 mice with a total of 12 tumors/group). Dox induction was achieved by replacing the regular diet (irradiated Teklad global rodent diet #2918, Harlan, Tampa, FL) with dox diet (200 mg/kg doxycycline, Bio-Serv, Frenchtown, NJ). IFN-α2b (4 × 104 IU in 200 µl) was given daily by s.c. injection. Some of the tumors were collected post-mortem for analysis.
2.6. Statistical analysis
Statistical analyses were performed using t-test with 95% confidence intervals. A difference in means with p ≤ 0.05 was considered statistically significant.
3. Results
3.1. Shp2 knockdown enhances IFN-α2b-stimulated STAT1 activation and inhibits Erk activation in A375 melanoma cells
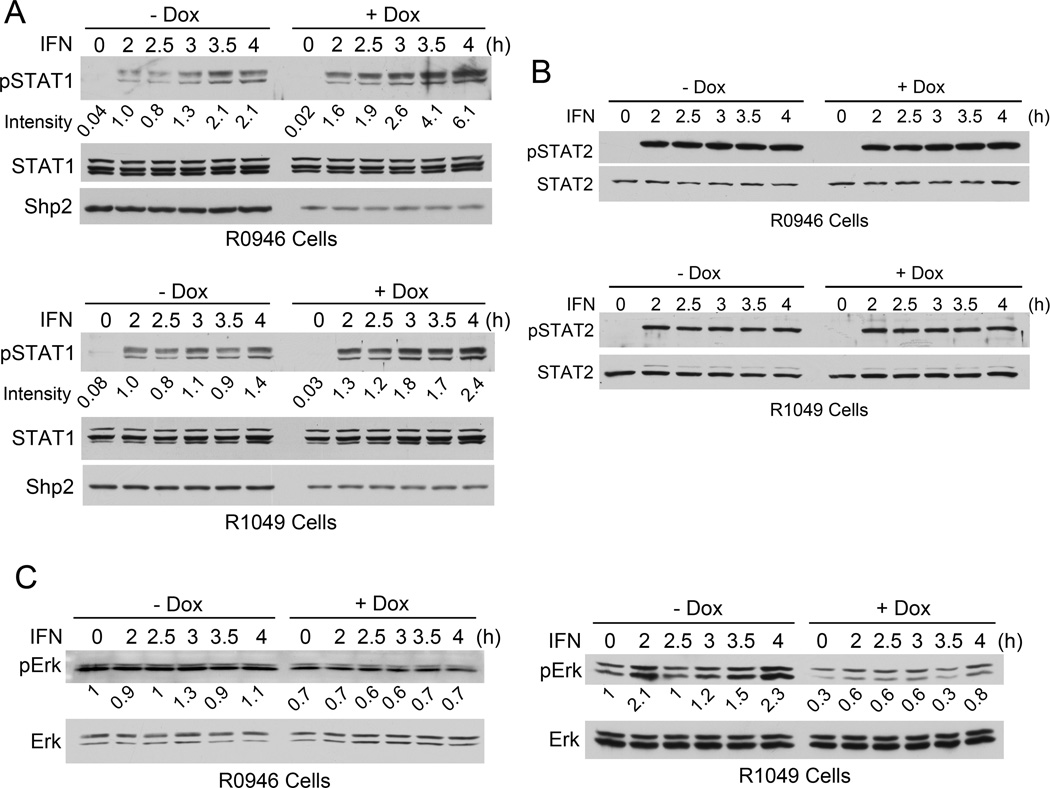
A375 cells display a modest response to IFN-α [4], suggesting that the IFN-α-stimulated Jak-STAT pathway in these cells is not defective but may be subject to negative regulation. Using pTRIPZ-based lentiviruses that contain two different dox-inducible Shp2 shRNAs R0926 and R1049 [20], we established stable A375-derived cell lines A375/R0946 and A375/R1049. Incubation of cells with dox (1–3 µg/ml) reduced the Shp2 protein by 68% in A375/R0946 cells and 42% in A375/R1049 cells (Fig. 1A). pSTAT1 was not detectable prior to IFN stimulation in these cells and was induced by IFN-α2b (1,000 IU/ml) (Fig. 1A). Shp2 knockdown alone did not induce pSTAT1. When stimulated with IFN-α2b, Shp2 knockdown cells consistently exhibited higher levels of pSTAT1 compared to their isogenic cells (Fig. 1A). In comparison, Shp2 knockdown did not affect IFN-α2b-induced STAT2 tyrosine phosphorylation (Fig. 1B).
