Abstract
Objective
Comparison of the efficacy of bleomycin over sodium tetradecyl sulfate (STS) when given intralesionally in the treatment of oral and maxillofacial venous malformation.
Methods
16 patients with venous malformation in craniofacial region were randomly divided into two groups of eight. Group 1 was given intralesional injection of bleomycin and group 2 was injected with STS. All the cases were evaluated for a minimum period of two and a maximum of 3 years.
Results
Efficacy of bleomycin was found to be superior to STS, when used as intralesional sclerotherapic agent. Most of the vascular lesions of group 1 resolved after first dose giving a cure rate of 87.5% and no recurrence was observed. Group 2 patients however, required 4–6, a mean of five repeated dosage of intralesional STS before their lesions started to resolve and three patients reported with recurrence within 2 years, giving an overall effective response rate of 62.5%.
Conclusion
Bleomycin under selected conditions appears to be an excellent therapy for treating soft tissue vascular lesions of low flow nature in craniofacial region. Predictable results were obtained with a high success rate. No systemic or pulmonary complications occurred.
Keywords: Vascular, Venous malformation, Maxillofacial
Introduction
Oral and maxillofacial hemangioma and vascular malformations account for nearly 60% of all the lesions in the body and are still great challenges for clinicians because of their unknown etiology and difficulty in management.
Successful therapy of these lesions now requires co-operative or multidisciplinary approaches including surgical procedures, embolization via interventional radiology, scleorosing therapy, laser therapy, psychological support and others.
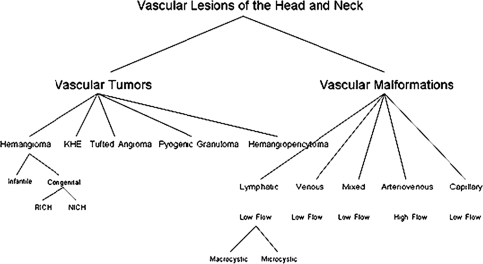
Hemangioma are tumors identified by rapid endothelial cell proliferation in early infancy, followed by involution over time; all other abnormalities are malformations resulting from anomalous development of vascular plexuses. The malformations have a normal endothelial cell growth cycle that affects veins, capillaries and lymphatics and these lesions do not involute [1] (Fig. 1).
Fig. 1.
Classification chart of vascular lesions of head and neck. RICH rapidly involuting congenital hemangioma, NICH non involuting congenital hemangioma, KHE kaposiform hemangioendothelioma
Proliferating hemangiomas have been shown to have estradiol-17 beta-receptors in the cytoplasm and corticosteroid treatment has been theorized to block these receptors [2]. Steroid treatment has become a first line of treatment for proliferating lesions.
A number of growth factors, including vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), transforming growth factor-β (TGF-β), and interleukin 6 (IL-6), have been demonstrated as regulators of angiogenesis [3]. The morbidity of oral and facial vascular lesions ranges from surface discoloration to life-threatening functional compromise of the airway or hemorrhage. Fatal spontaneous hemorrhage from jaw hemangiomas has been documented in 25 cases [4]. Significant morbidity can also occur from many of the treatment of hemangioma and biopsy of these lesions is also fraught with danger. Takahashi et al. [5] outlined a number of cellular markers that distinguish the phases of hemangioma, these markers include tissue metalloproteinase (TIMP-1), bFGF, proliferating cell nuclear antigen, type IV collagenase, VEGF, and urokinase. Difference between hemangioma and vascular malformation have been summarized in Table 1.
Table 1.
Comparative feature of vascular lesion [23]
| S. no | Hemangioma | Vascular malformation |
|---|---|---|
| 1. | Present at birth; most diagnosed by 1 year of age | Present at birth but often not diagnosed until second decade |
| 2. | Rapid growth until age 6–8 month then slows and involutes by 5–9 years | Slow growth with increase in size in response to infection, trauma, or hormonal fluctuation; does not involute |
| 3. | Neoplastic growth with increased endothelial cell turnover | Growth due to flow dynamics through the lesion and recruitment of collateral supply |
| 4. | Osseous involvement rare female–male ratio 5:1 usually low flow | Osseous involvement 35% female–male ratio 2:1 may be low flow (capillary, venous, lymphatic or high flow arterial or arteriovenous) |
| 5. | Frequently does not need treatment | Often requires treatment |
Venous malformations which are low flow vascular lesions are composed of large, irregular, deep dermal and subcutaneous blood-filled channels that impart a purplish discoloration to the overlying skin. Most are asymptomatic swellings, typically soft, poorly defined and readily blanch with compression giving them a characteristic “bag of worms” feel. They have no palpable thrill or audible bruit. The lesion may expand and darken with crying, when agitated or when placed in a dependent position (Fig. 2). Some lesions increase in size with compression of the ipsilateral jugular vein. Valsalva maneuver may also increase the size of the lesion. Venous malformations are present at birth although not all are clinically evident, their growth may become accelerated when the patient undergoes puberty or pregnancy with attendant hormonal changes. Low flow vascular lesion most commonly occurs in masseter, skin, lips, oral mucosa, and mandible. Mandibular lesions may present with loosening of teeth or bleeding. Lesions may also occur within orbit causing exophthalmos and vision changes.
