Abstract
During fear learning, anticipation of an impending aversive stimulus increases defensive behaviors. Interestingly, omission of the aversive stimulus often produces another response around the time the event was expected. This omission response suggests that the subject detected a mismatch between what was predicted and what actually occurred, thereby providing an indirect measure of cognitive expectancy. Here, we used functional magnetic resonance imaging to investigate whether omission-related brain activity reflects fear expectancy during learning and generalization of conditioned fear. During conditioning, a face expressing a moderate amount of fear (conditioned stimulus, CS+) signaled delivery of an aversive shock unconditioned stimulus (US), whereas the same face with a neutral expression was unreinforced. In a subsequent generalization test, subjects were presented with faces expressing more or less fear intensity than the CS+. Psychophysiological results revealed an increase in the skin conductance response (SCR) during learning when the US was omitted. Omission-related SCRs were also observed during the generalization test following the offset of high-but not low-intensity face expressions. Neuroimaging results revealed omission-related neural activity during learning in the anterior cingulate cortex, parietal cortex, insula, and striatum. These same regions also showed omission-related responses during the generalization test following highly expressive fearful faces. Finally, regression analysis on omission responses during the generalization test revealed correlations in offset-related SCRs and neural activity in the dorsomedial prefrontal cortex and posterior parietal cortex. Thus, converging psychophysiological and neural activity upon omission of aversive stimulation provides a novel metric of US expectancy, even to generalized cues that had no prior history of reinforcement.
Keywords: Pavlovian conditioning, fear generalization, electrodermal activity, orienting response, prediction error, functional magnetic resonance imaging
1. Introduction
Anticipating an aversive event frequently results in an increase in sympathetic arousal. In the laboratory study of fear learning, this anticipatory conditioned response (CR) is taken as evidence that a subject has learned the relationship between a neutral conditioned stimulus (CS) and delivery of an aversive unconditioned stimulus (US). But what happens when the US is omitted? The effects of US omission have been examined primarily for its role in extinction learning (Pavlov, 1927). Interestingly, an orienting response (OR) is generated at the time an anticipated US is typically delivered but unexpectedly absent, revealing that the subject detects a mismatch between the predicted and actual outcome (Sokolov, 1963). In this way, the omission-related OR provides an indirect measure of subjective processes like cognitive expectancy (Siddle and Lipp, 1997). The omission-related response has received little attention in neuroimaging studies of human fear learning. Here, we examined whether psychophysiological and neural activity associated with omission of an aversive US provides an index of expectancy during the acquisition and generalization of fear.
A motivation for examining activity associated with stimulus omission is that, unlike stimulus-specific responses, the omission response occurs in the absence of sensory stimulation (O’Gorman, 1973; Siddle, Remington, Kuiack, and Haines, 1983). Therefore, omission-responses are not constrained by arousal induced by processing the CS itself and may simply reflect cognitive states related to a perceived violation in outcome expectancy. This feature of the omission response may be of particular value in the study of fear generalization, wherein a number of physically different stimuli that have never directly predicted the US nonetheless evoke a fear response after acquisition training. Previous research has uncovered several factors influencing the generalized CR, including perceptual (Guttman and Kalish, 1956; Pavlov, 1927) or conceptual (Dunsmoor, Martin, and Labar, 2011a; Dunsmoor, White, and LaBar, 2011c; Razran, 1949) similarity to the CS, the physical intensity of the stimulus (Ghirlanda and Enquist, 2003), its emotional intensity (Dunsmoor, Mitroff, and LaBar, 2009) or learned equivalences through association with a common stimulus (Honey and Hall, 1989). Whether omission responses are concomitant with stimulus generalization, and are influenced by similar factors that affect the generalized CR, is unknown.
Omission related responses may provide an additional and complementary measure of learning and generalization that is not confounded by the myriad factors influencing cue-evoked anticipatory responses (e.g., the inherent fear-relevance of a CS that may determine conditioned responding (Öhman and Mineka, 2001) or variations in stimulus appearance, shape, or intensity that drive generalized responding). In this way, omission related activity may provide an unconfounded metric of cognitive expectancy, insofar as the magnitude of an omission response can be taken to reflect how strongly the subject had expected the US (Sokolov, 1963). We therefore hypothesized that omitting an aversive US would evoke an increase in psychophysiological and neural activity during learning and generalization testing when the US was expected relative to analogous time periods when the US was not expected. During functional magnetic resonance imaging (fMRI), subjects were presented with a range of faces of the same actor morphed between neutral and fearful endpoints before and after fear learning (see Figure 1). During fear learning, the middle face value along the neutral-to-fearful continuum (CS+) intermittently co-terminated with an electric shock US, whereas the most neutral face (CS−) was explicitly unreinforced.
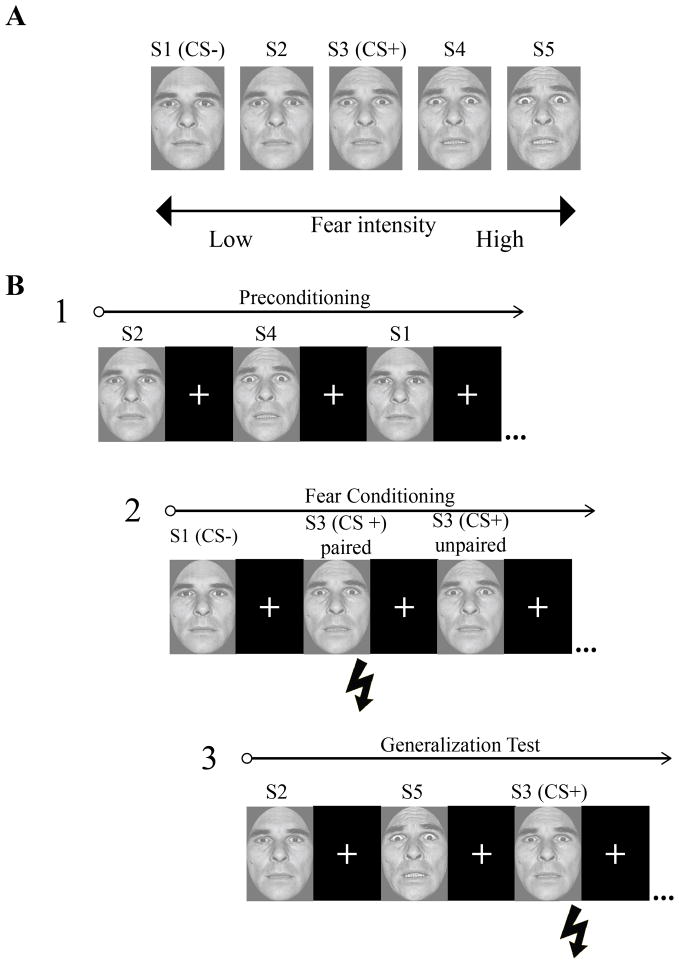
Figure 1.
Experimental paradigm. A) The conditioned and non-conditioned stimuli (S1–S5) consisted of a single identity morphed between neutral and fearful endpoints. The S1 and S2 were considered “low intensity” fearful faces and the S4 and S5 were considered “high intensity” fearful faces for the purpose of analysis. B) The experimental session included three phases: preconditioning, fear learning, and the generalization test. During fear learning, the US (pictured as a lightning bolt) followed the offset of CS+ paired trials and was omitted on CS+ unpaired trials. The US never followed the CS−. The CS+ was intermittently reinforced throughout the generalization test but never occurred following any of the other faces.
We first sought to identify psychophysiological and neural activity associated with the omission of the US during acquisition of conditioned fear when the US occurred with regularity following the CS+. We predicted increased skin conductance responses (SCRs) to the omission of the US following CS+ trials versus CS− trials for which the US had never occurred. This SCR finding would be in line with previous human electrodermal studies using non-aversive stimulus-stimulus associative learning procedures (Siddle, 1985; Siddle and Packer, 1987). We also predicted enhanced omission-related neural activity in regions important for detecting errors and signaling expectancy violations, including the dorsolateral prefrontal cortex (dlPFC), anterior cingulate cortex (ACC), and striatum (Botvinick, Cohen, and Carter, 2004; Schultz and Dickinson, 2000). Such findings would replicate the limited number of recent fMRI studies reporting brain activity indexing US omission following CS+ trials (Linnman, Rougemont-Bucking, Beucke, Zeffiro, and Milad, 2011; Spoormaker, Andrade, Schroter, Sturm, Goya-Maldonado, Samann, and Czisch, 2011a; Spoormaker, Schroter, Andrade, Dresler, Kiem, Goya-Maldonado, Wetter, Holsboer, Samann, and Czisch, 2011b).
