Abstract
Purpose
Respiratory papillomas, caused by human papillomaviruses types 6 and 11 (HPV6/11), are premalignant lesions with potential for malignant conversion. The cytokine and chemokine micromilieu of papillomas is TH2-like with a marked absence of IFN-γ expression. To illuminate why patients with recurrent respiratory papillomatosis (RRP) fail to effectively control their disease, we further investigated the suppressive cellular microenvironment in papillomas.
Experimental Design
CD4+CD25+CD127low/−Foxp3+ Tregs, and CD4+CD25−CD127low/−Foxp3− T-cells within papillomas were characterized and isolated. Their suppressor function was measured by inhibition of PBMC proliferation. Expression of PD-1, CD69, and Helios was identified on these T-cells. PD-L1, PD-L2, CCL17, and CCL22 mRNA was also identified in papillomas. by QPCR.
Results
Functional Tregs were markedly enriched in papillomas and strongly inhibited anti-CD3 and anti-CD28 antibody activated PBMC proliferation. The natural Treg marker Helios was reduced on Tregs from papillomas, indicating that the majority of Tregs in papillomas are adaptive. The majority of the papilloma-derived CD4+ T-cells expressed the CD4+CD25−CD127low/−Foxp3−PD1+CD69+ phenotype and failed to suppress PBMC proliferation, suggesting that they are chronically activated and exhausted. The Treg-attracting chemokine CCL22 was equally expressed by all laryngeal tissues examined. However CCL17 was robustly expressed by papillomas compared to unaffected laryngeal tissues from RRP patients and individuals without RRP. PD-L1 was elevated in papillomas compared to control laryngeal tissues.
Conclusions
Papilloma CD4+ T-cells are enriched with functional Tregs, and the adaptive Helios− Treg fraction was increased within the TH2-like papilloma micromilieu. CD4+CD25−CD127low/−Foxp3− T-cells failed to suppress PBMC proliferation and may be exhausted. The PD-1/PDL-1 pathway may represent an additional immunosuppressive mechanism that contributes to defective HPV6/11 clearance in RRP.
Keywords: Recurrent Respiratory Papillomatosis, Tregs, immune suppression, CCL17, PD-L1/PD-L2
INTRODUCTION
Recurrent respiratory papillomatosis (RRP) is characterized by the relentless recurrence of laryngeal papillomas that are induced by human papillomavirus (HPV) types 6 and 11. Laryngeal papillomas are premalignant lesions with potential for malignant conversion. This rare disease has an estimated incidence in the United States of 4.3/105 in children less than 14 years old, and of 1.8/105 in adults. The estimated cost of surgical treatment for RRP in the United States is greater than 100 million USD/year(1).
The strategic location of these lesions in the larynx and upper airway causes significant morbidity, and on occasion mortality. Patients with severe disease can require more than 150 general surgeries under general anesthesia to maintain a patent airway. The interval between surgical interventions can vary between patients ranging from three weeks to several years. Laryngeal disease can spread to the lower respiratory tract, and on occasion RRP can progress to malignancy (1, 2).
The persist recurrence of HPV 6/ 11 infection in these lesions highlights the failure of RRP patients to generate effective HPV-specific, innate and/or adaptive immune responses, although they demonstrate normal immune homeostasis, and respond normally to other infectious microbes(1). Thus, a better understanding of the HPV-specific, T-cell dysfunction in RRP that supports chronic HPV infection of laryngeal keratinocytes could provide a rationale to develop non-surgical strategies that would correct this defect.
Previously we described RRP as a TH2-like disease that is characterized by an imbalance in TH1-/TH2-like cytokine and chemokine expression (1–3). We identified a correlation between RRP disease severity and the expression of select class II MHC genes that likely regulate this imbalance(1–4) and predispose HPV6/11-infected individuals to develop RRP (1, 4). Recently, we described differences in the repertoire of killer cell immunoglobulin-like receptor (KIR) gene haplotypes in RRP as compared to controls(1). In addition, we also identified natural killer cell dysfunction in RRP (1), and differential expression of TH2-like polarizing immune response genes in papillomas compared to autologous unaffected laryngeal tissue(1) (5).
The objective of the present study was to further characterize the repertoire of T-cell subpopulations within tissue infiltrating lymphocytes (TILs) from respiratory papillomas that support chronic HPV 6/ 11 infection in these lesions.
In this communication we provide evidence for the first time that there is an enrichment of functional CD3+CD4+CD25+Foxp3+CD127low/− regulatory T-cells (Tregs)(6) that infiltrate HPV 6/ 11 induced, respiratory papillomas. We also identified the two chemokines (CCL17 and CCL22) that recruit Tregs into tissues where they are expressed(7). Additionally, we show that the majority of CD4+Foxp3− TILs (non-Tregs) in papillomas express a surface phenotype that is characteristic of exhausted T-cells (PD1+CD69+CD25−CD127−)(8–10), suggesting that this major T-cell fraction is chronically activated, quiescent, and unable to produce effector cytokines in response to these HPVs. Furthermore, we provide evidence that the PD-1/PD-L1 pathway(11) is likely active in RRP, given that papilloma-derived TILs express PD-1 strongly, and that papilloma tissue robustly expresses PD-L1, compared to control-derived laryngeal tissues. Taken together, these mechanisms may contribute to the immunosuppressive microenvironment in papillomas that prevent HPV-specific, effector TH1-like T-cell function in RRP.
MATERIALS AND METHODS
Subjects and index of disease severity
Thirty-seven randomly selected patients with RRP with varying disease severity were studied. Prior to enrollment, all patients signed informed consent that was approved by the Institutional Review Board at the North Shore-LIJ Health System. Table 1 shows the demographics of all patients with RRP included in this study.
Table 1.
| Patients | All | Severity Score | Male Gender | Juvenile Onset | Ethnicity** | ||
|---|---|---|---|---|---|---|---|
| W | B | A | |||||
| Mild/Moderate | 20 | 0.004–0.048 | 13 (65%) | 7 (35%) | 16 | 3 | 1 |
| Severe* | 17 | 0.061–0.685 | 11(65%) | 7(41%) | 16 | 1 | 0 |
includes three patients with tracheal involvement
W=White/Caucasian; B=Black/African-American; A=Asian
Heparinized blood and biopsies of autologous respiratory papillomas were obtained during surgical intervention. HPV typing was performed as previously described in detail (3, 12). Disease severity is defined by a composite score that represents the extent of disease and is inversely proportional to the frequency of recurrence(1). The composite score was used to define the overall severity for an individual patient. Three or more surgeries in the previous 12 months with a numeric score of ≥ 0.06, or the extension of disease into the trachea, was defined as severe. A severity score of < 0.06 was defined as mild-moderate disease(1). Severity scores for these study patients ranged from 0.004 to 0.685. Seventeen patients had mild/moderate RRP (score 0.004–0.048), and seventeen had severe disease (score 0.061–0.685).