Fig. 2.
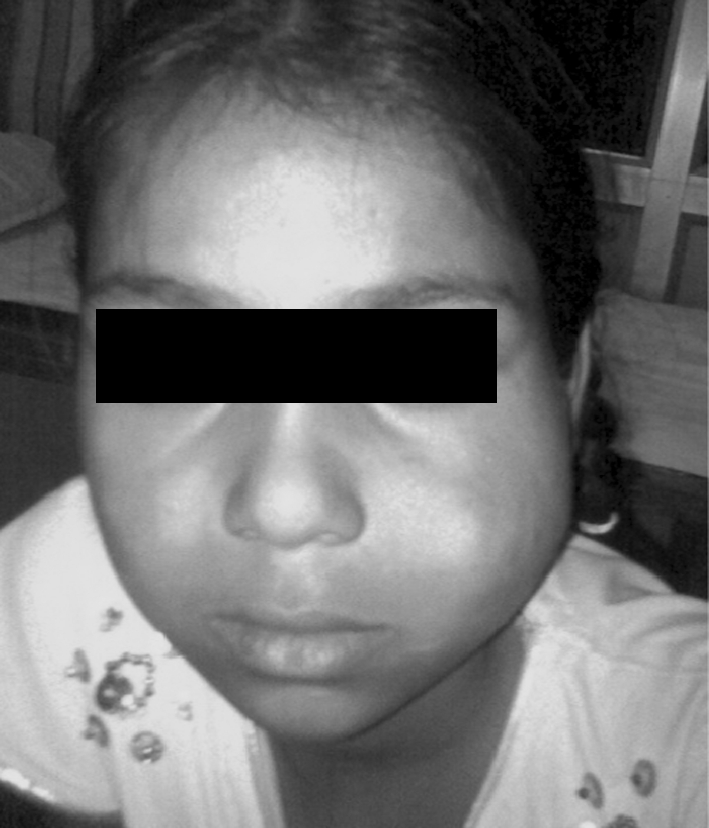
A typical clinical appearance of a venous malformation
Some lesions have palpable phleboliths within them, a diagnostic hallmark of the venous malformation. Phleboliths are often visible on plain film and are likely the result of sluggish, abnormal flow through the lesion’s ecstatic channels. MRI reveals a hyper intense lesion on T2, which enhances with contrast on T1 in the vascular channels and has flow voids where phleboliths are located [6, 7].
This prospective study evaluates the efficacy of intralesional bleomycin injection over sodium tetradecyl sulfate as an effective non surgical method to treat low flow vascular lesions in maxillofacial region.
Materials and Methods
16 patients both male and female between the age group of 8–24 years, with subcutaneous low flow vascular lesion diagnosed as venous malformation clinically and by Doppler study were taken from the out patient Department of Oral and Maxillofacial Surgery Mahranapratap Dental College and Hospital and Aanya Dental and Craniofacial Hospital Kanpur. The size of the lesions ranged from 6 × 10 mm to 21 × 24 mm as measured ultrasonographically. The cases were subdivided into two groups of eight each, group:-1 and group:-2.
Group 1 patient was given beomycin 8 mg, freshly prepared in 2 ml normal saline and 2% lidocaine 2 ml.
Group 2 patients were given 1 ml of 3% sodium tetradecyl sulfate and 2 ml of 2% lidocaine.
Evaluation Criteria
All the patients were evaluated for a minimum period of 2 years and a maximum of 3 years from January 2008 to January 2011. Clinical and radiologic evaluation was done for
Cured: tumor completely disappeared, the skin and mucous membrane color returned to normal for more than 2 years without recurrence following first dose.
