Abstract
Objective
This pilot study examined whether methylphenidate (MPH) was effective in enhancing cognitive performance and attention for children with sickle cell disease (SCD) with cerebrovascular complications who evidence attention problems.
Method
In this multisite, pilot study we evaluated two separate double-blind controlled clinical trials, including a laboratory trial of the short-term efficacy of MPH, with the second study a three-week home/school crossover trial evaluating the efficacy of MPH. The laboratory trial included 14 participants between the ages of 7 and 16 years. Assessments included measures of sustained attention, reaction time, executive functions, and verbal memory. The home/school trial included 20 participants. The outcome measures were parent and teacher ratings of attention. The first study compared MPH to placebo while the second trial compared placebo, low-dose MPH, and moderate-dose MPH.
Results
In the laboratory trial, significant effects were revealed for measures of memory and inhibitory control. Parent and teacher reports from the home/school trial indicate that moderate dose MPH produced superior improvement in attention relative to the placebo and low dose MPH.
Conclusion
Stimulant medication positively impacted select measures of memory and inhibitory control in some children with SCD. Attention, as rated by parent and teachers, was improved for a greater number of children and adolescents on higher doses of MPH relative to low-dose MPH and placebo. Stimulant medication may provide an effective intervention for some children with SCD and cerebrovascular complications who demonstrate attention problems.
Keywords: sickle cell disease, chronic illness, pilot study, intervention outcome
Impairments in attention and executive functions have been reported for children with sickle cell disease (SCD) and history of stroke,1–5 silent infarct,2,6–8 and elevated transcranial Doppler ultrasonography (TCD) velocities.9,10 Given the vulnerability of attention and executive functions in this pediatric population, children with SCD evidence similar neurocognitive impairments to their peers with attention-deficit/hyperactivity disorder (ADHD). Further, the structural central nervous system (CNS) impairments associated with SCD are similar to those of children and adolescents with ADHD, including abnormality in the regions of the frontal lobes2 and basal ganglia.11 Because children with SCD evidence similar deficits in attention to their peers with ADHD, it is posited that children with SCD may demonstrate the same therapeutic benefit from stimulant drug therapy as their peers with ADHD. This investigation is important considering that SCD and concomitant attention problems pose considerable risk for children and adolescents in the areas of cognitive and academic functioning.12
Methylphenidate (MPH) is a frequently prescribed stimulant medication for ADHD, releasing dopamine from presynaptic vesicles and thereby reducing dopamine reuptake.13 Among children with ADHD, strong dose-response relationships have been between MPH and symptoms of inattention, with improvements in many cognitive functions at higher doses in laboratory, school, and home situations.14 The most consistent and significant benefits of MPH have been demonstrated on measures of vigilance and sustained attention.14 Evidence of improved working memory, 15–17 paired-associate learning, and inhibitory control in response to MPH also has been reported.
Despite the compelling evidence of impairments in attention and concentration among children with SCD, to date no clinical trials have been conducted that examine pharmacologic management of the cognitive sequelae associated with this chronic disease. Thus, the aim of this multisite, randomized, placebo-controlled pilot study was to assess the efficacy of stimulant medication in enhancing attention, executive functions, and verbal memory among children with SCD who sustained neurological complications and who also evidenced impairments in attention and concentration. Two separate clinical trials were conducted, one of which was a laboratory trial evaluating the short-term efficacy of MPH. For the laboratory trial, it was hypothesized that MPH relative to placebo would result in improved attention, executive function, and verbal memory on laboratory-based measures. The second study evaluated the efficacy of long-acting MPH (three-week home/school crossover trial) based on parent and teacher behavioral ratings. For the home/school trial, it was hypothesized that relative to placebo, long-acting MPH would result in improvements on ecologically valid measures assessing sustained attention.
METHODS
Participants
Eligible participants ranged from 6 to 16 years of age and were primary English speakers, with a documented history in the medical record of cerebrovascular complications (i.e., abnormal MRI or MRA or abnormal transcranial Doppler ultrasonography). Clinically significant attention problems were operationally defined as a T-score ≥ 63 on either of the index scores (ADHD Index or Cognitive Problems/Inattention Index) on either the Conners’ Parent Rating Scale-Revised: Short Form (CPRS-R:S) or the Conners’ Teacher Rating Scale-Revised: Short Form (CTRS-R:S).18 In addition, clinically significant symptoms of ADHD were confirmed via the DSM-IV structured clinical interview, the National Institutes of Mental Health Diagnostic Interview Schedule for Children – Version IV (NIMH DISC-IV)19, with participants’ caregivers.
Exclusion criteria included known structural cardiac abnormalities or heart rhythm abnormalities, glaucoma, patient or family history of tic disorder or Tourette syndrome, uncontrolled seizures, uncorrected hypothyroidism, symptoms of affective and mood disorders, previous diagnosis of ADHD prior to the onset of neurological complications (e.g., stroke or silent infarct) as documented in the medical record or caregiver report, patient or family history of substance abuse, intellectual disability as determined by a full scale IQ (FSIQ) of less than 70 on the Wechsler Abbreviated Scale of Intelligence (WASI),20 or current use of psychotropic medications.
The study was approved by the Institutional Review Boards of the 5 participating sites (Cincinnati Children’s Hospital, Medical University of South Carolina (MUSC), University of Alabama at Birmingham, University of Mississippi Medical Center, Temple University). The Medical University of South Carolina enrolled participants in both the laboratory trial and home/school crossover trial. For those participants at MUSC who participated in both trials, enrollment in the home/school trial occurred immediately after participation in the one-day laboratory trial. Cincinnati Children’s Hospital enrolled participants only in the laboratory trial, while the University of Alabama at Birmingham and the University of Mississippi Medical Center enrolled participants only in the home/school crossover trial. Temple University did not enroll any participants, but served as the coordinating center for the study. Written informed consent was obtained from a legal guardian prior to participation in the study. For those participants ages 12 and older, informed assent also was obtained. Data collection occurred between November, 2006 and November, 2009.
