Abstract
Sugars and fats elicit innate and learned flavor preferences with the latter mediated by flavor-flavor (orosensory) and flavor-nutrient (post-ingestive) processes. Systemic dopamine (DA) D1 (SCH23390: SCH) and D2 (raclopride: RAC), but not opioid antagonists blocked the acquisition and expression of flavor-flavor preferences conditioned by sugars. In addition, systemic D1, but not D2 or opioid antagonists blocked the acquisition of flavor-nutrient preferences conditioned by intragastric (IG) sugar infusions. Given that DA antagonists reduce fat intake, the present study examined whether systemic D1 or D2 antagonists altered the acquisition and/or expression of conditioned flavor preferences (CFP) produced by pairing one novel flavor (CS+, e.g., cherry) with a 3.5% corn oil (CO: fat) solution relative to another flavor (CS−, e.g., grape) paired with a 0.9% CO solution. In an expression study, food-restricted rats were trained to drink either flavored 3.5% or 0.9% CO solutions on alternate days. Subsequent two-bottle tests with the CS+ and CS− flavors mixed in 0.9% CO solutions occurred 0.5 h after systemic administration of vehicle (VEH), SCH (50–800 nmol/kg) or RAC (50–800 nmol/kg). The rats displayed a robust CS+ preference following VEH treatment (87–88%) the expression of which was attenuated by treatment with moderate doses of RAC, and to a lesser degree, SCH. In an acquisition study, six groups of rats received VEH, SCH (25, 50, 200 nmol/kg) or RAC (50, 200 nmol/kg) 0.5 h prior to one-bottle training trials with CS+ flavored 3.5% and CS− flavored 0.9% (CS−) CO solutions. A seventh Limited VEH group was trained with its training intakes limited to that of the SCH and RAC groups. Subsequent two-bottle tests were conducted with the CS+ and CS− flavors presented in 0.9% CO without injections. Significant and persistent CS+ preferences were observed in VEH (75–82%), Limited VEH (70–88%), SCH25 (75–84%), SCH50 (64–87%), SCH200 (78–91%) and RAC200 (74–91%) groups. In contrast, the group trained with RAC50 displayed a significant initial CS+ preference (76%) which declined over testing to 61%. These data indicate limited DA D1 and D2 receptor signaling involvement in the expression and acquisition of a fat-CFP relative to previous robust effects for sugar-CFP.
Keywords: Corn Oil, SCH23390, Raclopride, Learning
Introduction
Fats and sugars are both recognized as contributing to the palatability of foods, overeating, and diet-induced obesity. In addition to the inherent hedonic properties associated with these nutrients, learning plays an important role in the preferences for fat- and sugar-rich foods (Sclafani, 1999). Preferences are based in part on learned associations between the various flavor elements in foods, flavor–flavor conditioning, and between flavor cues and postingestive consequences, flavor–nutrient conditioning. Animal research on conditioned flavor preferences (CFP) has focused almost exclusively on sugar and sweet taste, and the putative underlying roles of dopamine (DA) and opioid systems mediating its effects (Azzara et al., 2000, 2001; Baker et al., 2003, 2004; Holman, 1975; Hsiao and Smith, 1995; Yu et al., 1999, 2000a, 2000b). In this regard, flavor-flavor conditioning studies using either sucrose in sham-feeding rats (Yu et al., 1999, 2000a, 2000b) or fructose in real-feeding rats (Baker et al., 2003, 2004), identified a critical role for DA D1 and D2, but not opioid, receptor signaling in the acquisition and expression of these preferences. In contrast, flavor-nutrient conditioning studies using intragastric (IG) sucrose infusions (Azzara et al., 2000, 2001), identified a critical role for DA D1, but not D2 or opioid receptor signaling in the acquisition, and to a lesser degree, the expression of this preference. In addition, the acquisition of IG glucose flavor-nutrient preferences was blocked by DA D1 antagonism into the nucleus accumbens (NAc: Touzani et al., 2008), amygdala (AMY: Touzani et al., 2009) and medial prefrontal cortex (mPFC: Touzani et al., 2010a), indicating the existence of a distributed brain network mediating this response (see review: Touzani et al., 2010b). DA D1 and D2 antagonism in the NAc and AMY reduced, but did not eliminate the expression of fructose-conditioned flavor-flavor CFP. The acquisition of a fructose-CFP was completely blocked by DA D1 and D2 antagonism into the mPFC (Malkusz et al., 2010), but DA D1 and D2 antagonism in the NAc or AMY only hastened the extinction of these preferences (Bernal et al., 2008, 2009). In contrast, naltrexone administered into either the shell or core regions of the NAc failed to alter the expression of either fructose-conditioned flavor-flavor or IG glucose-conditioned flavor-nutrient CFP (Bernal et al., 2010).