Effective: the majority of tumor subsided following first dose and more injections required to produce cure.
Ineffective: tumor disappeared partially or recurrence within 2 years.
Number of injections required to produce cure.
Complications encountered.
The term recurrence was assigned to lesions in which skin or mucosal discoloration and palpable swelling reappeared following previous successful treatment.
Protocol
All the patients who underwent sclerotherapy were started on prophylactic antibiotic amoxicillin a day prior to the procedure which continued for next 4 days along with an analgesic, routine blood investigation, liver function test and chest radiograph were conducted before the patients were admitted to the hospital on day care basis. Under strict aseptic condition and operation theatre set up the skin overlying the lesion was scrubbed with surgical spirit, respective drugs were loaded in a 5 ml disposable syringe following which needle is inserted into the lesion via single prick and aspirated in two planes, gushing of blood in the barrel confirms presence within the lesion, pre-calculated amount of drug is injected in two or three planes circumlesionally and intralesionally. Pressure dressing is given for 5 min following needle exit. Monitoring 4 h after administration (dosing) includes the following:
Blood pressure check
Heart rate check
Temperature determination to evaluate for hypothermia
Observation for bronchospasm and SpO2
Injection if required were repeated after every 3 weeks and all the injections were given by a single operator to rule out individual variability, chest radiograph were repeated at the end of 1 year as a known complication of bleomycin is pulmonary fibrosis. Findings were noted down on a specially designed proforma and relevant statistics were applied to the data obtained.
Result
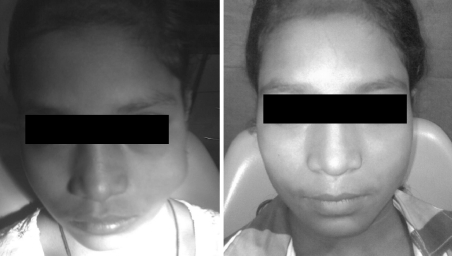
Group 1 (Figs. 3, 4, 5)
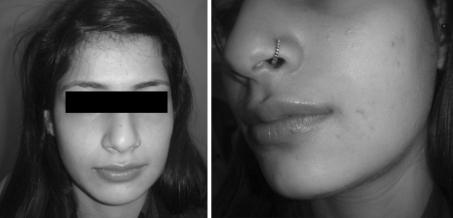
Fig. 3.
Pre and 2 year post op photograph of a patient with venous malformation treated with one injection of intralesional bleomycin
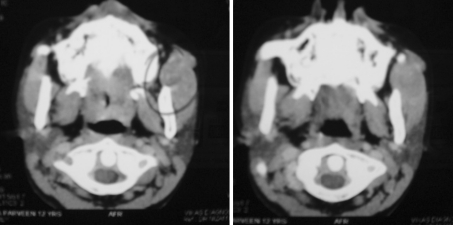
Fig. 4.
Pre and post op axial CT. Scan of patient in Fig. 7
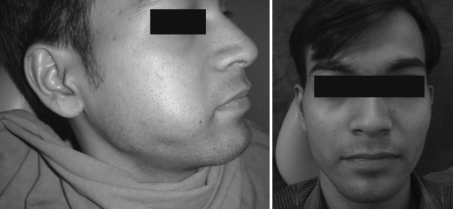
Fig. 5.
Pre and post op photograph of another patient treated with one injection of bleomycin
Lesions of seven patients in group 1 who received an intralesional injection of bleomycin disappeared, the color of the skin overlying the lesion became normal which before gave a bluish hue and size on palpation regressed considerably within 3 weeks of first dose of drug administration. There was no recurrence of the lesion in the time period in which patients were observed (2–3 years). These seven patients came under the category of cured. Giving a response rate of 87.5%.
One patient in group 1 required a second injection of bleomycin to obtain cure, after 3 weeks as the response obtained by first dose was very minimal and a considerable portion of the lesion persisted. Drug proved to be effective in 12.5% of cases, a total number of nine mean of 1.125 injections were required to produce cure.
Complications encountered in group 1 patients, included redness over the skin surrounding the lesion in two patients which persisted for 2–3 days, one patient complained of nausea and itching for 1 day following injection over the injected area, nearly all the patients complained of stretching sensation in the area surrounding the injection site 2–3 weeks following injection, which as we perceived may be due to the fibrosis induced by the drug. No other systemic or local complication like pulmonary fibrosis, ulceration or blistering was encountered (Table 2).