Fig. 1.
Shp2 knockdown enhances IFN-α2b-induced STAT1 Tyr-701 phosphorylation. A375/R0946 and A375/R1049 cells were cultured with or without dox for 6 days and stimulated with IFN (1,000 IU/ml) for the indicated times. Cell lysates were analyzed by immunoblotting to assess phosphorylation of STAT1 Tyr-701 (A), STAT2 Tyr-690 (B), and the activating, dual phosphorylation sites of Erk1/2 (C).
A375 cells have a high basal level of active Erk1/2 (Fig. 1C), possibly due to BRAF V600E mutation. IFN-α2b (1,000 IU/ml) did not consistently activate Erk1/2 in these cells. Nevertheless, Shp2 knockdown reduced the pErk1/2 level in both unstimulated and IFN-α2b-stimulated cells (Fig. 1C). Thus, Shp2 knockdown inhibits Erk1/2 activation in A375 cells.
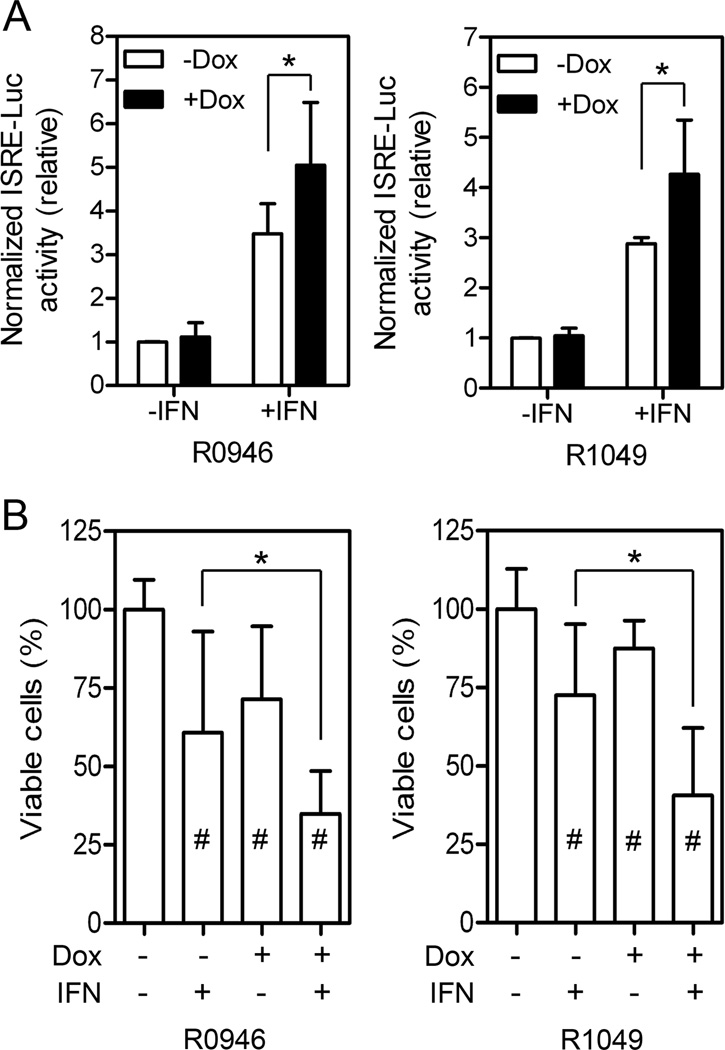
To determine if the increased STAT1 tyrosine phosphorylation resulted in higher IFN-α2b-stimulated ISRE-directed transcription activity, the ISRE luciferase report activity was measured. IFN-α2b activated the ISRE-Luc activity by 3.5- and 2.9-fold in A375/R0946 and A375/R1049 cells, respectively (Fig. 2A). When Shp2 was knocked down in these cells, the IFN-α2b-induced ISRE-Luc activity was further increased significantly to 5.1- and 4.3-fold, respectively.
Fig. 2.
Shp2 knockdown enhances IFN-α2b-induced ISRE-Luc reporter activity and growth inhibition. (A) ISRE-Luc reporter activity was measured as described in Materials and Methods. (B) Cells were cultured in 96-wells in medium with or without dox (3 µg/ml) and IFN-α2b (1,000 IU/ml) as indicated. Relative viable cell numbers were measured on Day 6. Data were from three independent experiments (n = 15). The mean ± SD are shown. *, p < 0.05. #, p <0.05 between the control (-Dox, -IFN) and the treated cells.