Our second goal was to investigate for the first time whether learning-induced omission responses extend to generalization trials for which the US had never actually been paired with the stimulus but may be expected nonetheless. Subsequent to acquisition training, subjects were presented with faces of the same actor containing more or less fear intensity than the CS+ during a test of stimulus generalization. We predicted increased SCRs following the offset of highly fearful expressions (but not low-intensity expressions), reflecting a violation in US expectancy to generalized threats as a function of emotional intensity (Dunsmoor et al., 2009). We also predicted that neural activity upon the offset of highly fearful expressions during the generalization test would overlap with US omission-related activity observed during learning, indicating that similar regions signal expectancy violations despite physical differences in the antecedent cue and reinforcement history. Finally, we examined whether offset-related neural activity correlates with offset-related SCRs during the generalization test, extending neuroimaging evidence for functional coupling between central and peripheral indices of fear learning (Dunsmoor, Prince, Murty, Kragel, and LaBar, 2011b; Knight, Nguyen, and Bandettini, 2005).
2. Materials and Methods
2.1 Subjects
Twenty-five healthy-right handed young adults participated in this study. Two subjects were not included in the final analysis due to excessive head motion (> 3 mm in any direction) and 9 subjects were not included due to a lack of SCR data (5 subjects lacked SCR data due to technical issues, and 4 subjects showed no measurable responses). Fourteen healthy right-handed subjects (7 females; age range = 19 to 30; median age = 22 yrs) were included in the final analysis. All participants provided written informed consent in accordance with the Duke University Institutional Review Board guidelines.
2.2 Stimulus material
Stimuli consisted of a male face morphed along a gradient from neutral-to-fearful taken from the Ekman pictures of facial affect (Ekman and Friesen, 1976) positioned in a full-frontal orientation and cropped to remove hair, ears, and neckline. Five morphs were created along the continuum using Morph-Man 2000 software (STOIK): 11.11% fear/88.88% neutral, 33.33% fear/66.66% neutral, 55.55% fear/44.44% neutral, 77.77% fear/ 22.22% neutral, and 100% fear (see also Graham, Devinsky, and LaBar, 2007; Thomas, De Bellis, Graham, and LaBar, 2007). These values were chosen based on our prior work using the same morph continuum (e.g. Thomas et al., 2007), which showed that subjects can readily discriminate between morph values even more subtle than those used in the present study (see Dunsmoor et al., 2011b for a more detailed discussion on this point). In this regard, generalization effects cannot be attributable to mere perceptual confusion. For clarity, these stimuli are labeled as S1, S2, S3, S4, and S5, respectively.
2.3 Experimental procedure
The imaging session consisted of three phases that occurred in the same order for each subject: preconditioning, fear learning, and the generalization test (Figure 1). A brief habituation phase consisting of one presentation of each of the five stimuli took place prior to the start of the preconditioning phase (data from these few habituation trials are not reported). Each stimulus presentation was 4 s in duration, during which time subjects rated whether or not the face was expressing fear (forced choice: yes/no) as quickly and accurately as possible by pressing one of two buttons (motor response was counterbalanced across subjects). A black screen with a white fixation cross followed the offset of the face stimulus during the intertrial interval (ITI). The lengths of the waiting period during the ITI were jittered according to an exponential distribution function. Preconditioning included a total of 9 trials of each of the 5 face stimuli (45 total trials), and the length of the waiting period during the ITI was jittered with an average length of 5 s (minimum 4 s). Subjects were not informed that the US would be absent during preconditioning. Fear learning followed preconditioning and included only two trial types: 16 trials of the S3 (CS+) and 16 trials of the S1 (CS−) with an average waiting period during the ITI of 11 s (minimum 9 s). The CS+ intermittently co-terminated with the US on 10 of 16 trials (partially reinforced, delay conditioning procedure), whereas the CS− served as a control stimulus and was never paired with the US. A 5-min break followed preconditioning and fear learning, during which time subjects passively viewed a silent video of a train traveling through British Columbia (High Ball Productions) while still inside the MR-scanner. FMRI acquisition did not occur during the video break. The generalization test followed fear learning and contained 9 trials of each of the 5 face stimuli (45 total trials) with an average waiting period during the ITI of 9 s (minimum 5 s). The S3 was intermittently paired with the US on 6 of 9 trials in a steady-state generalization test, which was employed in order to mitigate the potential effects of extinction over the course of an extended testing session (Blough, 1975; Honig and Urcuioli, 1981). The order of stimulus presentation was counterbalanced and pseudorandomized such that no more than two of the same face exemplars appeared consecutively. Subjects were not informed of the CS−US contingencies.
2.4 Psychophysiological measures, analysis, and shock administration
SCRs were acquired from the middle phalanx of the second and third digits on the non-dominant hand. The electric shock stimulator was attached to the right wrist and calibrated prior to the start of the experiment to a level deemed “highly annoying but not painful” using an ascending staircase procedure (Dunsmoor et al., 2009). Measurement of SCRs and shock administration were controlled by the MP-150 BIOPAC system (Goleta, CA) using MRI-compatible electrodes. A response was considered omission-related if the trough-to-peak response occurred 0.5 – 4 s following stimulus offset, lasted between 0.5 and 5 s, and was greater than 0.02 microSiemens. Trials that did not meet these criteria were scored as zero. It is important to note that defining omission responses in this way ensures that SCRs related to stimulus offset are distinct from cue-related SCRs. To normalize the SCR data, raw SCR values were range-corrected using each subject’s maximum SCR (Lykken and Venables, 1971), which was elicited in all cases by the US. These range-corrected values were square-root transformed to normalize the distribution. Statistical analysis of SCR data involved repeated-measures ANOVA and polynomial trend analysis with an alpha value of 0.05 (SPSS 15.0, Chicago, IL).
2.5 fMRI acquisition
Whole-brain functional imaging was conducted on a General Electric Signa EXCITE HD 3.0 Tesla MRI scanner. Blood oxygenation level-dependent (BOLD) functional images were acquired parallel to the AC-PC line using a SENSE™ spiral-in sequence: acquisition matrix, 64 x 64; field of view, 256 x 256; flip-angle, 60°; 34 slices with interleaved acquisition; slice thickness, 3.8 mm with no gaps between slices; in-plane resolution = 3.75 mm X 3.75 mm; repetition time, 2 s; echo time, 27 ms. Preprocessing was conducted using SPM8 (Wellcome Trust Center, www.fil.ion.ucl.ac.uk) implemented in Matlab (The Mathworks Inc, Natick MA). The first 4 functional images were removed from each scanning run to account for magnetic equilibration, and the remaining images were corrected for head motion using a center-of-mass movement threshold of 3 mm. Images were realigned, spatially normalized to the Montreal Neurological Institute (MNI) template, voxel sized resampled to 2 x 2 x 2 mm, and smoothed using an isotropic 8-mm3 Gaussian full-width half-maximum kernel. A high-pass filter of 128 s was applied to account for low-frequency drifts.
2.6 GLM Analysis
Analysis of the preprocessed data included a general linear model with separate regressors for each stimulus presented. The onset of each cue was set at the moment the stimulus appeared, and the hemodynamic response was modeled for each trial using a variable duration design that incorporates reaction time (Grinband, Wager, Lindquist, Ferrera, and Hirsch, 2008). Offset-related activity was modeled using an impulse (dirac) function corresponding to the moment the face was removed from the screen (i.e. when the absence of the US would be detected). The shock US was modeled as an impulse function and was included as a regressor of no interest. Six head movement parameters were also included as nuisance variables to account for subject motion. Events defined for fear learning included the following: CS− onset and offset, CS+ onset, CS+ unpaired offset (i.e. US omission), and CS+ paired offset (i.e. US presentation). Events defined for preconditioning and the generalization test included the onsets and offsets for each of the S1–S5 faces and the shock US. The generalization test analysis focused on the offsets for the low (S1, S2) and high (S4, S5) intensity faces, and stimulus onsets and offsets for the S3 were treated as covariates of no interest. Given that offset-related SCRs during the generalization test were differentiated as a function of low versus high intensity (see SCR results), the S1/S2 (low intensity) and S4/S5 (high intensity) stimuli were binned together for subsequent analyses.
Due to the inherent nature of the classical conditioning design, there is the potential for multicollinearity between these regressors (see also Linnman et al. (2011)). Namely, CS duration is constant; however, trial duration is jittered and a partial reinforcement schedule is used, which should reduce potential multicollinearity issues. Nonetheless we assessed collinearity of the design by pairwise correlations between regressors of interest for each subject. The offsets following CS− and CS+ unpaired trials (fear learning) were uncorrelated, as were the offsets between low and high intensity non-conditioned trials (generalization test): Pearson correlation coefficients between regressors ranged from −0.19 to −0.1. Collinearity was also low among the onsets versus offsets of the CS+ and CS− during fear learning (ranging from 0.08 to 0.31, median 0.16) and onsets versus offsets of high and low intensity non-conditioned trials during the generalization test (ranging from 0.09 to 0.37, median 0.24).