Table 2.
List of group 1 patient who were given IBI
| S. no | Site of lesion | Size of lesion in mm | Age/sex | No. of bleomycin injection | Result | Complications |
|---|---|---|---|---|---|---|
| 1. | Skin overlying Lt. zygoma | 12 × 18 | 13/F | 1 | Cured | Nausea, pruritits and stretching of overlying skin |
| 2. | Skin overlying Rt. angle of mandible | 6 × 10 | 15/F | 1 | Cured | Erythema of overlying skin |
| 3. | Upper labial mucosa | 8 × 9 | 15/M | 1 | Cured | Stretching sensation |
| 4. | Skin overlying Lt. buccinator | 21 × 24 | 19/F | 2 | Effective | Erythema of overlying skin and stretching sensation |
| 5. | Rt. buccal mucosa | 9 × 14 | 20/M | 1 | Cured | Nausea |
| 6. | Rt. buccal mucosa | 10 × 12 | 9/M | 1 | Cured | Nil |
| 7. | Skin overlying lower lip | 10 × 10 | 13/F | 1 | Cured | Nil |
| 8. | Lower labial mucosa | 8 × 11 | 14/m | 1 | Cured | Stretching sensation |
Group 2 (Figs. 6, 7)
Fig. 6.
Photograph of a patient with persistent discoloration of the skin and lesion after six STS. Injection
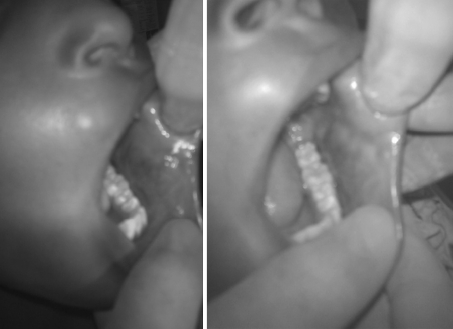
Fig. 7.
Photograph of an intraoral lesion pre-op and following 2 STS injection
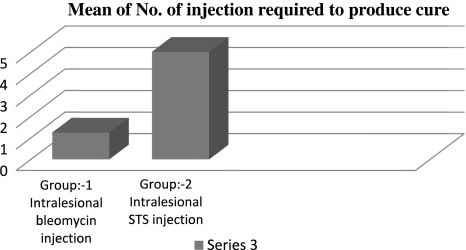
None of the eight patients in group 2 was cured following first intralesional injection of STS. All the cases required 4–6, a mean of five injections to obtain cure (Fig. 8). Three patients out of eight reported back with recurrence within 2 years.
Fig. 8.
Bar graph representation of comparison of mean No. of injection of bleomycin and STS. Required to produce cure
Five patients in group 2 came under the category of effective, giving an overall effective response rate of 62.5% and the drug was ineffective in 37.5% of cases.
Complications that were encountered in group 2 included cutaneous blister and ulcer formation, at injection site in two cases and scarring in one patient. Erythema appeared on skin overlying the lesion and injection site in three patients which persisted for 2–3 days. No other significant systemic or local complications were encountered (Table 3).
Table 3.
List of group 2 patient who were given STS
| S. no | Site of lesion | Size of lesion in mm | Age/Sex | No. of STS injections | Result | Complications |
|---|---|---|---|---|---|---|
| 1. | Lt. dorsal surface of tongue | 6 × 8 | 18/M | 6 | Ineffective | Recurrence in 18 months |
| 2. | Lower labial mucosa | 9 × 12 | 12/F | 4 | Effective | Ulceration of overlying mucosa |
| 3. | Skin overlying upper lip | 10 × 14 | 13/F | 6 | Effective | Scar formation at injection site |
| 4. | Skin overlying Rt. zygoma | 12 × 16 | 20/M | 6 | Effective | Erythema of overlying skin |
| 5. | Lt. buccal mucosa | 12 × 10 | 15/M | 4 | Effective | Blister formation at injection site |
| 6. | Skin overlying Lt. body of the mandible | 14 × 16 | 24/F | 5 | Effective | Erythema of overlying skin |
| 7. | Mucosa of anterior floor of the mouth | 8 × 8 | 8/M | 4 | Ineffective | Recurrence in 20 months |
| 8. | Skin overlying Rt. cheek | 10 × 12 | 13/F | 5 | Ineffective | Erythema of overlying skin recurrence in 22 months |
Discussion
The first tangible step towards better defining of these lesions and tailoring the treatment were made in the early 1980s. In 1982, Mulliken and Glowacki developed the first binary system of diagnosis based on histological characteristics, dividing these lesions into either hemangiomas or vascular malformations (Fig. 1).