Laboratory Trial Procedures
During the one-day laboratory MPH trial, participants received a physical examination by the study site pediatrician, including a comprehensive review of child and family history and review of systems. Blood pressure also was obtained both prior to and following completion of the laboratory trial. To ascertain adverse side effects, caregivers completed the Barkley’s Side Effects Rating Scale (SERS),21 which assesses 17 common adverse side effects of stimulant medication rated on a severity scale from 0 (absent) to 9 (severe).
During the laboratory trial, participants received one dose of placebo and one dose of active medication (methylphenidate, 20 mg), with the dose conditions counterbalanced across participants. Approximately 90 ± 30 minutes following ingestion of the first medication dose (i.e., placebo or active medication), under double-blind conditions, participants were administered a battery of neuropsychological measures that have demonstrated sensitivity to the effects of MPH among children with ADHD.22 The battery included: the Conners’ Continuous Performance Test-Second Edition (CPT-II)23 as a measure of sustained attention, reaction time, and inhibitory control; the Test of Everyday Attention for Children (TEA-Ch)24 to assess various aspects of executive functioning, including working memory, cognitive flexibility, and response inhibition; and the California Verbal Learning Test-Children’s Version as a measure of verbal rate of learning and recall (CVLT-C).25 To control for practice effects between test administrations within this repeated measures design, the parallel form of the TEA-Ch was used in the afternoon session. The CVLT-C does not have an alternate form; however, potential practice effects were equally balanced across groups. In addition, the CPT-II does not have an alternate version, but has been designed for repeated administrations.
Home/School Crossover Trial Procedures
Each participant received all three of the dose conditions: placebo, low dose (LD) long-acting MPH (Ritalin LA 10 mg), and moderate-dose (MD) long-acting MPH (Ritalin LA 20 mg). The order of treatments was randomized. All randomization was conducted by the pharmacist at the respective participating sites using a Latin square cross-over design to control for potential sequence confounds. The entire research team (investigators, physicians, research assistants, teachers, parents, and participating children) was blind with respect to dosage order during the home/school crossover trial. Each dose condition was administered Monday through Friday for 3 weeks. Each dose condition changeover took place after a weekend washout period.
Parents and teachers completed the CPRS-R:S/CTRS-R:S at the end of each of the three weeks, and the results were either mailed or faxed to the study coordinators of the respective study sites. The CPRS-R:S/CTRS-R:S is designed to assess symptoms and behaviors associated with ADHD. Internal consistency reliabilities for these rating scales range from .86 to .94 for the parent form, and .88 to .95 for the teacher form. Evidence for criterion-related validity includes significant correlations with the Conners’ CPT.26
The ADHD Index from the CPRS-R:S/CTRS-R:S was utilized in the data analyses because it represents a more sensitive measure of medication response, as compared to the Cognitive Problems/Inattention Index, which contains items that assess academic skills (e.g., “poor in spelling” and “not reading up to par”) and were not a focus of this investigation.
Statistical Analyses
In the laboratory trial, there were two conditions to which all participants were exposed: (1) treatment that consisted of two levels (placebo versus MPH), and (2) administration order (morning versus afternoon). Thus, data were presented as a Latin-Square design with a crossover measure (the morning-afternoon sequence or the afternoon-morning sequence). In order to control for the possibility of order effects, participants were randomized to sequence conditions and the trials were counterbalanced. Using the within-subject/repeated measures design, data were analyzed with repeated measures analysis of variance (ANOVA).27 Prior to computing the ANOVA, a Box’s M test of equality of covariance matrices, Mauchley’s test of sphericity, and tests of multicollinearity and singularity were performed. Pillai’s trace was employed as the test of the repeated-measure effect when the Box’s M test was significant and indicated an inequality of the variance-covariance matrices. 28
In order to measure the magnitude of change between the medication conditions and placebo for the home/school trial, we employed the Reliability Change Index (RCI).29 The RCI is used to determine whether individuals changed sufficiently across two occasions, such that change is unlikely to be due to simple measurement unreliability. Clinically significant change was deemed to occur when the posttreatment level of functioning resulted in a participant rated closer to the mean of the functional population than to the mean of the dysfunctional population.29 That level is a function of the initial standard deviation of the measure and its reliability. For the purposes of this investigation, an RCI score greater than 1.96 was established, indicating a less than 5% probability of finding a change in score of that magnitude by chance alone, given a test-retest reliability coefficient for the CPRS-R:S ADHD Index = .93, and for the CTRS-R:S ADHD Index = .80. In other words, participants with an RCI of greater than 1.96 were classified as improved.
