Abstract
To evaluate the applicability of high-dose conditioning, CD34 selection and enhanced natural killer (NK) cell alloreactivity reported as promising after haploidentical transplantation, we tested the same strategy for patients with advanced/high risk myeloid leukemia lacking either related or well-matched unrelated donors (URD). In a prospective multicenter clinical trial using pretransplant conditioning of thiotepa (5 mg/kg/day × 2), fludarabine (40 mg/mg/M2/day × 5) and total body radiation (800 cGy) plus thymoglobulin (2.5 mg/kg day × 2) and a CD34 selected filgrastim stimulated peripheral blood graft from a partial matched URD, we treated 24 patients. The patients (median age 40 (range 22–61)) were mismatched at 1–3/10 HLA loci with their donors; all were mismatched at HLA-C. Thirty-seven percent were ethnic or racial minorities. Twenty-one of 24 engrafted promptly with one primary graft failure and two early deaths. The cumulative incidence of Grade II–IV acute GVHD (34%, 95% confidence interval (CI), 14–54%), chronic GVHD (20%, 95% CI, 2–38%) and relapse (26%, 95% CI, 8–84%) were unaffected by KIR ligand donor:recipient mismatch (n = 5) vs. KIR ligand match (n = 19). Only three (12%) had Grade III–IV GVHD. Non-relapse occurred in 17% (95% CI, 30–31%) by 100 days and 35% (95% CI, 15–55%) by 1 year. Two year survival and leukemia-free survival were each 40% (95% CI, 21–59%) and was similar in KIR ligand matched or mismatched patients. Infections, mostly in the first two months, were frequent, and were the cause of death in five patients (35% of deaths). T cell recovery and NK cell proliferation and functional maturation were not altered by KIR ligand match or mismatch status. For these high risk patients, this high intensity regimen and T depleted approach yielded satisfactory outcomes, but logistical difficulties in arranging URD grafts for patients with high risk, unstable leukemia limited accrual. Improvements in peritransplant disease control and additional measures to augment the allogeneic graft vs. leukemia effect are still required.
Keywords: Transplantation, Unrelated donor, Leukemia, Mismatch
Introduction
The challenge of harnessing the potency of graft vs. leukemia to limit recurrence of advanced myeloid leukemia (AML) following allogeneic hematopoietic cell transplantation (HCT) remains difficult. Several strategies including large dose stem cell infusions from haploidentical or unrelated donors [1] and choosing a KIR ligand mismatched and thus natural killer (NK) cell alloreactive donor have been reported as important in experience from Perugia using T cell depleted grafts, [2,3] though not confirmed in other studies [4–7]. Additionally, intensive conditioning and careful patient selection based on performance status and limited leukemic burden at HCT has yielded encouraging outcomes [3]. Following unrelated donor (URD) HCT, we and others observed that utilization of donors with the KIR B haplotype and particularly those expressing favorable KIR loci have been associated with reduced relapse and improved leukemia-free survival [8–12]. To capitalize on this intensive conditioning regimen and study the role of KIR ligand mismatch and thus favorable alloreactivity reported with haploidentical transplantation, we designed a prospective multicenter trial testing this identical approach using CD34+ selected URD grafts and the value of KIR-ligand matched versus mismatched URD for patients lacking better matched family or URDs. We report patients’ outcomes and potential difficulties in applying this strategy to a broader leukemia population.
Patients and Methods
Eligible patients were those at participating centers (Washington University St. Louis; Moffitt Cancer Center, Tampa; Medical College of Wisconsin; Ohio State University; University of Pennsylvania; Emory University; Indiana University; University of Minnesota) with myeloid malignancies including acute myeloid leukemia (AML), chronic myelogenous leukemia (CML) or myelodysplastic syndrome (MDS) having either high-risk cytogenetic or molecular abnormalities in early remission or those with partial remission or advanced disease. Patients could have any marrow blast burden, but no more than 2,000/ul circulating blasts at the time of transplantation. Additionally, patients needed satisfactory multiorgan function, performance status (comorbidity index <=2) [13] and controlled pretransplant infections to be eligible for this myeloablative HCT. Active central nervous system (CNS) leukemia was not allowed unless treated with intrathecal or systemic therapy. Partially matched URD were used exclusively for this trial and thus patients with a closely matched related donor (HLA identical or 1 locus mismatched) and those with an HLA allele-matched (or single allele mismatch) URD were excluded. Donor selection was directed to identify those with KIR ligand mismatch [1,5,6] and thus to preferentially choose donor:recipient combinations with HLA C mismatch or Cw4, Bw4 or Bw6 mismatch as available. HLA-A11 alleles were not considered in the KIR-ligand matching algorithm. Other factors for donor selection including donor age, CMV serostatus, ABO match and previous alloimmunization through pregnancy were considered individually and not specified for this multicenter study. Beyond the 24 patients treated, 6 others consented to participate, but could not proceed to HCT for various reasons including progression of leukemia, donor unavailability or donor center non-participation. Some of these patients later had a transplant either off-study or using alternate techniques; their results are not included. All participating patients and donors exercised written informed consent for participation. Donor participation included consent for donating filgrastim-primed peripheral blood stem cells (PBSC) according to standard donor center and National Marrow Donor Program (NMDP) procedures as well as provision of a pre-filgrastim donor blood sample used for research studies associated with the protocol [14,15]. No other donor procedures were modified for this study. Institutional review boards (IRBs) at the NMDP and all participating donor and transplant centers approved the protocol which was registered as NCT00392782 at clinical trials.gov.