Kane et al. [8] developed a management algorithm that covers most of the current thinking regarding these tumors. If the tumor is a proliferating hemangioma, either observation or steroids are options. In lesions that are not proliferating, whether the lesion is involuting needs to be determined. Involuting lesions can be managed by observation. If the involution is incomplete and arrested, then the lesion can be managed the same as a low-flow vascular malformation.
If the lesion in question is determined to be a vascular malformation rather than a hemangioma, then its flow characteristics must be gauged. High-flow lesions require presurgical embolization followed by aggressive ablative therapy. Low-flow vascular malformations can be managed in numerous ways. For the easily collapsible lesions that are accessible, sclerotherapy, laser therapy, or cryotherapy are alternatives. For those that are not accessible, do not have compressible components, or are functionally compromising, ablative surgery is indicated [9].
STS is another commonly used sclerosant for vascular tumors. STS causes intimal inflammation, thrombus formation, and often permanent obliteration of the veins [9]. In animal studies, STS produces long-term arterial thrombosis in large arteries and marked inflammatory reactions in small vessels, with eventual replacement by connective tissue. In an early report on the use of STS in oral hemangiomas, Baurmash and Mandel [10] used 1% STS however, more recent reports use 3% solution [11–13].
Minkow et al. [11] used a technique of intralesionally injecting 0.1–0.5 ml of 3% STS into oral heamangiomas. Repeat injections were given at 2-week intervals. His study included 24 patients, 15 females and 9 males, with age group 11–79 years. Satisfactory results were reported in all patients, with minimal adverse effects and disappearance of the lesions without scarring. O’Donovan et al. [9] recommended 0.5–2.0 ml, 3% STS and manual compression of the lesions to ensure stasis. Kane et al. [8] recommended 3% STS used alone for oral hemangiomas but in combination with surgery for vascular malformations.
In a more recent study Khandpur S and Sharma VK performed sclerotherapy with 3% STS in 13 patients with venous malformations and micro cystic lymphatic malformation. They concluded that the lesions regressed by 90–100% in 11 cases after a mean of four injections, with no improvement in two cases. Complications included cutaneous blister formation, erosions, and crusting at injection site in seven cases and atrophic scarring in four patients [14].
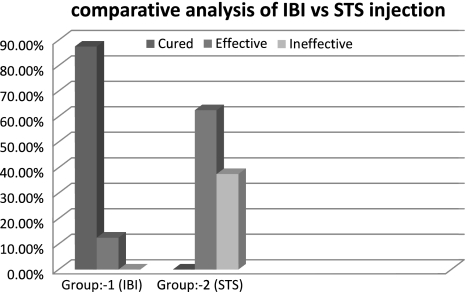
In accordance with the above mentioned studies we also used 3% sodium tetradecyl sulfate in eight patients. We obtained an effective response rate of 62.5% and a mean of five injections at an interval of 3 weeks were required to produce the response. Three patients returned with recurrence within 2 years giving an ineffective or failure rate of 37.5% which we found to be quite unacceptable considering the number of times patient has to undergo the injection procedure and overall time duration of treatment needed to treat a moderate sized low flow vascular lesion (Fig. 9). Complications encountered were minor as reported by other authors.
Fig. 9.
Bar graph comparative analysis between response rate of intralesional bleomycin and STS. Injection
Reports have been published on the safe and effective use of ethanol for the treatment of venous malformations [15, 16]. Ethanol is inexpensive and easy to administer. However, it is painful and requires general anesthesia. To enhance exposure of the lesion’s endothelium to the ethanol, limitation of venous outflow may be required (manual or rubber-band compression). Complications of ethanol sclerotherapy include pain, skin necrosis, transient neuropathy, bleeding, renal toxicity, cardiac arrest, and anaphylaxis [16, 17]. STS has a lower incidence of serious side effects when compared with ethanol but may be less effective as well [17].
