Abstract
Aims
This study aimed to determine the relative effectiveness of 12-months of Interim Methadone (IM; supervised methadone with emergency counseling only for the first 4 months of treatment), Standard Methadone treatment (SM; with routine counseling) and Restored Methadone treatment (RM: routine counseling with smaller caseloads).
Design
A randomized controlled trial was conducted comparing: IM, SM, and RM treatment. IM lasted for 4 months after which participants were transferred to SM.
Setting
The study was conducted in two methadone treatment programs in Baltimore, MD, USA.
Participants
The study included 230 adult methadone patients newly-admitted through waiting lists.
Measurements
We administered the Addiction Severity Index and a supplemental questionnaire at baseline, 4-, and 12-months post- baseline.
Measurements included retention in treatment, self-reported days of heroin and cocaine use, criminal behavior and arrests, and urine tests for heroin and cocaine metabolites.
Findings
At 12 months, on an intent-to-treat basis, there were no significant differences in retention in treatment among the IM, SM and RM groups (60.6%, 54.8% and 37.8%, respectively). Positive urine tests for the three groups declined significantly from baseline (ps<0.001 and 0.003, for heroin and cocaine metabolistes respectively) but there were no significant Group x Time interactions for these measures. Thirty-one percent of the sample reported at least one arrest during the year, but there were no significant between-group effects.
Conclusions
Limited availability of drug counseling services should not be a barrier to providing supervised methadone to adults dependent on heroin - at least for the first 4 months of treatment.
Keywords: Interim methadone, methadone treatment, counseling, heroin addiction treatment
INTRODUCTION
As of 2009, methadone treatment for opioid dependence was available in 65 countries.1 In virtually all studies, such treatment has been shown to reduce heroin use and mortality.2-5 The levels and type of psychosocial support provided along with methadone vary greatly across the world.6-8
In the US, from the beginning of methadone maintenance in the 1960s until the first decade of the 21st century, there were waiting lists for this treatment. Despite substantial expansion of methadone treatment programs (MTPs) over the 1970s, programs could not meet the demand for services. Starting in 1972, regulations at the US federal and state levels required, in addition to daily direct observation of methadone ingestion for periods of several months, some minimal level of psychosocial services to accompany methadone administration9 and this requirement probably contributed to waiting lists. These psychosocial services are not required to accompany methadone delivery in 13 countries in the European Union7 and Australia10 and are not required in the US for buprenorphine treatment.11
Because the availability of psychosocial services rather than methadone itself has often been the rate limiting step in providing treatment, researchers in the US have explored the question of whether supervised methadone alone even without psychosocial services might produce substantial benefits.12-15 During the height of the AIDS epidemic in New York City, Yancovitz and colleagues16 conducted a randomized trial of methadone alone (termed interim methadone by these authors) compared to 30 days of waiting list. This trial found that the group assigned to methadone alone had higher rates of treatment entry and lower rates of opioid positive urine tests as compared to the waiting list group. In 1993, US federal regulations were approved to permit the use of methadone alone for individuals on waiting lists under certain restrictive conditions, including limiting such treatment to 120 days, prohibiting any take home doses, limiting such treatment to not-for-profit programs and requiring prior state and federal approval.17 Subsequently this mechanism was seldom utilized outside of the context of research.13
In 2006, Schwartz and colleagues18, 19 compared interim methadone (IM) treatment to remaining on a waiting list in a random assignment study and found that the IM group was more than 3.5 times more likely to be enrolled in treatment and significantly less likely (p = .001) to have opioid positive urine tests at both 4 and 10 month follow-up. These findings, which were consistent with those of Mattick and co-workers’ review of opioid agonist treatment vs. no opioid agonist treatment5 left open the question of whether a 4-month period of supervised methadone with only the availability of emergency counseling (methadone alone) would be associated with inferior outcomes compared to standard methadone treatment with counseling. To deal with this question, we started the present random assignment study at two Baltimore MTPs (N =230) in which we compared initiating treatment with methadone alone (IM) to standard methadone (SM) with counseling and restored methadone (RM) with counseling provided by a counselor with a reduced case load. This question is distinct from that considered in the review by Amato and co-workers20 which compared outcomes in methadone treatment offering standard counseling to methadone treatment plus psychosocial interventions above and beyond methadone with standard counseling. We previously reported that at 4-month post-admission to these two sites, there were no significant differences among the groups in treatment retention, opioid or cocaine positive urine tests or self-reported criminal behavior.21 To examine whether there was a delayed impact of counseling, here we report on this study's outcomes at 1-year follow-up, 8 months after the IM group had been admitted to standard methadone with routine counseling.
METHODS
Participants
Individuals being placed on a waiting list for publicly-subsidized treatment availability at one of two methadone treatment programs (MTPs) in Baltimore, MD, USA were recruited for participation from May 2008 through January 2010. Inclusion criteria were: (1) being at least 18 years old; (2) being willing to provide informed consent; and (3) meeting US MTP admission criteria (i.e., at least one year of opioid dependence). Exclusion criteria were: (1) pregnancy or (2) acute medical or psychiatric conditions which the clinic staff believed required immediate intervention.
Setting
The study was conducted in two hospital-affiliated outpatient MTPs in Baltimore. The Institutional Review Boards (IRBs) of Friends Research Institute and the participating institutions approved the study and all participants provided informed consent.
