Abstract
Purpose
Our previous study revealed that 90% (47 of 52; 95% CI: 0.79–0.96) of Chinese never-smokers with lung adenocarcinoma harbor known oncogenic driver mutations in just four genes: EGFR, ALK, HER2, and KRAS. Here, we examined the status of known driver mutations specifically in female never-smokers with lung adenocarcinoma.
Experimental Design
Tumors were genotyped for mutations in EGFR, KRAS, ALK, HER2, and BRAF. Data on age, stage, tumor differentiation, histological subtypes, and molecular alterations were recorded from 349 resected lung adenocarcinomas from female never-smokers. We further compared the clinicopathological parameters according to mutational status of these genes.
Results
Two hundred and sixty-six (76.2%) tumors harbored EGFR mutations, 16 (4.6%) HER2 mutations, 15 (4.3%) EML4-ALK fusions, seven (2.0%) KRAS mutations, and two (0.6%) BRAF mutations. In univariate analysis, patients harboring EGFR mutations were significantly older (p<0.001), whereas patients harboring HER2 mutations were significantly younger (p=0.036). Higher prevalence of KRAS (p=0.028) and HER2 (p=0.021) mutations was found in invasive mucinous adenocarcinoma (IMA). The frequency of EGFR mutations was positively correlated with acinar predominant tumors (p=0.002). Multivariate analysis revealed that older age at diagnosis (p=0.013) and acinar predominant subtype (p=0.005) were independent predictors of EGFR mutations. Independent predictors of HER2 mutations included younger age (p=0.030) and IMA (p=0.017). IMA (p=0.006) and poor differentiation (p=0.028) were independently associated with KRAS mutations.
Conclusions
The frequency of driver mutations in never-smoking female lung adenocarcinoma varies with histological subtypes and age at diagnosis. These data have implications for both clinical trial design and therapeutic strategies.
Keywords: Lung adenocarcinoma, Female, Never smoker, EGFR mutation, HER2 mutation, Acinar, Mucinous, Age
Introduction
Lung cancer is the leading cause of death from malignant tumors in males, and the second leading cause of cancer death among females worldwide (1). Chinese females have a lung cancer incidence of 21.3 cases per 100,000 population (1). Histologically, lung cancer can be classified into small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC). The latter mainly contains three subtypes: adenocarcinoma, squamous cell carcinoma, and large cell carcinoma. Although tobacco smoking is the major risk factor for lung cancer (2–3), the disease also occurs in never-smokers, defined as those who smoked less than 100 cigarettes throughout their life (4). Never-smokers are more likely to be female and have tumors with adenocarcinoma histology (4–5). For example, up to 53% of lung cancers in women may not be caused by direct smoking (6).
Recently, adenocarcinomas in particular have been found to harbor recurrent driver mutations that can predict benefit from targeted therapies. The most prominent example involves epidermal growth factor receptor (EGFR) tyrosine kinase domain mutations that predict response to the EGFR tyrosine kinase inhibitors (TKIs), gefitinib and erlotinib (7–9). Other commonly reported driver mutations in lung adenocarcinoma involved genes such as KRAS, HER2, ALK, and BRAF. Tumors harboring HER2 mutations, ALK fusions, and BRAF mutations are sensitive to BIBW 2992 (10), crizotinib (11), and PLX4032 (12), respectively.
In 2011, a new multidisciplinary classification of lung adenocarcinoma was proposed by the International Association for the Study of Lung Cancer, American Thoracic Society, and European Respiratory Society (IASLC/ATS/ERS). In the new classification, the term bronchioloalveolar adenocarcinoma (BAC) was no longer used, and invasive adenocarcinomas were classified according to the predominant subtype (13). It is appealing to know the association between adenocarcinoma histological subtypes and prevalence of driver mutations.
Mutations in the tyrosine kinase domains of EGFR and HER2 are both more frequent in adenocarcinomas, never-smokers, females, and Asian patients (14–16). We previously showed that up to 90% (47 of 52; 95% confidence interval: 0.79–0.96) of lung adenocarcinoma from Chinese never-smokers harbor known oncogenic driver mutations in just 4 genes: EGFR, ALK, HER2, and KRAS (17). Here, we extended our comprehensive mutational analyses of EGFR, KRAS, ALK, HER2, and BRAF to 349 never-smoking women with lung adenocarcinoma, and examined correlations between molecular alterations and clinicopathological features.
Materials and Methods
Patients and samples
From October 2007 to July 2011, we consecutively procured lung tumors resected with curative intent at the Department of Thoracic Surgery, Fudan University Shanghai Cancer Center, Shanghai, China. Subjects eligible for this study had to meet the following criteria: pathologically confirmed lung adenocarcinoma; sufficient tissue for comprehensive mutational analyses. Patients who received neoajuvant chemotherapy were excluded. This research was approved by the Institutional Review Board of the Fudan University Shanghai Cancer Center, Shanghai, China. Written informed consent was obtained from all patients.
Mutational analyses
RNA was extracted as per standard protocol after frozen tumor specimens were dissected into TRIZOL (Invitrogen). Total RNA samples were reverse transcribed into complementary DNA (cDNA). EGFR (exons 18–22), HER2 (exons 18 to 21), KRAS (exons 2 to 3), and BRAF (exons 11 to 15) were amplified by polymerase chain reaction (PCR) using cDNA. Amplified products were analyzed by direct dideoxynucleotide sequencing. To identify EML4-ALK fusions, multiple 5’ primers were used along with a fixed 3’ primer localizing to ALK exon 20 in order to detect all known EML4 fusion variants as previously described (17). Note, in our previous study (17), we screened for more mutations; based upon results from the first 52 cases analyzed, subsequent tumors were analyzed for a more restricted set of mutations.