Isolation of PBMC and CD4+ T regulatory cells from respiratory papillomas and peripheral blood
Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Paque Plus gradient centrifugation (GE Healthcare). These PBMC were stained for surface and intracellular markers, or used as responders in the in vitro suppression assay described below. TILs were isolated from fresh papilloma biopsies by mechanical dissociation and the resulting cell suspension was passed through a 70 micron mesh filter. Tregs were isolated from TILs using a CD4+CD25+ Regulatory T-cell Isolation Kit (Miltenyi Biotec), as recommended by the manufacturer.
In addition to the isolation of cells by bead purification, effector memory CD3+CD4+CD127low/−CD25− T-cells (13) were isolated by cell sorting on a FACS Aria IIu flow cytometer. Minced papilloma tissue was filtered, washed, resuspended in sorting buffer (0.25%BSA/PBS with 1mM EDTA) and surface stained with antibodies to CD3-Pacific Blue, CD4-FITC, CD127-PerCP-Cy5.5, and CD25-PE. Aliquots of the sort-purified T-cell subpopulation were co-cultured with autologous PBMC in the suppression assay described below.
Antibody staining and flow cytometric analyses
PBMC and TILS were washed in PBS containing 2% FBS and 0.1% sodium azide and surface stained for Tregs using the following combinations of directly conjugated antibodies: anti-CD4 PerCP, anti-CD25 FITC, anti-CD127 PE (BD Biosciences). After washing, cells were stained for intracellular FoxP3 (anti-FoxP3-APC, clone PCH101, eBioscience) using the manufacturer's recommended procedure. Stained and washed cell suspensions were resuspended in 1% formaldehyde. Additional specimens were stained for surface expression with anti-PD-1 APC or PE-Cy7, anti-PD-L1 PE, anti-CD69 PE, (BD Biosciences), or intracellular anti-Helios PE, (Biolegend).
Samples were analyzed on a FACSCalibur or FACSCanto II flow cytometer (BD Biosciences). Single fluorochrome stained cells or antibody-capture beads were used to correct for spectral overlap. FlowJo version 7.2.5 (TreeStar) was used for compensation and data analysis. For determination of Treg frequencies, CD4+ cells with low side scatter were identified and then a forward vs. side scatter gate applied to isolate small lymphocytes and facilitate the exclusion of cellular debris and non-viable cells. Positive fluorescence staining was established using isotype and fluorescence minus one controls. Only biopsies that produced more than 1 × 103 CD4+ events on data analysis were included the study of Treg frequency. Studies evaluating PD-1, PD-L1 and Helios expression were stained as above and analyzed on the FACSCanto II using forward scatter area versus forward scatter height gating to remove cell aggregates, and Live/Dead Fixable Aqua Dead Cell Stain Kit (Invitrogen) to exclude non-viable cells.
Treg function measured by suppression of PBMC proliferation
Tregs isolated from the papillomas from three patients were tested for their ability to suppress autologous PBMC proliferation using a functional assay performed in vitro. In short, 5 × 103 PBMC were co-cultured with Tregs enriched from papilloma TILs or with CD4+ cells isolated from autologous PBMC and activated by 2μg/ml of anti-CD3 and 1μg/ml anti-CD28 (Pharmingen) monoclonal antibodies Cell suspensions were incubated for 6 days in 96-well, round bottom plates in RPMI supplemented with 10% bovine serum. One μCi of 3H-thymidine was added to each well for the last 18 hours of incubation. Plates were harvested as previously described(14). The proliferative responses were expressed as mean 3H-thymidine incorporated counts per minute (CPM) of triplicate wells minus background.
mRNA expression of CCL17, CCL22, and programmed cell death ligands, PD-L1 and PD-L2
To determine if papillomas expressed CCL17 and CCL22, known to be chemoattractants for Tregs(7), or expressed PD-L1 (CD274) and/or PD-L2 (CD273), the ligands for PD-1 (CD279)(11), we determined the relative expression of these genes by quantitative reverse transcriptase, real-time PCR. Relative levels of mRNA from papillomas (PAP, n=9), clinically normal laryngeal tissues from RRP patients (normal adjacent, NA, n=11), and controls without RRP (true normal, TN, n=6) were determined. Briefly, total RNA was isolated using nucleic acid affinity spin-columns (Qiagen) together with DNase-1 digestion. I-Script One-step RT-PCR for Probes (BioRad) was performed on an Applied Biosystems 7900HT thermocycler with gene-specific, intron-spanning, primers and either the appropriate FAM-labeled probe from the Universal Probe Library (Roche) or a FAM/TAMRA custom Taqman probe (Supplemental Table). The housekeeping gene (GAPDH) was used to normalize the input RNA. mRNA expression of CCL17, normalized to GAPDH, was calculated relative to the mean NA tissue, using the delta-delta CT method (5). Similarly, mRNA expression of PD-L1 and PD-L2, normalized to GAPDH, was calculated relative to TN PD-L1 expression. The expression efficiency of the PD-L1 and PD-L2 probe/primer sets were linearly efficient among the range of cycles measured in this assay, allowing for cross-primer relative expression calculations. Data from each group had similar standard deviations, confirmed by the method of Bartlett, and were Gaussian distributed, confirmed by the method of Kolmogrorov and Smirnov. Therefore ANOVA was utilized to analyze the three groups (TN, NA, PAP), and a Turkey-Kramer multiple comparison test and two-tailed unpaired t-tests were performed to compare between two groups.
Statistical analysis
All statistical analysis employed a two tailed, unpaired t-test performed by InStat (GraphPad). When the data was not normally distributed, it was log transformed, and if the standard deviation was different between groups, a Welch correction was applied.
Results
The frequency of Foxp3+CD127low/− Tregs are increased in CD4+ T-cells from papillomas compared to blood of patients with RRP
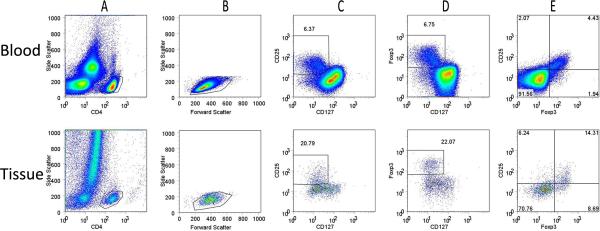
The flow cytometric strategy for identifying Tregs within TILs from a respiratory papilloma, and from the blood of the same patient, is shown in Figure 1. CD4+ Tregs were identified by their expression of Foxp3+CD127low/−, CD25+CD127low/−, or the co-expression of both Foxp3 and CD25 on CD4+ T-cells (Figure 1A–E). The fraction of Tregs identified by these surface markers in TILs from this papilloma was 3 fold greater than in the corresponding CD4+ T-cell fraction from the autologous PBMC (Figure 1C, D, top vs. bottom rows). Some Foxp3+CD127low/− CD4+ T-cells in the papilloma were negative, or showed reduced expression of CD25, in contrast to the strong CD25 co-expression by the majority of Foxp3+CD127low/−CD4+ cells in PBMC (Figure 1E).