Pretransplant conditioning replicated the regimen originally reported from Perugia [2,3] including thiotepa (5 mg/kg/day × 2, day −8,−7), fludarabine (40 mg/mg/M2/day × 5, days 7 to −3) and total body irradiation (TBI) 800 cGy in 1 or 2 fractions on day −9 (−10). The filgrastim-mobilized URD PBSC grafts were processed at the transplant center by immunomagnetic bead CD34 positive cell selection using the CliniMacs device using procedures approved under IDE 12840 from the US Food and Drug Administration for this study. Targeted graft yields were to retain >50% of the CD34+ cells collected and infuse grafts containing >75% CD34+ cells. Post-processing, grafts were required to include > 2 × 106 CD34+ cells/kg and < 3 × 104 CD3+ cells/kg prior to infusion. Processed cells were washed and administered intravenously on day 0 with no additional post-transplant GVHD prophylaxis. Rabbit anti-thymocyte globulin (Thymoglobulin, Genzyme Inc.) was administered (2.5 mg/kg day −3 and −2) for additional in vivo T cell depletion and host immunosuppression. Supportive care included individual room hospitalization, growth factor use, antibiotic therapy directed towards bacterial, fungal and viral pathogens per institutional guidelines as well as prospective monitoring for CMV and EBV infections to permit pre-emptive antiviral therapy as needed.
Post-transplant events were prospectively identified and reported to the central monitoring site, the University of Minnesota Masonic Cancer Center Clinical Trials Office. Engraftment was defined as the first of three consecutive measures of an absolute neutrophil count > 500/mcL and platelets > 20,000/mcL without a transfusion for 7 days. Acute and chronic GVHD were reported using conventional definitions. Because some patients (6 of 24) had transplantation with active leukemia or advanced MDS, documentation of the achievement of complete remission by day 28 was required. Subsequent post-transplant relapse documented by hematologic, marrow or extramedullary findings was calculated using the cumulative incidence method treating non-relapse mortality as a competing risk. Time to relapse or death (leukemia-free survival, LFS) and overall survival (OS) were calculated from the day of transplant using the Kaplan Meier estimator and 95% confidence intervals derived from the standard errors [16]. The cumulative incidence calculation [17] was applied for estimating the probability of acute and chronic GVHD and relapse treating non-event mortality as a competing risk and estimating non-relapse mortality (NRM) treating relapse as a competing risk. All calculations were performed using SAS and R software. Data was retrieved through the standardized baseline and follow-up reports to the Center for International Blood and Marrow Transplant Research (CIBMTR) and the NMDP plus supplemental monitoring for serious adverse events (SAEs) and infections using procedures specified for this prospective study protocol.
Results
Details of patients’ demographics, donor matching, diagnosis and disease status at transplant and graft characteristics are shown in Table 1. All 24 patients were adults with a median age of 40 and 13 were females. Seventeen (71%) patients had AML. Three were in relapse and 6 had intermediate/high grade MDS or CML beyond 1st chronic phase at transplantation. Three had pre-HCT fungal respiratory tract infections and 3 had pre-HCT performance scores of 60–80. Donors and recipients were mismatched at 1–3 HLA alleles (63% had 2–3 mismatches of 10 (HLA-A, B, C, DRB1, DQB1). Mismatches were at HLA-C (n=24, 100%); --B (10, 42%), and –A (3, 13%) and –DQB1 (5, 21%). Five of 24 (21%) had KIR ligand mismatch in the donor vs. host direction favoring NK cell alloreactivity.
Table 1.