Clinicopathological variables
Clinicopathological data collected for analyses included age at diagnosis, pathological TNM stage, tumor differentiation, and histological subtypes of adenocarcinoma according to the new IASLC/ATS/ERS multidisciplinary classification of lung adenocarcinoma (13). TNM stages were evaluated in accordance with the seventh edition of the lung cancer staging classification system (18).
Statistical methods
Pearson’s χ2 test (when no cell of a contingency table has expected count less than five) or Fisher’s exact test (when any cell of a contingency table has expected count less than five) was used to assess the association between two categorical variables. Independent sample t-test was applied to investigate correlation between a categorical variable and a continuous variable. For multivariate analyses, binary logistic regression model was employed. All the statistical analyses were performed in the SPSS for Windows (Version 16.0, Chicago, IL). P-values were two-tailed for all the tests. Statistical significance was set as p<0.05.
Results
Patient characteristics
A total of 349 never-smoking female lung adenocarcinoma cases met eligibility for this study. All patients were Chinese. A summary of patient characteristics was listed in Table 1. The median age at diagnosis was 58 years (range: 23–80). The number of patients in stages I–IV was 206, 33, 99, and 11 respectively. Seventy-seven (22.1%), 175 (50.1%), and 97 (27.8%) tumors were poorly, moderately, and well differentiated, respectively. The most common histological subtype was acinar predominant (52.4%), followed by papillary predominant (15.5%), solid predominant (13.2%), and lepidic predominant (9.7%). Other invasive types of adenocarcinoma included 14 (4.0%) invasive mucinous adenocarcinoma (IMA), six (1.7%) micropapillary predominant, and two (0.6%) enteric predominant. There were two (0.6%) adenocarcinoma in situ (AIS), and eight (2.3%) minimally invasive adenocarcinoma (MIA), all were nonmucinous. Detailed clinicopathological data for each patient was listed in Supplementary Table S1.
Table 1.
Demographics and clinicopathological features of 349 never-smoking female lung adenocarcinoma patients
| Variables | No. of patients | % |
|---|---|---|
| Age (years) | ||
| ≤30 | 3 | 0.9 |
| 31~40 | 13 | 3.7 |
| 41~50 | 60 | 17.2 |
| 51~60 | 147 | 42.1 |
| 61~70 | 82 | 23.5 |
| 71~80 | 44 | 12.6 |
| Mean ± SD (range) | 57.9 ± 10.3 (23–80) | |
| Stage | ||
| IA | 125 | 35.8 |
| IB | 81 | 23.2 |
| IIA | 26 | 7.4 |
| IIB | 7 | 2.0 |
| IIIA | 91 | 26.1 |
| IIIB | 8 | 2.3 |
| IV | 11 | 3.2 |
| Differentiation | ||
| Poor | 77 | 22.1 |
| Moderate | 175 | 50.1 |
| Well | 97 | 27.8 |
| Histological subtype | ||
| AISa | 2 | 0.6 |
| MIAb | 8 | 2.3 |
| Lepidic predominant | 34 | 9.7 |
| Acinar predominant | 183 | 52.4 |
| Papillary predominant | 54 | 15.5 |
| Micropapillary predominant | 6 | 1.7 |
| Solid predominant | 46 | 13.2 |
| IMAc | 14 | 4.0 |
| Enteric predominant | 2 | 0.6 |
| Total | 349 | |
AIS, adenocarcinoma in situ.
MIA, minimally invasive adenocarcinoma.
IMA, invasive mucinous adenocarcinoma.
Correlation between driver mutations and clinicopathological features
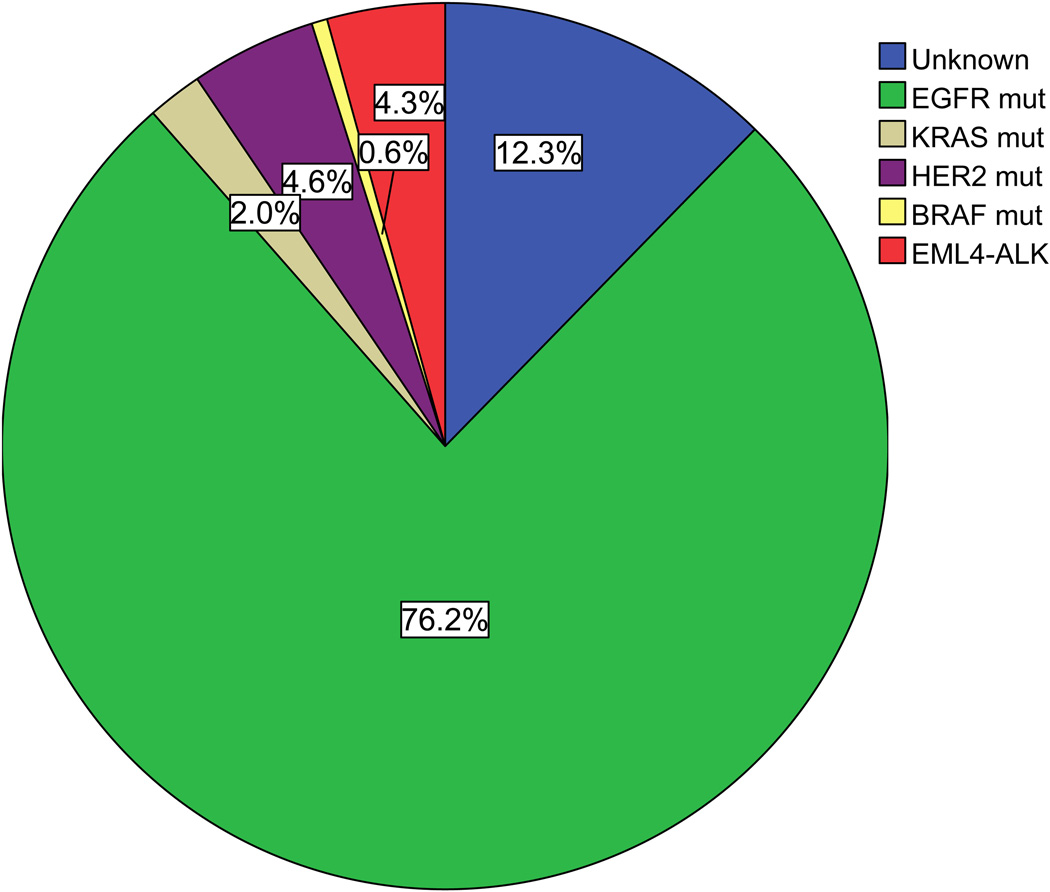
Two hundred and sixty-six (76.2%) tumors harbored EGFR mutations, 16 (4.6%) HER2 mutations, 15 (4.3%) EML4-ALK fusions, seven (2.0%) KRAS mutations, and two (0.6%) BRAF mutations (Figure 1). All were mutually exclusive. Only 43 (12.3%) cases did not have any of these mutations.