Figure 1. Flow cytometric identification of Tregs in papilloma TILs and peripheral blood.
PBMC and papilloma tissue were stained and analyzed for surface expression of CD4, CD25, CD127, and intracellular Foxp3. A representative staining of PBMC (top row) and TILs (bottom row) from a patient with RRP is shown. A) The ungated events (all cells) from these tissues; B) forward and side scatter characteristics of CD4+ gated cells; C–E) CD25, CD127, and Foxp3 expression of CD4+ lymphocytes. A gate is drawn around CD4+ cells which are CD25+CD127low/− (C) and the Foxp3+CD127low/− (D); E) The correlation of CD25+ and Foxp3+ on CD4+ T-cells. The percentages of CD4+ cells are indicated for each subset.
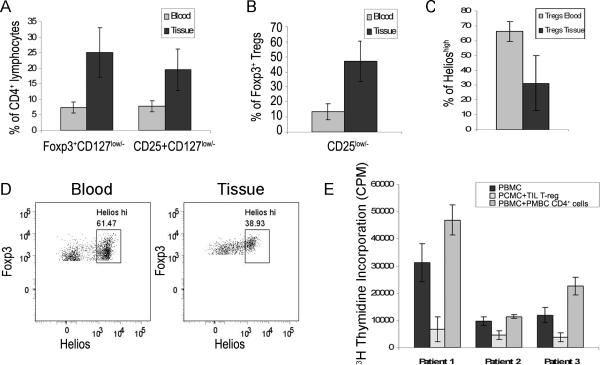
Figure 2A shows the composite results of 17 patients with RRP. Foxp3+ CD127low/− Tregs were increased in CD4+ TILs obtained from respiratory papillomas (25.0±8.0% of CD4+ T-cells), compared to CD4+ T cells from autologous PBMC (7.3±1.8% of CD4+ T-cells, p<0.0001). Similar frequencies were observed for the CD25+CD127low/− subset of CD4+ cells in the TILs compared to PBMC (19.5 ±6.7% and 7.8 ± 1.8%, respectively, p<0.0001). Tregs were increased in the tissue compared to blood of all patients studied, as demonstrated by a tissue to blood ratio that ranged from 1.7 to 6.9 (mean±SD=3.5 ± 1.4). The frequency of Foxp3+ Tregs in CD4+ TILs from papillomas obtained from 5 patients with mild/moderate disease vs. 12 patients with severe disease (27±11% vs. 24±7% respectively), did not predict disease severity. Additionally, the Foxp3+ CD127low/− CD4+ cell fraction with reduced expression of CD25 (Figure 2B) was prominent in TILs, but only a minor population in blood (46.7 ± 13.9% and 13.4 ± 5.4% of Foxp3+ CD4+ T-cells, respectively (p<0.0001) consistent with that reported in the blood of controls without RRP(15, 16)
Figure 2. Characterization of Suppressive Tregs isolated from the blood and tissue of patients with RRP.
A) The frequency of Foxp3+CD127low/− and CD25+CD127low/− cells in the CD4+ T-cell populations from papilloma tissues (TILs) (black bars, 25.0%±8.0% and 19.5%±6.7%, respectively), and autologous blood (grey bars, 7.3%±1.8% and 7.8%±1.8% respectively) was determined by flow cytometry. Both populations were significantly increased in the tissue when compared to autologous blood (n=17, p<0.0001, unpaired t-test with Welch correction) B) Percent of CD4+Foxp3+CD127low/− Tregs with reduced CD25 levels was 46.9%±13.5% in papilloma tissues (TILs) and 13.4%± 5.5% in the blood of patients with RRP (n=17, p<0.0001, unpaired t-test with Welch correction). C–D) Helios Expression on CD4+ T-cells isolated from laryngeal papilloma tissue and autologous blood. C) The composite results of six patients with RRP. Bright expression of Helios was detected on 66.1%±6.5% of Tregs isolated from the blood and on 31.2%±18.5% of papilloma-derived Tregs, p<0.005, two-tailed t-test. D) Representative example of Helios expression on CD4+Foxp3+CD127low/− T cells. High expression of Helios was detected on 61.47% and 38.93% of Tregs isolated from blood and tissue, respectively. E) Tregs enriched from papilloma-derived TILs suppress peripheral blood mononuclear cell proliferation (PBMC). PBMC (black bars), were cultured alone or with Tregs isolated from papilloma tissue (dotted bars), or with autologous CD4+ cells isolated from PBMC (gray bars). Suppression of PBMC proliferation by Tregs was observed in all 3 patients studied (76%, 53%, 69% respectively, dotted bars), while control PBMC cultures with added autologous CD4+ T-cells showed an anticipated increased in proliferation (grey bars).
The ratio of natural vs. adaptive Tregs is altered in papillomas
The transcription factor Helios has been recently been reported to distinguish nTregs from extra-thymically-derived adaptive Tregs (aTregs) and is expressed on 70% (on average) of Foxp3+ Tregs in human controls(17). Therefore we analyzed both the TILs and PBMC of 6 patients for Helios expression on CD3+CD4+ Foxp3+ cells. The frequency of Tregs with bright Helios expression was significantly reduced in papilloma TILs compared with blood (31.2 ± 18.51% TILs, vs 66.1 ± 6.5% blood, p<0.005) (Figure 2C, D). Concomitantly, there was a significant increase in the Helioslow/− Tregs subpopulation in papilloma TILs suggesting that within the papilloma microenvironment, there are more aTregs than in the blood.
Tregs isolated from papilloma TILs function by suppressing PBMC proliferation
The functional capacity of Tregs isolated from TILs was determined using an in vitro micro co-culture suppression assay (Figure 2E). CD25+CD4+ cells, enriched by magnetic bead selection from the papillomas of patients with RRP, were co-cultured with anti-CD3 and anti-CD28 activated, autologous PBMC. There was marked suppression of PBMC proliferation (66%±11.8) when autologous Tregs, enriched from TILs, were added. In contrast, when autologous, additional blood-derived CD4+ T-cells were added back as a control to similarly activated PBMC, proliferation was enhanced. The mean ± SD suppression ratios were calculated by dividing the CPM of PBMC alone, (black bars) by the CPM of PBMC with added TILs (dotted bars), or by the CPM of PBMC with added autologous CD4 cells (grey bars), (Figure 3E). Using these ratios, the TILs markedly suppressed PBMC proliferation (−3.3 ±1.3), while the autologous CD4 cells did not (1.5±0.4), (p=0.0033).