RESULTS
Consort Statement
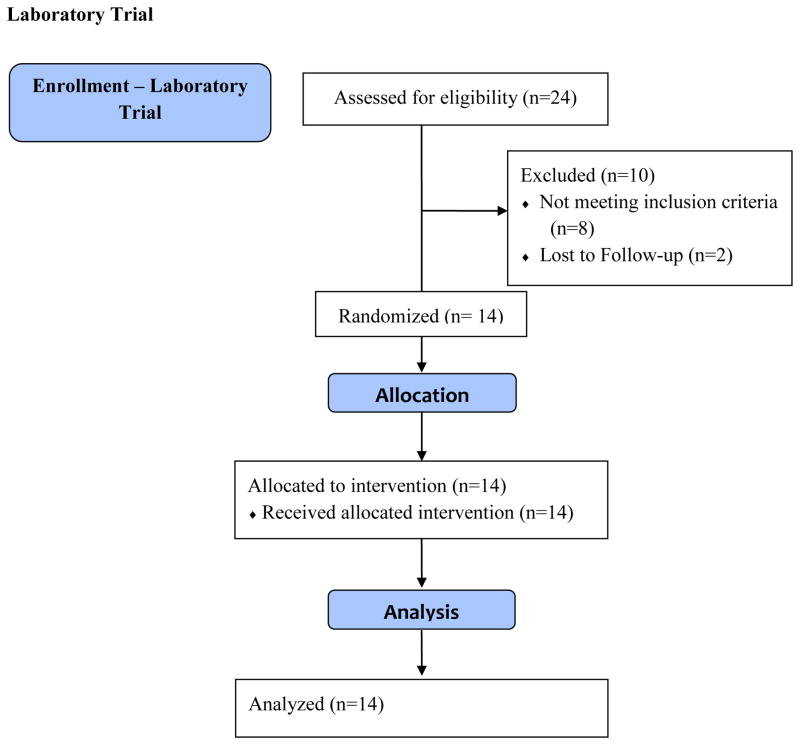
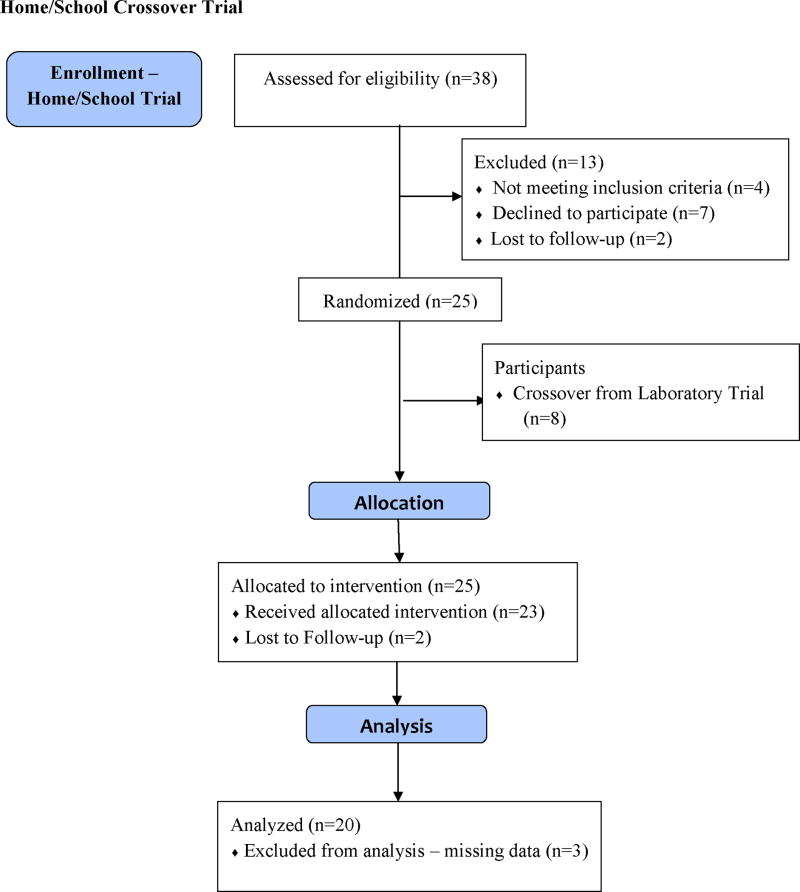
The flow of participants for the laboratory trial and home/school trial is presented in the Figure. For participants completing the trial at the respective sites, The Medical University of South Carolina randomized 11 participants to the laboratory trial and 8 participants to the home/school crossover trial. Cincinnati Children’s Hospital randomized 3 participants to the laboratory trial. The University of Mississippi Medical Center and the University of Alabama at Birmingham randomized 7 and 10 participants to the home/school crossover trial, respectively. Due to missing data for 3 participants, the final analysis for the home/school condition included a total of 20 participants.
FIGURE.
Flow Diagram for Laboratory and Home/School Crossover Trial
Participant Characteristics
The laboratory trial consisted of 14 African American participants (6 males, 8 females) between the ages of 7 and 16 years (M = 11.52 years; SD = 3.00), 13 of whom were diagnosed with the HbSS genotype, and 1 diagnosed with the HbSC genotype. Of the 14 participants, 13 received chronic transfusion, with average hemoglobin over the last 3 clinic visits between 7.3 and 11.2 (M = 9.33; SD = 1.01) and average hematocrit over the last 3 clinic visits between 20.4 and 35.4 (M = 27.90; SD = 3.60). The home/school crossover trial consisted of 20 African American participants (11 males, 9 females) between the ages of 6 and 16 years (M = 10.75 years; SD = 2.73), 16 of whom were diagnosed with the HbSS genotype, 2 diagnosed with the HbSC genotype, and 2 diagnosed with the HbSβ type. Of the 20 participants, 14 received chronic transfusion, with average hemoglobin over the last 3 clinic visits between 7.0 and 11.0 (M = 9.17; SD = 1.08) and average hematocrit over the last 3 clinic visits between 20.0 and 32.0 (M = 27.29; SD = 3.15). It should be noted that participants did not complete the one-day laboratory trial on the same day as a scheduled transfusion. Table 1 presents additional demographic and clinical characteristics of the sample participating in the laboratory and in the home/school crossover trial. In total, eight participants from the laboratory trial were immediately enrolled in the home/school trial, while three participants declined further participation. Comparison between study completers and those children that declined enrollment or were lost to follow-up did not reveal any statistically significant differences with respect to gender, ethnicity, age at study participation, chronic transfusion status, mean hemoglobin, or mean hematocrit level.