Measurement
Participants were assessed at baseline by research assistants (RAs) at MTP admission (in blinded fashion, prior to random assignment) and in an unblinded fashion at 4 and 12 months post-baseline. All participants were administered the Addiction Severity Index (ASI), an instrument that measures problems associated alcohol and drug use, medical, psychological, legal, family and social relationships, and employment status. 22 A composite score, ranging from 0 (no problem) to 1 (extreme problem), can be calculated for each problem area. In addition, for the present analysis, responses to a limited number of individual ASI items were examined, including the self-reported number of days in the 30 days prior to the interview of heroin and cocaine use, illegal activity and the amount of money spent on drugs and obtained from illegal activity. Self-reports of hospitalizations and arrests were obtained at each follow-up interview. For those individuals who missed their 12-month follow-up interview, incarceration data were obtained by searching the online publicly-available database of incarcerations.
Urine samples were analyzed by a certified laboratory using enzyme multiplied immunoassay testing (EMIT). For participants enrolled in treatment, urine specimens were collected by same gender clinical staff under direct observation following standard clinic procedures. RAs collected samples from participants who were not enrolled in treatment. Data were also gathered by the RAs from the participants’ clinical chart to assess the number of group and individual counseling sessions, the mean methadone dose and the number of days in treatment. Participants received $15 for their baseline assessment and $25 for each of three follow-up assessments (including a 2-month assessment not reported on here), for a maximum total of $90.
Random assignment
After they provided informed consent and completed their baseline assessment and their MTP admission procedures, participants were block randomized to study condition using a computer-generated procedure. At study onset, the project manager used this list to create a series of numbered cards with the study ID and assigned condition, inserting them into numbered, opaque envelopes. As participants were enrolled, the project manager gave the sealed envelopes to the RAs to open after informed consent and baseline interviews were complete. Participants at one MTP were assigned to Interim Methadone (IM) or Standard Methadone (SM) while those at the second MTP were randomly assigned to IM, SM, or a third condition intended to explore the impact of lower caseloads. The latter condition was termed Restored Methadone (RM) because it restored caseloads to those typical of the early days of MTPs in the US. MTP staff were not blind to condition post-randomization assignment.
IM was delivered under the US regulations which govern such treatment17 and consisted of methadone administered under direct observation seven days per week (no take home doses were permitted) for up to 120 days while awaiting an opening of a subsidized SM slot, a minimum of three drug tests, and emergency counseling only to deal with crises (i.e., no regularly scheduled counseling). Nearly all (94.5%) IM participants received IM for at least 110 days. SM treatment was provided by the participating clinics following their usual procedures. Therefore participants were able to earn take-home doses after a period of time demonstrating progress in treatment, such as negative drug tests and required attendance at scheduled counseling appointments. The counselor developed an individualized treatment plan for each patient, regularly scheduled required group and individual counseling sessions, and reviewed urine test results which were available on a more frequent basis than in IM. Counseling was delivered by the existing staff at the MTPs using approaches commonly in use throughout the US which include influences drawn from motivational interviewing, cognitive and 12-step approaches depending on the training and theoretical model employed by each counselor. Counselors also attempt to link patients to community services, such as job training or medical care. RM was available to randomly assigned participants at only one of the sites through the support of a local foundation. This condition offered all of the services of SM delivered by a counselor with no more than half the usual MTP caseload (i.e., not more than 25 patients at once).
There were no differences in dosing procedures across conditions and participants generally started with methadone 20 - 30 mg per day and gradually increased to a target to 60 mg to 80 mg with further adjustments as needed.
Statistical analysis
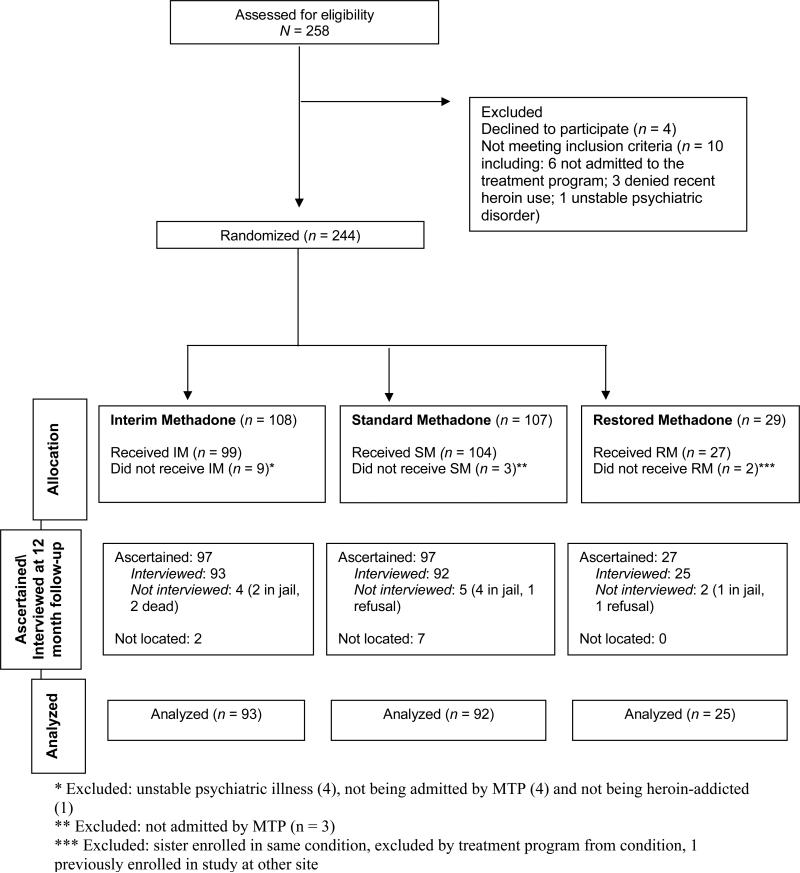
Data were analyzed using the sample of 230 participants who were randomly assigned to condition and received at least one dose of methadone. As previously reported, at the 4-month follow-up, 97 of the 99 IM participants (97.9%); 93 of the 104 SM participants (89.4%); and, 26 of 27 RM participants (96.3%) were interviewed.21 As shown in the Consort Diagram (see Figure 1), at 12 month follow-up, the whereabouts were ascertained for 97.9% of the IM; 93.3% of the SM; and, 100% of the RM participants, and 93.9%, 88.5% and 92.5% of the IM, SM, and RM participants, respectively, were interviewed.