IFN-α2b treatment or Shp2 knockdown by dox induction resulted in modest but statistically significant inhibition of A375/R0946 and A375/R1049 cell proliferation (Fig. 2B). It was reported that dox could affect adhesion and migration of melanoma cell lines B16F10, WM35, and WM451 [21]. We therefore tested whether dox could affect proliferation and morphology of A375 cells under our experimental conditions. Our results showed that dox alone did not affect cell proliferation or morphology of the parental A375 cells that do not contain a dox-inducible Shp2 shRNA (Supplementary Fig. 1). Thus, the growth inhibiting effect of dox on A375/R046 and A375/R1049 is mediated the dox-induced Shp2 shRNAs. Enhanced anti-proliferative response was detected in Shp2 knockdown cells treated with IFN-α2b. The combined anti-proliferative effect of Shp2 knockdown and IFN-α2b was synergistic based on the Bliss definition [19].
Very weak apoptotic markers (active caspase 3, cleaved PARP) were detected in A375 cells with induced Shp2 shRNAs or treated with IFN-α2b (1,000 IU/ml) under our cell culture conditions (DMEM/5% FBS). We have not consistently observed a dramatic increase in apoptosis markers by combination of Shp2 shRNAs and IFN-α2b under these cell culture conditions. Therefore, the observed inhibitory effect of combined Shp2 knockdown and IFN-α2b in cell culture is mainly anti-proliferative.
3.2. SPI-112Me increases IFN-α2b-induced STAT1 tyrosine phosphorylation and growth inhibition
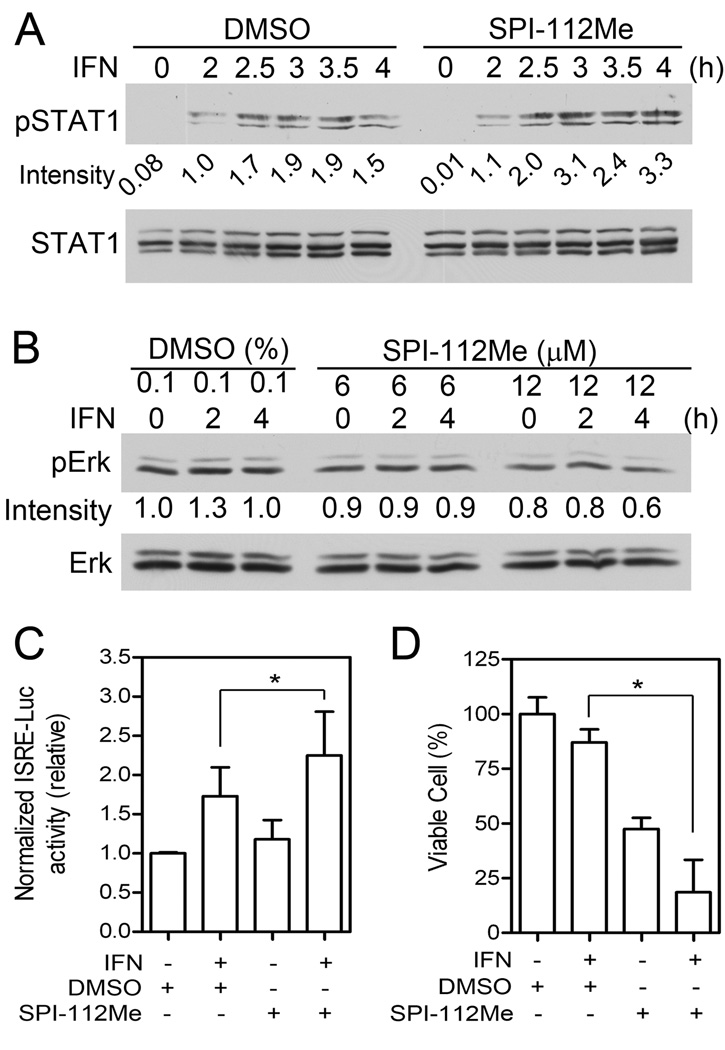
SPI-112Me is a cell permeable pro-drug of the Shp2 inhibitor SPI-112 [19,22]. To test if SPI-112Me could augment the IFN-α response, A375 cells were pre-treated with SPI-112Me or the vehicle and then stimulated with IFN-α2b. In the absence of SPI-112Me, the relative intensities of pSTAT1 signal in the IFN-α2b-stimulated cells were in the range of 1.0–1.9 (Fig. 3A). SPI-112Me by itself did not affect pSTAT1 level. However, SPI-112Me enhanced the IFN-α2b-stimulated pSTAT1, such that the relative intensities of IFN-α2b-stimulated pSTAT1 signal were in the range of 1.1–3.3 in cells treated SPI-112Me (Fig. 3A). Similar to Shp2 knockdown by shRNAs, SPI-112Me treatment reduced the pErk1/2 level, which is indicative of Shp2 inhibition (Fig. 3B). In the ISRE-Luc reporter assay, SPI-112Me treated cells had significantly higher IFN-α2b-stimulated ISRE luciferase activity (Fig. 3C). In cell proliferation assay, combined IFN-α2b and SPI-112Me treatment resulted in a synergistic increase in growth inhibition (Fig. 3D). These results suggest that SPI-112Me could increase the IFN-α2b-induced STAT1 and anti-proliferation activities in A375 melanoma cells.