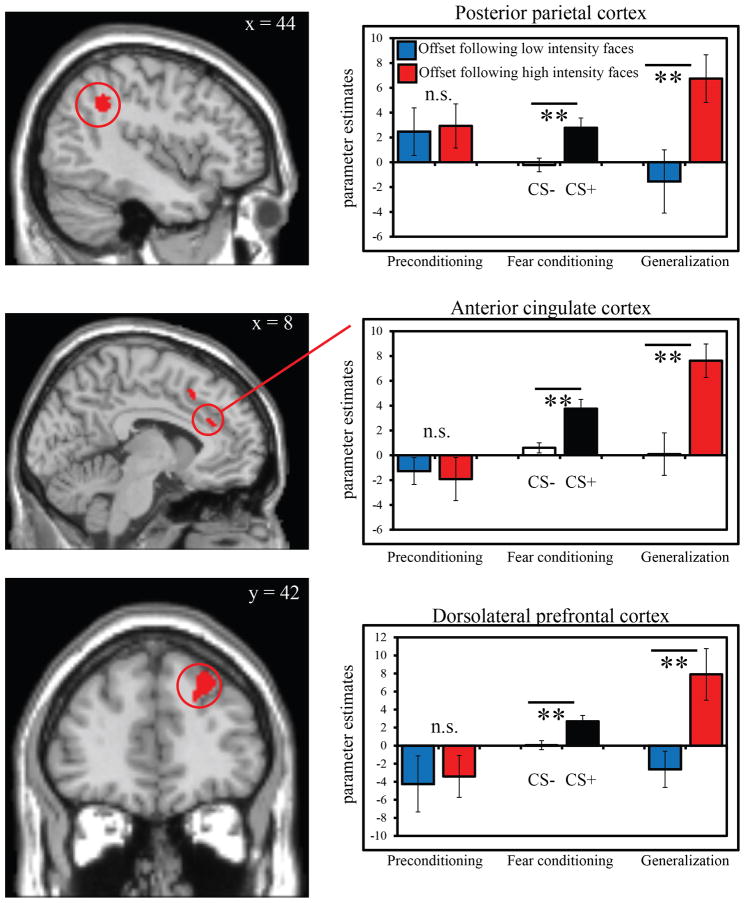
Second-level random-effects analysis for the fear learning phase included a 2 x 2 ANOVA using the factors of event time (onset, offset) and condition (CS+, CS−). Second-level random-effects analysis of the generalization test also employed a 2 x 2 ANOVA using event time (onset, offset) and condition (low intensity, high intensity) as factors. Group level analyses explored areas exhibiting differential increases in activity following CS+ unpaired versus CS− (fear learning) and following high versus low intensity non-conditioned stimuli (generalization test). We also explored regions showing an interaction between event time and condition. Activations from all second-level analyses were identified at p < .001, uncorrected, with a cluster extent threshold of 68 contiguous voxels. This cluster extent threshold was calculated using the REST AlphaSim utility (www.restfmri.net; toolkit V1.3) resulting in a cluster-correction of p < .05. AlphaSim (http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf) operates by conducting 1,000 Monte Carlo simulations to reject false positives based on cluster extent thresholds (Forman, Cohen, Fitzgerald, Eddy, Mintun, and Noll, 1995). For a priori regions of interest that did not survive cluster extent threshold, a small volume correction was applied using a family-wise error (FWE) correction of p < .05. Small volume correction for the caudate (Seymour, O’Doherty, Dayan, Koltzenburg, Jones, Dolan, Friston, and Frackowiak, 2004) was applied using anatomical masks from the Wake Forest PickAtlas toolbox (Maldjian, Laurienti, Kraft, and Burdette, 2003). Based on prior work showing activity in the midbrain (red nucleus) in response to an omitted shock, a 4 mm sphere was drawn around this region using the peak coordinates supplied by Linnman et al. (2011). The mean beta parameters from voxels in regions of interest (ROI) identified as overlapping between the fear learning and generalization test analyses were extracted and are plotted for illustrative purposes.
2.7 SCR regression analysis
A regression analysis was conducted examining correlations between generalized offset-related SCRs and brain activity following high- versus low-intensity faces. This analysis focused on regions exhibiting brain-behavior correlations in generalized offset activity during the early/middle trials (trials 1–6) of the generalization test, as SCR evidence for generalized offset responses was strongest during this phase. First, for each subject, normalized SCR values from trials 1–6 for the S1 and S2 (low intensity) were averaged and combined into a single value, as were values for the S4 and S5 (high intensity) trials. A second level multiple regression model in SPM8 was then conducted using each subjects SCR difference score (high minus low intensity offset-responses) as a covariate regressed against single subject (first level) brain imaging contrasts of the first 6 high minus the first 6 low intensity offsets. The mean parameter estimates from the functional ROIs were extracted and brain-behavior correlations were plotted for illustrative purposes only.
3. Results
Data reported here are from the omission responses only. For analyses relating to CS onsets from the same dataset, see Dunsmoor et al. (2011b).
3.1 Psychophysiological results
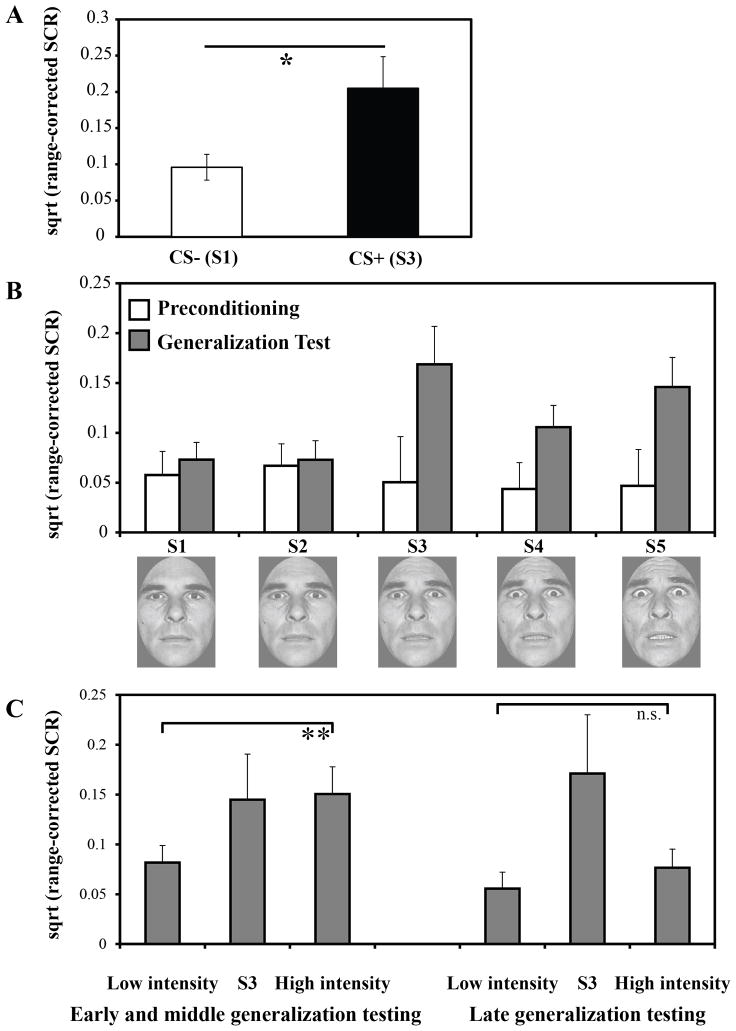
During fear learning, offset-related SCRs on CS+ unpaired trials were significantly greater than offset-related SCRs on CS− trials, t (13) = 2.39, p < 0.05 (Fig 2A). Repeated-measures ANOVA of omission responses during the generalization test, using the preceding stimulus (S1–S5) as a within-subjects factor, showed a main effect of intensity value, F (4, 52) = 3.78, p < 0.01, with a linear trend, p = 0.01, demonstrating that offset-related SCRs selectively increased as a function of fear intensity (Fig 2B, gray bars). This pattern of offset-related SCRs during the generalization test is consistent with SCRs elicited by the cue, such that generalized SCRs were lowest to the S1 and S2 and larger for the S4 and S5 (reported in Dunsmoor et al., 2011b). Notably, offset-related SCRs were low and undifferentiated prior to fear learning, F (4, 52) = 0.87, p = 0.53 (Fig. 2B, white bars). A stimulus (S1–S5) x phase (preconditioning, generalization test) ANOVA revealed a main effect of phase, F (1, 13) = 12.27, p < 0.001, as well as a phase x stimulus interaction, F (4, 52) = 4.36, p < 0.01. This pattern indicates that SCRs following stimulus presentation were dissociated as a function of the preceding stimulus type during conditioning (CS+ versus CS−) and the generalization test (high > low intensity faces).
Figure 2.
Omission-related SCRs. A) During fear learning, greater omission-related SCRs were exhibited following CS+ unpaired versus CS− trials. B) Omission-related responses were low and undifferentiated as a function of fear intensity value prior to fear learning (white bars) but increased following fear learning for the CS+ and high fear intensity trials (gray bars). C) The difference in offset SCRs between low (S1/S2) and high (S4/S5) fearful faces was greatest during the early and middle phase of the generalization test, and diminished by late generalization testing. Error bars reflect standard error (SEM). * p < 0.05, ** p < 0.01.
Analysis of offset responses over the course of the generalization test trials revealed a significant effect of fear intensity, F (3, 39) = 3.84, p < 0.05, as well as a fear intensity x trial interaction, F (24, 312) = 1.78, p < 0.05. As shown in Figure 2C, offset responses were differentiated during the early and middle phases (trials 1–6) of the generalization test following high intensity faces, t (13) = 2.90, p < 0.01, but offset-related SCRs were undifferentiated during later generalization testing (trials 7–9), p > 0.5.