Figure 3. Differential CCL-17 mRNA expression in laryngeal tissues.
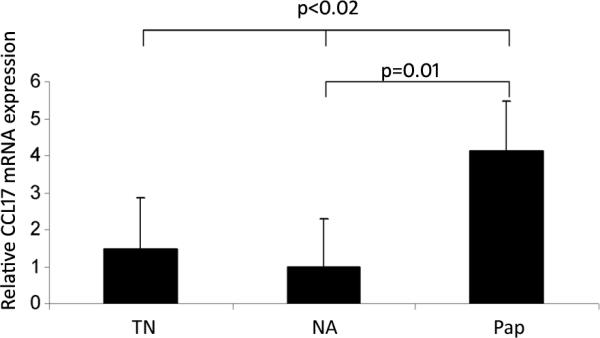
Total mRNA from laryngeal tissue obtained from true normal tissues (TN), clinically normal adjacent laryngeal tissue (NA) from patients with RRP, and from respiratory papillomas (PAP) were compared using reverse transcriptase, real time PCR, and expressed as a relative mRNA concentration (ΔΔCt), after normalized to a housekeeping gene (GAPDH), and also standardized to NA tissue. Papilloma (PAP) CCL-17 mRNA expression was increased between groups (p<0.02, ANOVA) and between NA and PAP (p=0.01, t-test).
Treg-attracting chemokine CCL17 is differentially expressed by papillomas
To determine if the increase of Tregs in papillomas was the result of the expression of chemokines known to attract Tregs into tissues(18), we compared CCL17 and CCL22 mRNA expression in 9 papillomas (PAP), 11 clinically normal laryngeal tissues from RRP patients (NA), and 6 control laryngeal tissues (TN) from patients without RRP (Figure 3). All three types of tissues expressed equivalent amounts of CCL22 mRNA (data not shown). In contrast, the expression of CCL17 mRNA was up-regulated in papillomas, compared to both types of control laryngeal tissues from individuals without RRP (Figure 3). Thus, Tregs may be recruited to papillomas by CCR4 receptor engagement of an increasing gradient of CCL17 that is differentially expressed by papillomas, together with normal constitutive robust expression of CCL22.
CD4+ “non-Tregs” in TILs from papillomas show reduced CD127 expression and lack suppressor function
We observed that the majority of the CD4+ T-cells in TILs from papillomas were CD4+Foxp3−CD25− (non-Tregs), and had low or absent expression of CD127 (49.2 ± 7.4%) (Figure 4). In comparison, the majority of CD4+ non-Tregs in the blood were CD127+, and only a minor subgroup of these cells lacked CD127 (22.2 ± 5.4%, p<0.001). CD127 is constitutively expressed on naïve T-cells, is down-regulated upon T-cell activation, and then re-expressed on a subset of effector T-cells that ultimately differentiate into memory T-cells(19). The absence of CD127 expression on T-cells has been associated with T-cell exhaustion in patients with chronic infection or tumors(8, 19), while absent CD127, Foxp3 and CD25 expression is also a characteristic of effector Tr1-like cells(20, 21). Therefore we asked if this major T-cell subpopulation in TILs (CD4+CD25low/−CD127low/−) could also suppress PBMC proliferation. This purified cell fraction from TILs was co-cultured with anti-CD3 and anti-CD28 antibody activated autologous PBMC to determine if they could suppress PBMC proliferation. CD4+CD25low/−CD127low/− T-cells from papilloma-derived TILs from 2 different RRP patients did not suppress PBMC proliferation (data not shown). However, in a parallel experiment, CD4+CD25low/−CD127low/− T-cells from the blood of a control without RRP showed 40% suppression of autologous PBMC proliferation. Thus, CD4+CD25low/−CD127low/− T-cells in control blood contain a subpopulation of functional Tr1-like effector cells, as described(6, 20, 21), whereas T-cells with this phenotype in TILs from papillomas do not contain sufficient Tr1-like cells to show suppression in-vitro.
Figure 4. The major CD4+Foxp3− population in TILs (non-Tregs) have reduced expression of CD127.
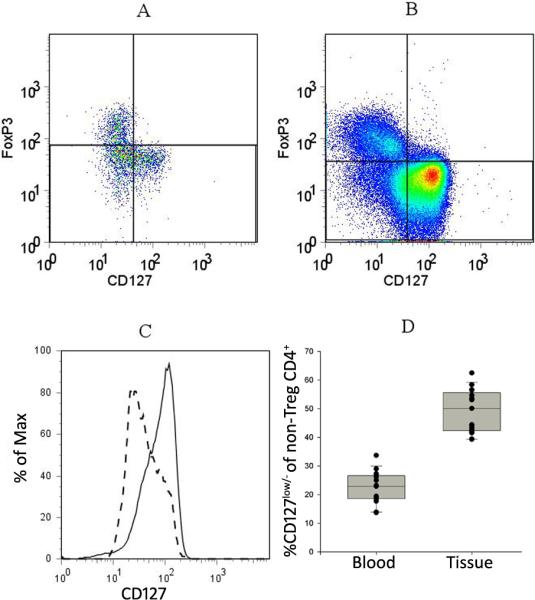
Papilloma tissues and PBMC were stained for CD4, CD25, CD127 and Foxp3 and analyzed by flow cytometry. A representative analysis of the expression of Foxp3 and CD127 on the CD4+ cells from papilloma tissue (A) and blood (B) from a patient with RRP are shown. Foxp3− “non-Tregs” are represented in the lower two quadrants. (C) The CD127 expression of CD4+ “non-Tregs” (lower two quadrants) in papilloma tissue (dashed line) and autologous blood (solid line). (D) Frequency of CD127low/− cells within the CD4+Foxp3− “non-Tregs” from the blood (22.2 ± 5.4%) and the tissue (49.2 ± 7.4 %) of 17 patients (p = <0.0001, two-tailed t-test). Expression of CD127 greater than that of Foxp3+cells was considered positive.