Fig. 3.
SPI-112Me enhances IFN-α2b-activated STAT1 activation, inhibits Erk1/2, and increases the IFN-α2b-induced cell growth inhibition activity. A375 cells were pre-treated with 12 µM (A) or the indicated concentrations (B) of SPI-112Me or mock-treated with the vehicle overnight and then stimulated with IFN-α2b (1,000 IU/ml) for the indicated time. Cell lysates were analyzed by immunoblotting with the indicated antibodies (A, B). (C) ISRE-Luc reporter activity was determined as described in the Materials and Methods. (D) Cells were cultured in 96-well plates, treated with IFN-α2b (1,000 IU/ml), SPI-112Me (6 µM) or the vehicle. Relative cell numbers were measured on Day 6. Data were from two independent experiments (n=6). *, p < 0.05.
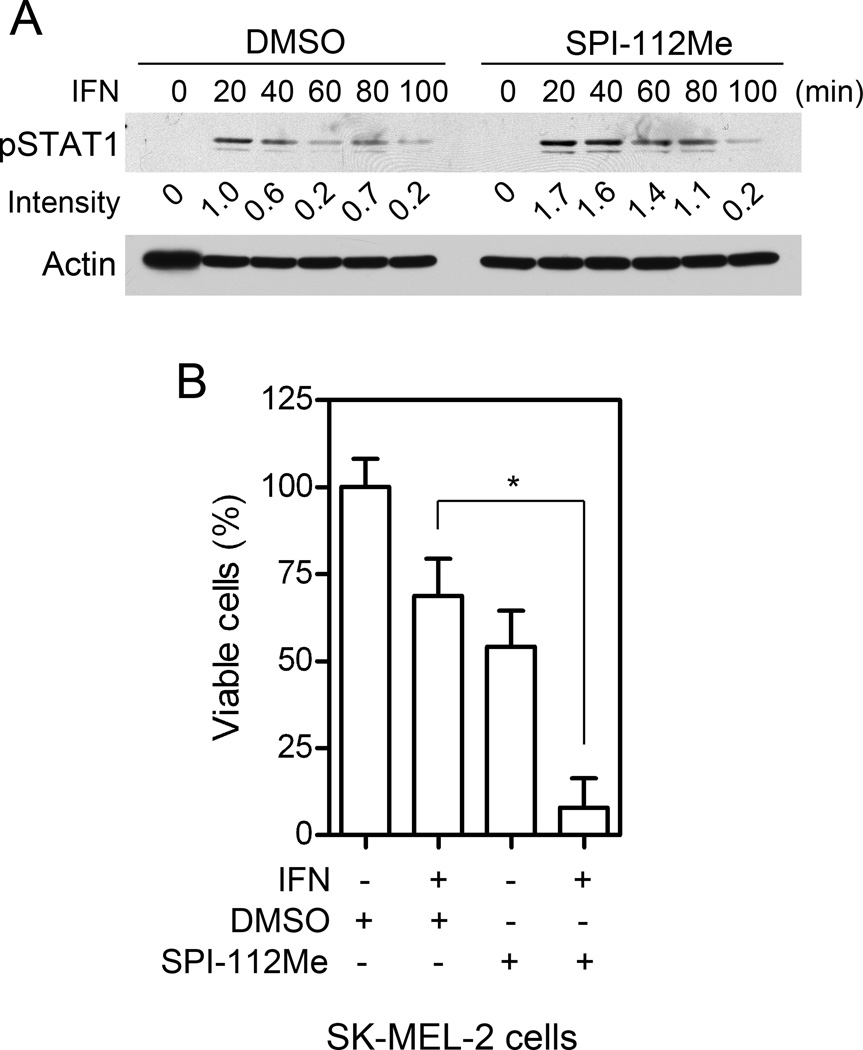
To determine whether the effects of the Shp2 inhibitor also occur in other melanoma cells, we treated SK-MEL-2 cells with SPI-112Me and IFN-α2b. As shown in Fig. 4A, enhanced IFN-induced STAT1 tyrosine phosphorylation was observed in SK-MEL-2 cells pre-treated with SPI-112Me. In the cell proliferation assay, the combined IFN-α2b and SPI-112Me treatment again caused a synergistic increase in growth inhibition (Fig. 4B).