To examine the relationship between generalized cue-evoked and offset-related SCRS, we conducted a trial by trial analysis of all generalization test trials in which an offset-related response was evoked. We did not find a trial by trial correlation in the magnitude of the cue-evoked SCR and offset-related SCR, p > .1. This finding suggests that the offset response does not provide redundant information (i.e., is merely a second CR), but instead reflects a different behavioral component related to the omission of the US.
3.2 Whole-brain fMRI analyses
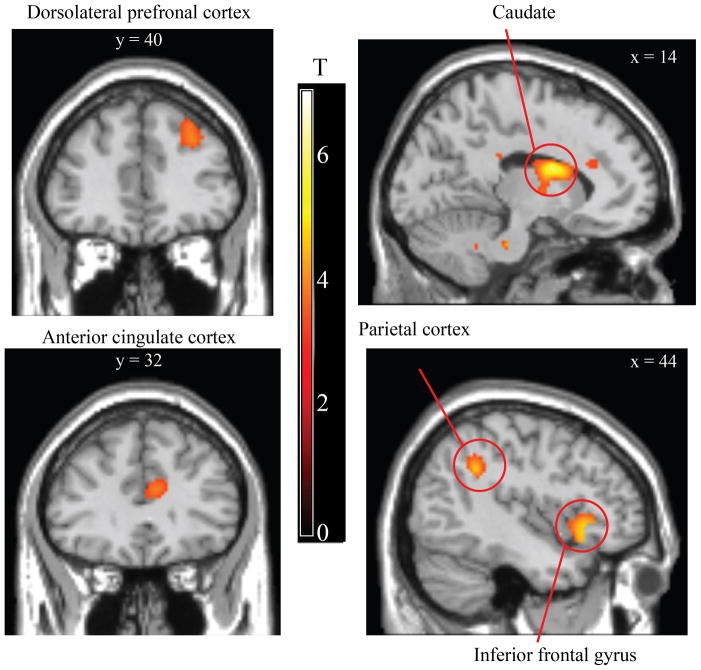
Whole-brain analysis of fear learning focused on the difference in brain activity at cue offset on trials in which the US was expected but did not occur relative to when it was not expected (CS+ unpaired offset > CS− offset). This analysis revealed activation in the ACC, dlPFC, posterior parietal cortex (PPC), and striatum (Table 1 and Fig 3). Greater offset-related responses following CS− trials relative to CS+ trials were observed in the middle temporal gyrus (Table 1). The event time (onsets, offsets) by condition (CS+ unpaired offset, CS− offset) ANOVA revealed a positive interaction (CS− onset > CS+ onset and CS+ offset > CS− offset) in dlPFC and ACC (Supplemental Table 1). See also Supplemental Table 2 for analysis of CS+ onsets versus CS+ unpaired offsets.
Table 1.
Regions exhibiting offset-related activity during fear learning following CS+ unpaired vs. CS− trials. Activations identified at cluster correction p < .05.
| Region | Hemisphere | MNI coordinates | # of voxels | Peak T | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Offsets: CS+ unpaired > CS− | ||||||
| Caudate | Right | 10 | 4 | 18 | 1041 | 6.04 |
| Thalamus | Right | 6 | −4 | 2 | 4.66 | |
| Anterior cingulate | Right | 8 | 18 | 28 | 4.15 | |
| Putamen | Right | 26 | 14 | −18 | 1091 | 5.42 |
| Putamen | Right | 26 | 22 | 0 | 4.89 | |
| Insula | Right | 42 | 20 | −4 | 4.76 | |
| Insula | Left | −40 | 18 | −6 | 284 | 5.32 |
| Insula | Left | −34 | 4 | −6 | 3.99 | |
| Superior frontal gyrus | Right | 4 | 18 | 64 | 275 | 5.15 |
| Supramarginal gyrus | Right | 44 | −48 | 34 | 429 | 4.8 |
| Supramarginal gyrus | Right | 52 | −46 | 36 | 4.36 | |
| Inferior parietal lobule | Right | 54 | −36 | 40 | 3.88 | |
| Putamen | Left | −22 | 12 | −16 | 161 | 4.79 |
| Middle frontal gyrus | Right | 36 | −4 | 42 | 216 | 4.36 |
| Middle frontal gyrus | Right | 34 | 0 | 52 | 3.84 | |
| Superior frontal gyrus | Right | 26 | 42 | 42 | 232 | 4.16 |
| Middle frontal gyrus | Right | 28 | 40 | 34 | 4 | |
| Superior frontal gyrus | Right | 20 | 44 | 30 | 3.84 | |
| Middle frontal gyrus | Right | 62 | −40 | 6 | 308 | 4.12 |
| Inferior frontal gyrus | Right | 52 | 4 | 18 | 244 | 3.89 |
| Inferior frontal gyrus | Right | 52 | 12 | 24 | 3.67 | |
| Offsets: CS− > CS+ unpaired | ||||||
| Paracentral lobule | Left | −10 | −32 | 74 | 317 | 4.88 |
| Paracentral lobule | Middle | 2 | −28 | 78 | 3.62 | |
| Medial frontal gyrus | Right | 12 | −24 | 58 | 127 | 4.71 |
| Precentral gyrus | Right | 12 | −22 | 72 | 3.95 | |
| Postcentral gyrus | Right | 32 | −30 | 72 | 156 | 4.37 |
| Postcentral gyrus | Right | 30 | −40 | 66 | 3.35 | |
| Middle temporal gyrus | Left | −60 | −10 | −20 | 98 | 4.19 |
| Middle temporal gyrus | Left | −42 | −68 | 26 | 84 | 4.06 |
Figure 3.
Differential offset-related brain activity during fear learning. Enhanced offset-related brain activity following CS+ unpaired trials versus CS− trials was observed in the dorsolateral prefrontal cortex, anterior cingulate cortex, caudate, parietal cortex, and inferior frontal gyrus. A full set of coordinates is available in Table 1. Activation is thresholded at p < 0.001, uncorrected, for illustrative purposes.
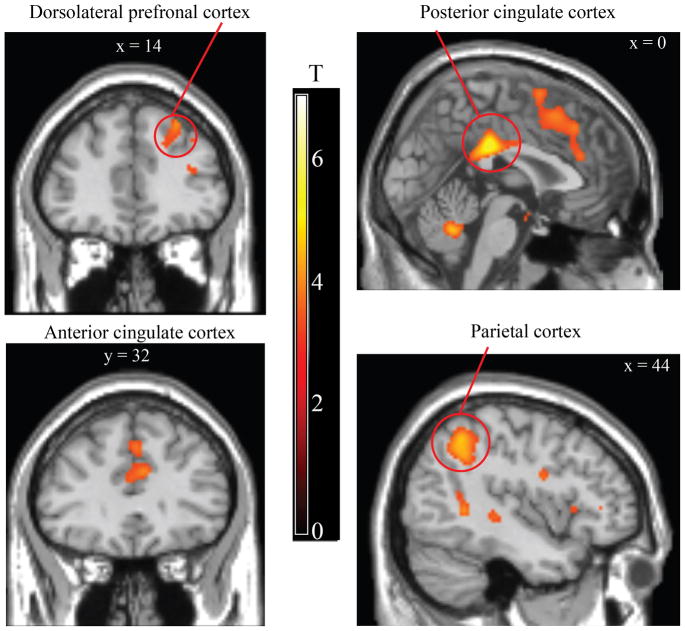
We next compared activity during the generalization test following high intensity versus low intensity faces that did not predict shock. We observed activations in ACC, posterior cingulate cortex, PPC, and insula (Table 2 and Fig 4). Small volume correction revealed activations in the caudate and midbrain. Several of these regions overlapped with those identified from the fear learning analysis (Table 3 and Fig 5). The event time (onsets, offsets) by condition (high, low intensity non-conditioned trials) ANOVA revealed a positive interaction (low intensity onset > high intensity onset and high intensity offset > low intensity offset) in the dlPFC, posterior cingulate gyrus, and parietal cortex (Supplemental Table 3). An analysis of offset-related responses during preconditioning revealed no regions exhibiting greater offset-related activity following high versus low intensity trials (or vice-versa) and no interaction between event time and condition, suggesting that differential neural activity emerged as a consequence of fear learning.
Table 2.