Figure 1.
Consort Diagram
Tests of differences at 12 months among the three conditions were conducted using oneway analysis of variance for methadone dose and analysis of variance for the number of counseling sessions under the assumptions that these variables followed a Poisson distribution. Separate χ2 goodness-of-fit tests were utilized to examine differences in treatment retention (yes v. no) among the conditions at 4 and 12 months.
Analyses of self-reported days of drug use and criminal activity utilized separate GEE models under the assumption that these outcome measures followed a Poisson distribution. Opiate and cocaine drug testing results also were analyzed with a GEE model under the assumption that these variables followed a binomial distribution. A general linear mixed model approach was utilized to assess differences among the three groups on the seven ASI Composite scores.
RESULTS
Participant Characteristics
As shown in Table 1, the 230 study participants had a mean (SD) age of 43.2 years (8.0); 161 (70%) were males, 178 (77.4%) were African American, 49 (21.3%) were white, and 31 (13.5%) were married. There were no significant differences among the groups of participants assigned to IM, SM, or RM in terms of those demographic variables, or in terms of their mean years of education (11.3 [1.9]) or percent employed during the 30 days prior to baseline (32.6%). The total sample reported using heroin and cocaine on 29.1 (0.3) and 5.8 (9.1) days, respectively, preceding the baseline interview and 97.4% and 50.0% of the urine samples were positive for opiates and cocaine, respectively. There were no significant differences among the groups in any of these variables as well.
Table 1.
Participant Characteristics at Baseline
| Variable* | Total (N = 230) | Interim (n = 99) | Standard (n = 104) | Restored (n = 27) |
|---|---|---|---|---|
| Age, Mean (SD) | 43.2 (8.0) | 43.6 (8.2) | 43.5 (7.7) | 40.7 (7.8) |
| Male, Number (%) | 161 (70.0) | 69 (69.7) | 74 (71.2) | 18 (66.7) |
| Race, Number (%) | ||||
| Black | 178 (77.4) | 78 (78.8) | 79 (76.0) | 21 (77.8) |
| White | 49 (21.3) | 19 (19.2) | 24 (23.1) | 6 (22.2) |
| Native-American | 2 (0.9) | 1 (1.0) | 1 (1.0) | 0 |
| Asian | 1 (0.4) | 1 (1.0) | 0 | 0 |
| Married, Number (%) | 31 (13.5) | 12 (12.1) | 16 (15.4) | 3 (11.1) |
| Employed in last 30 days, Number (%) | 75 (32.6) | 27 (27.3) | 42 (40.4) | 6 (22.2) |
| Years of education, Mean (SD) | 11.3 (1.9) | 11.3 (1.6) | 11.3 (1.9) | 11.1 (2.6) |
| Lifetime months of incarceration, Mean (SD) | 50.6 (67.3) | 51.8 (71.2) | 49.5 (65.6) | 50.2 (61.1) |
| Lifetime number of arrests, Mean (SD) | 10.8 (10.7) | 11.6 (12.4) | 10.5 (9.1) | 8.9 (9.7) |
| Prior MTP episode, (yes), Number (%) | 107 (46.5) | 53 (53.5) | 39 (37.5) | 15 (55.6) |
| Days of hospitalization/last 12 months, Mean (SD) | 0.85 (3.83) | 1.27 (5.19) | 0.6 (2.55) | 0.30 (0.87) |
Values are mean (SD), except for gender, race, marital and employment status, and prior MTP episode, which are expressed as number of participants (%).
Note: There were no statistically significant differences among groups on any of the variables reported in this table (all ps > .05), except for prior MTP episodes (p = 0.044).
Treatments
Average methadone dose
The mean dose (standard deviation) in milligrams (mgs) for the total sample at 12 months was 84.2 (22.8), and the mean dose for the IM, SM and RM groups were respectively, 91.8 (21.3), 79.4 (22.3), and 64.7 (15.6) mgs. The difference in dose among the three groups was significant (p<.001).
Counseling sessions
There was a significant Group main effect for the number of individual counseling sessions received in each condition between 4 and 12 months (p<.001). The IM condition received a mean (SE) of 7.9 (0.70) individual sessions over the 8-month period, while the SM condition received a mean (SE) of 5.8 (.58) individual sessions. The RM condition received the most individual sessions, a mean (SE) of 14.2 (1.79) sessions.
The Group main effect was not significant for the number of group counseling sessions received in each condition between 4 and 12 months (p=.50). The IM condition received a mean (SE) of 3.8 (.63) group sessions, the SM condition received a mean (SE) of 4.6 (.68), and RM received a mean of 3.3 (1.13) group sessions.