Patient Demographics
| N (%) | |
|---|---|
| Total | 24 |
| Male | 11 (46%) |
| Age in years | |
| Median (range), (IQR) | 39.7 (21.9–60.5), (29.7–53.0) |
| Race/Ethnicity | |
| Hispanic | 2 (8%) |
| Non-hispanic White | 15 (63%) |
| African American | 6 (25%) |
| Asian | 1 (4%) |
| Diagnosis | |
| AML | 17 (71%) |
| CR1 | 10 (42%) |
| High risk^ | 6(25%) |
| CR2 | 4 (21%) |
| Relapse | 3 (8%) |
| MDS (advanced) | 3 (13%) |
| CML | 4 (17%) |
| Chronic Phase 1 | 1 (4%) |
| Accelerated/CP2 | 3 (13%) |
| Karnofsky* | |
| 90–100 | 19 (79%) |
| 60–80 | 3 (13%) |
| Pre-HCT fungal infection | 3 (13%) |
| CMV Serostatus* | |
| Recipient positive | 13 (54%) |
| Recipient negative/Donor positive | 3 (13%) |
| Recipient negative/Donor negative | 7 (29%) |
| Weight in kilograms | |
| Median (range) | 95 (48–168) |
| CD34+ cells infused (×106/kg)* | |
| Median (range), (IQR) | 4.4 (1.2–9.7), (2.9–6.0) |
| CD3+ cells infused (×104/kg)* | |
| Median (range), (IQR) | 2.8 (1–8.4), (1.7–4.2) |
| HLA locus match | |
| 7/10 | 3 (13%) |
| 8/10 | 12 (50%) |
| 9/10 | 9 (38%) |
| HLA mismatch locus | |
| -A | 3 (13%) |
| -B | 10 (42%) |
| -C | 24 (100%) |
| -DRB1 | 0 |
| -DQB1 | 5 (21%) |
| KIR-ligand | |
| Matched | 19 (79%) |
| Mismatch (GVH direction) | 5 (21%) |
Molecular or cytogenetic high risk
With reported data Interquartile range (IQR)
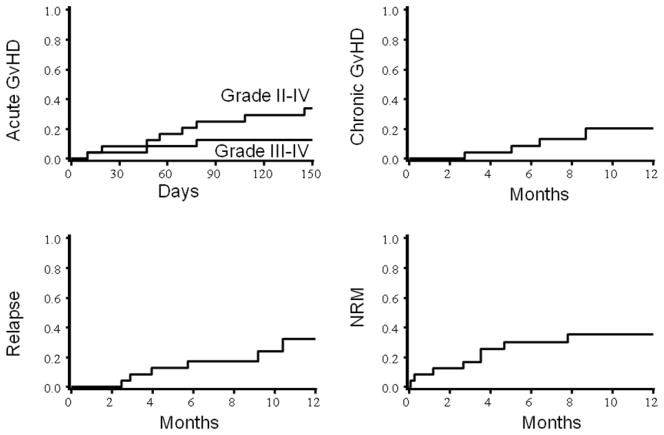
Of the 24 patients 21 had prompt donor engraftment at a median of 13 days (range 9–27) post-HCT. Platelet recovery (to > 20,000/mcL) occurred in 17 of 23 evaluable at a median of 18 days (range 12–34) post-HCT. Primary graft failure occurred in 1 patient who had active leukemia at transplantation; 2 others had early deaths. Two patients had secondary donor cell reinfusions for incomplete hematologic recovery and/or persistent leukemia and 1 recovered following a second allograft. As shown in Table 2, only 10 patients developed acute GVHD (two had maximum Grade I, five Grade II, three Grade III–IV). As shown in Figure 1, the cumulative incidence of Grade II–IV acute GVHD was 34% (95% confidence interval (CI), 14–54%). Only 4 (20% (95% CI, 2–38%) had developed chronic GVHD by 1 year post-HCT. Six had a post-transplant relapse at a median of 5 (2.5–11) months post-transplantation. Relapse incidence at 2 years was not lower in recipients of KIR-ligand (KIR-L) mismatched than KIR-L matched donor grafts (KIR-L matched: 22% (95% CI 4–40%); mismatched 40% (95% CI 1–79), p= 0.82). Transplant-related, non-relapse mortality (NRM, death during continuous post-transplant remission) occurred in 8 patients, 17% (95% CI, 30–31%) by 100 days and 35% (95% CI, 15–55%) by 1 year. The NRM incidence was similar in recipients transplanted in remission or during relapse (not shown). These deaths were attributable to infection (viral (HHV-6, CMV), candida, E. Coli), pneumonitis, septic shock, CNS injury due to hypotension plus hypoxia and renal failure.
Table 2.