Figure 1.
Frequency of driver mutations in lung adenocarcinoma from female never-smokers. The majority (87.7%) of patients harbored a known mutation in EGFR, HER2, ALK, KRAS or BRAF.
Among EGFR tyrosine kinase domain mutations, 124 were exon 19 deletions, 111 were L858R, eight were G719X mutations in exon 18, and 10 were insertions in exon 20. Five T790M were detected in chemotherapy-naïve patients (four concurrent with L858R, one with G719S). Other aberrations were composed of 709ET=>D, E709K, F723I, L692V, and V689L in exon 18, K757M, 746 ELREAT ins L, 745–746 ins IPVAM, and 746–747 ins GVV in exon 19, S784Y, I768S, V774M, P776L, and S768I in exon 20, and L861Q, L833V, and V834L in exon 21.
All the 16 HER2 mutations were exon 20 insertions. EML-ALK fusions involved six V1, four V2, two V3a/b, and three other fusion variants. KRAS mutations included three G12C, two G12D, one G12V, and one G12A. The two BRAF alterations were both V600E mutations.
As shown in Table 2, patients with EGFR mutations were significantly older than EGFR wild-type patients (mean age, 58.9 versus 54.5 years; p<0.001), whereas patients harboring HER2 mutations were significantly younger than those who did not (mean age, 52.6 versus 58.1 years; p=0.036). There were no significant differences in the distributions of disease stage, or tumor differentiation. Clinicopathological characteristics among EGFR mutation subtypes were not statistically different (Supplementary Table S2). Since the number of cases with KRAS or BRAF mutations was low, we only listed individual patient characteristics for these patients in Supplementary Table S1.
Table 2 .
The association between clinicopathological characteristics and mutational status of EGFR, HER2, and EML4-ALK in 349 never-smoking women with lung adenocarcinoma
| Clinicopathological variables |
EGFR | HER2 | EML4-ALK | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Muta | Wildb | P | Mut | Wild | P | Mut | Wild | P | |
| Age (years) | |||||||||
| Mean | 58.9 | 54.5 | 52.6 | 58.1 | 54.5 | 58.0 | |||
| SD | 9.7 | 11.4 | <0.001 | 6.6 | 10.4 | 0.036 | 8.8 | 10.3 | 0.190 |
| Differentiation | |||||||||
| Poor | 51 | 26 | 5 | 72 | 4 | 73 | |||
| Moderate | 138 | 37 | 7 | 168 | 8 | 167 | |||
| Well | 77 | 20 | 0.066 | 4 | 93 | 0.634 | 3 | 94 | 0.786 |
| Stage | |||||||||
| I | 166 | 40 | 8 | 198 | 6 | 200 | |||
| II | 24 | 9 | 1 | 32 | 3 | 30 | |||
| III | 68 | 31 | 7 | 92 | 5 | 94 | |||
| IV | 8 | 3 | 0.116 | 0 | 11 | 0.653 | 1 | 10 | 0.150 |
| Stage I | |||||||||
| IA | 99 | 26 | 5 | 120 | 5 | 120 | |||
| IB | 67 | 14 | 0.533 | 3 | 78 | 1.000 | 1 | 80 | 0.407 |
Mut, mutant type.
Wild, wild type.