Regions exhibiting greater offset-related activity during the generalization test following high versus low intensity faces. Activations identified at cluster correction p < .05.
| Region | Hemisphere | MNI coordinates | # of voxels | Peak T | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Offsets: High intensity > Low intensity trials | ||||||
| Posterior cingulate | Middle | 4 | −28 | 32 | 1026 | 5.7 |
| Posterior cingulate | Left | −14 | −28 | 32 | 5.53 | |
| Posterior cingulate | Right | 20 | −22 | 38 | 4.22 | |
| Cerebellum | Left | −14 | −82 | −38 | 1462 | 5.58 |
| Cerebellum | Left | −14 | −58 | −32 | 5.38 | |
| Cerebellum | Left | −38 | −62 | −36 | 5.31 | |
| Caudate tail | Left | −28 | −30 | 26 | 211 | 5.49 |
| Middle frontal gyrus | Right | 34 | 4 | 50 | 288 | 5.03 |
| Precentral gyrus | Right | 42 | 0 | 24 | 3.76 | |
| Precentral gyrus | Right | 32 | 0 | 32 | 3.58 | |
| Inferior parietal lobule | Right | 42 | −52 | 46 | 820 | 4.65 |
| Supramarginal gyrus | Right | 58 | −54 | 38 | 4.28 | |
| Superior temporal gyrus | Right | 48 | −44 | 26 | 4.25 | |
| Cerebellum | Right | 16 | −72 | −38 | 185 | 4.43 |
| Cerebellum | Right | 14 | −76 | −26 | 3.99 | |
| Superior frontal gyrus | Right | 24 | 42 | 46 | 111 | 4.41 |
| Middle frontal gyrus | Right | 26 | 50 | 32 | 3.31 | |
| Precuneus | Right | 12 | −66 | 42 | 311 | 4.28 |
| Middle frontal gyrus | Right | 48 | 20 | 28 | 129 | 4.26 |
| Middle temporal gyrus | Right | 62 | −52 | 6 | 169 | 4.22 |
| Parahippocampal gyrus | Right | 42 | −50 | 2 | 4.16 | |
| Middle frontal gyrus | Right | 34 | 44 | 12 | 172 | 4.22 |
| Middle frontal gyrus | Right | 34 | 46 | 20 | 3.98 | |
| Middle frontal gyrus | Right | 28 | 48 | 2 | 3.93 | |
| Superior frontal gyrus | Middle | 0 | 18 | 50 | 633 | 4.16 |
| Anterior cingulate | Middle | 4 | 34 | 24 | 4.14 | |
| Medial frontal gyrus | Middle | 0 | 30 | 42 | 4 | |
| * Caudate | Right | 8 | 8 | 14 | 21 | 3.73 |
| * Midbrain | Middle | −6 | −18 | −8 | 28 | 2.96 |
| Offsets: Low intensity > High intensity trials | ||||||
| No significant clusters of activation | ||||||
Small volume correction p < .05, family wise error.
Figure 4.
Differential offset-related brain activity during the generalization test. Enhanced offset-related brain activity following high versus low intensity faces was observed in the caudate, anterior cingulate cortex, posterior cingulate cortex, and parietal cortex. A full set of coordinates is available in Table 2. Activation is thresholded at p < 0.001, uncorrected, for illustrative purposes.
Table 3.
Regions of overlap between the fear learning (offsets: CS+ unpaired > CS− trials) and generalization test (offsets: high > low intensity) analyses.
| Region | Hemisphere | Center of Mass | # of voxels | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Supramarginal gyrus | Right | 45.1 | −48 | 35.7 | 151 |
| Middle frontal gyrus | Right | 34 | −0.2 | 46.5 | 76 |
| Superior frontal gyrus | Right | 22.8 | 42.8 | 39.9 | 73 |
| Anterior cingulate | Middle | 1.6 | 16.1 | 44 | 63 |
| Anterior cingulate | Middle | 4 | 33.1 | 23.5 | 23 |
| Inferior frontal gyrus | Right | 49.5 | 18.1 | 25.4 | 29 |
| Inferior parietal lobule | Right | 52.7 | −47.9 | 52.2 | 25 |
| Superior frontal gyrus | Middle | 1.8 | 20.4 | 54.5 | 16 |
Figure 5.
Regions of overlap between the fear learning and generalization test analyses. Regions commonly activated at stimulus offset following CS+ > CS− and high > low intensity non-conditioned faces, including the anterior cingulate gyrus, posterior parietal cortex, and dorsolateral prefrontal cortex. See Table 3 for a full list of regions of overlap. Bar graphs demonstrate that prior to fear learning, offset-related activity was undifferentiated within these regions. During fear learning, offset-related activity selectively increased following CS+ vs. CS− trials. This pattern was evident during the generalization test as well for highly fearful faces that had never been reinforced. Error bars reflect standard error (SEM). ** p < 0.01, n.s. = non-significant.
3.3 Brain-behavior correlations in generalized offset responses
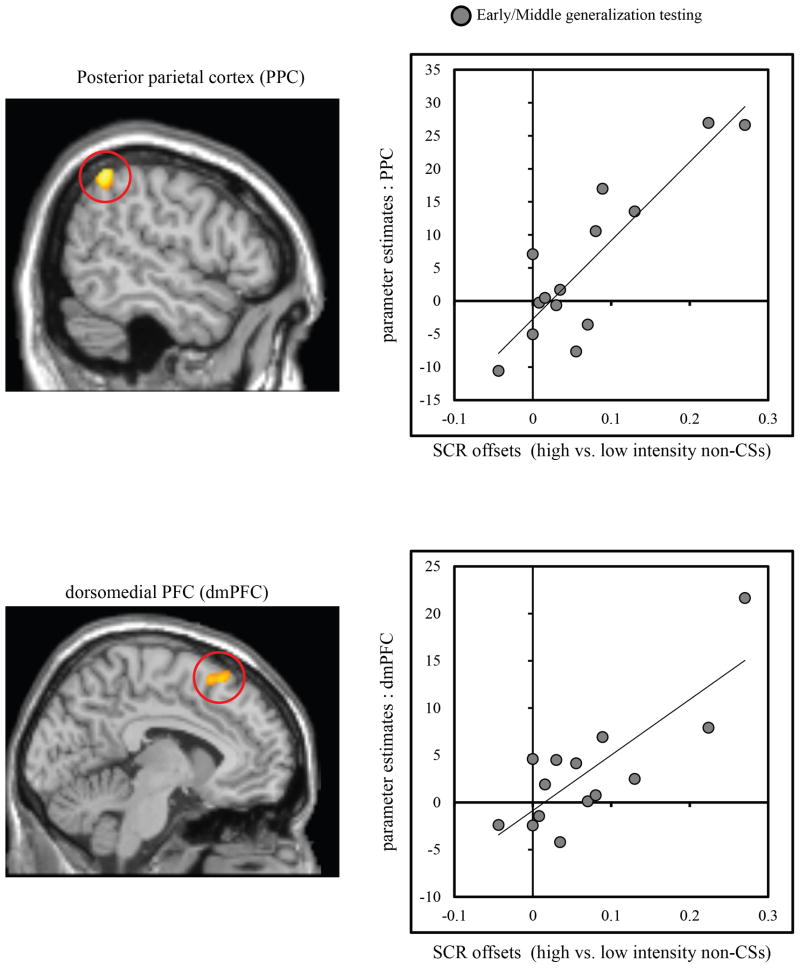
Brain activity in the PPC (x = −54, y = −52, z = 52; 78 voxels), and dorsomedial PFC (dmPFC) (x = 6, y = 18, z = 58; 91 voxels), was positively correlated with the difference in offset-related responses between high vs. low intensity faces during the early/middle runs of the generalization test, when SCR evidence of generalized offset responses were strongest (Fig 6).
Figure 6.
Regression analysis relating fMRI and SCR indices of offset activity during generalization testing as a function of fear intensity. Correlations were conducted across subjects. During the early and middle runs of the generalization test when differences in offset-related SCRs were strongest, activity in the posterior parietal cortex (PPC), and dorsomedial prefrontal cortex (dmPFC) tracked the difference in offset-related SCRs following high versus low intensity faces (gray circles).
4. Discussion
Studies of conditioned fear learning have almost universally focused on activity related to processing cues predictive of an aversive event. Here, we provide evidence that activation related to the omission of an aversive event reveals the effects of learning and generalization of conditioned fear. During learning, the omission of an expected shock US resulted in enhanced SCRs around the time that the electrical stimulation was typically delivered. Neural activity indexing US omission was observed in the anterior cingulate gyrus, dlPFC, parietal cortex, and striatum. We show here for the first time that these omission-related autonomic and brain activation patterns later generalize to faces containing greater fear intensity than the CS+, suggesting a transfer of expectancy to physically distinct stimuli that had never directly predicted the US. The transfer occurred along a gradient of emotional expression intensity. These observations demonstrate that neurophysiological activity associated with the omission of an expected aversive event provides a unique metric to investigate fear expectancy, even in cases when the US had never been delivered in conjunction with the stimulus.