Outcomes after allogeneic HCT (N = 24)
| N died | 100 day survival | 2 year survival | ||
|---|---|---|---|---|
| Survival | 14 | 71% (53–89%) | 40% (21–59%) | |
| N events | 100 day LFS | 2 year PFS | ||
| Leukemia-Free Survival | 14 | 75% (53–88%) | 40% (21–59%) | |
| 100 day Relapse | 2 year Relapse | |||
| Relapse/Progression | 6 | 8% (0–19%) | 26% (8–44%) | |
| 100 day NRM | 1 year NRM | |||
| Non-Relapse Mortality | 8 | 17% (3–31%) | 34% (15–53%) | |
| Grade II–IV acute GVHD | 8 | 34% (14–54%) | ||
| Maximum grade acute GVHD | N (%) | |||
| Grade | None | 14 (58%) | ||
| I | 2 (8%) | |||
| II | 5 (21%) | |||
| III | 2 (8%) | |||
| IV | 1 (4%) | |||
| 1 year chronic GVHD | ||||
| Chronic GVHD | 4 | 20% (2–38%) | ||
Shown are the number (%) and Kaplan Meier (survival, LFS) or cumulative incidence (Relapse, GVHD, NRM) estimates of post-HCT events (± 95% confidence intervals).
Figure 1.
Cumulative incidence of acute and chronic GVHD, relapse and NRM
Infections
Sixteen of 24 patients developed one or more grade 3–5 infections following HCT. These included 16 early bacterial infections (2 E. Coli; 4 staphylococci; 3 streptococci, 5 enterococci; 1 bacillus sp.), 8 viral infections (3 polyomavirus; 4 Herpes simplex; 1 CMV). and 2 systemic fungal infections (both candidemia) plus later infections as shown in Table 3A. The day 100 cumulative incidence of CMV infection was 42% (95%CI 22–62). One patient had multiorgan CMV disease; the others were detected in blood alone. Of these infections, 5 were life-threatening and 4 were the primary attributable causes of death (Table 3B). Beyond 6 months post HCT, infections were reported in only a minority of evaluable patients.
Table 3A.
Infections (Severity grades 3–5*)
| N evaluable | Patients with Infections N (%) | |
|---|---|---|
| 1st 4 weeks | ||
| Bacterial | 24 | 16 (67%) |
| Gram negative | 1 | |
| Gram positive | 13 | |
| C. difficile | 2 | |
| Viral | 8 (33%) | |
| Polyoma | 3 | |
| HSV | 4 | |
| CMV | 1 | |
| Fungal | 2 (8%) | |
| Candida | 2 | |
| 5–8 weeks | ||
| Bacterial | 20 | 5 (25%) |
| Gram negative | 1 | |
| Gram positive | 4 | |
| Viral | 6 (30%) | |
| HSV | 1 | |
| CMV | 3 | |
| EBV | 1 | |
| Adenovirus | 1 | |
| Fungal | 0 | |
| 9–12 weeks | ||
| Bacterial | 20 | 6 (30%) |
| Gram negative | 1 | |
| Gram positive | 5 | |
| Viral | 5 (25%) | |
| HSV | 0 | |
| CMV | 4 | |
| EBV | 0 | |
| Adenovirus | 1 | |
| HHV-6 | 1 | |
| Fungal | 2 (10%) | |
| Candida | 2 | |
| 13–24 weeks | ||
| Bacterial | 16 | 4 (25%) |
| Gram negative | 0 | |
| Gram positive | 4 | |
| Viral | 3 (19%) | |
| HSV | 0 | |
| CMV | 2 | |
| EBV | 0 | |
| Adenovirus | 0 | |
| HHV-6 | 1 | |
| Fungal | 1 (6%) | |
| Yeast, nos | 1 | |
Grade 3 (requiring parenteral antimicrobial therapy; Grade 4 (life-threatening) and Grade 5 (fatal) infections reported over time.