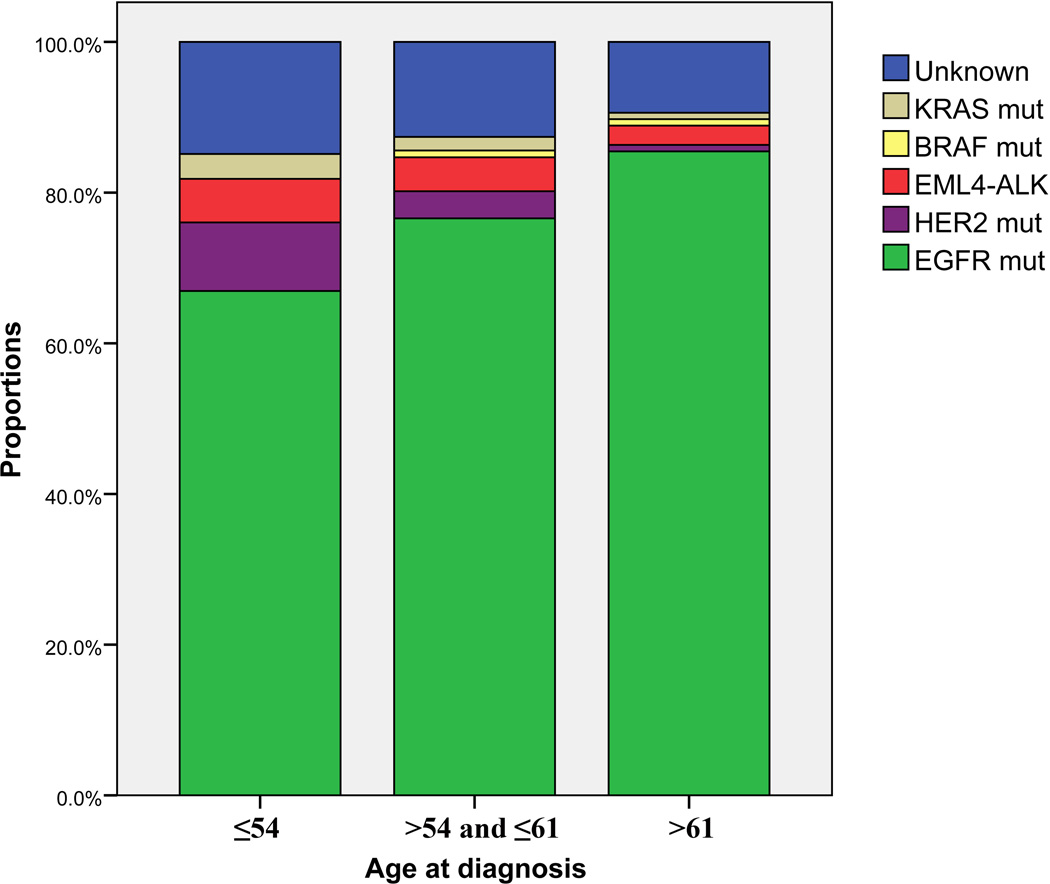
Based on the findings of correlation between age and the presence of driver mutations, we further equally stratified patients into three categories according to age at diagnosis: ≤54, >54 and ≤61, and >61 years old. The three age groups contained 121 (34.7%), 111 (31.8%), and 117 (33.5%) patients, respectively. Figure 2 showed the distribution of molecular drivers among various age groups. Although more than half of the patients harbored EGFR mutations in each category, the frequency of EGFR mutation increased significantly from 66.9% in patients ≤54 years to 76.6% in patients >54 and ≤61 years, and 85.5% in those >61 years (p=0.004). The mutation rate of HER2 was 9.1% in patients ≤54 years, whereas only 3.6% of patients >54 and ≤61 years, and one patient (0.9%) in the oldest category harbored HER2 mutations (p=0.008). The incidences of KRAS mutation, EML4-ALK fusion, and BRAF mutation, respectively, were low and comparable among the age categories.
Figure 2.
Distribution of driver mutations in three age categories. The mutation rate of EGFR increased progressively with age, from 66.9% in patients ≤54 years to 85.5% in cases >61 years (p=0.004). The frequency of HER2 mutations reached 9.1% in the youngest one-third of patients, and declined significantly with increasing age (p=0.008).
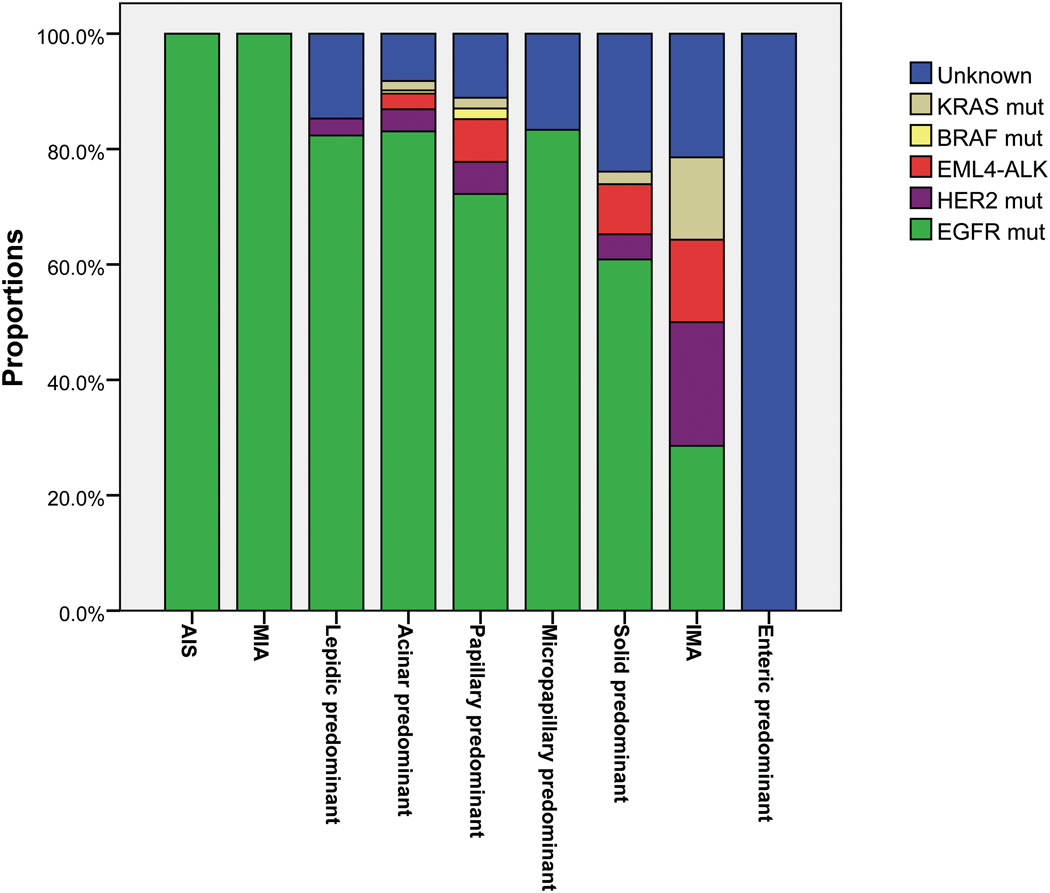
The distribution of driver mutations according to adenocarcinoma histological subtypes was shown in Figure 3. We compared frequency of driver mutations in each individual subtype to all other subtypes (Supplementary Table S3). All the AIS and MIA harbored EGFR mutations. The frequency of EGFR mutations was positively correlated with acinar predominant tumors (83.1% versus 68.7%; p=0.002), and negatively with invasive mucinous adenocarcinoma (28.6% versus 78.2%; p<0.001) and solid predominant tumors (60.9% versus 78.5%; p=0.009). Significantly higher prevalence of KRAS (14.3% versus 1.5%; p=0.028) and HER2 (21.4% versus 3.9%; p=0.021) mutations was found in IMA.
Figure 3.
Distribution of driver mutations according to adenocarcinoma histological subtypes. All the adenocarcinoma in situ (AIS) and minimally invasive adenocarcinoma (MIA) harbored EGFR mutations. Significantly higher frequency of KRAS (14.3% versus 1.5%; p=0.028) and HER2 (21.4% versus 3.9%; p=0.021) mutations was found in invasive mucinous adenocarcinoma (IMA). Frequency of EGFR mutations was positively associated with acinar predominant tumors (83.1% versus 68.7%; p=0.002), and negatively with IMA (28.6% versus 78.2%; p<0.001) and solid predominant tumors (60.9% versus 78.5%; p=0.009).