TABLE 1.
Demographic and Clinical Characteristics of Children with Sickle Cell Disease in the Laboratory Trial (N = 14) and Home/School Crossover Trial (N = 20)
| Laboratory Trial | Home/School Crossover Trial | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | N | % | Mean | SD | N | % | |
| WASI FSIQ | 93.57 | 14.02 | 86.58 | 9.89 | ||||
| Repeat a grade | 5 | 36 | 10 | 50 | ||||
| Special Education | 7 | 50 | 9 | 45 | ||||
| Mother’s level of Education | ||||||||
| Did not complete high school | 1 | 7 | 2 | 10 | ||||
| Completed high school | 6 | 43 | 7 | 35 | ||||
| Some college/technical school | 3 | 21 | 3 | 15 | ||||
| Graduate degree | 2 | 14 | 3 | 15 | ||||
| Unknown | 2 | 14 | 5 | 25 | ||||
| Father’s level of Education | ||||||||
| Did not complete high school | 1 | 7 | 1 | 5 | ||||
| Completed high school | 7 | 50 | 8 | 40 | ||||
| Some college/technical school | 0 | 0 | 0 | 0 | ||||
| Graduate degree | 2 | 14 | 0 | 0 | ||||
| Unknown | 4 | 29 | 11 | 55 | ||||
| Family Income | ||||||||
| Less than $10,000 | 4 | 29 | 5 | 25 | ||||
| $10,000 to $19,000 | 1 | 7 | 3 | 15 | ||||
| $20,000 to $30,000 | 5 | 36 | 2 | 10 | ||||
| $31,000 to $40,000 | 2 | 14 | 1 | 5 | ||||
| More than $40,000 | 1 | 7 | 3 | 15 | ||||
| Unknown | 1 | 7 | 6 | 30 | ||||
WASI FSIQ = Wechsler Abbreviated Scale of Intelligence – full scale intelligence quotient.
Laboratory Trial
The results of the laboratory trial are summarized in Table 2. The majority of parameters on the CVLT-C reached statistical significance with improved rates of verbal learning and recall occurring when children received MPH as compared to placebo. Specifically, significant treatment effects for MPH were found for CVLT-C, Trials 1-5 (F[1,12] = 52.67, p = .001); Trial 1 Free Recall (F[1,12] = 57.79, p = .001); Trial 5 Free Recall (F[1,12] = 141.06, p = .001); Short-Delay Free Recall (F[1,12] = 14.26, p = .003); Short-Delay Cued Recall (F[1,12] = 9.42, p = .01); Long-Delay Free Recall (F[1,12] = 17.08, p = .001); and, Long Delay Cued Recall (F[1,12] = 42.0, p = .001). Effect sizes for these findings, as denoted by partial eta squared (i.e., partial η2), ranged from medium to very large (.44 – .92).30 No significant effects were found for Perseverations or Recognition False Positives on the CVLT-C.
TABLE 2.
Mean Values and Group Differences for Placebo and Methylphenidate (MPH) in the Laboratory Trial
| Treatment Effect | |||||||
|---|---|---|---|---|---|---|---|
| Placebo | MPH | ||||||
| Variable | M | SD | M | SD | F | p | Partial η2 |
| CVLT-C Trials 1-5-T scorea | 55.29 | 10.4 | 68.7 | 8.9 | 52.67 | .001† | .814 |
| CVLT-C Trial 1 Free Recall-Z Scoreb | 0.58 | 1.00 | 3.36 | 1.22 | 57.79 | .001† | .828 |
| CVLT-C Trial 5 Free Recall-Z Score | .029 | 1.12 | 3.36 | 1.22 | 141.06 | .001† | .922 |
| CVLT-C Short-Delay Free Recall | 0.14 | 1.23 | 1.18 | 0.64 | 14.26 | .003† | .543 |
| CVLT-C Short-Delay Cued Recall | .018 | 1.12 | 0.79 | 1.40 | 9.42 | .01* | .440 |
| CVLT-C Long-Delay Free Recall | 0.46 | 1.13 | 1.00 | 0.88 | 17.084 | .001† | .587 |
| CVLT-C Long Delay Cued Recall | 0.14 | 1.10 | 1.00 | 0.83 | 42.0 | .001† | .778 |
| CVLT-C Perseverations | 1.07 | 1.55 | 1.46 | 2.22 | 0.88 | .367 | .068 |
| CVLT-C Recognition False Positivesc | −0.29 | 0.85 | −0.79 | 1.37 | 2.78 | .163 | .188 |
| TEA-Ch Sky Search, Number of correctly identified targets | 11.0 | 2.88 | 10.3 | 2.20 | 1.02 | .333 | .078 |
| TEA-Ch Sky Search, Time per target | 7.93 | 3.00 | 8.71 | 3.60 | 2.66 | .129 | .181 |
| TEA-Ch Sky Search, Attention score | 8.50 | 3.18 | 8.71 | 3.60 | 0.24 | .633 | .020 |
| TEA-Ch Score! | 7.71 | 3.69 | 8.80 | 3.97 | 1.47 | .248 | .109 |
| TEA-Ch Creature Counting, total correct | 8.64 | 3.84 | 16.1 | 24.2 | 0.90 | .361 | .070 |
| TEA-Ch Creature Counting, timing score | 25.00 | 40.21 | 27.14 | 39.11 | 3.36 | .092 | .219 |
| TEA-Ch Sky Search DT! | 12.14 | 25.20 | 6.57 | 4.80 | 0.69 | .422 | .054 |
| CPT-II # Omissions | 52.58 | 14.37 | 46.70 | 6.94 | 3.97 | .069 | .249 |
| CPT-II # Commissions | 46.67 | 13.36 | 38.71 | 13.39 | 14.77 | .002† | .552 |
| CPT-II Hit Reaction Time (RT) | 52.29 | 11.92 | 49.15 | 11.86 | 2.69 | .127 | .183 |
| CPT-II Variability | 51.00 | 11.51 | 45.6 | 9.21 | 2.76 | .123 | .187 |
| CPT-II Detectability (d′) | 50.13 | 9.90 | 40.39 | 13.82 | 10.57 | .007† | .468 |
| CPT-II Response Style (β) | 52.72 | 14.75 | 55.43 | 19.85 | 0.12 | .731 | .010 |
| Examiner SERS Total Score | 32.07 | 44.24 | 24.79 | 40.51 | 0.82 | .383 | .064 |
| Caregiver SERS Total Score | 18.93 | 18.55 | 14.07 | 25.14 | 1.22 | .291 | .092 |
T-scores have a normative mean score = 50 and an SD = 10; higher scores are better.