Survival and Leukemia Free Survival
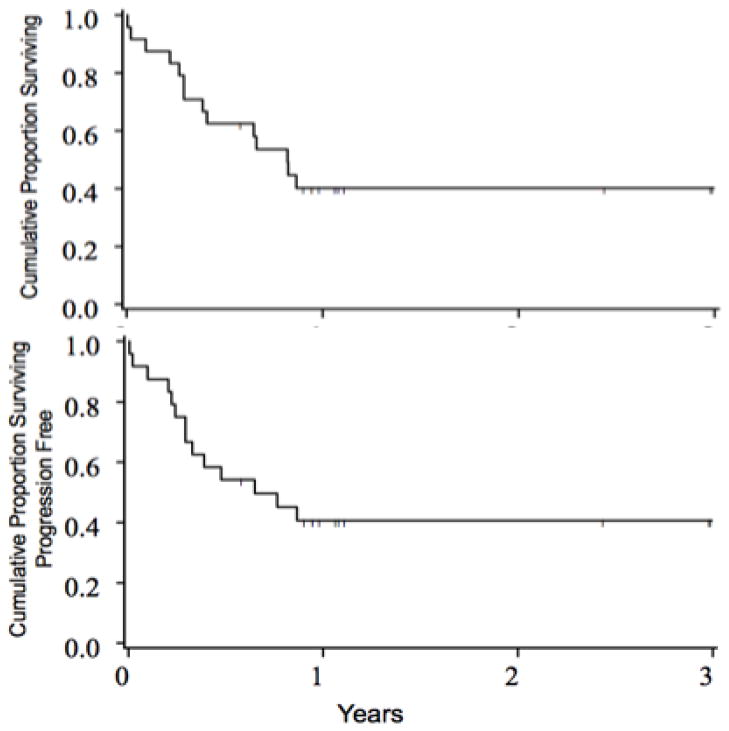
At median follow-up of 13 (7–37) months, 10 of 24 patients survive with an estimated 2 year survival of 40% (95% CI, 21–59%), Figure 2A. Relapse was the primary cause in 6 (43%) deaths (Table 3B). No patients surviving in remission beyond 12 months have had subsequent post-transplant relapse. In the 6 patients with persisting or recurring leukemia post-HCT, the median survival from HCT is 9 (3–11) months. All 10 of the survivors remain in complete remission and thus two year leukemia free survival is also 40% (95% CI, 21–59%), 2B. Survival and leukemia free survival at 2 years were identical at 47% (95% CI, 24–67%) for those transplanted in remission and 20% (95% CI, 1–58%) for those not in remission at HCT, p=0.68. KIR-L mismatch (MM) in the graft versus host direction was present in 5 of 24 patients, but had no notable impact on survival: 3 of 5 KIR-L MM recipients survive (median 13 months, range 12–38) versus 7 of 19 KIR-L matched (median 13 months, range 7–36), p=0.29. Similarly, 2 year LFS was similar in KIR-L matched (35% (95% CI, 15–56)) and KIR-L MM (60%, 13–88), p=0.63).
Figure 2.
Survival (A) and Leukemia Progression-Free Survival (B) after partial matched URD HCT
Immune Recovery after HCT
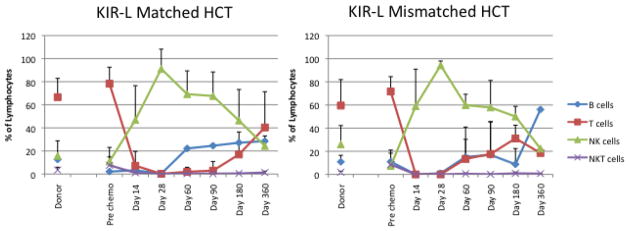
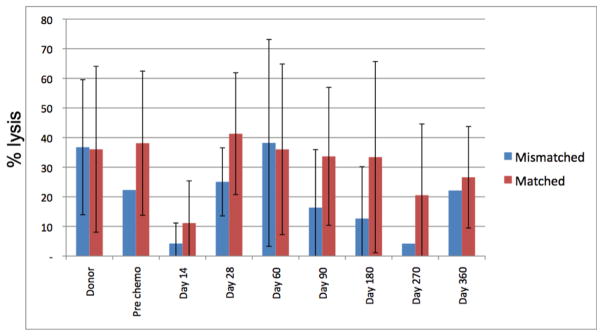
To address the hypothesis that KIR-L MM would enhance alloreactivity, we also monitored lymphoid phenotypic and functional recovery after HCT. Post-transplant recovery of both B cell and T cell subsets were delayed and did not reach donor or recipient pre-HCT levels by day + 360, but did not vary according to KIR-L matched vs. MM grafts (Fig 2A, B). NK cell numbers increased early (by day +14) and were sustained until day +180 when they reached levels similar to those seen in the donor (and recipient) pre-HCT (Figure 3). There was no accelerated recovery of any cell subset in HCTs using KIR-L MM donors (Fig 2A, B). Recovery of KIR+ (>30%) NK cells occurred in half the patients by day +180 and was similar in those with KIR-L match or MM donor grafts (data not shown). NK cell cytolytic capacity (assessed by K562 cytolysis in vitro, 4) reached normal levels by day +180 and was also unaffected by HCT using KIR-L M or MM donor grafts. Recovery of these educated, KIR+ NK cells was not, however, associated with better protection against leukemia relapse, infection or TRM (data not shown).
Figure 3.
Lymphocyte recovery after HCT. Circulating lymphocyte subsets (T cells, B cells, NK cells) in KIR-ligand matched (A) and KIR-ligand mismatched (B) HCT. The recovery pattern and timing was similar in both cohorts and recovered to near normal (similar to pre-HCT donor and recipient measures) by 6–9 months after HCT. Subset numbers were similar in both cohorts studied. Absolute lymphocyte numbers were not available at all timepoints thus absolute lymphocyte subset numbers are not shown.