PD-1 and CD69 are highly expressed on TILs from papillomas
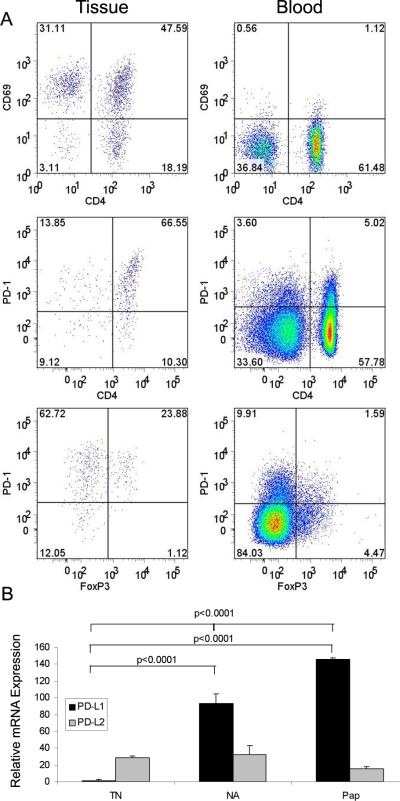
To determine if the PD-1/PD-L1/L2 pathway was relevant in the immunosuppressive micromilieu present in papillomas, TILs from 7 patients were stained with anti-PD-1 antibodies and analyzed by flow cytometry. PD-1 was expressed on 85%±7% of the CD3+ T-cells, and on 92%±4% of CD4+ T-cells from these papillomas (Figure 5A). In addition, the intensity of expression of PD-1 on TILs was much greater than that observed on T-cells from the blood. The majority of TILs also expressed CD69, another marker of activation (9). These results suggest that the CD4+ effector T-cells within papilloma TILs are chronically activated as continuous engagement of the TCR is necessary for maintaining a high PD-1 expression(9). The majority of TILs did not express CD25 and therefore they may be in an exhausted state with reduced capacity to proliferate and produce cytokines, as has been shown for CD8+PD-1+ T-cells during chronic infection (10). Thus, in addition to the decreased CD127 expression that was observed on the majority of CD4+ TILs, the papilloma microenvironment also contributes to effector dysfunction by up-regulating PD-1 expression on T-cells.
Figure 5. Papilloma TILs have high expression of CD69, PD-1, and PD-L1.
A) TILs and autologous PBMC were stained with antibodies to CD3, CD4, and CD69 (row 1), or CD3, CD4, CD127, Foxp3, and PD-1 (rows 2 and 3). Representative samples from 2 patients with RRP (A top row or B–C middle and lower rows) are shown. Gating was performed using CD3+ T-cells (rows 1 and 2) and CD3+4+ T-cells (row 3). B) Total mRNA normal laryngeal tissue from individuals without RRP (TN; n=6), clinically normal adjacent laryngeal tissue from patients with RRP (NA; n=11), and from respiratory papillomas (PAP; n=9) were compared using reverse transcriptase, real time PCR. Relative mRNA expression (ΔΔCt), was normalized to GAPDH mRNA, a housekeeping gene, and standardized to PD-L1 TN. PD-L1 was upregulated in both NA and PAP compared to TN (p<0.0001, ANOVA and post-hoc t-tests). There was no significant difference in PD-L2 mRNA levels between the 3 different laryngeal tissues.
PD-L1 and PD-L2 are expressed by papillomas
To determine if the engagement of PD-1 that is highly expressed on the majority of CD3+ TILs could represent a mechanism that induces T-cell exhaustion in papillomas, we analyzed PD-L1 and PD-L2 mRNA extracted from papilloma tissue, clinically normal laryngeal tissue, and control laryngeal tissues from individuals without RRP. PD-L1 mRNA in papillomas (PAP, n=9) was relatively expressed 145 times more than laryngeal tissues from individuals without RRP (TN, n=6) and 93 times more than clinically normal laryngeal tissues from RRP patients (NA, n=11) (p<0.0001 ANOVA, and p<0.0001 unpaired two-tailed t-test for TN vs. NA, and TN vs. PAP) (Figure 5B). In contrast, all three types of laryngeal tissues expressed PD-L2 mRNA equivalently, and at relative expression levels 15–32 times that of PD-L1 in TNs. Thus, PD-1 ligation on CD3+ TILs by PD-L1 expressed by papillomas may represent an additional inhibitory pathway that blocks effector T-cell function in these lesions as previously reported in other systems(8).
Discussion
There has been intense interest in the role of Tregs and their respective subpopulations in both murine and human disease. Increased Treg numbers and function have been linked to chronic bacterial, parasitic, and viral (HIV, HCV, EBV) infection, while a low frequency and/or reduced Treg function, have been linked to the resolution of acute infection and the reestablishment of tumor immunity in various cancer, as recently reviewed(22). Most relevant to our studies are recent descriptions of Tregs in chronic HPV-infection of the human cervix, specifically cervical intraepithelial neoplasia (CIN) and cervical cancer caused by HPV16 and 18. These studies showed that the frequency of Tregs increased with HPV-induced cervical disease (23, 24).
In this communication, we provide evidence for the first time that there is an increased frequency of functional Foxp3+ CD127low/− Tregs within the total CD4+ TIL population within HPV6/11-induced respiratory papillomas. The frequency of these Tregs within respiratory papillomas did not predict disease severity, unlike that reported in cervical neoplasia (23, 24). Thus, an increased frequency of functional Tregs likely contributes to the chronicity of HPV-induced RRP, but may not greatly influence the severity of this disease.
The increased frequency of Tregs has been shown to contribute to the suppression of infiltrating cytotoxic T-cells in human cancerous lesions allowing these tumors to escape immunosurveillence (7). Several types of CD4+ regulatory T-cells have been described, including CD4+ natural Tregs (nTregs) that express the transcription factor Forkhead box P3 (Foxp3) and the high affinity IL-2 receptor α-subunit (CD25), but fail to express the α-subunit of the high affinity receptor for IL-7 (CD127). They are phenotypically defined as CD4+ CD25+Foxp3+CD127low/− T-cells (6). nTregs develop within the thymus and exert their suppressive activity by several mechanisms as recently reviewed (25). Adaptive Tregs (aTregs) share the same phenotype as nTregs(26), however they may also show reduced or absent CD25 and Foxp3 as described in Tr1-like CD4+ T-cells (6, 20, 21). aTregs are generated in the periphery from naïve TH0-like T-cells following antigen exposure and a TH2-like cytokine micromilieu, and they suppress T-cell function by their production of immunosuppressive cytokines such as IL-10 and TGFβ (26).
Tregs may accumulate within papillomas either by internal proliferation, as reported in other tissues (26), or by their continued ingress from the blood because they express CCR4, and papillomas express the CCR4 ligands CCL17 and CCL22, that would enable the retention of Tregs in these tissues (7, 18). While a detailed analysis of Treg trafficking was not the focus of our studies, it is interesting to speculate from our data that the robust and comparable expression of CCL22 by papillomas, clinically normal autologous laryngeal tissues, and laryngeal tissues from controls without RRP, is important in regulating upper airway homeostasis by the recruitment of anti-inflammatory Tregs into the airway (18). In contrast, recent observations in breast and ovarian cancer showed that CCL22 was differentially expressed by tumors compared to normal tissue (27, 28). Similar observations were also described in the nasal mucosa of patients with allergic rhinitis, compared to non-atopic controls (29). These differences suggest that the effect of CCL22 is site-specific, and may elicit beneficial (airway patency), or harmful outcomes (malignancy), by attracting Tregs and/or TH2-like T-cells into different tissues.