Bleomycin is an antineoplastic antibiotic. The drug is active against gram-positive and gram-negative bacteria and fungi, but its cytotoxicity precludes its use as an anti-infective agent. The precise mechanism(s) of action of bleomycin is not fully known. Several studies in Escherichia coli and HeLa cells suggest that the drug inhibits the incorporation of thymidine into DNA. In these in vitro studies, DNA synthesis was found to be inhibited to a greater extent than was RNA or protein synthesis. Bleomycin also appears to labialize the DNA structure, resulting in scission of both single- and double-stranded DNA. The drug has no immunosuppressive activity in mice. T Muir and associates reported a response rate of 98% with no systemic or pulmonary complication; they used a maximum dose of 3 mg/kg of body weight [18]. J. W. Zheng and Z. Y. Zhang [19] reviewed various modalities of sclerotherapy and emphasized on multidisciplinary approach to treat vascular malformation.
In a case study of 37 patient of head and neck vascular malformation (VM), using pre and post sclerotherapy MR imaging, Spence et al. [20] reported that Percutaneous sclerotherapy by using bleomycin is a safe technique to objectively decrease size and subjectively alleviate symptoms of facial VMs. Subjective clinical improvement is not always associated with visual size reduction on MR imaging. Hou et al. [21] reported 84% cure rate following first injection of bleomycin in treatment of facial VM. Sainsbury et al. [22] in a study of 164 patients reported an overall response rate of 93.3% and a complete cure rate of 82.7% following one intralesional bleomycin injection, they highly recommend its use in facial VMs.
In our study 8 mg of bleomycin was injected intralesionally in 8 patients with moderate sized low flow vascular lesion. We obtained a cure response rate of 87.5% following administration of first dose, a mean of 1.125 injections which was in agreement with few of the above mentioned studies. Only one patient out of eight required a second injection to obtain cure (Fig. 8). The complication encountered was again minimal.
When compared with STS the efficacy of bleomycin proved to be much higher as only 1.125 injections were required in contrast to five injections of STS to produce cure, that not only reduces the treatment time and cost but also avoids the traumatic experience of repeated injections to the patient, as most of such patients are young adults or child. No ineffective response or failure due to recurrence was noted in case of intralesional bleomycin when compared to STS injection in which a failure rate of 37.5% was obtained due to recurrence which is quite unacceptable (Fig. 9). Safety standards of both the drugs were good as no serious systemic or local complications were encountered during this study.
Conclusion
We highly recommend the use of intralesional bleomycin sclerotherapy in moderate sized low flow vascular malformation of craniofacial region, as marked superior result were obtained over STS therapy. Predictable results were obtained with a high success rate. No systemic or pulmonary complications occurred.
Future Directions
Very few or no valid study has been conducted till date comparing the efficacy of various sclerosing agents, larger sample size studies are required to compare the results obtained by us.
References
- 1.Mulliken JB, Glowacki J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg. 1982;69(3):412–422. doi: 10.1097/00006534-198203000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Sasaki GH, Pang CY, Wittliff JL. Pathogenesis and treatment of infant skin strawberry hemangiomas: clinical and in vitro studies of hormonal effects. Plast Reconstr Surg. 1984;73(3):359–370. doi: 10.1097/00006534-198403000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Folkman J, Klagsbrun M. Angiogenic factors. Science. 1987;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- 4.Yih WY, Ma GS, Merrill RG, Sperry DW. Central hemangioma of the jaws. J Oral Maxillofac Surg. 1989;47(11):1154–1160. doi: 10.1016/0278-2391(89)90005-0. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi K, Mulliken JB, Kozakewich HP, Rogers RA, Folkman J, Ezekowitz RA. Cellular markers that distinguish the phases of hemangioma during infancy and childhood. J Clin Invest. 1994;93(6):2357–2364. doi: 10.1172/JCI117241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson PL, Eckard DA, Brecheisen MA, Girod DA, Tsue TT. Percutaneous ethanol sclerotherapy of venous malformations of the tongue. AJNR Am J Neuroradiol. 2002;23(5):779–782. [PMC free article] [PubMed] [Google Scholar]