Discussion
We report leukemia free survival in this series of high risk leukemia patients receiving CD34+ cell-selected grafts from partially matched URD after myeloablative conditioning. Investigators from Perugia reported promising findings in a series of reports stemming from the early 1990’s using this high intensity conditioning, large dose progenitor cell infusions and a T cell depleted graft, preferentially collected from a KIR ligand mismatched and thus alloreactive haploidentical donor [1–3]. In similar patients, they described 28% 5 year event free survival, with better results (up to 67%) for patients treated during remission using NK alloreactive donors, but less favorable outcomes for those with active relapse (6–34% based upon donor NK cell alloreactivity) [3]. Based on these encouraging results, we tested the identical conditioning regimen, immunosuppression and CD34+ graft selection in this prospective series of high risk myeloid leukemia patients lacking an HLA-identical sibling or well-matched URD. In the current series, we observed favorable achievement of complete remission, even for those treated during relapse and modest NRM. Leukemia-free survival was encouraging with 40% of patients alive and in remission beyond 2 years post-transplantation, similar to other reports of PBSC HLA matched and HLA-C mismatched transplants [18].
Compared to the Perugia series, these URD recipients were somewhat older, had no exposure to any tolerizing effects of non-inherited maternal antigens and would not, of course, have inherited any other immunologically pertinent genetic elements that could modulate the hazards of GVHD in a haploidentical related donor HCT. Some of these factors may explain, at least in part, the observed higher rates of GVHD than those reported from Perugia.
Transplantation for patients with advanced, high risk myeloid leukemia remains challenging [18]. Adverse cytogenetics, advanced remission or active leukemia at HCT remain as major barriers to success [19]. A recent CIBMTR report documented favorable HCT outcomes for AML patients transplanted during relapse, but only for younger patients (under age 35) who had low blast burden, and HLA-identical sibling or well-matched URDs [19]. However, for older patients or those with advanced disease, intensified conditioning has not yielded improved outcomes [19]. In contrast, harnessing the potent alloreactivity of the graft either through T replete or NK cell alloreactive, T cell-depleted grafts offers somewhat better results [3]. T-cell depletion may be essential as residual donor T cells may confound the favorable impact of NK alloreactivity [21–23].
However, exercising this option for patients with advanced or unstable leukemia was daunting. Orchestrating the logistics to enroll patients with suitable performance status and minimal disease burden or remission was difficult; particularly coordinating the timing of transplantation to best meet the patients’ needs. Addionally, the delivered CD34 graft dose is restricted by donor registry (NMDP) standards and thus grafts might be smaller than those received from haploidentical family donors. We restricted eligibility for this trial to patients lacking an HLA identical sibling (approximately 1/3 of patients) or an HLA allele-matched URD (40–75% of patients based on their ethnicity, racial group), leaving only a minority of patients potentially eligible for consideration [24,25]. Umbilical cord blood (UCB) or haploidentical family transplants may be another option, potentially more quickly available [26,27], but for haploidentical transplants, published success beyond the Perugia experience has been limited [28], but are now being explored within the BMT CTN [31]. UCB grafts have rarely been used for AML in relapse.
In this trial we observed satisfactory clinical results including some control of active leukemia, but no suggestion that KIR ligand mismatch was associated with improved outcome or less risk of relapse. Due to limited accrual we were unable to definitively address these questions. Though early but not all reports suggested the possibility [29,30], we did not observe that KIR-L mismatch was associated with improved NK reconstitution following transplantation. Similar to several retrospective analyses [5–7,10,12], our data suggest that additional factors beyond the KIR ligand mismatch model are operative in these transplants.
Substantially larger number of subjects, perhaps with more uniform disease status, will be required to satisfactorily probe these hypotheses which were derived from the earlier, large haploidentical HCT experience. Nonetheless, outcomes in these high risk and advanced patients, with limited post-transplant GVHD-related morbidity, suggest that elements of this approach should be further investigated. Enhancements needed for timely logistics in coordinating donor availability and patient readiness suitable for unstable, high risk AML remain to be refined. Prompt initiation of search and donor identification soon after diagnosis can enhance donor availability at the right time to allow patients to receive a transplant when they best need it. Further investigations to enhance donor graft alloreactivity mediated either by T cells or NK cells might better capitalize on GVL to limit relapse and improve survival for high risk AML.
Figure 4.
Lymphocyte cytolysis of K562 in post-HCT samples. Shown are the % lysis (effector:target 20:1) of K562 targets by lymphocytes collected from HCT donor and recipients over time post-HCT. Recovery of lytic activity was good beyond day 28 post HCT and was similar in recipients of KIR-ligand matched and mismatched HCT.
