Abstract
Cutaneous malignant melanoma is rapidly increasing in the developed world and continues to be a challenge in the clinic. Although extensive epidemiological evidence points to solar ultraviolet radiation (UV) as the major risk factor for melanoma, there is a significant gap in our knowledge about how this most ubiquitous environmental carcinogen interacts with the largest organ of the mammalian body (skin) at the microenvironmental and molecular level. We review some recent advances that have started to close this gap.
Introduction
Cutaneous malignant melanoma remains one of the few malignancies that exhibit positive rates of increase in the developed world (1). In the last few decades, the incidence of melanoma has accelerated to increasingly become a major disease of young women. In fact, according to the latest Surveillance Epidemiology and End Results (SEER) data (1975-2008), melanoma is now the most prevalent cancer in women under the age of 40, surpassing even breast cancer. The major etiological risk factor for melanoma is thought to be exposure to intermittent erythemogenic doses of ultraviolet radiation (UV), especially during childhood and early adolescence years (2). We experimentally validated these UV-associated risks in the hepatocyte growth factor/scatter factor transgenic (HGF/SF-Tg) mouse model, which features constitutive signaling through the receptor tyrosine kinase MET (3). We also produced in vivo experimental evidence that sunscreen, when applied as directed, inhibits melanoma (4). Notably, in the albino HGF/SF-Tg mouse, UVB was highly melanomagenic, but UVA was not (5). Unfortunately, the molecular mechanisms underlying UVB-driven melanomagenesis have remained elusive.
The UV spectrum of sunlight is divided into three regions*: UVA (320-400 nm wavelength), UVB (290-320 nm), and UVC (200-290 nm). Terrestrial UVC is biologically irrelevant, as it is almost completely absorbed by the stratospheric ozone layer. Both UVA and UVB reach the earth’s surface and have deleterious effects on nucleic acids and proteins. UVB is considered to be more carcinogenic than UVA, as it directly causes two types of DNA lesions: cyclobutane pyrimidine dimers (CPD), formed between adjacent thymine (T) or cytosine (C) residues, and 6-pyrimidine 4-pyrimidone photoproducts (6-4PP) (6). The CPDs are more abundant, more carcinogenic, and less efficiently repaired. These UVB-induced lesions give rise to DNA mutations hallmarked by C→T and CC→TT transitions, the so-called “UVB signature mutations” (6). On the other hand, UVA mutates DNA indirectly, thought to be mediated through generation of reactive oxygen species via absorption by endogenous photosensitizers (6).
There is a clear association between UV-induced DNA damage and skin cancer. In non-melanoma skin cancer (NMSC), e.g. squamous cell carcinoma and basal cell carcinoma, UVB signature DNA mutations have been found in several genes and are considered to play an essential role (7). The most well characterized example is the tumor suppressor p53, which exhibits UVB signature mutations in a large majority of NMSC and appears to be an initiating event (7). In contrast, although several melanoma susceptibility genes have been identified, no definitive UVB-induced driver mutations have been observed in melanoma. CDKN2A, a bona fide melanoma susceptibility gene, harbors mutations at dipyrimidine sites, but many of these genetic alterations are not UVB signature mutations. C→T transition mutations in CDKN2A are also observed in cancers of internal organs that are protected from sun exposure, e.g. gastric adenocarcinomas, calling into question a central role of these mutations in UV-induced melanomagenesis (8). BRAF, a part of the RAS signaling pathway and critical for the cellular response to growth signals, has been shown to be mutated in a majority of melanomas, but these mutations are not UVB signature mutations (9). Similarly, mutations in NRAS are found in melanomas but do not carry UVB signatures (9). Although a small percentage of melanomas have UVB signature mutations in p53, these mutations are considered late events during the progression of melanoma to a higher grade, and are not involved in the initiation stages. Thus, evidence so far for the presence of UVB-generated signature mutations in melanoma that could be ascertained as the driver mutations has been less than compelling (9). UVB exposure is undoubtedly mutagenic, and signature mutations are starting to be uncovered; for example, UVB-induced mutations in PTEN gene have been reported in melanomas arising in Xeroderma Pigmentosum patients (10), and whole genome and exome sequencing methodologies have revealed large-scale UVB-type mutational signatures in melanoma tumors and cell lines (11-12). Nevertheless, strong evidence that UV can also induce immunosuppression and inflammation (1, 13) has fueled the notion that these processes work in concert with DNA damage in the initiation and/or progression of melanoma. There is strong support for the notion that UV is a complete carcinogen, acting with respect to melanoma as both an initiator, through genotoxicity, and a promoter, through immunosuppression.