Multivariate analyses of predictors of driver mutations
Correlations among EGFR, HER2, and KRAS mutations with clinicopathological features were further evaluated by logistic regression analysis incorporating patient age, TNM stage, tumor differentiation, and histological subtypes. We used median age (58 years) as cutoff value. Odds ratios (OR), 95% confidence intervals (CI), and P-values were listed in Table 3. Older age at diagnosis (OR=1.93, 95% CI: 1.15–3.24; p=0.013) and acinar predominant subtype (OR=2.10, 95% CI: 1.25–3.52; p=0.005) were independent predictors of EGFR mutations. Independent predictors of HER2 mutations included younger age at diagnosis (OR=4.17, 95% CI: 1.15–15.11; p=0.030) and IMA subtype (OR=5.81, 95% CI: 1.37–24.72; p=0.017). IMA subtype (OR=14.30, 95% CI: 2.15–95.10; p=0.006) and poor differentiation (OR=6.91, 95% CI: 1.24–38.60; p=0.028) were independently associated with KRAS mutations.
Table 3.
Multivariate analyses of factors that might affect the presence of EGFR, HER2 or KRAS mutations
| EGFR mutation | ||||
|---|---|---|---|---|
| Variable | Category | OR | 95% CI | P |
| Age | ≥58 years/<58 years | 1.93 | 1.15–3.24 | 0.013 |
| Stage | I+II/III+IV | 1.46 | 0.84–2.54 | 0.180 |
| Differentiation | Poor/Moderate+Well | 0.64 | 0.35–1.16 | 0.144 |
| Histological subtype | Acinar predominant/Othersa | 2.10 | 1.25–3.52 | 0.005 |
| HER2 mutation | ||||
| Variable | Category | OR | 95% CI | P |
| Age | <58 years/≥58 years | 4.17 | 1.15–15.11 | 0.030 |
| Stage | I+II/III+IV | 0.72 | 0.24–2.15 | 0.551 |
| Differentiation | Poor/Moderate+Well | 1.65 | 0.50–5.42 | 0.408 |
| Histological subtype | IMA/Othersb | 5.81 | 1.37–24.72 | 0.017 |
| KRAS mutation | ||||
| Variable | Category | OR | 95% CI | P |
| Age | <58 years/≥58 years | 2.32 | 0.42–12.98 | 0.337 |
| Stage | I+II/III+IV | 1.16 | 0.22–6.10 | 0.858 |
| Differentiation | Poor/Moderate+Well | 6.91 | 1.24–38.60 | 0.028 |
| Histological subtype | IMA/Othersb | 14.30 | 2.15–95.10 | 0.006 |
Others, subtypes other than acinar predominant.
Others, subtypes other than invasive mucinous adenocarcinoma.