- 7.Rahbar R, McGill T, Mulliken J. Vascular tumors and malformations of the head and neck. In: Cummings CW, editor. Otolaryngology-head and neck surgery. Philadelphia: Mosby; 2005. pp. 4013–4026. [Google Scholar]
- 8.Kane WJ, Morris S, Jackson IT, et al. Significant hemangiomas and vascular malformations of the head and neck: clinical management and treatment outcomes. Ann Plast Surg. 1995;35(2):133–143. doi: 10.1097/00000637-199508000-00003. [DOI] [PubMed] [Google Scholar]
- 9.O’Donovan JC, Donaldson JS, Morello FP, Pensler JM, Vogelzang RL, Bauer B. Symptomatic hemangiomas and venous malformations in infants, children, and young adults: treatment with percutaneous injection of sodium tetradecyl sulfate. AJR Am J Roentgenol. 1997;169(3):723–729. doi: 10.2214/ajr.169.3.9275886. [DOI] [PubMed] [Google Scholar]
- 10.Baurmash H, Mandel L. The nonsurgical treatment of hemangioma with Sotradecol. Oral Surg Oral Med Oral Pathol. 1963;16:777–782. doi: 10.1016/0030-4220(63)90313-X. [DOI] [PubMed] [Google Scholar]
- 11.Minkow B, Laufer D, Gutman D. Treatment of oral hemangiomas with local sclerosing agents. Int J Oral Surg. 1979;8(1):18–21. doi: 10.1016/S0300-9785(79)80034-4. [DOI] [PubMed] [Google Scholar]
- 12.Govrin-Yehudain J, Moscona AR, Calderon N, Hirshowitz B. Treatment of hemangiomas by sclerosing agents: an experimental and clinical study. Ann Plast Surg. 1987;18(6):465–469. doi: 10.1097/00000637-198706000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Baurmash H, DeChiara S. A conservative approach to the management of orofacial vascular lesions in infants and children: report of cases. J Oral Maxillofac Surg. 1991;49(11):1222–1225. doi: 10.1016/0278-2391(91)90422-I. [DOI] [PubMed] [Google Scholar]
- 14.Khandpur S, Sharma VK. Utility of intralesional sclerotherapy with 3% sodium tetradecyl sulphate in cutaneous vascular malformations. Dermatol Surg. 2010;36(3):340–360. doi: 10.1111/j.1524-4725.2009.01440.x. [DOI] [PubMed] [Google Scholar]
- 15.Siniluoto TM, Svendsen PA, Wikholm GM, Fogdestam I, Edstrom S. Percutaneous sclerotherapy of venous malformations of the head and neck using sodium tetradecyl sulphate (sotradecol) Scand J Plast Reconstr Surg Hand Surg. 1997;31(2):145–150. doi: 10.3109/02844319709085481. [DOI] [PubMed] [Google Scholar]
- 16.Legiehn GM, Heran MK. Venous malformations: classification, development, diagnosis, and interventional radiologic management. Radiol Clin North Am. 2008;46(3):545–597. doi: 10.1016/j.rcl.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Acevedo JL, Shah RK, Brietzke SE. Nonsurgical therapies for lymphangiomas: a systematic review. Otolaryngol Head Neck Surg. 2008;138(4):418–424. doi: 10.1016/j.otohns.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 18.Muir T, Kirsten M, Fourie P, Dippenaar N, Ionescu GO. Intralesional bleomycin injection (IBI) treatment for haemangiomas and congenital` vascular malformations. Pediatr Surg Int. 2004;12(19):766–773. doi: 10.1007/s00383-003-1058-6. [DOI] [PubMed] [Google Scholar]
- 19.Zheng JW, Zhang ZY, Zhu HG, Wang YA (2006) Przegląd Flebologiczny 14(5):193–209 © Blackhorse Scientific Publishers, 2006
- 20.Spence J, Krings T, terBrugge KG, da Costa LB, Agid R. Interventional percutaneous sclerotherapy for facial venous malformations: subjective clinical and objective MR imaging follow-up results. Am J Neuroradiol. 2010;31:955–960. doi: 10.3174/ajnr.A1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hou R, Guo J, Hu K, Yang Y, Wang L, Kong L, Liu G, Lei D. A clinical study of ultrasound-guided intralesional injection of bleomycin A5 on venous malformation in cervical-facial region in China. J Vasc Surg. 2010;51(4):940–945. doi: 10.1016/j.jvs.2009.11.038. [DOI] [PubMed] [Google Scholar]
- 22.Sainsbury DCG, Kessell G, Fall AJ, Muir T. Intralesional bleomycin injection treatment for vascular Birthmarks: a 5-year experience at a single United Kingdom Unit. Plast Reconstr Surg. 2011;127(5):2031–2044. doi: 10.1097/PRS.0b013e31820e923c. [DOI] [PubMed] [Google Scholar]
- 23.Fonseca RJ (2000) Oral and maxillofacial surgery, vol 5. Philadelphia, W.B. Saunders Company, p 433