Z-scores have a normative mean score = 0.0 and an SD = 1.0; higher scores are better.
Recognition false positives are scored in a manner that a higher score is worse; to be consistent, these scores were reversed scored such that a higher score was better.
P < .05.
P < .01
On the CPT-II, significant treatment effects for MPH were found for Commission Errors (F[1,12] = 14.77, p = .002) and Detectability (F[1,12] = 10.57, p = .007), with effect sizes for both measures categorized as within the small to medium range.30 Thus, following MPH administration, children displayed improved inhibitory control and improved ability to distinguish target from non-target stimuli (i.e., detectability). No significant effects were found on measures of Omission Errors, Hit Reaction Time (RT), Variability, or Response Style on the CPT-II. No significant treatment effects were obtained on any parameters of the TEA-Ch. In addition, no order effects were found to be significant, and are therefore not reported.
Of the adverse side effects reported for participants receiving MPH in the laboratory trial, the majority were considered to be non-serious and mild to moderate in intensity. The average total SERS score (range 0 – 153) when receiving MPH was 20.3, while the average score for placebo was 12.7. The most frequently reported adverse side effects in the placebo and MPH conditions were decreased appetite (43%), social withdrawal (36%), and tics or nervous movements (29%). Of particular relevance to this patient population, pain (e.g., “pain crises”) was not reported by any of the participants’ caregivers. When receiving MPH, 7 out of 14 participants had at least one item out of the 17 total items that was rated as a 7 or higher (severe), one participant had at least one score between 4 to 6 (moderate), and the remaining 6 participants had scores on all items that were 3 or less (mild). When receiving placebo, 3 out of 14 participants had at least one item that was rated as a 7 or higher (severe), eight participants had at least 1 score between 4 to 6 (moderate), and the remaining 3 participants had scores on all items that were 3 or less (mild).
Home/School Crossover Trial
RCI analyses comparing the Parent-rated ADHD Index scores for low dose MPH compared to placebo revealed that 4 out of 20 children evidenced improvement on low dose MPH as compared to placebo (20%; Table 3). Thus, the 20% improvement rate is higher than the anticipated 5% rate established by the RCI. When comparing the ADHD Index scores for moderate dose MPH to placebo, 5 out of 20 children evidenced improvement on moderate dose MPH as compared to placebo (25%). Again, the 25% improvement rate is higher than the anticipated 5% rate established by the RCI. When comparing the ADHD Index scores for moderate dose MPH to low dose MPH, 6 out of 20 children evidenced improvement on moderate dose MPH as compared to low dose MPH (30%). The 30% improvement rate is much higher than the anticipated 5% rate established by the RCI. When examining how many children improved on any dose relative to placebo, findings indicate that 10 out of 20 children improved on parent ratings. A repeated measures analysis of variance determined that mean ADHD Index scores are not statistically different between medication conditions for the Parent rating form (F(2, 38) = .370, p = .69, η2= .02).
TABLE 3.
Percentage of Participants in the Respective Conditions Demonstrating Clinically Significant Change (Reliability Change Index) on Parent and Teacher Ratings on the ADHD Index of the CPRS:S/CTRS:S
| T-Score ADHD Index | Percent demonstrating clinically significant change* | |
|---|---|---|
| Parent Report | ||
| Placebo | 64.3 | |
| Low Dose | 63.5 | |
| Moderate Dose | 62.6 | |
| Low Dose vs. Placebo | 20% | |
| Moderate Dose vs. Placebo | 25% | |
| Moderate Dose vs. Low Dose | 30% | |
| Teacher Report | ||
| Placebo | 68.7 | |
| Low Dose | 66.5 | |
| Moderate Dose | 64.9 | |
| Low Dose vs. Placebo | 10% | |
| Moderate Dose vs. Placebo | 20% | |
| Moderate Dose vs. Low Dose | 10% | |
RCI values were compared 1.96 (P < .05) in determining a clinically significant change from baseline.