Treatment retention
At 12 months, 127 (55.2%) of participants in the total sample were retained in their original MTP. As shown in Table 2, there were no significant differences among the three groups in rates of retention in the original program (60.6%, 54.8%, and 37.0% for the IM, SM, and RM groups, respectively; p>.05).
Table 2.
Outcomes at baseline and 4- and 12-month follow-up for total sample (N = 230)
| Variable* | Interim (n =99) | Standard (n = 104) | Restored (n = 27) | Group × Time Interaction | p |
|---|---|---|---|---|---|
| Drug and criminal activity | |||||
| Days of heroin use/last 30 days, Mean (SE) | χ2(4) = 2.9 | 0.57 | |||
| Baseline | 29.2 (0.30) | 29.1 (0.38) | 29.1 (0.51) | ||
| 4-month | 2.6 (0.53) | 3.7 (0.83) | 2.8 (1.0) | ||
| 12-Month | 4.4 (0.98) | 6.2 (1.2) | 6.9 (2.4) | ||
| Days of cocaine use/last 30 days, Mean (SE) | χ2(4) = 3.9 | 0.42 | |||
| Baseline | 5.4 (0.83) | 6.2 (0.97) | 6.0 (1.8) | ||
| 4-month | 1.6 (0.38) | 2.8 (0.72) | 1.4 (0.78) | ||
| 12-Month | 1.8 (0.62) | 2.9 (0.74) | 1.0 (0.82) | ||
| Days of illegal activity/last 30 days, Mean (SE) | χ2(4) = 3.6 | 0.46 | |||
| Baseline | 10.4 (1.3) | 9.4 (1.2) | 10.2 (2.4) | ||
| 4-month | .48 (0.29) | 1.1 (0.47) | 1.1 (1.1) | ||
| 12-Month | .96 (0.47) | 2.0 (0.66) | 1.3 (1.2) | ||
| Illegal income/last 30 days, Mean (SE) | χ2(4) = 34.6 | <0.001 | |||
| Baseline | 657 (127) | 475 (106) | 443 (148) | ||
| 4-month | 9 (3) | 341 (288) | 114 (113) | ||
| 12-Month | 27 (12) | 55 (19) | 14 (12) | ||
| Money spent on drugs/last 30 days, Mean (SE) | χ2(4) = 4.8 | 0.31 | |||
| Baseline | 962 (100) | 927 (72) | 1256 (127) | ||
| 4-month | 58 (10) | 80 (21) | 55 (16) | ||
| 12-month | 89 (33) | 132 (29) | 188 (71) | ||
| Opiate positive test, Mean (SE) | χ2(4) = 1.0 | 0.91 | |||
| Baseline | 0.97 (0.02) | 0.98 (0.01) | 0.96 (0.04) | ||
| 4-month | 0.46 (0.05) | 0.52 (0.05) | 0.40 (0.10) | ||
| 12-Month | 0.46 (0.05) | 0.48 (0.05) | 0.51 (0.11) | ||
| Cocaine positive test, Mean (SE) | χ2(4) = 5.6 | 0.23 | |||
| Baseline | 0.49 (0.05) | 0.54 (0.05) | 0.37 (0.09) | ||
| 4-Month | 0.33 (0.05) | 0.44 (0.05) | 0.23 (0.09) | ||
| 12-Month | 0.39 (0.05) | 0.36 (0.05) | 0.32 (0.10) | ||
| Benzodiazepine positive test (Mean (SE) | χ2(4) = 1.4 | 0.85 | |||
| Baseline | 0.19 (0.04) | 0.22 (0.4) | 0.26 (0.08) | ||
| 4-month | 0.16 (0.04) | (0.20 (0.04) | 0.16 (0.08) | ||
| 12-Month | 0.17 (0.04) | 0.15 (0.04) | 0.23 (0.09) | ||
| ASI Composite | |||||
| Medical, Mean (SE) | F(4, 402) = 0.75 | 0.56 | |||
| Baseline | 0.09 (0.02) | 0.08 (0.02) | 0.08 (0.05) | ||
| 4-month | 0.013 (0.03) | 0.10 (0.03) | 0.19 (0.06) | ||
| 12-month | 0.19 (0.03) | 0.12 (0.03) | 0.12 (0.06) | ||
| Employment, Mean (SE) | F(4, 397) = 0.76 | 0.55 | |||
| Baseline | 0.84 (0.03) | 0.74 (0.02) | 0.66 (0.05) | ||
| 4-month | 0.86 (0.03) | 0.79 (0.03) | 0.57 (0.05) | ||
| 12-month | 0.86 (0.03) | 0.76 (0.03) | 0.62 (0.06) | ||
| Alcohol, Mean (SE) | F(4, 369) =0.96 | 0.43 | |||
| Baseline | 0.06 (0.01) | 0.06 (0.01) | 0.04 (0.02) | ||
| 4-month | 0.05 (0.01) | 0.04 (0.01) | 0.01 (0.02) | ||
| 12-month | 0.08 (0.01) | 0.05 (0.01) | 0.00 (0.03) | ||
| Drug, Mean (SE) | F(4, 344) = 0.83 | 0.51 | |||
| Baseline | 0.35 (0.01) | 0.35 (0.01) | 0.34 (0.01) | ||
| 4-month | 0.08 (0.01) | 0.10 (0.01) | 0.11 (0.02) | ||
| 12-month | 0.08 (0.01) | 0.10 (0.01) | 0.12 (0.03) | ||
| Legal, Mean (SE) | F(4, 511) = 0.48 | 0.75 | |||
| Baseline | 0.24 (0.03) | 0.25 (0.03) | 0.20 (0.05) | ||
| 4-month | 0.03 (0.01) | 0.06 (0.01) | 0.01 (0.02) | ||
| 12-month | 0.04 (0.02) | 0.07 (0.02) | 0.07 (0.03) | ||
| Family/Social, Mean (SE) | F(4, 468) = 0.44 | 0.78 | |||
| Baseline | 0.04 (0.01) | 0.04 (0.01) | 0.06 (0.02) | ||
| 4-month | 0.02 (0.01) | 0.02 (0.01) | 0.02 (0.02) | ||
| 12-month | 0.02 (0.01) | 0.02 (0.01) | 0.01 (0.02) | ||