Rodents are attracted to the flavor of fat (e.g., corn oil) and nonnutritive fat substitutes (e.g., mineral oil, sefose) from an early age which may be mediated in part by taste receptors for fatty acids (Ackroff & Sclafani, 2009; Passilly-Degrace et al., 2009). In addition, both the postingestive actions and orosensory properties of fat are rewarding and condition a CS+ flavor preference (Sclafani, 1999; Ackroff & Sclafani, 2009). DA mediation of the rewarding effect of fat flavor is suggested by the findings that corn oil sham-feeding promotes NAc DA release (Liang et al., 2006), and DA D1 and D2 antagonists suppress the sham-feeding response to corn oil and real-feeding of fats in rats (Baker et al., 2001; Davis et al., 2006; Rao et al., 2008; Weatherford et al., 1988; 1990). In inbred mice, strain differences were observed in the ability of the D1 antagonist, SCH23390 to significantly reduce fat intake whereas the D2 antagonist, raclopride, had minimal effects on fat intake (Dym et al., 2010). Furthermore, DA D2, but not D1 receptor antagonism suppressed operant responding for corn oil in mice (Yoneda et al., 2007), whereas D1, but not D2 receptor antagonism attenuated place preference conditioning by corn oil intake (Imaizumi et al., 2000).
The present study examined whether systemic administration of DA D1 and D2 receptor antagonists (SCH23390 and raclopride, respectively) would attenuate flavor preference conditioning by corn oil ingestion. The first experiment revealed that a flavor (e.g., cherry) paired with a higher (3.5%) concentration of a corn oil (CO: fat) solution conditioned a flavor preference relative to a flavor (e.g., grape) paired with a lower (0.9%) concentration of a CO solution. Consequently we used this paradigm to investigate the effects of DA receptor antagonism on corn oil-CFP. To this end, SCH22390 or raclopride, at different doses, was injected systemically either prior to testing (expression) or training (acquisition) sessions. Based on the observed reductions in corn oil sham-feeding produced by both SCH23390 and raclopride (Weatherford et al., 1988; 1990), we expected that both DA D1 and D2 receptors antagonists would impair both the acquisition and expression of corn oil-CFP.
Methods
Subjects
Male Sprague-Dawley rats (260–300 g, Charles River Laboratories, Wilmington, MA) were housed individually in wire mesh cages and maintained on a 12:12 h light/dark cycle with chow (5001, PMI Nutrition International, Brentwood, MO) and water available ad libitum, except as noted below. The experimental protocols were approved by the Queens College Institutional Animal Care and Use Committee (Protocol 69) certifying that all subjects and procedures are in compliance with the National Institutes of Health Guide for Care and Use of Laboratory Animals.
Test Solutions
The training fluids consisted of 3.5% and 0.9% corn oil (CO: Sigma Chemical Co., St. Louis, MO) flavored with 0.05% unsweetened grape or cherry Kool-Aid (General Foods, White Plains, NY) and prepared as suspensions using 0.3% xanthan gum (Sigma). These concentrations are isocaloric to 8% and 2% sugar solutions used in another flavor conditioning study (Sclafani and Ackroff, unpublished). Half of the rats in each group had the cherry flavor added to the 3.5% CO and the grape flavor added to the 0.9% CO; the flavors were reversed for the remaining rats. In the two-bottle preference tests, the cherry and grape flavors were each presented in 0.9% CO. The CO + Kool-Aid + gum mixtures are hereafter referred to as solutions. The flavored training solutions are referred to as the CS+/3.5% CO and CS−/0.9% CO. The flavored 0.9% solutions used in the two bottle tests are referred to as CS+ and CS−. All testing took place in the rat’s home cage during the mid-light phase of the light:dark cycle. Two weeks before testing began, the rats were placed on a food restriction schedule that maintained their body weights at 85–90% of their ad libitum level. The rats were initially trained to drink an unflavored 0.2% saccharin solution from sipper tubes during daily 2-h sessions. The sipper tube was mounted on the front of the cage held by a taut steel spring, and was positioned 3–6 cm above the cage floor. This training procedure was repeated daily until all rats approached the sipper tubes with short (< 1 min) latency, typically within three days. The limited food rations were given 30 min after each training session.