RCI analyses comparing Teacher-rated ADHD Index scores for low dose MPH compared to placebo revealed that 2 out of 20 (10%) children evidenced improvement on low dose MPH as compared to placebo. Thus, the 10% improvement rate is higher than the anticipated 5% rate established by the RCI. When comparing the ADHD Index scores for moderate dose MPH to placebo, 4 out of 20 (20%) children evidenced improvement on moderate dose MPH as compared to placebo. The 20% improvement rate is higher than the anticipated 5% rate established by the RCI. When comparing the ADHD Index scores for moderate dose MPH to low dose MPH, 2 out of 20 (10%) children evidenced improvement on moderate dose MPH as compared to low dose MPH. The 10% improvement rate is higher than the anticipated 5% rate established by the RCI. Further examination of the teacher rating data indicates that 6 out of 20 children improved on any dose relative to placebo. A repeated measures analysis of variance determined that mean ADHD Index scores are not statistically different between medication conditions for the Teacher rating form (F(2, 38) = 1.53, p = .23, η2 = .07).
DISCUSSION
This is the first known randomized, double-blind, placebo-controlled pilot study designed to examine the efficacy of stimulant drug therapy for children with sickle cell disease (SCD) categorized by attentional problems. Our first hypothesis, that stimulant drug therapy would enhance performance on laboratory measures of attention, executive function, and working memory, was partially supported. Specifically, the results of our laboratory trial indicate that, compared to placebo, treatment with MPH significantly improved select measures of verbal memory and inhibitory control but not measures of executive function for children with SCD. These findings are in accord with the extant literature for children with ADHD that reveals improved inhibitory control with stimulant medication,31,32 but limited evidence for improvement on laboratory measures of executive functions.33,34
Of importance in this investigation is the finding of significant improvement on multiple measures of memory. Specifically, positive effects were noted on measures of verbal rate of learning and recall. Although the magnitude of change could reflect, in part, practice effects, the cross-over design substantiates the statistically significant improvement produced by the MPH condition, as the benefit of repeated practice was equal across groups. These results are in accord with a number of studies that report improvements in verbal learning and memory among children with ADHD when treated with MPH.15–17 The data from this study are encouraging as they suggest that learning and memory, skills that are often impaired among children with SCD12, may be improved as a result of methylphenidate, a frequently employed stimulant, in a similar manner to children with ADHD.
Results from the laboratory trial indicate that while MPH did improve inhibitory control, it did not result in enhanced performance on specific parameters of sustained attention. Our failure to obtain significant medication effects on laboratory measures of sustained attention is surprising. However, the laboratory setting, characterized by significant structure, including the one-to-one support of an examiner, likely produces an environment that results in a low base rate of problems with sustained attention and concentration. In general, ADHD is a disorder in which behavior is contextually specific. Thus, it is likely that the results obtained during our laboratory trial emanate from floor effects at baseline, where medication may have exerted little effect on sustained attention.
An alternative explanation for the failure to obtain effects on select parameters of sustained attention during the laboratory trial may relate to the dose of stimulant medication administered to participants. That is, all participants received a standard dose of MPH (20mg), which for some children may not have yielded optimal therapeutic benefit. In the clinical setting, standard practice typically involves prescription of the lowest dose of standard immediate-release stimulant medication (i.e., 5 mg), followed by titration of dosage until therapeutic changes in behavior are evident, as reported by either parents or teachers on behavioral ratings, with the fewest adverse side effects.35 Thus, it is plausible that some of the study participants would have achieved optimal therapeutic response at different doses. It is also plausible that MPH was not the optimal stimulant medication for all participants in our investigation. For example, many children with ADHD - Predominantly Inattentive Type, a subtype of ADHD similar to the sample in this investigation, demonstrate greater benefit from amphetamines as compared to MPH.
In contrast to the laboratory trial, the three-week home/school crossover trial yielded evidence for improvement on behavioral ratings of sustained attention as rated by teachers for a sub-sample of the study. Specifically, utilizing the Reliability Change Index (RCI) to assess magnitude of change, findings from the home/school crossover trial revealed that the moderate dose long-acting MPH condition produced superior improvement for some of the participants than either the placebo or the low dose long-acting MPH, as assessed by parent and teacher ratings of sustained attention. These findings are consistent with the compelling evidence for the efficacy of stimulant drug therapy on parent and teacher observations and behavioral ratings for children with ADHD.36 These data also are in accord with those studies of children with ADHD where higher doses of stimulant medication have been demonstrated to improve behavior as rated by caregivers and teachers.14 Moreover, our findings reveal a clinically significant difference between the moderate dose and low dose MPH for some of the children in the sample. Although the reason for these differences is unclear, it remains the goal of future studies to elucidate factors underlying the difference in dose response curves of stimulant medication for various populations of children with neurological impairments and concomitant attention deficits.
The contribution of our investigation must be interpreted within the limitations of the present study design. First, this pilot study is characterized by a small sample size that may have mitigated sufficient power, thereby diminishing significant effects that otherwise may have been detected with a larger sample. Unfortunately, small sample sizes, in part due to a low incidence chronic illness, often characterize investigations of SCD. As such, additional research will need to include collaborative multi-site clinical samples that ensure larger cohorts of children with SCD. Second, because the ADHD Index of the Conners’ Parent and Teacher forms was used as the sole outcome variable for the home/school crossover trial, we cannot rule out the possibility of floor effects occurring for some participants, which would serve to reduce the effect sizes of the measures used to assess the efficacy of methylphenidate. Third, the uniform dosing of MPH for all participants in both the laboratory and home/school crossover trial may have yielded subclinical effects for some participants, especially when considering the broad age and weight range of our sample. Future investigations that employ a trial whereby MPH is titrated for each participant to his/her optimal therapeutic dosage may yield more robust results. Moreover, while no participants were currently being treated with stimulant medication at study entry, systematic data on prior treatment history with stimulant medication was not explicitly collected, leaving open the possibility that past use of stimulant medication by participants could have influenced ratings by parents or teachers.