| Psychiatric, Mean (SE) | F(4, 373) = 0.67 | 0.61 | |||
| Baseline | 0.04 (0.01) | 0.03 (0.01) | 0.01 (0.02) | ||
| 4-month | 0.05 (0.01) | 0.02 (0.01) | 0.01 (0.02) | ||
| 12-month | 0.06 (0.02) | 0.06 (0.02) | 0.02 (.03) | ||
| Retention in Original Treatment Program | χ2(2) | P | |||
| 4 month, Mean (%) | 91 (91.9) | 84 (80.8) | 24 (88.9) | 5.6 | 0.06 |
| 12 month, Mean (%) | 60 (60.6) | 57 (54.8) | 10 (37.0) | 4.8 | 0.09 |
Note: Due to missing urine samples, the ns for opioid, cocaine, and benzodiazepine urine tests are as follows: At Baseline, n = 228 (2 missing); at 4-Month follow-up, n = 214 (2 missing); and at 12-Month follow-up, n = 200 (10 missing).
Serious adverse events
Serious adverse events were defined for this study by the FRI IRB as including hospitalization (regardless of study-relatedness) and death. As this was a study of counseling level and not of medication, non-serious adverse events were not systematically recorded. SAEs are shown in Table 3 divided by those which occurred during the first 4 months of the study when no counseling was provided to the IM group and the subsequent 8 months during which all participants had counseling. As reported elsewhere,21 there were 18 SAEs – all hospitalizations – during the first four months of the study of which, 11 were in the IM, 6 in the SM and 1 in the RM conditions, respectively. In the subsequent 8 months of follow-up, there were a total of 22 SAEs (12 in IM, 6 in SM and 4 in RM conditions). None of these SAEs were considered definitely, probably or possibly study-related to counseling condition by the IRB or the study's external medical reviewer. Two of the 22 SAEs were deaths (gastric hemorrhage and pneumonia) that were considered unrelated to the study. Both of the deaths occurred while in treatment during the 11th month post-baseline in the group that originally started in the IM condition.
Table 3.
Total Number of Serious Adverse Events (on an intent-to-treat basis)
| Specific type of SAE | Interim (n = 99) | Standard (n = 104) | Restored (n = 27) |
|---|---|---|---|
| 1. During IM Phase of Study (First 120 Days) | |||
| Accident (car accident, assault, fall) | 1 | 1 | 0 |
| Cardio-vascular (Stroke?) | 0 | 1 | 0 |
| Gastrointestinal (hepatitis) | 1 | 0 | 0 |
| Genito-urital (kidney stones) | 0 | 1 | 0 |
| Muscular-skeletal (muscle strain) | 1 | 0 | 0 |
| Respiratory (asthma) | 2 | 0 | 0 |
| Infection (cellulitis) | 2 | 2 | 0 |
| Neurological (seizure) | 1 | 0 | 1 |
| Other (cyst, dehydration, breast cancer) | 3 | 1 | 0 |
| Total | 11 | 6 | 1 |
| 2. During Second Phase of Study (Days 121-365) | |||
| Accident (car accident, assault, fall) | 0 | 1 | 1 |
| Cardio-vascular (angina, hypertension) | 2 | 1 | 0 |
| Detoxification (benzodiazepines) | 1 | 0 | 0 |
| Gastrointestinal (constipation, hepatitis, bleeding, ulcers) | 2a | 1 | 0 |
| Genito-urital (uterine fibroids, kidney stones) | 0 | 2 | 1 |
| Muscular-skeletal (arthritis, muscle strain) | 1 | 0 | 0 |
| Respiratory (asthma, bronchitis, emphysema) | 2 | 0 | 1 |
| Infection (cellulitis, pneumonia) | 2b | 0 | 0 |
| Childbirth (and complications) | 1 | 1 | 1 |
| Neurological (seizure, stroke?) | 1 | 0 | 0 |
| Total | 12 | 6 | 4 |
Note: There was a total of 40 SAEs. Thirty-two participants reported at least one SAE, 8 of whom reported 2 SAEs each. The number of participants experiencing at least one SAE in the IM, SM and RM conditions was 19, 9 and 4, respectively.
Death from gastric hemorrhage at day 304 post-enrollment
Death from pneumonia at day 334 post-enrollment
Arrests
There was no significant Group main effect (p > 0.6) with regard to whether the participants had been arrested during the 12 months from study entry to final follow-up. The percentage of the sample arrested during the one year period was 30.6%.