Discussion
While the incidence of lung cancer in men seems to have reached a plateau, lung cancer rates in women are still rising (1). In parallel, translational research has progressed to the point where we can define the disease at a clinically relevant molecular level. Previous studies have demonstrated distinct molecular features in Asian female never-smokers with lung adenocarcinoma (14–16). However, our study is the first to clarify the spectrum of well-identified oncogenic driver mutations specifically in a large set of lung adenocarcinoma from East Asian never-smoking women, and to analyze correlations between driver mutations and clinicopathological characteristics, especially histological subtypes of adenocarcinoma in line with the new IASLC/ATS/ERS classification of lung adenocarcinoma.
There have been few studies examining the relationship between age at diagnosis and the status of multiple driver mutations concurrently in lung cancer. In those that studied associations with just EGFR mutations, the data are conflicting (19–21). Two studies retrospectively genotyped lung cancer specimens for EGFR from large clinical trials, and revealed that EGFR mutations were associated with younger age of onset (20–21). However, these trials mainly enrolled Caucasian patients. Moreover, a considerable percentage of patients in both studies were males, smokers, or diagnosed with non-adenocarcinoma histology (20–21). This age discrepancy could be partly due to the fact that female lung cancer patients, who are enriched for EGFR mutations, tend to be younger at diagnosis than male patients (22). More recently, a study on 98 Korean females with NSCLC stratified patients into 5-year age groups and showed that EGFR mutation rate significantly increased with age (19), which is consistent with our results. Never-smokers and adenocarcinomas comprised, respectively, 84% and 83% of this population (19). Collectively, these data along with ours highlight again that never-smoking women with lung adenocarcinoma represent a unique subset of patients with the disease.
HER2 mutations were found to be more prevalent in East Asian ethnicity, adenocarcinoma histology, females, and never-smokers (14–15). According to a meta-analysis of published studies, HER2 mutations were present in a small proportion of NSCLC patients, namely 2.1% and, 1.5% of Asian and, Caucasian cases, respectively (23). Consistent with our data, this study also showed that HER2 mutated patients were significantly younger at diagnosis (23). Here, we report a mutation rate of 4.6% in Chinese never-smoking females with adenocarcinoma. When limited to the youngest one-third of patients, the frequency of HER2 mutation was 9.1% in all the patients, and 27.5% in EGFR wild-type patients. These data have implications for clinical trials seeking to enrich for patients with HER2 mutant tumors.
To our knowledge, however, a mechanistic basis to explain an association between the occurrence of driver mutations and age at diagnosis is unclear. As this relationship was revealed in female never-smokers, female hormones might play a role. Estrogens potentially played a role in promoting lung cancer (24). Increased estrogen levels were significantly associated with poorer survival among lung cancer patients (25). Immunohistochemical studies showed that EGFR mutations correlated with higher expression of estrogen receptors (26–27). In a cohort study, women experiencing a longer fertile life (measured as period from age of menarche to age of menopause or age at diagnosis) were found to have a higher proportion of EGFR mutations (28). Although these data are not sufficient to support our findings, future studies on female hormones might help to elucidate the mechanisms underlying the relationship between somatic mutations and age at diagnosis in lung adenocarcinoma from female never-smokers.
With regard to histologic molecular correlations, previous studies revealed a high prevalence of EGFR mutations in adenocarcinomas formerly classified as nonmucinous BAC or with nonmucinous BAC patterns (29–30) which probably fell into AIS, MIA, and lepidic predominant subtype in the new classification (13). Our study showed that all the AIS and MIA (all were nonmucinous in this study), and 82.4% (28 out of 34) of lepidic predominant adenocarcinoma harbored EGFR mutations, while only one HER2 mutation and no other driver mutations were detected in lepidic predominant adenocarcinoma, although statistical significance was not reached, probably due to the limited numbers of these subtypes. A negative correlation between EGFR mutations and solid subtype had also been reported (31–32). Motoi and colleagues (32) showed that EGFR mutations were present in 9.1% (3 out of 33) of the acinar subtype, without statistical significance. However, we could not find a related study enrolling East Asian patients. To our knowledge, our study is the first to report a positive association between EGFR mutations and the acinar predominant subtype.
It has been well demonstrated that invasive mucinous adenocarcinoma (formerly mucinous BAC) is correlated with an absence of EGFR mutations and the presence of KRAS mutations (29, 33–35). The present study confirmed these molecular histological correlations. Casali and colleagues (35) reported that HER2/neu immunohistochemical expression significantly characterized mucinous BAC. However, they did not analyze HER2 mutation in their study (35). Our study is the first to show that IMA subtype was significantly correlated with the presence of HER2 mutations. A possible explanation is the high frequency of HER2 mutations in our series of nearly 350 East Asian female never-smoking lung adenocarcinoma patients which contributed to achieving statistical significance. Together with the findings that EGFR mutations were frequently detected in AIS, MIA, and lepidic predominant adenocarcinomas, our study confirmed the distinctive molecular nature between formerly mucinous BAC and adenocarcinoma formerly classified as nonmucinous BAC or with a nonmucinous BAC component, thus supporting the removal of the term BAC in the new classification (13).
In addition to our previous study (17), we also detected two V600E BRAF mutations in lung adenocarcinoma from never-smoking women, accounting for a mutation rate of 0.6%. Paik and colleagues (36) screened 697 patients with lung adenocarcinoma for BRAF mutations, and found that this molecular alteration was only present in current or former smokers. However, Marchetti and colleagues (37) also detected V600E BRAF mutations in never-smokers, and revealed that this mutation subtype was more prevalent in females than in males.
A major limitation of this study was the lack of survival data. Since the cases were collected from 2007 to 2011, survival data of these patients were far from maturity. However, it would be of interest to evaluate the prognostic impact of clinical variables, histological patterns, and mutation types, and further research in this aspect is warranted.