Interestingly, we found CCL17 to be differentially expressed by papillomas in RRP, compared to both, autologous laryngeal tissue, and control laryngeal tissue from patients without RRP (Figure 3). These results suggest that active HPV infection in the larynx induces CCL17 expression and likely supports the infiltration of both TH2-like T-cells (1–4) and Tregs into papillomas.
There are several mechanisms in respiratory papillomas that could induce the generation and/or expansion of Tregs. We previously showed that IL-10 and TGF-β are expressed in papilloma lesions (1). The involvement of these cytokines in generating, activating and expanding Tregs from naïve CD4+ T-cells that differentiate into Foxp3 expressing aTreg following engagement of their T-cell receptors has been documented (7, 30). Of interest, recent studies have shown that prostaglandin E2 (PGE2) can induce Foxp3 expression and enhance the suppressive activity of CD4+CD25+ Tregs, and that these responses could be reversed by COX-2 inhibition (31, 32). Respiratory papilloma and their surrounding uninfected laryngeal tissues from patients with RRP have elevated COX-2 expression(33). Thus, the laryngeal micromilieu in RRP is a rich source of PGE2 that can further support Treg function.
Our studies also showed that there are a substantial number of Tregs in papilloma CD4+ TILs that have reduced or negative CD25 expression. These Tregs have been reported to function similarly to their CD25high Treg counterparts, although less efficiently (6). In studies using FoxP3-GFP knock-in mice, the expression of Foxp3-GFP correlated with suppressor activity irrespective of CD25 expression(34). It has also been suggested that CD4+Foxp3+CD25− Tregs can show functional plasticity and can loose Foxp3 expression, while CD4+Foxp3+CD25high T cells represent a more stable Foxp3+ Treg population (35, 36). Stable Foxp3 expression depends on the DNA methylation status of the Treg-specific determining region of the Foxp3 gene(37). Demethylation of the Foxp3 locus and high expression of the Ikaros transcription factor Helios have recently been reported to be a specific “signature” of thymic-derived nTregs(37, 38). From our studies, the CD4+ Foxp3+CD127low/− T cells in papilloma TILs include both Helioshigh, nTregs, and Helioslow/−, aTregs (Figure 2C, D). Importantly, the frequency of Helioshigh Tregs in papillomas was lower than in the blood of the same RRP patient (Figure 2C, D). This suggests that the papilloma microenvironment is conducive to de novo generation of aTregs from TH0-like T-cells. Novel therapies aimed at destabilization of Foxp3 expression on Tregs in papillomas may benefit RRP patients by blocking Treg function and thereby restoring effective TH1-like responses in these premalignant lesions.
Our studies also showed that a large population of CD4+Foxp3− cells (non-Tregs) in papilloma TILs lacked, or had reduced CD127 and CD25 expression. Expression of IL-7 and IL-2 receptors regulate naïve and memory T cell homeostasis, proliferation, and differentiation(39). The function of CD3+CD4+CD25−CD127−Foxp3− T-cells is varied. This population can include Tr1-like T-cells, and previously activated but exhausted effector memory T-cells (6, 8, 10, 20, 21). The expansion of this population has been shown to be an index of dysfunction in CD4+ cells from HIV-infected individuals(13) and was linked to enhanced, tumor-specific, CD4+ T-cell apoptosis(19). The CD3+CD4+CD25−CD127− fraction isolated from papilloma TIL did not suppress autologous PBMC proliferation. Additionally, in a single experiment, this TIL fraction failed to express IL-10, TGF-β, or IFN-γ mRNA after anti-CD3 and anti-CD28 antibody stimulation (data not shown). Thus, we suspect that this TIL subpopulation contains chronically activated T cells that express CD69+ and PD-1high, and represent dysfunctional effector T-cells, as described in other diseases (9, 19).
Our observations that CD3+ T-cells isolated from papillomas expressed PD-1 brightly (Figure 5a), but not PD-L1, identified by flow cytometry (data not shown), and that papillomas express both PD-1 ligands (PD-L1, PD-L2) (Figure 5b), suggest that the PD-1/PD-L1/L2 pathway may also contribute to the immunosuppressive milieu in papillomas. PD-L1 is expressed on a wide variety of tissues and tumors and both PD-L1 and PD-L2 are expressed on professional antigen presenting cells(11). High levels of PD-L1 expressed by tumor cells have been correlated with an unfavorable prognosis in cancer(40). PD-1 is a major regulator of CD8+ T-cell exhaustion and viral control in chronic infection and tumor progression, and it has become an important potential therapeutic target in these diseases (41, 42). However, the PD-1/ PD-1L pathway in CD4+ effector T-cell and Treg function is more controversial, and the mechanisms that regulate exhaustion in PD1+CD4+ and PD1+CD8+ T cells may differ between the these two cell types in adaptive cellular immunity (9, 19, 43–45). In addition, conflicting reports of the role of PD-1 on CD4+ Treg function suggested that PD-1- PD-L1 ligation on CD4+ Tregs supported Treg stability and expansion (40, 46–48), while others suggested that this pathway inhibited Treg expansion and suppressive function (49, 50). Further studies are needed to determine how nTreg or aTreg stability, expansion, and function, are modulated by interactions between PD-1 and its ligands in RRP and in various tumors at different anatomic sites.
In our studies, CD4+ non-Treg T cells from HPV6/11-induced papillomas showed up-regulated PD-1 expression along with expression or absence of other markers of chronic activation and terminal differentiation (CD25−CD127−CD69+). Continued interaction with PD-1 ligands within papillomas may therefore block effector T-cell function and/or induce their apopotosis. As a consequence, these T-cells within papillomas are likely unable to expand into polyfunctional T helper cells that could control HPV6/11 infection.
In summary, we demonstrate that the overwhelming majority of CD4+ cells in papillomas are either actively involved in immune suppression, or are chronically stimulated and are likely exhausted effector T-cells that fail to respond to HPV/tumor antigen exposure. Better understanding of PD-1 expressing TILs, and the identification of the antigen(s) that induce T-cell exhaustion in papillomas, could help develop novel strategies to reverse T-cell exhaustion and restore TH1-like function in RRP. Clinical trials using PD-1/PD-L1 blockade in cancer and infectious diseases should be monitored closely for their applicability in RRP. Additional characterization of the interactive cycle of cells and antigens within papillomas that polarize immune responses towards HPV6/11 tolerance may provide the rationale to develop novel immune-based therapies that can restore effective T-cell clearance of these viruses.
Statement of Translational Relevance.