4.1 Offset-related SCRs
During fear learning, offset-related SCRs were enhanced following the CS+unpaired (when the US was expected but omitted) relative to the offset of the CS− (when the US was neither presented nor expected). Previous studies of omission responses using non-aversive associative learning procedures have shown that the omission of a second stimulus from a stimulus-stimulus pair (e.g. light-tone pair) results in an increase in SCRs around the time the second stimulus was expected (Siddle, 1985; Siddle and Lipp, 1997), which is likely due to an expectation that the second stimulus should follow the first stimulus (Siddle, 1991). In support of the expectancy model, Siddle and colleagues have found that the removal of the second stimulus lowers subjective expectancy measures (Siddle, Booth, and Packer, 1987) and results in a large increase in SCRs to the stimulus when it is later reintroduced, as a measure of dishabituation (Siddle, 1991). In the present study, greater offset responses were also found following high versus low intensity fearful faces during the generalization test, despite the fact that the US had never been paired with these faces. This pattern of offset-related SCRs compliments the pattern of cue-evoked responses reported previously (Dunsmoor et al., 2011b), such that both the cue-evoked and omission responses showed an asymmetrical gradient biased towards stimuli of high emotional intensity (Ghirlanda, 2002), as opposed to a symmetrical bell-shaped gradient indicative of similarity-based generalization (Honig and Urcuioli, 1981). The former gradient is characteristic of an intensity-based generalization gradient that is often found along dimensions of increasing physical intensity (Ghirlanda, 2002; Ghirlanda and Enquist, 2003), such as increases in brightness or loudness. Importantly, a trial-by-trial analysis showed no correlation between the magnitude of the CR and omission responses, suggesting that omission responses are not simply indexing a second CR. Instead, we interpret the omission response as an index of US expectancy violation that is not entirely captured by the cue-evoked response, which may instead index an associative or attentional relationship with the prediction of the impending US. It is also noteworthy that in a retrospective test of CS+ awareness a majority of subjects (71%, 10 out of 14) mistakenly identified the more fearful S4 as the CS+ (reported in Dunsmoor et al. (2011b)). As this bias to identify a fearful face as the CS+ was revealed following the generalization test, it will be interesting to examine expectancy during the course of generalization testing in future studies in order to directly assess the relationship between subjective expectancy and omission-related activity.
4.2 Offset-related fMRI responses
The brain regions identified as showing enhanced activity at cue offset during fear learning and generalization falls under a collection of areas implicated in detecting and signaling error. For instance, the anterior cingulate cortex has consistently been implicated in tasks involving the detection of conflict and error (Botvinick et al., 2004). In rodents, the medial PFC region is involved in predictive fear learning, such that reversible inactivation of the rodent medial PFC prevents learning from prediction errors (PE) in a blocking paradigm (Furlong, Cole, Hamlin, and McNally, 2010). Activity was also observed in the parietal cortex, a region generally implicated in attentional processing (Posner and Petersen, 1990), including attention to unpredictable events (Hahn, Ross, and Stein, 2007). The parietal cortex may be important for learning from surprise, as lesions of cholinergic neurons in the posterior parietal cortex in rodents impairs surprise-induced associative learning (Bucci, 2009; Maddux, Kerfoot, Chatterjee, and Holland, 2007) – learning based on the allocation of attention towards cues that are not consistently predictive of a US (Pearce and Hall, 1980). Finally, the striatum is strongly implicated in coding reward-related PEs, and several human neuoroimaging studies have found that activity in the striatum tracks PE signals computed from reinforcement learning algorithms during reward-related (Daw, Gershman, Seymour, Dayan, and Dolan, 2011; O’Doherty, Dayan, Friston, Critchley, and Dolan, 2003) and aversive learning (Delgado, Li, Schiller, and Phelps, 2008; Schiller, Levy, Niv, LeDoux, and Phelps, 2008; Seymour et al., 2004) tasks.
Several of the brain areas showing enhanced offset-related activity during learning and generalization in the present study have been identified in recent reports examining omission of an aversive US during fear learning, including the caudate, putamen, inferior frontal gyrus, and insula (Linnman et al., 2011; Spoormaker et al., 2011a; Spoormaker et al., 2011b). Enhanced neural responses to a non-delivered shock on CS+ versus CS− trials has been interpreted to reflect anticipation for an impending shock, as opposed to a PE signal per se (Linnman et al., 2011). Our present findings are consistent with the interpretation that omission signals reflect US expectancy, and, importantly, extend this finding to include a class of generalized threats that have no direct history of reinforcement.
4.3 Generalized offset-related SCR regression analysis
Generalized offset-related SCRs following highly fearful faces were associated with increased activity in the dmPFC and PPC. The dmPFC and PPC are important for allocating attention and learning from PEs (Bucci, 2009; Maddux et al., 2007; McNally, Johansen, and Blair, 2011). In addition, activity in these regions replicates reports examining offset-related activity during fear acquisition (Spoormaker et al., 2011a). Notably, correlations between SCRs and neural activity were detected during the early/middle phases of the generalization test, when subjects exhibited larger offset-related SCRs following fearful faces and when subjects may have been more likely to expect the US. At the end of generalization testing, offset-related SCRs following high fearful faces diminished and were undifferentiated from SCRs produced following low intensity faces. This decrease in offset-related SCRs may have resulted from subjects’ realization that highly fearful faces ultimately were never paired with shock during the generalization test. Of note, the use of fear-relevant stimuli in this study may have helped sustain omission responding during the extended generalization test, whereas omission responses may be weaker and dissipate more quickly along non-intensity sensory dimensions (e.g., wavelengths of light or frequency of sound). In this study, we utilized a dimension of fear-relevant stimuli because they provide an ecologically valid source for the transfer of fear expectancy (Öhman and Mineka, 2001); but the use of fear-relevant stimuli may present an inherent potential for confounding arousal induced by learning from that induced by the fear-relevant qualities of the cues. Importantly, we show here that omission-related responses provides a complementary measure to verify the effects of fear learning that mitigates sensory confounds related to processing fear-relevant cues. Interestingly, subjects in this study consisted of psychologically healthy adults, which raises the intriguing possibility that different levels of sustained omission responses over a longer duration generalization test may relate to individual differences in anxiety levels, even when the stimulus dimension is not inherently fear-relevant [e.g. geometric shapes (Lissek, Rabin, Heller, Lukenbaugh, Geraci, Pine, and Grillon, 2010)].
4.4 Effects of US omission on orienting and information processing
These results are interpreted within theoretical models of the orienting response (Sokolov, 1963). In the Sokolovian model, ORs are induced by novel or significant stimuli, thereby enabling an organism to allocate information-processing resources towards meaningful stimuli in the environment. Typically, an organism will habituate to stimulation that is repeated or fully predicted but will show an orienting response when there is a perceptible change in stimulation or stimulation occurs unexpectedly. Sokolov (1963) proposed a mechanism that detects the difference between the stored history of stimulation and current input; namely, habituation occurs when the input matches the stored representation but an OR is produced when there is a discrepancy. In this way, the omission of a regularly repeating stimulus would act as a surprising event that could produce an OR. The magnitude of the OR thus provides a relative measure for whether (or how much) the US was expected at a given point in time. This model provides a simple framework for interpreting the production of offset-responses on certain trials as reflecting a violation in outcome expectancy. Of note, the task demands in the present study were not concerned with predicting the US, thus showing that orienting and neural activity at stimulus offset can be found under incidental processing conditions.
In a similar way, the omission of an expected US may reflect enhanced attentional processing important for conditioned learning. For instance, human electrodermal studies by Siddle and colleagues (Siddle, 1991; Siddle and Lipp, 1997) have interpreted omission effects as a result of associatively-generated priming (Wagner, 1978; 1981). In this way, the CS acts as a retrieval cue to prime the representation for the US. A US that is reliably signaled demands less attentional processing, as evidenced by a decrease in the unconditioned response to expected versus unexpected USs (Domjan, 2005; Dunsmoor, Bandettini, and Knight, 2008; Knight, Waters, King, and Bandettini, 2010). Conversely, a primed US that is omitted causes a conflict with expectations leading to an increase in orienting when the stimulus is omitted, an increase in responding to the stimulus itself when it is reintroduced (Siddle and Lipp, 1997), and a change in attention to the preceding CS (Pearce and Hall, 1980). Importantly, some associative learning models have proposed that stimuli other than the CS+ may act as strong retrieval cues for the US if they contain elements that are more predictive of the US (McLaren and Mackintosh, 2002). In the context of the present study, faces of greater emotional expression than the CS+ may have primed a more salient representation of the US than faces of the same identity that were less expressive. Consequently, the absence of the US on high intensity non-conditioned trials was surprising despite the fact the US had never actually occurred on those trials. In contrast, the low intensity faces provided poor retrieval cues for the US.
4.5 Do generalized offset-related signals reflect PE?
As an alternative interpretative framework for these findings, it is possible that the absence of the US induced a negative PE that may have effects on learning and behavior. Under this framework, learning is guided in large part by the difference between what is predicted by a CS and what actually occurs (Rescorla and Wagner, 1972). The omission of an expected US comes as a surprising event that conflicts with predictions, thereby reducing the predictive value of the CS. Based on previous findings implicating the PFC, striatum, and parietal cortex in learning from PEs, it is reasonable to interpret omission-related neural activity observed in this study in a similar computational framework of conditioned learning. Notably, there are issues regarding the overall similarity of negative PEs in aversive versus rewarded learning (Schultz, 2010). Perhaps the most obvious point of departure is the fact that omitting an aversive stimulus could be construed as rewarding. In this way, the omission of an expected US (negative PE) could be interpreted as an unexpected reward (positive PE). It should be noted, however, that neuroimaging evidence suggests PE signals in the striatum track appetitive learning and aversive learning with primary and secondary reinforcers (Delgado et al., 2008; Seymour, O’Doherty, Koltzenburg, Wiech, Frackowiak, Friston, and Dolan, 2005), suggesting that some neural systems are involved in signaling PEs across both domains.