In summary, through analyses of 349 Chinese female never-smokers with lung adenocarcinoma, we found that the frequency of driver mutations varies with histological subtypes and age at diagnosis. Given the potential effectiveness of TKIs, our findings have implications for translational research as well as clinical therapeutic strategies.
Translational Relevance.
We carried out comprehensive mutational analyses of EGFR, KRAS, ALK, HER2, and BRAF on lung adenocarcinomas from 349 Chinese never-smoking women, and examined correlations between molecular alterations and clinicopathological features. In logistic regression analyses, older age at diagnosis (p=0.013) and acinar predominant subtype (p=0.005) were independent predictors of EGFR mutations. Independent predictors of HER2 mutations consisted of younger age at diagnosis (p=0.030) and invasive mucinous adenocarcinoma (IMA) subtype (p=0.017). IMA subtype (p=0.006) and poor differentiation (p=0.028) were independently associated with KRAS mutations. HER2 mutations are present in a small proportion of NSCLC patients. However, in our study, when limited to the youngest one-third of patients, the frequency of HER2 mutations was 9.1% in all the patients, and 27.5% in EGFR wild-type patients. These data may help inform both clinical trial design and therapeutic strategies for the treatment of never smoking women with lung adenocarcinoma.
Supplementary Material
Acknowledgements
The authors thank Qiong Lu, Shilei Liu, and Hongbin Ji for excellent technical support.
Grant Support
This work was supported by the funds from Key Construction Program of the National “985” Project (985-YFX0102), the Wu Jieping Medical Foundation (320.6740.10020), the National Natural Science Foundation of China (81050022, 81172218, 81101761, 81101760), the Science and Technology Commission of Shanghai Municipality (09JC1416302 and 08411961700), the Shanghai Municipal Health Bureau (2011-206), and the US National Institutes of Health (NCI R01-CA121210).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Doll R, Peto R, Wheatley K, Gray R, Sutherland I. Mortality in relation to smoking, 40 years' observations on male British doctors. BMJ. 1994;309:901–911. doi: 10.1136/bmj.309.6959.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daff ME, Doll R, Kennaway EL. Cancer of the lung in relation to tobacco. Br J Cancer. 1951;5:1–20. doi: 10.1038/bjc.1951.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers--a different disease. Nat Rev Cancer. 2007;7:778–790. doi: 10.1038/nrc2190. [DOI] [PubMed] [Google Scholar]
- 5.Toh CK, Lim WT. Lung cancer in never-smokers. J Clin Pathol. 2007;60:337–340. doi: 10.1136/jcp.2006.040576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 7.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 8.Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 9.Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, et al. EGF receptor gene mutations are common in lung cancers from "never smokers" and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Gre`ve J, Decoster L, De Mey J. Clinical activity of BIBW 2992, an irreversible inhibitor of EGFR/HER1 and HER2 in adenocarcinoma of the lung with mutations in the kinase domain of HER2neu. J Thorac Oncol. 2010;5:S90. [Google Scholar]
- 11.Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–285. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shigematsu H, Takahashi T, Nomura M, Majmudar K, Suzuki M, Lee H, et al. Somatic mutations of the HER2 kinase domain in lung adenocarcinomas. Cancer Res. 2005;65:1642–1646. doi: 10.1158/0008-5472.CAN-04-4235. [DOI] [PubMed] [Google Scholar]
- 15.Stephens P, Hunter C, Bignell G, Edkins S, Davies H, Teague J, et al. Lung cancer: intragenic ERBB2 kinase mutations in tumours. Nature. 2004;431:525–526. doi: 10.1038/431525b. [DOI] [PubMed] [Google Scholar]
- 16.Shigematsu H, Lin L, Takahashi T, Nomura M, Suzuki M, Wistuba II, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97:339–346. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- 17.Sun Y, Ren Y, Fang Z, Li C, Fang R, Gao B, et al. Lung adenocarcinoma from East Asian never-smokers is a disease largely defined by targetable oncogenic mutant kinases. J Clin Oncol. 2010;28:4616–4620. doi: 10.1200/JCO.2010.29.6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest. 2009;136:260–271. doi: 10.1378/chest.08-0978. [DOI] [PubMed] [Google Scholar]
- 19.Choi YH, Lee JK, Kang HJ, Lee TS, Kim HR, Kim CH, et al. Association between age at diagnosis and the presence of EGFR mutations in female patients with resected non-small cell lung cancer. J Thorac Oncol. 2010;5:1949–1952. doi: 10.1097/jto.0b013e3181f38816. [DOI] [PubMed] [Google Scholar]
- 20.Eberhard DA, Johnson BE, Amler LC, Goddard AD, Heldens SL, Herbst RS, et al. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol. 2005;23:5900–5909. doi: 10.1200/JCO.2005.02.857. [DOI] [PubMed] [Google Scholar]
- 21.Bell DW, Lynch TJ, Haserlat SM, Harris PL, Okimoto RA, Brannigan BW, et al. Epidermal growth factor receptor mutations and gene amplification in non-small-cell lung cancer: molecular analysis of the IDEAL/INTACT gefitinib trials. J Clin Oncol. 2005;23:8081–8092. doi: 10.1200/JCO.2005.02.7078. [DOI] [PubMed] [Google Scholar]