Respiratory papillomas induced by human papillomaviruses 6 and 11 are premaligant lesions with potential for malignant conversion and cause significant morbidity and on occasion mortality Standard therapy for recurrent respiratory papillomatosis (RRP) is surgical extirpation, that significantly impacts on lifestyle (>150 surgeries), and medical costs (>100 million US dollars yearly). The cytokine/chemokine micromilieu of papillomas is TH2-like with markedly reduced IFN-γ. We further investigated the microenvironment in papillomas towards ultimately developing a non-surgical, medical treatment for RRP. Our studies show for the first time that papillomas contain an enrichment of functional CD4+ regulatory T-cells (Tregs), express Treg/TH2-like-tropic chemokines (CCL17), and contain T-cells that express CD69 and PD-1, a phenotype of chronically activated and exhausted T-cells. PD-1L that induces effector T-cell dysfunction is also expressed in papillomas. These studies provide potential new targets for future medical therapies that can reverse the immunosuppressive micromilieu in papillomas by restoring effective TH1-like anti-HPV responses.
Acknowledgments
The authors wish to thank Virginia Mullooly, R.N. for coordinating the collection of patient samples, the residents from the Department of Otolaryngology, and the anesthesiologists who obtained blood from RRP patients.
Grant Support: This work is supported by a grant from the National Institute of Dental & Craniofacial Research/National Institutes of Health, Grant R0 1DE017227 (to V.R.B.).
Footnotes
Disclosure of potential conflicts of interest: none
References
- 1.Bonagura VR, Hatam LJ, Rosenthal DW, de Voti JA, Lam F, Steinberg BM, et al. Recurrent respiratory papillomatosis: a complex defect in immune responsiveness to human papillomavirus-6 and -11. APMIS. 2010;118:455–70. doi: 10.1111/j.1600-0463.2010.02617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonagura VR, Hatam L, DeVoti J, Zeng F, Steinberg BM. Recurrent respiratory papillomatosis: altered CD8(+) T-cell subsets and T(H)1/T(H)2 cytokine imbalance. Clin Immunol. 1999;93:302–11. doi: 10.1006/clim.1999.4784. [DOI] [PubMed] [Google Scholar]
- 3.DeVoti JA, Steinberg BM, Rosenthal DW, Hatam L, Vambutas A, Abramson AL, et al. Failure of gamma interferon but not interleukin-10 expression in response to human papillomavirus type 11 e6 protein in respiratory papillomatosis. Clin Diagn Lab Immunol. 2004;11:538–47. doi: 10.1128/CDLI.11.3.538-547.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonagura VR, Vambutas A, DeVoti JA, Rosenthal DW, Steinberg BM, Abramson AL, et al. HLA alleles, IFN-gamma responses to HPV-11 E6, and disease severity in patients with recurrent respiratory papillomatosis. Hum Immunol. 2004;65:773–82. doi: 10.1016/j.humimm.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 5.DeVoti JA, Rosenthal DW, Wu R, Abramson AL, Steinberg BM, Bonagura VR. Immune dysregulation and tumor-associated gene changes in recurrent respiratory papillomatosis: a paired microarray analysis. Mol Med. 2008;14:608–17. doi: 10.2119/2008-00060.DeVoti. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–11. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishikawa H, Sakaguchi S. Regulatory T cells in tumor immunity. Int J Cancer. 2010;127:759–67. doi: 10.1002/ijc.25429. [DOI] [PubMed] [Google Scholar]
- 8.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–9. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 9.Wang SF, Fouquet S, Chapon M, Salmon H, Regnier F, Labroquère K, et al. Early T cell signalling is reversibly altered in PD-1+ T lymphocytes infiltrating human tumors. PLoS One. 2011;6:e17621. doi: 10.1371/journal.pone.0017621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–44. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–42. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maran A, Amella CA, Di Lorenzo TP, Auborn KJ, Taichman LB, Steinberg BM. Human papillomavirus type 11 transcripts are present at low abundance in latently infected respiratory tissues. Virology. 1995;212:285–94. doi: 10.1006/viro.1995.1486. [DOI] [PubMed] [Google Scholar]
- 13.Dunham RM, Cervasi B, Brenchley JM, Albrecht H, Weintrob A, Sumpter B, et al. CD127 and CD25 expression defines CD4+ T cell subsets that are differentially depleted during HIV infection. J Immunol. 2008;180:5582–92. doi: 10.4049/jimmunol.180.8.5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonagura VR, Nordli DR, Pernis B. Antibodies against monomorphic determinants of the alpha chain of class I human leukocyte antigens inhibit the reaction of human lymphocytes to mitogen and tetanus toxoid. Immunol Invest. 1985;14:233–43. doi: 10.3109/08820138509076147. [DOI] [PubMed] [Google Scholar]
- 15.Bonelli M, Savitskaya A, Steiner CW, Rath E, Smolen JS, Scheinecker C. Phenotypic and functional analysis of CD4+ CD25− Foxp3+ T cells in patients with systemic lupus erythematosus. J Immunol. 2009;182:1689–95. doi: 10.4049/jimmunol.182.3.1689. [DOI] [PubMed] [Google Scholar]
- 16.Ahmadzadeh M, Felipe-Silva A, Heemskerk B, Powell DJ, Wunderlich JR, Merino MJ, et al. FOXP3 expression accurately defines the population of intratumoral regulatory T cells that selectively accumulate in metastatic melanoma lesions. Blood. 2008;112:4953–60. doi: 10.1182/blood-2008-06-163048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184:3433–41. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sather BD, Treuting P, Perdue N, Miazgowicz M, Fontenot JD, Rudensky AY, et al. Altering the distribution of Foxp3(+) regulatory T cells results in tissue-specific inflammatory disease. J Exp Med. 2007;204:1335–47. doi: 10.1084/jem.20070081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding ZC, Blazar BR, Mellor AL, Munn DH, Zhou G. Chemotherapy rescues tumor-driven aberrant CD4+ T-cell differentiation and restores an activated polyfunctional helper phenotype. Blood. 2010;115:2397–406. doi: 10.1182/blood-2009-11-253336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Häringer B, Lozza L, Steckel B, Geginat J. Identification and characterization of IL-10/IFN-gamma-producing effector-like T cells with regulatory function in human blood. J Exp Med. 2009;206:1009–17. doi: 10.1084/jem.20082238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Couper KN, Blount DG, Wilson MS, Hafalla JC, Belkaid Y, Kamanaka M, et al. IL-10 from CD4CD25Foxp3CD127 adaptive regulatory T cells modulates parasite clearance and pathology during malaria infection. PLoS Pathog. 2008;4:e1000004. doi: 10.1371/journal.ppat.1000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allan SE, Broady R, Gregori S, Himmel ME, Locke N, Roncarolo MG, et al. CD4+ T-regulatory cells: toward therapy for human diseases. Immunol Rev. 2008;223:391–421. doi: 10.1111/j.1600-065X.2008.00634.x. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi A, Weinberg V, Darragh T, Smith-McCune K. Evolving immunosuppressive microenvironment during human cervical carcinogenesis. Mucosal Immunol. 2008;1:412–20. doi: 10.1038/mi.2008.33. [DOI] [PubMed] [Google Scholar]