Fig. 4.
SPI-112Me enhances IFN-α2b responses in SK-MEL-2 cells. (A) SK-MEL-2 cells were treated with SPI-112Me and/or IFN-α2b and cell lysates were analyzed by immunoblotting with the indicated antibodies. (B) SK-MEL-2 cell proliferation was assayed in 96-well plate cultures. Experiments were performed similar to that described in Fig. 3.
3.3. Shp2 inhibition enhances the anti-tumor efficacy of IFN-α2b in human melanoma xenografts in immune deficient mice
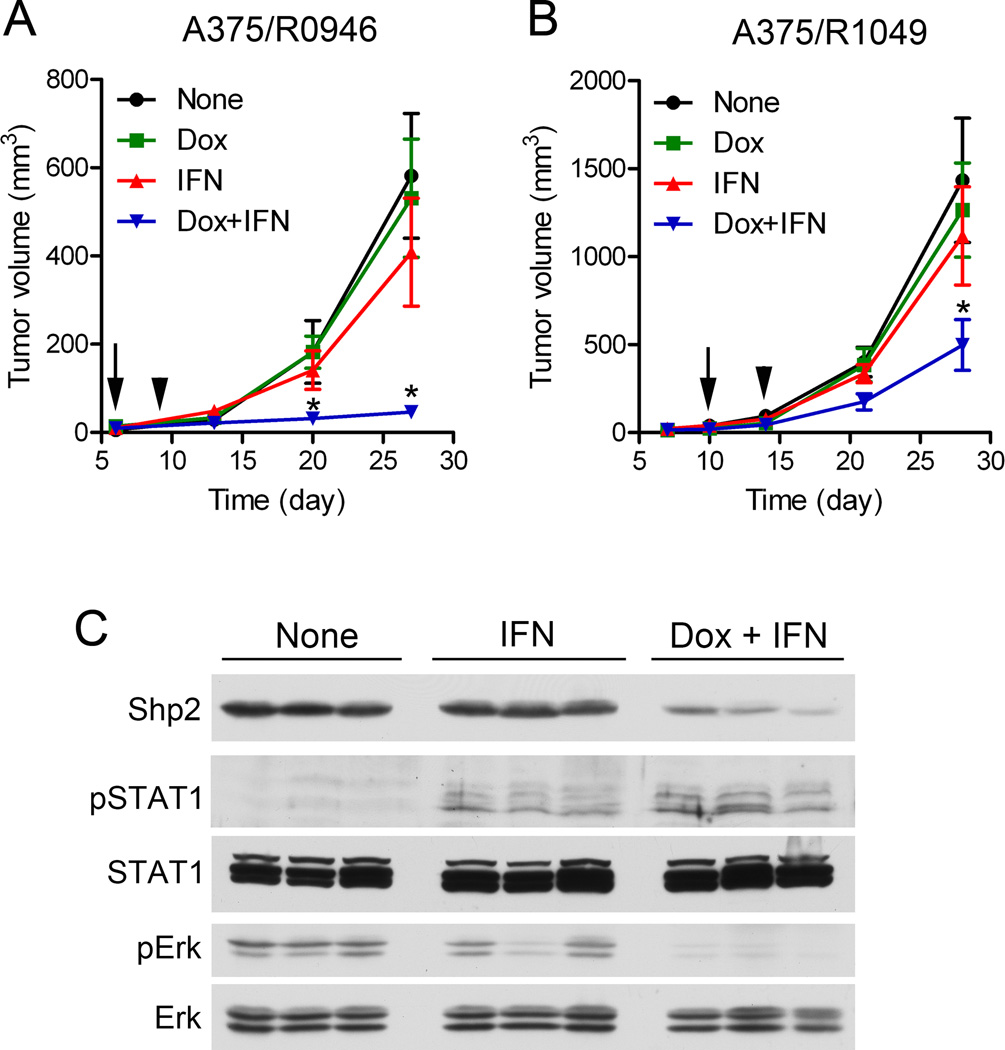
To assess whether Shp2 inhibition could suppress A375 melanoma tumor growth or enhance the anti-tumor effect of IFN-α2b, we first tested the effect of Shp2 knockdown on A375/R0946 tumor growth. A375/R0946 cells were inoculated s.c. into nude mice. Mice bearing small tumors of similar size were selected and treated with either: a) dox by feeding with dox diet to induce Shp2 shRNA expression, b) s.c injection of IFN-α2b (4 × 104 IU/day), c) the combination of dox diet and IFN-α2b, or d) untreated (Fig. 5A). Knockdown of Shp2 by dox induction did not reduce A375/R0946 tumor growth. IFN-α2b treated mice had smaller tumors but the difference in the average tumor volumes at the endpoint between untreated mice and IFN-α2b-treated mice were not statistically significant (p = 0.364). In contrast, the combination of dox and IFN-α2b treatments markedly suppressed A375/R0946 tumor growth (p = 0.001 at the end point).