It is well described that UV initiates in the skin a profound and immediate p53-dependent stress response, as well as a variety of inflammatory mediators and paracrine factors, notably but not exclusively from keratinocytes, which profoundly alter melanocyte function. Ultimately, the combined effect of these stress responses is a rapid onset of cell cycle arrest and DNA repair mechanisms. While several studies have examined the immediate genomic response of UV-induced stress in several skin cell types, the long-term persistent response in melanocytes beyond the initial 48 hours had not been investigated. We hypothesized that novel clues to the molecular mechanism(s) underlying UV-induced melanomagenesis would be found within the persistent genomic response of melanocytes to UV radiation.
We reckoned that any relevant analysis of the UV-response by melanocytes had to be performed in situ, within their natural physiologic and morphologic microenvironment. The considerable technical challenge was to devise a means to study a cell type in vivo that constitutes a tiny fraction of the cellular milieu of the mammalian skin, and bears no exclusive cell surface markers. To circumvent this problem we developed a mouse model in which melanocytes can be both in vivo imaged and highly purified by virtue of tetracycline-inducible, melanocyte-specific GFP expression (iDct-GFP mice) (14-15). The faithful and rapid on-demand GFP labeling of melanocytes within the iDct-GFP mouse provided an invaluable tool to begin to explore melanocyte and melanoma biology in vivo.
Key Findings
Our previous studies had shown that UVB, but not UVA, was melanomagenic in our albino HGF/SF-Tg mouse model (5). We reasoned that waveband-specific tumorigenicity could be explained by differential cellular/molecular responses of melanocytes to different wavelengths. We therefore used the iDct-GFP mouse to examine the responses to UVB vs. UVA irradiation of melanocytes residing within their natural morphologic and physiologic microenvironment. These studies showed that the answers to many of the questions regarding the role of UV in melanomagenesis lie not only in the penchant of UV for inducing DNA mutations, but also in its ability to provoke interactions between melanocytes and elements of the microenvironment to regulate remodeling of UV-damaged skin.
It was clear that UVB was far more effective at inducing gene expression response in melanocytes than UVA, consistent with their respective melanomagenic potential in the HGF/SF-Tg mouse model. UVB induced melanocyte activation, characterized by increased proliferation and migration towards the epidermis. GFP-labeled melanocytes were FACS-isolated and subjected to microarray analysis of gene expression. In addition to an expected early stress response that waned, a delayed response was seen, which was largely comprised of interferon-gamma (IFN-γ) response genes. Antibody-mediated blockade of IFN-γ, but not IFN-γ, abolished the UVB-induced melanocyte activation, suggesting that the IFN-γ-induced gene signature was responsible for the activation of melanocytes. Immunohistochemical and flow cytometric analyses identified a subset of infiltrated macrophages as the source of IFN-γ. When the UV-recruited skin macrophages were isolated and admixed with melanoma cells transplanted in syngeneic mice, tumor growth was significantly enhanced. The biologically active agent responsible for this effect was found to be IFN-γ, as the enhanced growth was mitigated by systemic anti-IFN-γ antibody treatment.