Treatment outcomes
Participant outcomes data reported at baseline, 4-, and 12-month follow are shown in Table 2.
ASI Composite scores
There were no significant Treatment Group X Time interactions for any Composite scores (ps>0.05). Time main effects showed statistically significant changes in the Drug (p<0.001), Legal (p<0.001) and Family/Social (p=0.013) Composite scores, all of which decreased from baseline to 12 month follow-up.
Drug use outcomes
Heroin
There were significant Time main effects in both self-reported heroin use (p<0.001) and opioid-positive drug tests (p<0.001) showing marked reductions from baseline to 12 month follow-up. There were no significant Treatment Group X Time interaction effects or Treatment Group main effects in self-reported days of heroin use or in the percentage of participants with opioid-positive drug tests (ps>0.05).
Cocaine
Significant Time main effects were found in both self-reported cocaine use (p<0.001) and cocaine-positive drug tests (p<0.003) showing a reduction from baseline to 12 month follow-up, albeit less striking than the reduction in opiate use. There were no significant Treatment Group X Time interaction effects or Treatment Group main effects in self-reported cocaine use or in the percentage of participants with cocaine-positive drug tests (ps>0.05) or with benzodiazepine-positive drug tests (p>0.05).
Criminal behavior
Significant Time main effects were found in criminal activity, money spent on drugs, and illegal income (ps<0.001) with reductions from baseline to 12 month follow-up. Of these three criminal behavior outcome measures, only self-reported illegal income showed significant Treatment Group X Time interaction effects (p<0.001).
Status of those not in treatment
Of the 103 participants who were no longer in the original MTP, at the 12-month follow-up, 29 reported being enrolled in another drug treatment program. Of these 29, 24 were in another MTP (including 13 who had been transferred seamlessly to another MTP and 11 who had been discharged from the original program and re-entered another MTP), 1 entered buprenorphine treatment, 2 entered a residential program, 1 entered a drug-free outpatient program, and 1 entered a transitional recovery house. Therefore, in one sense, about 67% of the original sample was in treatment at 12-month follow-up.
DISCUSSION
Despite a significant treatment discontinuation rate, on an intent-to-treat basis, all groups in this study, showed significant improvements in the primary outcome measure (opioid positive urine tests) from baseline to 12 months. Although one group (IM) received only emergency counseling during the first 4 months of treatment, there were no statistically significant Group x Time differences. The high participant follow-up rate (96.1% ascertained and 91.3% interviewed) lends confidence to a conclusion that the findings were reflective of the fact that at 12 months there were no differences in the study sample among the three treatment groups.
In these two MTPs, the level of counseling provided was generally low. More frequent or more structured individual counseling (such as cognitive-behavioral therapy) may have had greater impact.23 Since counselors vary in their effectiveness, it is possible that while some counselors provided beneficial effects, these were largely offset by the minimal or even negative impact of others.24 It may also be the case that until their lives become more stable, newly-admitted heroin users may not be ready to benefit from counseling or that methadone itself has such powerful impact on the use of illicit heroin, that additional improvements with low levels of counseling could not be shown. Lastly, since most participants had been in MTPs previously they may have been relatively refractory to counseling or believed that an additional counseling experience had little new to offer.
These findings showing no significant differences between IM and SM are in contrast to the widely cited paper by McLellan et al.15 that found that male veterans randomly assigned to methadone with emergency counseling only had higher rates of opiate and cocaine positive urine tests as compared to standard methadone or enhanced methadone treatment accompanied by the availability of medical/psychiatric treatment, employment counseling and family therapy. That study was conducted in a well-staffed program with masters’ level counselors and was conducted among male veterans and hence might not be generalizable to a more heterogeneous community sample such as in our study. The mean methadone dose was not reported and although the target dose (60mg - 90mg) was reported, it is uncertain whether a lower dose of methadone in that study may have made it possible to see an effect of counseling.
In the present study, the IM group did as well as the SM group, despite not being permitted by US regulations to have the incentive to earn take-home doses contingent on negative drug tests during the first 4 months of IM, an incentive that has been proven to increase negative drug test results.25 The average methadone dose for the IM group was significantly greater than the dose for the group that received counseling at both the 4 and 12-month follow-up. We have no data-based explanation for this difference, although it may have been related to the ability of IM participants to request a dose increase directly through nursing rather than through their counselors.
Serious adverse events
Heroin-dependent individuals have high levels of serious health problems and are frequent users of inpatient hospitalization.26, 27 This was found to be the case in the present study. Among its 230 participants, there were a total of 40 SAEs during the 12 months of the study, including 38 hospitalizations and 2 deaths. None of the SAEs were judged by the IRB and an independent medical reviewer to be study related and, as described above, they were of the usual type of events that might occur in this population (see Table 3). Even for patients who remained in treatment for the entire year, there were a number of SAEs. Although these SAEs were unrelated to the study, some might have been prevented with better access to primary care.
The present study's findings with respect to drug counseling and MTPs could be limited to Baltimore or to this specific population within Baltimore. It is also possible that the level of services (e.g., one counseling session every other week) was too low or of the wrong type or quality. Although our findings may be atypical, they are congruent with those of other studies12-14 where patients who were randomly assigned to limited or virtually no counseling fared as well as those receiving counseling as usual in methadone treatment.