Another issue with using a negative PE framework to interpret the present results concerns offset-related activity during generalization. Specifically, it is not entirely clear from classical reinforcement learning models how stimuli with no history of reinforcement come to elicit a PE. One possibility is that US expectancy (however it is acquired) is sufficient to evoke a PE in the absence of direct experience. In line with this possibility, Tremblay et al. (1998) showed that neuronal activity involved in building up outcome expectancies during training generalizes to novel stimuli when the same task structure is used. Initially, monkeys trained to make a movement (or withhold a movement) showed activation in striatal neurons in response to a cue signaling a rewarding outcome. When monkeys were then presented with novel cues, activity in dopaminergic neurons was at first observed on trials in which monkeys made both correct and incorrect movements but, over time, became selective to rewarded trials as behavior improved (Tremblay et al., 1998). These findings suggest that animals may have an expectancy bias following reinforcement learning (at least with appetitive cues) and expect the US by default (Schultz, Tremblay, and Hollerman, 2003). Granting the task demands in the present fear learning study did not require an update in decision making or instrumental behavior, this explanation may describe why subjects routinely expected the shock following cues that had never before predicted the US.
Given the functional significance of PEs for reducing the predictive value of the CS (i.e. fear extinction), one would expect a reduction in fear during the generalization test to non-conditioned cues that never signal the US. The SCR results are in line with this notion, such that omission-related SCRs diminished for all non-conditioned stimuli by late generalization testing, but remained relatively robust following unpaired CS+ trials. Importantly, the effect of PEs on the production of omission-related activity may vary from study to study based on methodology. The present study used procedures derived from the animal literature specifically intended to extend the duration of responding during a relatively lengthy generalization test: these include the use of intermittent reinforcement during acquisition (Guttman and Kalish, 1956; Skinner, 1950) and steady-state reinforcement of the CS+ throughout the generalization test (Blough, 1975). Both these procedures are necessary to delay extinction but may have important consequences on expectancy for the US. For instance, partial CS−US reinforcement has been shown to differentially affect brain activity during fear acquisition relative to continuous CS−US reinforcement (Dunsmoor, Bandettini, and Knight, 2007), and some dopamine neurons respond maximally to cues with uncertain outcomes (Fiorillo, Tobler, and Schultz, 2003). Therefore, since subjects learned that the CS+ is itself an imperfect predictor for the US, omission of the US on non-conditioned trials may not immediately impact contingency awareness (i.e. knowledge that only the CS+ predicts the US). Alternatively, continuous CS−US pairing during learning may result in a rapid decrease in omission responses on all unpaired trials. Another issue regarding generalization testing concerns which features of the CS+ are manipulated, i.e. the stimulus dimension. In this study, the identity of a face CS was kept constant while degrees of emotional intensity were varied, which lead to a bias in responding to stimuli of high intensity. We have shown previously, however, that discriminatory learning procedures affect the subsequent generalization gradient; differential fear learning involving a CS− of higher intensity than the CS+ leads to a sharper generalization gradient (Dunsmoor et al., 2009). In all, the PE signals in regulating learning and behavior during stimulus generalization testing may be dependent on experience with the CS−US contingencies and the generalization test procedures employed, including the dimension along which generalization testing is conducted and discriminations acquired during initial learning (see Honig and Urcuioli, 1981 for review). Further studies are needed to address whether omission signals during stimulus generalization conform to PE-based learning models. In particular, it will be important to demonstrate the effects of PEs on updating behavior and neural activity on a trial-by-trial manner.
4.7 Conclusions
In conclusion, the results presented here indicate that US expectancy is revealed through responses generated when a stimulus terminates and no stimulation is delivered. By detailing the effects of learning and generalization on the omission response, the present study provides novel directions for future research on fear learning. Findings from the present study suggest that components of the fear learning process related to stimulus omission can be used to index expectancy in situations that have never actually lead to an aversive outcome but induce fear nonetheless. Importantly, these effects were observed despite the fact that subjects were not explicitly instructed to predict the outcome on each trial, suggesting that robust omission-related activity is evoked in cases when stimulus-stimulus learning is implicit. These results have practical applications for understanding clinical disorders marked by heightened overexpression of fear responses. For instance, consistently expecting a negative outcome following stimuli that do not actually present a threat is symptomatic of certain anxiety disorders, such as posttraumatic stress disorder and panic disorder (Lissek, Powers, McClure, Phelps, Woldehawariat, Grillon, and Pine, 2005). Research will be needed to address whether sustained omission-related behavioral and neural activity throughout generalization testing is characteristic of clinical anxiety and is a potential marker of aberrant emotion regulation processes.
Supplementary Material
Highlights.
Skin conductance responses increase at the time of an expected but omitted shock
Omission-related SCRs also observed during stimulus generalization
Brain activity associated with stimulus omission in striatum, parietal cortex, and ACC
These same regions showed omission-related activations during a generalization test
Acknowledgments
The authors are grateful to Vishnu Murty for helpful comments. This work was supported by NSF grant 0745919 and NIH grants R01 DA027802 and F31 MH090682.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blough DS. Steady-state data and a quantitative model of operant generalization and discrimination. Journal of Experimental Psychology. 1975;104:3–21. [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Sciences. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Bucci DJ. Posterior parietal cortex: an interface between attention and learning? Neurobiol Learn Mem. 2009;91:114–120. doi: 10.1016/j.nlm.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw ND, Gershman SJ, Seymour B, Dayan P, Dolan RJ. Model-based influences on humans’ choices and striatal prediction errors. Neuron. 2011;69:1204–1215. doi: 10.1016/j.neuron.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Li J, Schiller D, Phelps EA. The role of the striatum in aversive learning and aversive prediction errors. Philosophical Transactions of the Royal Society B-Biological Sciences. 2008;363:3787–3800. doi: 10.1098/rstb.2008.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domjan M. Pavlovian conditioning: A functional perspective. Annual Review of Psychology. 2005;56:179–206. doi: 10.1146/annurev.psych.55.090902.141409. [DOI] [PubMed] [Google Scholar]
- Dunsmoor JE, Bandettini PA, Knight DC. Impact of continuous versus intermittent CS−UCS pairing on human brain activation during Pavlovian fear conditioning. Behavioral Neuroscience. 2007;121:635–642. doi: 10.1037/0735-7044.121.4.635. [DOI] [PubMed] [Google Scholar]
- Dunsmoor JE, Bandettini PA, Knight DC. Neural correlates of unconditioned response diminution during Pavlovian conditioning. Neuroimage. 2008;40:811–817. doi: 10.1016/j.neuroimage.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor JE, Martin A, Labar KS. Role of conceptual knowledge in learning and retention of conditioned fear. Biol Psychol. 2011a doi: 10.1016/j.biopsycho.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor JE, Mitroff SR, LaBar KS. Generalization of conditioned fear along a dimension of increasing fear intensity. Learning & Memory. 2009;16:460–469. doi: 10.1101/lm.1431609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor JE, Prince SE, Murty VP, Kragel PA, LaBar KS. Neurobehavioral mechanisms of human fear generalization. Neuroimage. 2011b;55:1878–1888. doi: 10.1016/j.neuroimage.2011.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor JE, White AJ, LaBar KS. Conceptual similarity promotes generalization of higher order fear learning. Learn Mem. 2011c;18:156–160. doi: 10.1101/lm.2016411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Measuring facial movement. Environmental Psychology and Nonverbal Behavior. 1976;1:56–75. [Google Scholar]
- Fiorillo CD, Tobler PN, Schultz W. Discrete coding of reward probability and uncertainty by dopamine neurons. Science. 2003;299:1898–1902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Furlong TM, Cole S, Hamlin AS, McNally GP. The role of prefrontal cortex in predictive fear learning. Behav Neurosci. 2010;124:574–586. doi: 10.1037/a0020739. [DOI] [PubMed] [Google Scholar]