Erythemal UVB induces overtly contrasting microenvironmental responses in neonatal and adult skins. Neonatal mouse skin is characterized by robust macrophage infiltration, whereas the adult mouse skin exhibits a rapid but short-lived neutrophil influx, severe enough to disrupt cycling hair follicles (16). These qualitative differences coincide with the fact that UVB is melanomagenic in neonatal mice but not in adults (3).
We have proposed that the UVB-induced melanomagenic mechanisms operate within the immunoediting paradigm proposed by Schreiber and colleagues (17). The UVB-activated mutant melanocytes and/or their progenitors may be able to evade immune-mediated elimination and persist during an extended equilibrium phase before evolving into clinically significant melanoma. UVB-induced melanocytic proliferation might aid in the fixation of UVB-induced mutations as well as accumulation of non-UVB-type mutations. In addition, migration of melanocytes outside of their privileged niche could facilitate neonate-specific UVB-associated long-term tolerance to melanocytic antigens by promoting aberrant interactions between melanocytes and their inflammatory microenvironment. Moreover, inflammation-associated epigenetic alterations in transformed cells and macrophages can set forth enduring biological effects.
The most notable discovery from our analysis of in vivo UV-irradiated melanocytes was that, rather than deterring melanomagenesis, physiologically relevant levels of IFN-γ actually promoted survival of melanocytes within this hostile inflammatory microenvironment, as well as melanoma cells within tumors. Antibody-mediated systemic blockade experiments demonstrating the importance of IFN-γ in UVB-induced melanocyte activation and mouse melanoma development strongly support the notion that the cellular effects of IFN-γ are context-dependent, and can be anti-tumorigenic or pro-tumorigenic (18).
Significance & Implications
Immunosuppressive cell networks and factors clearly play a significant role in the failure of anti-tumor immune responses and currently available therapies to eradicate melanoma. We propose that the same immunoevasive/survival mechanisms noted in UVB-irradiated neonatal skin can be exploited by melanoma, and likely contribute to the selection of the aggressive and therapeutically resistant melanoma phenotype. The importance of IFN-γ noted in UVB-induced melanocyte activation in the neonatal skin fuels speculation that IFN-γ signaling plays a critical early step in subsequent susceptibility to melanomagenesis. It is noteworthy that the interferon “survival” signature seen in the UVB-activated melanocytes (15) includes several genes known to be involved in immunoevasion, including non-classical major histocompatibility complex (MHC) class Ib antigens. For example, H2-T23 (Qa-1; mouse homolog of HLA-E) plays a vital role in suppression of NK and cytotoxic T lymphocytes via binding with the CD94/NKG2 family of receptors, leading to T cell anergy (19). Several MHC I subclass H2-Q genes (functional homologs of HLA-G) also exhibited UVB-induced upregulation in melanocytes (15). Indeed, the human MHC class Ib molecules HLA-G and HLA-E have been implicated in the immune escape of human melanoma (20-21). The UVB-induced melanocyte survival signature also features the complement isoforms C4A and C4B, which are involved in suppression of systemic autoimmunity (22). CTLA4 is another potent immune evasion facilitator highly upregulated in neonatal melanocytes in response to UVB, as well as in transplanted melanoma cells admixed with macrophages isolated from UVB-irradiated neonatal skin (15). CTLA4 negatively regulates T cell immune response via auto-attenuation of effector T cell activation as well as by positive regulation of the suppressor function of regulatory T cells (Treg) (23). Interestingly, CTLA4 has been shown to play a role in Treg-mediated immunosuppression induced by UV radiation (24). CTLA4-blocking monoclonal antibody, considered prototypical immunotherapeutic agent, has been reported to induce long-term regression of metastatic melanoma and improved survival of patients (25).
Melanocytes are built for enhanced survival, to withstand both UV exposure ensuring the continued synthesis of melanin, and the chemical stresses associated with the synthesis of melanin itself. Therefore, the discovery of the upregulation of a variety of immunoevasive molecules in melanocytes in the aftermath of UVB irradiation has important implications. These elements may play an integral role in further protecting melanocytes from eradication by the UVB-induced inflammatory response, otherwise designed to remodel all damaged portions of the skin. Melanoma could take advantage of this built-in circuitry to develop into one of the most evasive cancers. The fact that this circuitry converges on IFN-γ epitomizes the importance of this pathway to melanocytic survival mechanisms. At the same time, it represents an exciting new class of potential anti-melanoma preventive and/or therapeutic targets.