Adverse side effect data were collected for the laboratory trial, but not for the home/school crossover trial. The most frequently reported adverse side effects in the laboratory trial, decreased appetite (43%), social withdrawal (36%), and tics or nervous movements (29%), are generally equivalent to findings from a study that investigated side effects of methylphenidate in children with ADHD and reported the following prevalence rates: decreased appetite (56%), social withdrawal (28%), and tics or nervous movements (28%).37 Future investigations should further evaluate the safety of stimulant medication in this population by gathering data across multiple sources including parents, teachers, and health care providers. Finally, this investigation relied on laboratory tests and behavioral ratings, measures that are ecologically limited in assessing cognitive behaviors. Future research should also include observations of behavior, including natural observations within the classroom setting.
While we took several safety precautions, including exclusion of children with known cardiac complications, evaluation of all study participants at study entry by attending pediatricians, obtaining pre- and post-trial blood pressures, and monitoring of drug adverse side effects, future investigations should focus specifically on the safety of MPH in this population of children. Given the potential for hypertension and cardiac complications among children with SCD, the practitioner cannot be too judicious when prescribing stimulant medication to children and adolescents with SCD. While caution in prescribing stimulant medication is imperative, our data do lend preliminary support for MPH as a promising pharmacological intervention for some children with SCD who struggle with symptoms of inattention and poor concentration. It is hoped that research efforts in this area will continue so as to improve the quality of life of children and adolescents with SCD.
Footnotes
Financial Disclosure: Ronald Brown served as a consultant for Shire Pharmaceuticals. He has received research support from Shire Pharmaceuticals. The other authors report no conflicts of interest or financial relationships relevant to this article to disclose.
Contributor Information
Brian Daly, Department of Psychology, Drexel University, Philadelphia, PA
Mary C. Kral, Department of Pediatrics, Medical University of South Carolina, Charleston, SC
Ronald T. Brown, Provost Office, Wayne State University, Detroit, MI
David Elkin, Division of Psychology, University of Mississippi Medical Center, Jackson, MS
Avi Madan-Swain, Department of Pediatrics, Children’s Hospital, Birmingham, AL
Monica Mitchell, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH
Lori Crosby, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH
David DeMatteo, Department of Psychology, Drexel University, Philadelphia, PA
Angela LaRosa, Department of Pediatrics, Medical University of South Carolina, Charleston, SC
Sherron Jackson, Department of Pediatrics, Medical University of South Carolina, Charleston, SC
References
- 1.Armstrong FD, Thompson RJ, Wang W, et al. Cognitive functioning and brain magnetic resonance imaging in children with sickle cell disease. Pediatrics. 1996;97:864–70. [PubMed] [Google Scholar]
- 2.Brown RT, Davis PC, Lambert R, et al. Neurocognitive functioning and magnetic resonance imaging in children with sickle cell disease. J Pediatr Psychol. 2000;25:503–13. doi: 10.1093/jpepsy/25.7.503. [DOI] [PubMed] [Google Scholar]
- 3.Cohen MJ, Branch WB, McKie VC, et al. Neuropsychological impairment in children with sickle cell anemia and cerebrovascular accidents. Clin Pediatr. 1994;33:517–524. doi: 10.1177/000992289403300902. [DOI] [PubMed] [Google Scholar]
- 4.Schatz J, Craft S, Koby M, et al. Neuropsychological deficits in children with sickle cell disease and cerebral infarction: Role of lesion site and location. Child Neuropsychol. 1999;5:92–103. [Google Scholar]
- 5.White DA, Salorio CF, Schatz J, et al. Preliminary study of working memory in children with stroke related to sickle cell disease. J Clin Exp Neuropsychol. 2000;22:257–64. doi: 10.1076/1380-3395(200004)22:2;1-1;FT257. [DOI] [PubMed] [Google Scholar]
- 6.DeBaun MR, Schatz J, Siegel MJ, et al. Cognitive screening examinations for silent cerebral infarcts in sickle cell disease. Neurology. 1998;50:1678–82. doi: 10.1212/wnl.50.6.1678. [DOI] [PubMed] [Google Scholar]
- 7.Schatz J, Brown RT, Pascual JM, et al. Poor school and cognitive functioning with silent cerebral infarcts and sickle cell disease. Neurology. 2001;56:1109–11. doi: 10.1212/wnl.56.8.1109. [DOI] [PubMed] [Google Scholar]
- 8.Watkins KE, Hewes DK, Connelly A, et al. Cognitive deficits associated with frontal-lobe infarction in children with sickle cell disease. Dev Med Child Neurol. 1998;40:536–43. doi: 10.1111/j.1469-8749.1998.tb15412.x. [DOI] [PubMed] [Google Scholar]
- 9.Kral MC, Brown RT, Nietert PJ, et al. Transcranial doppler ultrasonography and neurocognitive functioning in children with sickle cell disease. Pediatrics. 2003;112:324–31. doi: 10.1542/peds.112.2.324. [DOI] [PubMed] [Google Scholar]
- 10.Kral MC, Brown RT. Transcranial doppler ultrasonography and executive dysfunction in children with sickle cell disease. J Pediatr Psychol. 2004;29:185–95. doi: 10.1093/jpepsy/jsh020. [DOI] [PubMed] [Google Scholar]