This study's results have implications for jurisdictions where regulations and accrediting guidelines require documenting that counseling has been provided. Currently, in the US programs may feel obliged to extrude those who are non adherent with respect to counseling regimens and to deny treatment to those who cannot pay for the bundled services.28 In Baltimore and elsewhere, it is not uncommon for patients to be involuntarily discharged from MTPs for continued drug use, non-adherence to scheduled counseling sessions, or non-payment of nominal fees. While some posit that discharging poorly performing patients may be better for the clinic as a whole, 29 this practice is not recommended by the World Health Organization8 and it has been shown to be potentially harmful to the discharged patient. 30,31
During the course of the study, the participating MTPs had to suspend new admissions to their programs for many weeks in response to counselor shortages in order to avoid caseloads, which would exceed state and federal regulations. The study's findings, if generalizable, suggest that limited availability of scheduled drug counseling services should not be a barrier to providing supervised methadone to those dependent on heroin—at least for the first 4 months. In the US, removing these barriers may require a change in regulations or accreditation standards. Whether methadone with only the availability of emergency counseling would be effective over a longer period is not answerable with these data. However, more research is needed, since budgetary pressures are likely to increase the focus on which components of addictions treatments contribute significantly to treatment outcomes.
Acknowledgements
We thank Dr. John Urbaitis and Suzanne Harrison at the Sinai Hospital Addiction Recovery Program, Dr. Eric Weintraub, Jewell Benford, and Wayne Clemons at the University of Maryland Drug Treatment Center and Mr. Robert Embry of the Abell Foundation. Robert P. Schwartz, M.D., the research study's Principal Investigator, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding/Support: This research was supported by NIDA grant 2R01 DA 13636 entitled “Entry into Comprehensive Methadone Treatment via Interim Opioid Maintenance” and by the Abell Foundation which supported the counselor in the Restored condition.
Footnotes
Conflict of Interest Declaration: None
Conflict of Interest and Financial Disclosure
The authors report no conflict of interest or financial interest regarding this study.
Contributor Information
Robert P. Schwartz, Friends Research Institute, Inc. 1040 Park Avenue, Suite 103 Baltimore, MD 21201
Sharon M. Kelly, Friends Research Institute, Inc. 1040 Park Avenue, Suite 103 Baltimore, MD 21201
Kevin E. O'Grady, University of Maryland, 1147 Biology/Psychology Bldg. College Park, MD 20742
Devang Gandhi, University of Maryland School of Medicine, 110 S. Paca Street, Baltimore, MD 21201
Jerome H. Jaffe, Friends Research Institute, 1040 Park Avenue, Suite 103 Baltimore, MD 21201 University of Maryland School of Medicine, 110. S. Paca Street, Baltimore, MD 21201.
REFERENCES
- 1.World Health Organization . WHO Prisons and Health Facts and Figures. WHO; Denmark: 2011. [April 19, 2011]. Available at: http://www.euro.who.int/en/what-we-do/health-topics/health-determinants/prisons-and-health/facts-and-figures (archived by Webcite at http://www.webcitation.org/5yG7d9Dnt) [Google Scholar]
- 2.Clausen T, Anchersen K, Waal H. Mortality prior to, during and after opioid maintenance treatment (OMT): a national prospective cross-registry study. Drug Alcohol Depend. 2008;94:151–157. doi: 10.1016/j.drugalcdep.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Degenhardt L, Randall D, Hall W, Law M, Butler T, Burns L. Mortality among clients of a state-wide opioid pharmacotherapy program over 20 years: risk factors and lives saved. Drug Alcohol Depend. 2009;105:9–15. doi: 10.1016/j.drugalcdep.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 4.Gibson A, Degenhardt L, Mattick RP, Ali R, White J, O'Brien S. Exposure to opioid maintenance treatment reduces long-term mortality. Addiction. 2008;103:462–468. doi: 10.1111/j.1360-0443.2007.02090.x. [DOI] [PubMed] [Google Scholar]
- 5.Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009;(3):CD002209. doi: 10.1002/14651858.CD002209.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gossop M, Grant M. A six country survey of the content and structure of heroin treatment programmes using methadone. Br J Addict. 1991;86:1151–1160. doi: 10.1111/j.1360-0443.1991.tb01883.x. [DOI] [PubMed] [Google Scholar]
- 7.European Monitoring Centre for Drugs and Drug Addiction . Annual Report: The State of the Drugs Problem in Europe. EMCDDA; Portugal: 2010. [April 7, 2011]. Available at: http://www.emcdda.europa.eu/online/annual-report/2010 (archived by Webcite at http://www.webcitation.org/5yG72bp1G) [Google Scholar]
- 8.World Health Organization . Guidelines for the Psychosocially Assisted Pharmacological Treatment of Opioid Dependence. World Health Organization Press; Geneva, Switzerland: 2009. [PubMed] [Google Scholar]
- 9.Institute of Medicine . In: Federal Regulation of Methadone Treatment. Retting RA, Yarmolinsky A, editors. National Academy Press; Washington, DC: 1995. [PubMed] [Google Scholar]
- 10.Farrell M, Ward J, Mattick R, Hall W, Stimson GV, des Jarlais D, et al. Methadone maintenance treatment in opiate dependence: a review. BMJ. 1994;309:997–1001. doi: 10.1136/bmj.309.6960.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drug Abuse Treatment Act . Drug Addiction Treatment Act of 2000, SEC. 3502. Amendment to Controlled Substance Act. US Department of Health and Human Services; Rockville, MD: 2000. [Google Scholar]
- 12.Calsyn DA, Wells EA, Saxon AJ, Jackson TR, Wrede AF, Stanton V, et al. Contingency management of urinalysis results and intensity of counseling services have an interactive impact on methadone maintenance treatment outcome. J Addict Dis. 1994;13:47–63. doi: 10.1300/j069v13n03_05. [DOI] [PubMed] [Google Scholar]
- 13.Gruber VA, Delucchi KL, Kielstein A, Batki SL. A randomized trial of 6-month methadone maintenance with standard or minimal counseling versus 21-day methadone detoxification. Drug Alcohol Depend. 2008;94:199–206. doi: 10.1016/j.drugalcdep.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Senay EC, Jaffe JH, diMenza S, Renault PF. A 48-week study of methadone, methadyl acetate and minimal services. In: Fisher S, Freeman A, editors. Opiate Dependence: Origins and Treatment. Halstead Press; New York, NY: 1973. pp. 185–201. [Google Scholar]
- 15.McLellan AT, Arndt IO, Metzger DS, Woody GE, O'Brien CP. The effects of psychosocial services in substance abuse treatment. JAMA. 1993;269:1953–1959. [PubMed] [Google Scholar]
- 16.Yancovitz SR, Des Jarlais DC, Peyser NP, Drew E, Friedmann P, Trigg HL, et al. A randomized trial of an interim methadone maintenance clinic. Am J Public Health. 1991;81:1185–1191. doi: 10.2105/ajph.81.9.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Federal Register Title 21, Codified at 58 CFR §495. 1993 pt 291. U.S.A.