Fig. 5.
Effects of Shp2 knockdown on A375 melanoma tumor growth. (A, B) Cells (1 × 106 cells/each) were injected s.c. on both flanks of each mouse on Day 0 (2 sites/mouse). Tumor sizes were measured on the indicated days. Each group has six mice bearing 12 tumors of similar sizes at the start of dox induction on Day 6 (A375/R0946 tumors) or Day 10 (A375/R1049 tumors) as indicated by the arrow. Arrowhead indicates the starting date of IFN-α2b treatment (Day 9 and 14, respectively as shown in the graphs). Mice were euthanized at the end points (Day 27 or Day 28 as shown in the graphs). Mean ± SEM are shown. *, p < 0.05. (C) Immunoblotting analysis of A375/R1049 tumor samples.
To determine if the enhanced anti-melanoma effect also occurs with a different Shp2 shRNA, A375/R1049 tumor growth assay was performed (Fig. 5B). Similar to A375/R0946 tumors, dox induction had little effect on A375 tumor growth even though Shp2 was knockdown in the tumors of mice fed with the dox diet (Fig. 5C). IFN-α2b treatment alone did not significantly reduced tumor growth (p = 0.490). Combination of dox induction and IFN-α2b treatment again significantly suppressed A375/R1049 tumor growth (p = 0.025 at the end point).
In this experiment, mice in the IFN-treated and the dox plus IFN-treated groups were given a final IFN-α2b injection 2 h before euthanasia. Tumor samples were collected post mortem and analyzed by immunoblotting (Fig. 5C). pSTAT1 was detected in tumor samples from IFN-α2b-treated mice. The pSTAT1 level was further increased in two of three tumor samples from dox plus IFN-treated mice while lower levels of pErk1/2 was present in these tissues.
4. Discussion
Poor response to IFN-α limits the clinical benefits of IFN-α2b therapy in melanoma patients. Given that IFN-α signaling is mediated by STAT1/STAT2 tyrosine phosphorylation, it is subject to negative regulation by PTPs. Inhibition of specific PTPs that negatively regulate the Jak-STAT pathway is a potential approach to enhance IFN-α-induced responses in melanoma. Using molecular biology approach, we provide evidence here that Shp2 inhibition can enhance the anti-tumor activity of IFN-α2b in melanoma. These results establish Shp2 as a critical negative regulator of the anti-tumor effect of IFN-α in melanoma.
IFN-α2b induces tyrosine phosphorylation of both STAT1 Tyr-701 and STAT2 Tyr-690 in A375 melanoma cells (Fig. 1). Our data show that Shp2 only affects IFN-α2b-induced STAT1 Tyr-701 phosphorylation in these cells. This observation is similar to that observed in EGF-stimulated A431 cells, which showed specificity of Shp2 towards STAT1 among STAT1, STAT3, and STAT5 [15]. Nevertheless, although Shp2 inhibition did not affect IFN-α2b-induced STAT2 tyrosine phosphorylation, it was sufficient to enhance the anti-tumor activity of IFN-α2b. This observation suggests that STAT1 tyrosine phosphorylation is a limiting factor of the IFN-α2b responses in A375 melanoma.