IFN-γ-associated survival mechanisms operational in neonatal melanocytes may be recapitulated in melanoma, contributing to the selection of more aggressive and therapeutically resistant phenotypes. The relevance of this finding to human melanoma is supported by detection of macrophage-associated IFN-γ expression in most human melanoma samples examined (15). Moreover, a clinical trial has shown that IFN-γ may have adverse effects on melanoma patients with respect to relapse and mortality (26). We have provided evidence that IFN-γ signaling can facilitate melanoma progression, a significant discovery considering that high-dose IFN-γ is used as an adjuvant in melanoma treatment regimens, although often with limited success.
We observed that a substantial proportion of human melanomas harbor IFN-γ-producing macrophages, consistent with the proposed role for infiltrating macrophages within the IFN-γ-driven pro-tumorigenic microenvironment created in UVB-irradiated skin. Although strong data implicate lymphocytic infiltration in primary melanoma as a favorable prognostic marker (27), little is known about the prognostic significance of macrophage infiltration. The prospect of validating IFN-γ+ macrophages as a new and simple cellular marker of poor prognosis deserves further investigation. It is noteworthy that IFN-γ has already been implicated in serum as an independent prognostic indicator for disease recurrence in melanoma patients (28). Moreover, recently Cho et al. have shown that IFN-γ decreases the susceptibility of B16 melanoma cells to CD8+ T cell-mediated cytolysis (29).
Pro-tumorigenic inflammatory elements represent promising targets of anti-cancer therapeutics. We have discovered IFN-γ to be the driver of novel cellular/molecular inflammatory mechanisms that may underlie the initiation, immunoevasion/survival and outgrowth of UVB-induced melanoma. These studies strongly suggest that IFN-γ/IFN-γR, or its downstream pathway members, represent not only promising prognostic markers, but may well prove to be efficacious therapeutic targets for human melanoma. Our findings also raise the likely possibility that tools designed to enable study of the biology and pathobiology of melanocytes in their natural microenvironment will continue to generate novel mechanistic and therapeutic insights into melanomagenesis.
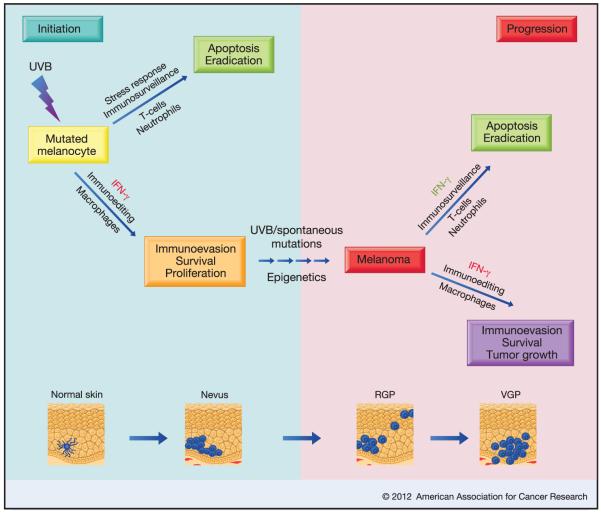
A proposed model depicting how IFN-γ may play dual contrasting roles during the initiation as well as progression phases of melanomagenesis. IFN-γ may be involved in the survival and immunoevasion of UV-damaged melanocytes, leading to their entry into the equilibrium phase of the immunoediting paradigm. These mutated melanocytes would be prone to accumulating further mutations and/or epigenetic modulations and transformation. During the progression phase of the tumorigenic process, IFN-γ would aid either the immunosurveillance programs, leading to elimination of the tumor, or an immunoediting program that would help the burgeoning tumor evade anti-tumor immune response. Whether IFN-γ would act as a good guy (green) or a bad guy (red), may depend on the contexts of microenvironmental factors, tumor-specific antigenicity and signal intensity.