- 11.Pegelow CH, Macklin EA, Moser FG, et al. Longitudinal changes in brain magnetic resonance imaging findings in children with sickle cell disease. Blood. 2002;99:3014–18. doi: 10.1182/blood.v99.8.3014. [DOI] [PubMed] [Google Scholar]
- 12.Berkelhammer LD, Williamson AL, Sanford SD, et al. Neurocognitive sequelae of pediatric sickle cell disease: A review of the literature. Clin Neuropsychol. 2007;13:120–31. doi: 10.1080/09297040600800956. [DOI] [PubMed] [Google Scholar]
- 13.Guevara J, Lozano P, Wickizer T, et al. Psychotropic medication use in a population of children who have attention-deficit/hyperactivity disorder. Pediatrics. 2002;109:733–39. doi: 10.1542/peds.109.5.733. [DOI] [PubMed] [Google Scholar]
- 14.Brown RT, Daly BP. Neuropsychological effects of stimulant medication on children’s learning and behavior. In: Reynolds CR, Fletcher-Janzen E, editors. Handbook of clinical child neuropsychology. 3. New York: Plenum; 2009. pp. 529–580. [Google Scholar]
- 15.Mehta MA, Goodyer IM, Sahakian BJ. Methylphenidate improves working memory and set-shifting in AD/HD: Relationships to baseline memory capacity. J Child Psychol Psychiatry. 2004;45:293–305. doi: 10.1111/j.1469-7610.2004.00221.x. [DOI] [PubMed] [Google Scholar]
- 16.Murray DW, Childress A, Giblin J, et al. Effects of OROS methylphenidate on academic, behavioral, and cognitive tasks in children 9 to 12 years of age with attention-deficit/hyperactivity disorder. Clin Pediatr. 2011;50:308–320. doi: 10.1177/0009922810394832. [DOI] [PubMed] [Google Scholar]
- 17.Wigal SB, Wigal T, Schuck S, et al. Academic, behavioral, and cognitive effects of OROS methylphenidate on older children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopahrmacol. 2011;21:121–131. doi: 10.1089/cap.2010.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conners CK. Conners’ Rating Scale – Revised. New York: Multi-Health Systems, Inc; 2000a. [Google Scholar]
- 19.Shaffer D, Fisher P, Lucas CP, et al. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Wechsler D. The Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- 21.Barkley RA, McMurray MB, Edlbrock CS, et al. Side effects of methylphenidate in children with Attention Deficit Hyperactivity Disorder: A systematic, placebo-controlled evaluation. Pediatrics. 1990;86:184–192. [PubMed] [Google Scholar]
- 22.Brown RT, Sammons M. Pediatric psychopharmacology: A review of new developments and recent research. Prof Psychol Res Pract. 2002;33:135–47. [Google Scholar]
- 23.Conners CK. Conners’ Continuous Performance Test. 2. New York: Multi-Health Systems, Inc; 2000b. [Google Scholar]
- 24.Manly T, Robertson IH, Anderson V, et al. TEA-Ch: The test of everyday attention for children. Bury St. Edmunds, UK: Thames Valley Test Company Limited; 1999. [Google Scholar]
- 25.Delis D, Kramer JH, Kaplan E, et al. California Verbal Learning Test-Children’s Edition. New York: Harcourt, Brace, Jovanovich, Inc; 1994. [Google Scholar]
- 26.Conners CK. The Conners’ Continuous Performance Test. Toronto, Ontario: Multi-Health Systems; 1995. [Google Scholar]
- 27.Tabachnick BG, Fidell LS. Computer assisted research design and analysis. Boston: Alyn and Bacon; 2001. [Google Scholar]
- 28.Stevens JP. Applied multivariate statistics for the social sciences. 4. New Jersey: Lawrence Erlbaum; 2002. [Google Scholar]
- 29.Jacobson NS, Truax P. Clinical significance: A statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. 1991;59:12–9. doi: 10.1037//0022-006x.59.1.12. [DOI] [PubMed] [Google Scholar]
- 30.Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- 31.Aron AR, Dowson JH, Sahakian BJ, et al. Methylphenidate improves response inhibition in adults with Attention-Deficit/Hyperactivity Disorder. Biol Psychiatry. 2003;54:1465–68. doi: 10.1016/s0006-3223(03)00609-7. [DOI] [PubMed] [Google Scholar]
- 32.Tannock R, Carr R, Chajczyk D, et al. Effects of methylphenidate on inhibitory control in hyperactive children. J Abnorm Child Psychol. 1989;17:473–91. doi: 10.1007/BF00916508. [DOI] [PubMed] [Google Scholar]
- 33.Coghill DR, Rhodes SM, Matthews K. The neuropsychological effects of chronic methylphenidate on drug-naive boys with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;62:954–62. doi: 10.1016/j.biopsych.2006.12.030. [DOI] [PubMed] [Google Scholar]
- 34.Willcutt EG, Doyle AE, Nigg JT, et al. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry. 2005;57:1336–46. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 35.American Academy of Pediatrics and National Initiative for Children’s Healthcare Quality. Stimulant medication management information. [cited 2010 Oct 10]. Available from: http://www.nccpeds.com/adhd_toolkit.htm.
- 36.Brown RT, Amler RW, Freeman WS, et al. Treatment of Attention-Deficit/Hyperactivity Disorder: Overview of the evidence. Pediatrics. 2005;115:749–57. doi: 10.1542/peds.2004-2560. [DOI] [PubMed] [Google Scholar]
- 37.Efron D, Jarman F, Barker M. Side effects of methylphenidate and dexamphetamine in children with attention deficit hyperactivity disorder: A double-blind, crossover trial. Pediatrics. 1997;100:662–666. doi: 10.1542/peds.100.4.662. [DOI] [PubMed] [Google Scholar]