- 24.Loddenkemper C, Hoffmann C, Stanke J, Nagorsen D, Baron U, Olek S, et al. Regulatory (FOXP3+) T cells as target for immune therapy of cervical intraepithelial neoplasia and cervical cancer. Cancer Sci. 2009;100:1112–7. doi: 10.1111/j.1349-7006.2009.01153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–32. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bour-Jordan H, Bluestone JA. Regulating the regulators: costimulatory signals control the homeostasis and function of regulatory T cells. Immunol Rev. 2009;229:41–66. doi: 10.1111/j.1600-065X.2009.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faget J, Biota C, Bachelot T, Gobert M, Treilleux I, Goutagny N, et al. Early detection of tumor cells by innate immune cells leads to Treg recruitment through CCL22 production by tumor cells. Cancer Res. 2011 doi: 10.1158/0008-5472.CAN-11-0573. [DOI] [PubMed] [Google Scholar]
- 28.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Nat Med. United States: 2004. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival; pp. 942–9. [DOI] [PubMed] [Google Scholar]
- 29.Yanai M, Sato K, Aoki N, Takiyama Y, Oikawa K, Kobayashi H, et al. The role of CCL22/macrophage-derived chemokine in allergic rhinitis. Clin Immunol. 2007;125:291–8. doi: 10.1016/j.clim.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 30.Liu VC, Wong LY, Jang T, Shah AH, Park I, Yang X, et al. Tumor evasion of the immune system by converting CD4+CD25− T cells into CD4+CD25+ T regulatory cells: role of tumor-derived TGF-beta. J Immunol. 2007;178:2883–92. doi: 10.4049/jimmunol.178.5.2883. [DOI] [PubMed] [Google Scholar]
- 31.Sharma S, Yang SC, Zhu L, Reckamp K, Gardner B, Baratelli F, et al. Tumor cyclooxygenase-2/prostaglandin E2-dependent promotion of FOXP3 expression and CD4+ CD25+ T regulatory cell activities in lung cancer. Cancer Res. 2005;65:5211–20. doi: 10.1158/0008-5472.CAN-05-0141. [DOI] [PubMed] [Google Scholar]
- 32.Baratelli F, Lin Y, Zhu L, Yang SC, Heuzé-Vourc'h N, Zeng G, et al. Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cells. J Immunol. 2005;175:1483–90. doi: 10.4049/jimmunol.175.3.1483. [DOI] [PubMed] [Google Scholar]
- 33.Lucs AV, Wu R, Mullooly V, Abramson AL, Steinberg BM. Constitutive Overexpression of the Oncogene Rac1 in the Airway of Recurrent Respiratory Papillomatosis Patients is a Targetable Host-Susceptibility Factor. Mol Med. 2011 doi: 10.2119/molmed.2011.00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–41. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 35.Komatsu N, Mariotti-Ferrandiz ME, Wang Y, Malissen B, Waldmann H, Hori S. Heterogeneity of natural Foxp3+ T cells: a committed regulatory T-cell lineage and an uncommitted minor population retaining plasticity. Proc Natl Acad Sci U S A. 2009;106:1903–8. doi: 10.1073/pnas.0811556106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martínez-Llordella M, Ashby M, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10:1000–7. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huehn J, Polansky JK, Hamann A. Epigenetic control of FOXP3 expression: the key to a stable regulatory T-cell lineage? Nat Rev Immunol. 2009;9:83–9. doi: 10.1038/nri2474. [DOI] [PubMed] [Google Scholar]
- 38.Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184:3433–41. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657–79. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- 40.Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–29. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wherry EJ, Ahmed R. Memory CD8 T-cell differentiation during viral infection. J Virol. 2004;78:5535–45. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–7. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 43.Porichis F, Kwon DS, Zupkosky J, Tighe DP, McMullen A, Brockman MA, et al. Responsiveness of HIV-specific CD4 T cells to PD-1 blockade. Blood. 2011;118:965–74. doi: 10.1182/blood-2010-12-328070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chapon M, Randriamampita C, Maubec E, Badoual C, Fouquet S, Wang SF, et al. Progressive upregulation of PD-1 in primary and metastatic melanomas associated with blunted TCR signaling in infiltrating T lymphocytes. J Invest Dermatol. 2011;131:1300–7. doi: 10.1038/jid.2011.30. [DOI] [PubMed] [Google Scholar]
- 45.Reiley WW, Shafiani S, Wittmer ST, Tucker-Heard G, Moon JJ, Jenkins MK, et al. Distinct functions of antigen-specific CD4 T cells during murine Mycobacterium tuberculosis infection. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1006298107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Periasamy S, Dhiman R, Barnes PF, Paidipally P, Tvinnereim A, Bandaru A, et al. Programmed death 1 and cytokine inducible SH2-containing protein dependent expansion of regulatory T cells upon stimulation With Mycobacterium tuberculosis. J Infect Dis. 2011;203:1256–63. doi: 10.1093/infdis/jir011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou Q, Munger ME, Highfill SL, Tolar J, Weigel BJ, Riddle M, et al. Program death-1 signaling and regulatory T cells collaborate to resist the function of adoptively transferred cytotoxic T lymphocytes in advanced acute myeloid leukemia. Blood. 2010;116:2484–93. doi: 10.1182/blood-2010-03-275446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang HY, Lee DA, Peng G, Guo Z, Li Y, Kiniwa Y, et al. Tumor-specific human CD4+ regulatory T cells and their ligands: implications for immunotherapy. Immunity. 2004;20:107–18. doi: 10.1016/s1074-7613(03)00359-5. [DOI] [PubMed] [Google Scholar]
- 49.Franceschini D, Paroli M, Francavilla V, Videtta M, Morrone S, Labbadia G, et al. PD-L1 negatively regulates CD4+CD25+Foxp3+ Tregs by limiting STAT-5 phosphorylation in patients chronically infected with HCV. J Clin Invest. 2009;119:551–64. doi: 10.1172/JCI36604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karim R, Jordanova ES, Piersma SJ, Kenter GG, Chen L, Boer JM, et al. Tumor-expressed B7-H1 and B7-DC in relation to PD-1+ T-cell infiltration and survival of patients with cervical carcinoma. Clin Cancer Res. 2009;15:6341–7. doi: 10.1158/1078-0432.CCR-09-1652. [DOI] [PubMed] [Google Scholar]