Footnotes
There is no standard definition for these wavebands, and one may find in the scientific literature other definitions such as the CIE (Commission Internationale d’Eclairage) recommended 100-280 nm for UVC, 280-315 nm for UVB and 315-400 nm for UVA.
Bibliography
- 1.Tran TT, Schulman J, Fisher DE. UV and pigmentation: molecular mechanisms and social controversies. Pigment Cell Melanoma Res. 2008;21:509–16. doi: 10.1111/j.1755-148X.2008.00498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett DC. Ultraviolet wavebands and melanoma initiation. Pigment Cell Melanoma Res. 2008;21:520–4. doi: 10.1111/j.1755-148X.2008.00500.x. [DOI] [PubMed] [Google Scholar]
- 3.Noonan FP, Recio JA, Takayama H, Duray P, Anver MR, Rush WL. Neonatal sunburn and melanoma in mice. Nature. 2001;413:271–2. doi: 10.1038/35095108. [DOI] [PubMed] [Google Scholar]
- 4.Klug HL, Tooze JA, Graff-Cherry C, Anver MR, Noonan FP, Fears TR, et al. Sunscreen prevention of melanoma in man and mouse. Pigment Cell Melanoma Res. 2010;23:835–7. doi: 10.1111/j.1755-148X.2010.00756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Fabo EC, Noonan FP, Fears T, Merlino G. Ultraviolet B but not ultraviolet A radiation initiates melanoma. Cancer Res. 2004;64:6372–6. doi: 10.1158/0008-5472.CAN-04-1454. [DOI] [PubMed] [Google Scholar]
- 6.Black HS, deGruijl FR, Forbes PD, Cleaver JE, Ananthaswamy HN, deFabo EC, et al. Photocarcinogenesis: an overview. J Photochem Photobiol B. 1997;40:29–47. doi: 10.1016/s1011-1344(97)00021-3. [DOI] [PubMed] [Google Scholar]
- 7.Melnikova VO, Ananthaswamy HN. Cellular and molecular events leading to the development of skin cancer. Mutat Res. 2005;571:91–106. doi: 10.1016/j.mrfmmm.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 8.Chen YJ, Chang JG, Shih LS, Chen PH, Endo M, Whang-Peng J, et al. Frequent detection of aberrant RNA transcripts of the CDKN2 gene in human gastric adenocarcinoma. Int J Cancer. 1997;71:350–4. doi: 10.1002/(sici)1097-0215(19970502)71:3<350::aid-ijc7>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 9.Hocker T, Tsao H. Ultraviolet radiation and melanoma: a systematic review and analysis of reported sequence variants. Hum Mutat. 2007;28:578–88. doi: 10.1002/humu.20481. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Digiovanna JJ, Stern JB, Hornyak TJ, Raffeld M, Khan SG, et al. Evidence of ultraviolet type mutations in xeroderma pigmentosum melanomas. Proc Natl Acad Sci U S A. 2009;106:6279–84. doi: 10.1073/pnas.0812401106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei X, Walia V, Lin JC, Teer JK, Prickett TD, Gartner J, et al. Exome sequencing identifies GRIN2A as frequently mutated in melanoma. Nat Genet. 2011;43:442–6. doi: 10.1038/ng.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pleasance ED, Cheetham RK, Stephens PJ, McBride DJ, Humphray SJ, Greenman CD, et al. A comprehensive catalogue of somatic mutations from a human cancer genome. Nature. 2010;463:191–6. doi: 10.1038/nature08658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Fabo EC, Noonan FP. Mechanism of immune suppression by ultraviolet irradiation in vivo. I. Evidence for the existence of a unique photoreceptor in skin and its role in photoimmunology. J Exp Med. 1983;158:84–98. doi: 10.1084/jem.158.