- 22.Radzikowska E, Glaz P, Roszkowski K. Lung cancer in women: age, smoking, histology, performance status, stage, initial treatment and survival. Population-based study of 20 561 cases. Ann Oncol. 2002;13:1087–1093. doi: 10.1093/annonc/mdf187. [DOI] [PubMed] [Google Scholar]
- 23.Tomizawa K, Suda K, Onozato R, Kosaka T, Endoh H, Sekido Y, et al. Prognostic and predictive implications of HER2/ERBB2/neu gene mutations in lung cancers. Lung Cancer. 2011 doi: 10.1016/j.lungcan.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 24.Stabile LP, Davis AL, Gubish CT, Hopkins TM, Luketich JD, Christie N, et al. Human non-small cell lung tumors and cells derived from normal lung express both estrogen receptor alpha and beta and show biological responses to estrogen. Cancer Res. 2002;62:2141–2150. [PubMed] [Google Scholar]
- 25.Olivo-Marston SE, Mechanic LE, Mollerup S, Bowman ED, Remaley AT, Forman MR, et al. Serum estrogen and tumor-positive estrogen receptor-alpha are strong prognostic classifiers of non-small-cell lung cancer survival in both men and women. Carcinogenesis. 2010;31:1778–1786. doi: 10.1093/carcin/bgq156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nose N, Sugio K, Oyama T, Nozoe T, Uramoto H, Iwata T, et al. Association between estrogen receptor-beta expression and epidermal growth factor receptor mutation in the postoperative prognosis of adenocarcinoma of the lung. J Clin Oncol. 2009;27:411–417. doi: 10.1200/JCO.2008.18.3251. [DOI] [PubMed] [Google Scholar]
- 27.Raso MG, Behrens C, Herynk MH, Liu S, Prudkin L, Ozburn NC, et al. Immunohistochemical expression of estrogen and progesterone receptors identifies a subset of NSCLCs and correlates with EGFR mutation. Clin Cancer Res. 2009;15:5359–5368. doi: 10.1158/1078-0432.CCR-09-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsuo K, Ito H, Yatabe Y, Hiraki A, Hirose K, Wakai K, et al. Risk factors differ for non-small-cell lung cancers with and without EGFR mutation: assessment of smoking and sex by a case-control study in Japanese. Cancer Sci. 2007;98:96–101. doi: 10.1111/j.1349-7006.2006.00347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakuma Y, Matsukuma S, Yoshihara M, Nakamura Y, Noda K, Nakayama H, et al. Distinctive evaluation of nonmucinous and mucinous subtypes of bronchioloalveolar carcinomas in EGFR and K-ras gene-mutation analyses for Japanese lung adenocarcinomas: confirmation of the correlations with histologic subtypes and gene mutations. Am J Clin Pathol. 2007;128:100–108. doi: 10.1309/WVXFGAFLAUX48DU6. [DOI] [PubMed] [Google Scholar]
- 30.Marchetti A, Martella C, Felicioni L, Barassi F, Salvatore S, Chella A, et al. EGFR mutations in non-small-cell lung cancer: analysis of a large series of cases and development of a rapid and sensitive method for diagnostic screening with potential implications on pharmacologic treatment. J Clin Oncol. 2005;23:857–865. doi: 10.1200/JCO.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 31.Ding L, Getz G, Wheeler DA, Mardis ER, McLellan MD, Cibulskis K, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455:1069–1075. doi: 10.1038/nature07423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Motoi N, Szoke J, Riely GJ, Seshan VE, Kris MG, Rusch VW, et al. Lung adenocarcinoma: modification of the 2004 WHO mixed subtype to include the major histologic subtype suggests correlations between papillary and micropapillary adenocarcinoma subtypes, EGFR mutations and gene expression analysis. Am J Surg Pathol. 2008;32:810–827. doi: 10.1097/PAS.0b013e31815cb162. [DOI] [PubMed] [Google Scholar]
- 33.Finberg KE, Sequist LV, Joshi VA, Muzikansky A, Miller JM, Han M, et al. Mucinous differentiation correlates with absence of EGFR mutation and presence of KRAS mutation in lung adenocarcinomas with bronchioloalveolar features. J Mol Diagn. 2007;9:320–326. doi: 10.2353/jmoldx.2007.060182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tam IY, Chung LP, Suen WS, Wang E, Wong MC, Ho KK, et al. Distinct epidermal growth factor receptor and KRAS mutation patterns in non-small cell lung cancer patients with different tobacco exposure and clinicopathologic features. Clin Cancer Res. 2006;12:1647–1653. doi: 10.1158/1078-0432.CCR-05-1981. [DOI] [PubMed] [Google Scholar]
- 35.Casali C, Rossi G, Marchioni A, Sartori G, Maselli F, Longo L, et al. A single institution-based retrospective study of surgically treated bronchioloalveolar adenocarcinoma of the lung: clinicopathologic analysis, molecular features, and possible pitfalls in routine practice. J Thorac Oncol. 2010;5:830–836. doi: 10.1097/jto.0b013e3181d60ff5. [DOI] [PubMed] [Google Scholar]
- 36.Paik PK, Arcila ME, Fara M, Sima CS, Miller VA, Kris MG, et al. Clinical characteristics of patients with lung adenocarcinomas harboring BRAF mutations. J Clin Oncol. 2011;29:2046–2051. doi: 10.1200/JCO.2010.33.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marchetti A, Felicioni L, Malatesta S, Sciarrotta MG, Guetti L, Chella A, et al. Clinical Features and Outcome of Patients With Non-Small-Cell Lung Cancer Harboring BRAF Mutations. J Clin Oncol. 2011 doi: 10.1200/JCO.2011.35.9638. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.