- Ghirlanda S. Intensity generalization: Physiology and modelling of a neglected topic. Journal of Theoretical Biology. 2002;214:389–404. doi: 10.1006/jtbi.2001.2452. [DOI] [PubMed] [Google Scholar]
- Ghirlanda S, Enquist M. A century of generalization. Animal Behaviour. 2003;66:15–36. [Google Scholar]
- Graham R, Devinsky O, LaBar KS. Quantifying deficits in the perception of fear and anger in morphed facial expressions after bilateral amygdala damage. Neuropsychologia. 2007;45:42–54. doi: 10.1016/j.neuropsychologia.2006.04.021. [DOI] [PubMed] [Google Scholar]
- Grinband J, Wager TD, Lindquist M, Ferrera VP, Hirsch J. Detection of time-varying signals in event-related fMRI designs. Neuroimage. 2008;43:509–520. doi: 10.1016/j.neuroimage.2008.07.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman N, Kalish HI. Discriminability and stimulus-generalization. Journal of Experimental Psychology. 1956;51:79–88. doi: 10.1037/h0046219. [DOI] [PubMed] [Google Scholar]
- Hahn B, Ross TJ, Stein EA. Cingulate activation increases dynamically with response speed under stimulus unpredictability. Cereb Cortex. 2007;17:1664–1671. doi: 10.1093/cercor/bhl075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey RC, Hall G. Acquired equivalence and distinctiveness of cues. Journal of Experimental Psychology-Animal Behavior Processes. 1989;15:338–346. [PubMed] [Google Scholar]
- Honig WK, Urcuioli PJ. The legacy of Guttman and Kalish (1956) - 25 years of research on stimulus-generalization. Journal of the Experimental Analysis of Behavior. 1981;36:405–445. doi: 10.1901/jeab.1981.36-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DC, Nguyen HT, Bandettini PA. The role of the human amygdala in the production of conditioned fear responses. Neuroimage. 2005;26:1193–1200. doi: 10.1016/j.neuroimage.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Knight DC, Waters NS, King MK, Bandettini PA. Learning-related diminution of unconditioned SCR and fMRI signal responses. Neuroimage. 2010;49:843–848. doi: 10.1016/j.neuroimage.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnman C, Rougemont-Bucking A, Beucke JC, Zeffiro TA, Milad MR. Unconditioned responses and functional fear networks in human classical conditioning. Behav Brain Res. 2011 doi: 10.1016/j.bbr.2011.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Powers AS, McClure EB, Phelps EA, Woldehawariat G, Grillon C, Pine DS. Classical fear conditioning in the anxiety disorders: a meta-analysis. Behaviour Research and Therapy. 2005;43:1391–1424. doi: 10.1016/j.brat.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Lissek S, Rabin S, Heller RE, Lukenbaugh D, Geraci M, Pine DS, Grillon C. Overgeneralization of Conditioned Fear as a Pathogenic Marker of Panic Disorder. American Journal of Psychiatry. 2010;167:47–55. doi: 10.1176/appi.ajp.2009.09030410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykken DT, Venables PH. Direct measurement of skin conductance: a proposal for standardization. Psychophysiology. 1971;8:656–672. doi: 10.1111/j.1469-8986.1971.tb00501.x. [DOI] [PubMed] [Google Scholar]
- Maddux JM, Kerfoot EC, Chatterjee S, Holland PC. Dissociation of attention in learning and action: Effects of lesions of the amygdala central nucleus, medial prefrontal cortex, and posterior parietal cortex. Behavioral Neuroscience. 2007;121:63–79. doi: 10.1037/0735-7044.121.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- McLaren IPL, Mackintosh NJ. Associative learning and elemental representation: II. Generalization and discrimination. Animal Learning & Behavior. 2002;30:177–200. doi: 10.3758/bf03192828. [DOI] [PubMed] [Google Scholar]
- McNally GP, Johansen JP, Blair HT. Placing prediction into the fear circuit. Trends Neurosci. 2011 doi: 10.1016/j.tins.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty JP, Dayan P, Friston K, Critchley H, Dolan RJ. Temporal difference models and reward-related learning in the human brain. Neuron. 2003;38:329–337. doi: 10.1016/s0896-6273(03)00169-7. [DOI] [PubMed] [Google Scholar]
- O’Gorman JG. Change in stimulus conditions and the orienting response. Psychophysiology. 1973;10:465–470. doi: 10.1111/j.1469-8986.1973.tb00533.x. [DOI] [PubMed] [Google Scholar]
- Öhman A, Mineka S. Fears, phobias, and preparedness: Toward an evolved module of fear and fear learning. Psychological Review. 2001;108:483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned Reflexes. London: Oxford University Press; 1927. [Google Scholar]
- Pearce JM, Hall G. A model for Pavlovian learning: variations in the effectiveness of conditioned but not of unconditioned stimuli. Psychol Rev. 1980;87:532–552. [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Razran G. Stimulus generalization of conditioned responses. Psychological Bulletin. 1949;46:337–365. doi: 10.1037/h0060507. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. Appleton-Century-Crofts; 1972. [Google Scholar]
- Schiller D, Levy I, Niv Y, LeDoux JE, Phelps EA. From Fear to Safety and Back: Reversal of Fear in the Human Brain. Journal of Neuroscience. 2008;28:11517–11525. doi: 10.1523/JNEUROSCI.2265-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Dopamine signals for reward value and risk: basic and recent data. Behav Brain Funct. 2010;6:24. doi: 10.1186/1744-9081-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dickinson A. Neuronal coding of prediction errors. Annu Rev Neurosci. 2000;23:473–500. doi: 10.1146/annurev.neuro.23.1.473. [DOI] [PubMed] [Google Scholar]
- Schultz W, Tremblay L, Hollerman JR. Changes in behavior-related neuronal activity in the striatum during learning. Trends Neurosci. 2003;26:321–328. doi: 10.1016/S0166-2236(03)00122-X. [DOI] [PubMed] [Google Scholar]
- Seymour B, O’Doherty JP, Dayan P, Koltzenburg M, Jones AK, Dolan RJ, Friston KJ, Frackowiak RS. Temporal difference models describe higher-order learning in humans. Nature. 2004;429:664–667. doi: 10.1038/nature02581. [DOI] [PubMed] [Google Scholar]
- Seymour B, O’Doherty JP, Koltzenburg M, Wiech K, Frackowiak R, Friston K, Dolan R. Opponent appetitive-aversive neural processes underlie predictive learning of pain relief. Nat Neurosci. 2005;8:1234–1240. doi: 10.1038/nn1527. [DOI] [PubMed] [Google Scholar]
- Siddle DA. Effects of stimulus omission and stimulus change on dishabituation of the skin conductance response. J Exp Psychol Learn Mem Cogn. 1985;11:206–216. doi: 10.1037//0278-7393.11.2.206. [DOI] [PubMed] [Google Scholar]
- Siddle DA. Orienting, habituation, and resource allocation: an associative analysis. Psychophysiology. 1991;28:245–259. doi: 10.1111/j.1469-8986.1991.tb02190.x. [DOI] [PubMed] [Google Scholar]
- Siddle DA, Lipp OV. Orienting, habituation, and information processing: The effects of omission, the role of expectancy, and the problem of dishabituation. In: Lang PJ, Simons RF, Balaban M, editors. Attention and Orienting. London: Lawrence Erlbaum Associates; 1997. pp. 23–40. [Google Scholar]
- Siddle DA, Packer JS. Stimulus omission and dishabituation of the electrodermal orienting response: the allocation of processing resources. Psychophysiology. 1987;24:181–190. doi: 10.1111/j.1469-8986.1987.tb00276.x. [DOI] [PubMed] [Google Scholar]
- Siddle DA, Remington B, Kuiack M, Haines E. Stimulus omission and dishabituation of the skin conductance response. Psychophysiology. 1983;20:136–145. doi: 10.1111/j.1469-8986.1983.tb03279.x. [DOI] [PubMed] [Google Scholar]
- Siddle DAT, Booth ML, Packer JS. Effects of stimulus preexposure on omission responding and omission-produced dishabituation of the human electrodermal response. Quarterly Journal of Experimental Psychology Section B-Comparative and Physiological Psychology. 1987;39:339–363. [Google Scholar]
- Skinner BF. Are theories of learning necessary? Psychological Review. 1950;57:193–216. doi: 10.1037/h0054367. [DOI] [PubMed] [Google Scholar]
- Sokolov EN. Perception and the conditioned reflex. Oxford: Pergamon; 1963. [Google Scholar]
- Spoormaker VI, Andrade KC, Schroter MS, Sturm A, Goya-Maldonado R, Samann PG, Czisch M. The neural correlates of negative prediction error signaling in human fear conditioning. Neuroimage. 2011a;54:2250–2256. doi: 10.1016/j.neuroimage.2010.09.042. [DOI] [PubMed] [Google Scholar]
- Spoormaker VI, Schroter MS, Andrade KC, Dresler M, Kiem SA, Goya-Maldonado R, Wetter TC, Holsboer F, Samann PG, Czisch M. Effects of rapid eye movement sleep deprivation on fear extinction recall and prediction error signaling. Hum Brain Mapp. 2011b doi: 10.1002/hbm.21369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas LA, De Bellis MD, Graham R, LaBar KS. Development of emotional facial recognition in late childhood and adolescence. Developmental Science. 2007;10:547–558. doi: 10.1111/j.1467-7687.2007.00614.x. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Hollerman JR, Schultz W. Modifications of reward expectation-related neuronal activity during learning in primate striatum. Journal of Neurophysiology. 1998;80:964–977. doi: 10.1152/jn.1998.80.2.964. [DOI] [PubMed] [Google Scholar]
- Wagner AR. Expectancies and the priming of STM. In: Hulse SH, Fowler H, Honig WK, editors. Cognitive processes in animal behavior. Hillsdale, NJ: Erlbaum; 1978. pp. 177–209. [Google Scholar]
- Wagner AR. SOP: A model of automatic memory processing in animal behavior. In: Spear NE, Miller RR, editors. Information processing in animals: Memory mechanisms. Hillsdale, NJ: Erlbaum; 1981. pp. 5–47. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.