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaidi MR, Hornyak TJ, Merlino G. A genetically engineered mouse model with inducible GFP expression in melanocytes. Pigment Cell Melanoma Res. 2011;24:393–4. doi: 10.1111/j.1755-148X.2011.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zaidi MR, Davis S, Noonan FP, Graff-Cherry C, Hawley TS, Walker RL, et al. Interferon-gamma links ultraviolet radiation to melanomagenesis in mice. Nature. 2011;469:548–53. doi: 10.1038/nature09666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolnicka-Glubisz A, Damsker J, Constant S, Corn S, De Fabo E, Noonan F. Deficient inflammatory response to UV radiation in neonatal mice. J Leukoc Biol. 2007;81:1352–61. doi: 10.1189/jlb.1206729. [DOI] [PubMed] [Google Scholar]
- 17.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836–48. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 18.Zaidi MR, Merlino G. The two faces of interferon-γ in cancer. Clin Cancer Res. 2011;17:6118–24. doi: 10.1158/1078-0432.CCR-11-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wischhusen J, Waschbisch A, Wiendl H. Immune-refractory cancers and their little helpers--an extended role for immunetolerogenic MHC molecules HLA-G and HLA-E? Semin Cancer Biol. 2007;17:459–68. doi: 10.1016/j.semcancer.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Rebmann V, Wagner S, Grosse-Wilde H. HLA-G expression in malignant melanoma. Semin Cancer Biol. 2007;17:422–9. doi: 10.1016/j.semcancer.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 21.Derre L, Corvaisier M, Charreau B, Moreau A, Godefroy E, Moreau-Aubry A, et al. Expression and release of HLA-E by melanoma cells and melanocytes: potential impact on the response of cytotoxic effector cells. J Immunol. 2006;177:3100–7. doi: 10.4049/jimmunol.177.5.3100. [DOI] [PubMed] [Google Scholar]
- 22.Chen Z, Koralov SB, Kelsoe G. Complement C4 inhibits systemic autoimmunity through a mechanism independent of complement receptors CR1 and CR2. J Exp Med. 2000;192:1339–52. doi: 10.1084/jem.192.9.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–5. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 24.Schwarz A, Beissert S, Grosse-Heitmeyer K, Gunzer M, Bluestone JA, Grabbe S, et al. Evidence for functional relevance of CTLA-4 in ultraviolet-radiation-induced tolerance. J Immunol. 2000;165:1824–31. doi: 10.4049/jimmunol.165.4.1824. [DOI] [PubMed] [Google Scholar]
- 25.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyskens FL, Jr., Kopecky KJ, Taylor CW, Noyes RD, Tuthill RJ, Hersh EM, et al. Randomized trial of adjuvant human interferon gamma versus observation in high-risk cutaneous melanoma: a Southwest Oncology Group study. J Natl Cancer Inst. 1995;87:1710–3. doi: 10.1093/jnci/87.22.1710. [DOI] [PubMed] [Google Scholar]
- 27.Oble DA, Loewe R, Yu P, Mihm MC., Jr. Focus on TILs: prognostic significance of tumor infiltrating lymphocytes in human melanoma. Cancer Immun. 2009;9:3. [PMC free article] [PubMed] [Google Scholar]
- 28.Porter GA, Abdalla J, Lu M, Smith S, Montgomery D, Grimm E, et al. Significance of plasma cytokine levels in melanoma patients with histologically negative sentinel lymph nodes. Ann Surg Oncol. 2001;8:116–22. doi: 10.1007/s10434-001-0116-3. [DOI] [PubMed] [Google Scholar]
- 29.Cho HI, Lee YR, Celis E. Interferon gamma limits the effectiveness of melanoma peptide vaccines. Blood. 2011;117:135–44. doi: 10.1182/blood-2010-08-298117. [DOI] [PMC free article] [PubMed] [Google Scholar]