- 18.Schwartz RP, Highfield DA, Jaffe JH, Brady JV, Butler CB, Rouse CO, et al. A randomized controlled trial of interim methadone maintenance. Arch Gen Psychiatry. 2006;63:102–109. doi: 10.1001/archpsyc.63.1.102. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz RP, Jaffe JH, Highfield DA, Callaman JM, O'Grady KE. A randomized controlled trial of interim methadone maintenance: 10-Month follow-up. Drug Alcohol Depend. 2007;86:30–36. doi: 10.1016/j.drugalcdep.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 20.Amato L, Minozzi S, Davoli M, Vecchi S, Ferri MM, Mayet S. Psychosocial combined with agonist maintenance treatments versus agonist maintenance treatments alone for treatment of opioid dependence. Cochrane Database Syst Rev. 2008;(4):CD004147. doi: 10.1002/14651858.CD004147.pub2. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz RP, Kelly SM, O'Grady KE, Gandhi D, Jaffe JH. Interim methadone treatment compared to standard methadone treatment: 4-Month findings. J Subst Abuse Treat. 2011 doi: 10.1016/j.jsat.2011.01.008. [doi:278810.271016/j.physletb.272003.278810.278071] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, et al. The fifth edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 23.Carroll KM, Rounsaville BJ, Nich C, Gordon LT, Wirtz PW, Gawin F. One-year follow-up of psychotherapy and pharmacotherapy for cocaine dependence. Delayed emergence of psychotherapy effects. Arch Gen Psychiatry. 1994;51:989–997. doi: 10.1001/archpsyc.1994.03950120061010. [DOI] [PubMed] [Google Scholar]
- 24.McLellan AT, Woody GE, Luborsky L, Goehl L. Is the counselor an “active ingredient” in substance abuse rehabilitation? An examination of treatment success among four counselors. J Nerv Ment Dis. 1988;176:423–430. doi: 10.1097/00005053-198807000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Stitzer ML, Iguchi MY, Felch LJ. Contingent take-home incentive: effects on drug use of methadone maintenance patients. J Consult Clin Psychol. 1992;60:927–934. doi: 10.1037//0022-006x.60.6.927. [DOI] [PubMed] [Google Scholar]
- 26.McCarty D, Perrin NA, Green CA, Polen MR, Leo MC, Lynch F. Methadone maintenance and the cost and utilization of health care among individuals dependent on opioids in a commercial health plan. Drug Alcohol Depend. 2010;111:235–240. doi: 10.1016/j.drugalcdep.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petry NM, Roll JM, Rounsaville BJ, Ball SA, Stitzer M, Peirce JM, et al. Serious adverse events in randomized psychosocial treatment studies: safety or arbitrary edicts? J Consult Clin Psychol. 2008;76:1076–1082. doi: 10.1037/a0013679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reisinger HS, Schwartz RP, Mitchell SG, Peterson JA, Kelly SM, O'Grady KE, et al. Premature discharge from methadone treatment: patient perspectives. J Psychoactive Drugs. 2009;41:285–296. doi: 10.1080/02791072.2009.10400539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Center for Substance Abuse Treatment . Medication-Assisted Treatment for Opioid Addiction in Opioid Treatment Programs. Substance Abuse and Mental Health Services; Rockville, MD: 2005. [Treatment Improvement Protocol (TIP) Series 43. DHHS Publication No. (SMA) 05-4048].
- 30.Woody GE, Kane V, Lewis K, Thompson R. Premature deaths after discharge from methadone maintenance: a replication. J Addict Med. 2007;1:180–185. doi: 10.1097/ADM.0b013e318155980e. [DOI] [PubMed] [Google Scholar]
- 31.Zanis DA, Woody GE. One-year mortality rates following methadone treatment discharge. Drug Alcohol Depend. 1998;52:257–260. doi: 10.1016/s0376-8716(98)00097-0. [DOI] [PubMed] [Google Scholar]