Table 3B.
Primary Cause of Death
| N (%) | |
|---|---|
| Relapse | 6 (43%) |
| Bacterial Infection | 1 (7%) |
| Fungal Infection | 1 (7%) |
| Viral Infection | 2 (14%) |
| Other Infection | 1 (7%) |
| Neurotoxicity (sepsis; hypotension | 2 (14%) |
| Renal Failure | 1 (7%) |
Grade 3 (requiring parenteral antimicrobial therapy; Grade 4 (life-threatening) and Grade 5 (fatal) infections reported over time.
Acknowledgments
The investigators express their appreciation for assistance from Genzyme, Inc. and Miltenyi Corp. who provided partial research support and graft processing materials for the study. Support from 2PO1 CA111412 from the National Cancer Institute and the collaboration of personnel at the NMDP and CIBMTR and the participating donor centers were also essential for successful completion of the trial.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 2.Aversa F, Terenzi A, Tabilio A, et al. Full haplotype-mismatched hematopoietic stem-cell transplantation: a phase II study in patients with acute leukemia at high risk of relapse. J Clin Oncol. 2005;23:3447–3454. doi: 10.1200/JCO.2005.09.117. [DOI] [PubMed] [Google Scholar]
- 3.Ruggeri L, Mancusi A, Capanni M, et al. Donor natural killer cell allorecognition of missing self in haploidentical hematopoietic transplantation for acute myeloid leukemia: challenging its predictive value. Blood. 2007;110:433–440. doi: 10.1182/blood-2006-07-038687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giebel S, Locatelli F, Lamparelli T, et al. Survival advantage with KIR ligand incompatibility in hematopoietic stem cell transplantation from unrelated donors. Blood. 2003;102:814–819. doi: 10.1182/blood-2003-01-0091. [DOI] [PubMed] [Google Scholar]
- 5.Davies S, Ruggeri L, DeFor T, et al. Evaluation of KIR ligand incompatibility in mismatched unrelated donor hematopoietic transplants. Blood. 2002;100:3825–3827. doi: 10.1182/blood-2002-04-1197. [DOI] [PubMed] [Google Scholar]
- 6.Farag SS, Bacigalupo A, Eapen M, et al. The effect of KIR ligand incompatibility on the outcome of unrelated donor transplantation: a Report from the Center for International Blood and Marrow Transplant Research, the European Blood and Marrow Transplant Registry, and the Dutch Registry. Biol Blood Marrow Transplant. 2006;12:876–884. doi: 10.1016/j.bbmt.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Miller JS, Cooley S, Parham P, et al. KIR ligands are associated with less relapse and increased graft-versus-host disease (GVHD) following unrelated donor allogeneic HCT. Blood. 2007;109:5058–5061. doi: 10.1182/blood-2007-01-065383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooley S, Trachtenberg E, Bergemann TL, et al. Donors with Group B KIR haplotypes improve relapse-free survival after unrelated hematopoietic cell transplantation for acute myelogenous leukemia. Blood. 2009;113:726–732. doi: 10.1182/blood-2008-07-171926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooley S, Weisdorf DJ, Guethlein LA, et al. Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood. 2010;116:2411–2419. doi: 10.1182/blood-2010-05-283051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cook MA, Milligan DW, Fegan CD, et al. The impact of donor KIR and patient HLA-C genotypes on outcome following HLA-identical sibling hematopoietic stem cell transplantation for myeloid leukemia. Blood. 2004;103:1521–1526. doi: 10.1182/blood-2003-02-0438. [DOI] [PubMed] [Google Scholar]
- 11.Hsu KC, Keever-Taylor CA, Wilton A, et al. Improved outcome in HLA-identical sibling hematopoietic stem-cell transplantation for acute myelogenous leukemia predicted by KIR and HLA genotypes. Blood. 2005;105:4878–4884. doi: 10.1182/blood-2004-12-4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beelen DW, Ottinger HD, Ferencik S, et al. Genotypic inhibitory killer immunoglobulin-like receptor ligand incompatibility enhances the long-term antileukemic effect of unmodified allogeneic hematopoietic stem cell transplantation in patients with myeloid leukemias. Blood. 2005;105:2594–600. doi: 10.1182/blood-2004-04-1441. [DOI] [PubMed] [Google Scholar]