Although Shp2 knockdown reduced pErk1/2 level and had some effect on A375 cell growth in vitro, Shp2 knockdown did not inhibit A375 tumor growth in vivo. It is possible that multiple genetic lesions in these melanoma cells have rendered these cells less dependent on the Shp2-Erk1/2 pathway for tumor growth in vivo. This implies that inhibition of Shp2 alone is not sufficient to yield clinical benefit in melanoma. Importantly, our data show that Shp2 inhibition can increase the anti-tumor effect of IFN-α2b in melanoma. Thus, Shp2 inhibition will be best used in the setting of combination with IFN-α2b to enhance the IFN-α2b response in melanoma.
Developing PTP inhibitors as novel anti-cancer drugs is an emerging field [13,14,23]. Enhanced IFN-α2b-induced STAT1 activation and anti-proliferative activity were observed with the Shp2 inhibitor SPI-112Me in A375 and SK-MEL-2 cells in cell cultures. However, SPI-112Me has aqueous solubility issue that requires further improvement before it can be tested in vivo. Thus, our promising results with SPI-112Me in vitro await the development of suitable drug formulations and further optimization of this and other Shp2 inhibitors to improve their bioavailability and pharmacokinetic properties for in vivo evaluation in animal models. Meanwhile, our validation of Shp2 as a molecular target for enhancing the anti-melanoma activity of IFN-α2b should stimulate the development of Shp2 inhibitors.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grant P01 CA118210 and a Pilot Research Award of Bankhead-Coley Melanoma Pre-SPORE Program of the Moffitt Cancer Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
None declared.
References
- 1.Gogas HJ, Kirkwood JM, Sondak VK. Chemotherapy for metastatic melanoma: time for a change? Cancer. 2007;109:455–464. doi: 10.1002/cncr.22427. [DOI] [PubMed] [Google Scholar]
- 2.Sabel MS, Sondak VK. Pros and cons of adjuvant interferon in the treatment of melanoma. Oncologist. 2003;8:451–458. doi: 10.1634/theoncologist.8-5-451. [DOI] [PubMed] [Google Scholar]
- 3.McLoughlin JM, Zager JS, Sondak VK, Berk LB. Treatment options for limited or symptomatic metastatic melanoma. Cancer Control. 2008;15:239–247. doi: 10.1177/107327480801500307. [DOI] [PubMed] [Google Scholar]
- 4.Lesinski GB, Trefry J, Brasdovich M, Kondadasula SV, Sackey K, Zimmerer JM, Chaudhury AR, Yu L, Zhang X, Crespin TR, Walker MJ, Carson WE., 3rd Melanoma cells exhibit variable signal transducer and activator of transcription 1 phosphorylation and a reduced response to IFN-alpha compared with immune effector cells. Clin Cancer Res. 2007;13:5010–5019. doi: 10.1158/1078-0432.CCR-06-3092. [DOI] [PubMed] [Google Scholar]
- 5.Taniguchi T, Takaoka A. A weak signal for strong responses: interferon-alpha/beta revisited. Nat Rev Mol Cell Biol. 2001;2:378–386. doi: 10.1038/35073080. [DOI] [PubMed] [Google Scholar]
- 6.Chan G, Kalaitzidis D, Neel BG. The tyrosine phosphatase Shp2 (PTPN11) in cancer. Cancer Metastasis Rev. 2008;27:179–192. doi: 10.1007/s10555-008-9126-y. [DOI] [PubMed] [Google Scholar]
- 7.Cunnick JM, Meng S, Ren Y, Desponts C, Wang HG, Djeu JY, Wu J. Regulation of the mitogen-activated protein kinase signaling pathway by SHP2. J Biol Chem. 2002;277:9498–9504. doi: 10.1074/jbc.M110547200. [DOI] [PubMed] [Google Scholar]
- 8.Ren Y, Meng S, Mei L, Zhao ZJ, Jove R, Wu J. Roles of Gab1 and SHP2 in paxillin tyrosine dephosphorylation and Src activation in response to epidermal growth factor. J Biol Chem. 2004;279:8497–8505. doi: 10.1074/jbc.M312575200. [DOI] [PubMed] [Google Scholar]