- 13.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)–specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karanes C, Nelson GO, Chitphakdithai P, et al. Twenty years of unrelated donor hematopoietic cell transplantation for adult recipients facilitated by the National Marrow Donor Program. Biol Blood Marrow Transplant. 2008;14:8–15. doi: 10.1016/j.bbmt.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 15.MacMillan ML, Davies SM, Nelson GO, et al. Twenty years of unrelated donor bone marrow transplantation for pediatric acute leukemia facilitated by the National Marrow Donor Program. Biol Blood Marrow Transplant. 2008;14:16–22. doi: 10.1016/j.bbmt.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 17.Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Stat Med. 1997;16:901–910. doi: 10.1002/(sici)1097-0258(19970430)16:8<901::aid-sim543>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 18.Woolfrey A, Klein JP, Haagenson M, Spellman S, Petersdorf E, Oudshoorn M, Gajewski J, Hale GA, Horan J, Battiwalla M, Marino SR, Setterholm M, Ringden O, Hurley C, Flomenberg N, Anasetti C, Fernandez-Vina M, Lee S. HLA-C Antigen Mismatch Is Associated with Worse Outcome in Unrelated Donor Peripheral Blood Stem Cell Transplantation. Biol Blood Marrow Transplant. 2011;17:885–892. doi: 10.1016/j.bbmt.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duval M, Klein JP, He W, et al. Hematopoietic stem-cell transplantation for acute leukemia in relapse or primary induction failure. J Clin Oncol. 2010;28:3730–3738. doi: 10.1200/JCO.2010.28.8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta V, Tallman MS, Weisdorf DJ. Allogeneic hematopoietic cell transplantation for adults with acute myeloid leukemia: myths, controversies, and unknowns. Blood. 2011;117:2307–2318. doi: 10.1182/blood-2010-10-265603. [DOI] [PubMed] [Google Scholar]
- 21.Bishara A, De Santis D, Witt CC, et al. The beneficial role of inhibitory KIR genes of HLA class I NK epitopes in haploidentically mismatched stem cell allografts may be masked by residual donor-alloreactive T cells causing GVHD. Tissue Antigens. 2004;63:204–211. doi: 10.1111/j.0001-2815.2004.00182.x. [DOI] [PubMed] [Google Scholar]
- 22.Cooley S, McCullar V, Wangen R, et al. KIR reconstitution is altered by T cells in the graft and correlates with clinical outcomes after unrelated donor transplantation. Blood. 2005;106:4370–4376. doi: 10.1182/blood-2005-04-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Ianni M, Falzetti F, Carotti A, et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011;117:3921–3928. doi: 10.1182/blood-2010-10-311894. [DOI] [PubMed] [Google Scholar]
- 24.Flomenberg N, Baxter-Lowe LA, Confer D, et al. Impact of HLA class I and class II high-resolution matching on outcomes of unrelated donor bone marrow transplantation: HLA-C mismatching is associated with a strong adverse effect on transplantation outcome. Blood. 2004;104:1923–1930. doi: 10.1182/blood-2004-03-0803. [DOI] [PubMed] [Google Scholar]
- 25.Lee SJ, Klein J, Haagenson M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110:4576–4583. doi: 10.1182/blood-2007-06-097386. [DOI] [PubMed] [Google Scholar]
- 26.Willemze R, Rodrigues CA, Labopin M, et al. KIR-ligand incompatibility in the graft-versus-host direction improves outcomes after umbilical cord blood transplantation for acute leukemia. Leukemia. 2009;23:492–500. doi: 10.1038/leu.2008.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brunstein C, Wagner JE, Weisdorf DJ, et al. Negative effect of KIR alloreactivity in recipients of umbilical cord blood transplantation depends on transplantation conditioning intensity. Blood. 2009;22:5628–5634. doi: 10.1182/blood-2008-12-197467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kasamon YL, Luznik L, Leffell MS, et al. Nonmyeloablative HLA-haploidentical bone marrow transplantation with high-dose posttransplantation cyclophosphamide: effect of HLA disparity on outcome. Biol Blood Marrow Transplant. 2010;16:482–489. doi: 10.1016/j.bbmt.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shilling HG, McQueen KL, Cheng NW, Shizuru JA, Negrin RS, Parham P. Reconstitution of NK cell receptor repertoire following HLA-matched hematopoietic cell transplantation. Blood. 2003;101:3730–3740. doi: 10.1182/blood-2002-08-2568. [DOI] [PubMed] [Google Scholar]
- 30.Stern M, de Angelis C, Urbani E, et al. Natural killer-cell KIR repertoire reconstitution after haploidentical SCT. Bone Marrow Transplant. 2010;45:1607–1610. doi: 10.1038/bmt.2010.19. [DOI] [PubMed] [Google Scholar]
- 31.Brunstein CG, Fuchs EJ, Carter SL, et al. Blood and Marrow Transplant Clinical Trials Network. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011 Jul 14;118(2):282–8. doi: 10.1182/blood-2011-03-344853. [DOI] [PMC free article] [PubMed] [Google Scholar]