- 9.Chan RJ, Feng G-S. PTPN11 is the first identified proto-oncogene that encodes a tyrosine phosphatase. Blood. 2007;109:862–867. doi: 10.1182/blood-2006-07-028829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ren Y, Chen Z, Chen L, Woods NT, Reuther GW, Cheng JQ, Wang HG, Wu J. Shp2E76K mutant confers cytokine-independent survival of TF-1 myeloid cells by up-regulating Bcl-XL. J Biol Chem. 2007;282:36463–36473. doi: 10.1074/jbc.M705789200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bentires-Alj M, Paez JG, David FS, Keilhack H, Halmos B, Naoki K, Maris JM, Richardson A, Bardelli A, Sugarbaker DJ, Richards WG, Du J, Girard L, Minna JD, Loh ML, Fisher DE, Velculescu VE, Vogelstein B, Meyerson M, Sellers WR, Neel BG. Activating mutations of the noonan syndrome-associated SHP2/PTPN11 gene in human solid tumors and adult acute myelogenous leukemia. Cancer Res. 2004;64:8816–8820. doi: 10.1158/0008-5472.CAN-04-1923. [DOI] [PubMed] [Google Scholar]
- 12.Ren Y, Chen Z, Chen L, Fang B, Win-Piazza H, Haura EB, Koomen JM, Wu J. Critical role of Shp2 in tumor growth involving regulation of c-Myc. Genes Cancer. 2010;1:994–1007. doi: 10.1177/1947601910395582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scott LM, Lawrence HR, Sebti SM, Lawrence NJ, Wu J. Targeting protein tyrosine phosphatases for anticancer drug discovery. Curr Pharm Des. 2010;16:1843–1862. doi: 10.2174/138161210791209027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang ZX, Zhang ZY. Targeting PTPs with small molecule inhibitors in cancer treatment. Cancer Metastasis Rev. 2008;27:263–272. doi: 10.1007/s10555-008-9113-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu TR, Hong YK, Wang XD, Ling MY, Dragoi AM, Chung AS, Campbell AG, Han ZY, Feng GS, Chin YE. SHP-2 is a dual-specificity phosphatase involved in Stat1 dephosphorylation at both tyrosine and serine residues in nuclei. J Biol Chem. 2002;277:47572–47580. doi: 10.1074/jbc.M207536200. [DOI] [PubMed] [Google Scholar]
- 16.Yi T, Pathak MK, Lindner DJ, Ketterer ME, Farver C, Borden EC. Anticancer activity of sodium stibogluconate in synergy with IFNs. J Immunol. 2002;169:5978–5985. doi: 10.4049/jimmunol.169.10.5978. [DOI] [PubMed] [Google Scholar]
- 17.Pathak MK, Yi T. Sodium stibogluconate is a potent inhibitor of protein tyrosine phosphatases and augments cytokine responses in hemopoietic cell lines. J Immunol. 2001;167:3391–3397. doi: 10.4049/jimmunol.167.6.3391. [DOI] [PubMed] [Google Scholar]
- 18.Singh S, Davis R, Alamanda V, Pireddu R, Pernazza D, Sebti S, Lawrence N, Chellappan S. Rb-Raf-1 interaction disruptor RRD-251 induces apoptosis in metastatic melanoma cells and synergizes with dacarbazine. Mol Cancer Ther. 2010;9:3330–3341. doi: 10.1158/1535-7163.MCT-10-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen L, Pernazza D, Scott LM, Lawrence HR, Ren Y, Luo Y, Wu X, Sung SS, Guida WC, Sebti SM, Lawrence NJ, Wu J. Inhibition of cellular Shp2 activity by a methyl ester analog of SPI-112. Biochem Pharmacol. 2010;80:801–810. doi: 10.1016/j.bcp.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ren Y, Chen Z, Chen L, Fang B, Win-Piazza H, Haura E, Koomen JM, Wu J. Critical role of Shp2 in tumor growth involving regulation of c-Myc. Genes Cancer. 2010;1:994–1007. doi: 10.1177/1947601910395582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun T, Zhao N, Ni CS, Zhao XL, Zhang WZ, Su X, Zhang DF, Gu Q, Sun BC. Doxycycline inhibits the adhesion and migration of melanoma cells by inhibiting the expression and phosphorylation of focal adhesion kinase (FAK) Cancer Lett. 2009;285:141–150. doi: 10.1016/j.canlet.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Lawrence HR, Pireddu R, Chen L, Luo Y, Sung SS, Szymanski AM, Yip ML, Guida WC, Sebti SM, Wu J, Lawrence NJ. Inhibitors of Src homology-2 domain containing protein tyrosine phosphatase-2 (Shp2) based on oxindole scaffolds. J Med Chem. 2008;51:4948–4956. doi: 10.1021/jm8002526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hellmuth K, Grosskopf S, Lum CT, Wurtele M, Roder N, von Kries JP, Rosario M, Rademann J, Birchmeier W. Specific inhibitors of the protein tyrosine phosphatase Shp2 identified by high-throughput docking. Proc Natl Acad Sci U S A. 2008;105:7275–7280. doi: 10.1073/pnas.0710468105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.