Experiment 1
Eight naïve male rats were given ten 1-bottle training sessions (2 h/day) with 24 ml of the CS+/3.5% CO solution presented on odd-numbered days, and 24 ml of the CS−/0.9% CO solution presented on even-numbered days. On days 9 and 10, the rats had access to a second sipper tube containing water. This familiarized the rats to the presence of two sipper tubes used during the choice tests; water intake was negligible in these training trials. Training intakes were limited to 24 ml/session to minimize the difference between CS+/3.5% CO and CS−/0.9% CO intakes. The left-right position of the CS and water sipper tubes was counterbalanced over the two days. Following training, the rats were given eight two-bottle choice test sessions (2 h/day) with unlimited access to the CS+ vs. CS− solutions. Intakes during training and testing in this and all subsequent experiments were measured by weighing (0.1 g) the bottles before and after the 2 h sessions. Because some of the effects could be potentially small, care was also taken to minimize spillage. After initially weighing each bottle, it was gently shaken to insure appropriate flow of the viscous corn oil solutions. Any effluent from the bottle (~ 0.5–1.0 g) was collected and appropriate spillage adjustments were made to obtain an accurate pre-weight measurement. The sipper tube was occluded when the bottles were placed onto the cage and subsequently removed. The taut steel spring prevented movement of the bottles during the sessions. Visual inspection of the bottles during the study revealed minimal if any spillage because of the viscosity of the solutions. The session length of 2 h was identical to that previously used in assessing fructose-CFP (Baker et al., 2003, 2004).
Experiment 2
Expression Procedure
Twenty-two naïve male rats received the identical training procedure described above with the following modifications. Following training, all rats were given ten daily two-bottle choice test sessions (2 h/day) with the CS+ and CS− solutions. Thirty min prior to the two-bottle test sessions, all rats were given vehicle (1 ml 0.9% saline/kg body weight, sc) injections for the first two sessions. Two groups of 11 rats matched for their CS+ preference under vehicle injections received sc treatment with four doses (50, 200, 400 and 800 nmol/kg) of SCH23390 or raclopride prior to two-bottle test sessions; half of the rats were tested with an ascending dose order, and the remaining rats were tested with a descending dose order. The rats were tested in two consecutive daily sessions at each drug dose with the left-right position of the CS+ and CS− solutions counterbalanced across sessions to control for position effects. The antagonist dose range was identical to that used in our prior conditioning studies (Azzara et al., 2001; Baker et al., 2003; Yu et al., 2000a, 2000b).
Experiment 3
Acquisition Procedure
Seven groups of naïve male rats were matched for their intakes of an unflavored 0.2% saccharin solution prior to training. The rats were given ten 1-bottle training sessions (2 h/day, 24 ml) with the CS+/3.5% CO solution presented on odd-numbered sessions, and the CS−/0.9% CO solution presented on even-numbered sessions. The first group (VEH, n=8) received vehicle (1 ml 0.9% saline/ kg body weight, sc) injections 30 min prior to each training session. The second (SCH25, n=9), third (SCH50, n=8) and fourth (SCH200, n=11) groups received daily sc injections of SCH23390 at respective doses of 25, 50 and 200 nmol/kg 30 min prior to each training session. The fifth (RAC50, n=9) and sixth (RAC200, n=7) groups received daily sc injections of raclopride at respective doses of 50 and 200 nmol/kg 30 min prior to each training session. Because SCH23390 and raclopride reduce corn oil intake, the seventh group (Limited VEH) of ten rats received daily sc injections of vehicle 30 min prior to each training session, and their intakes were limited to approximate the reduced intakes observed in the drug groups. These doses were similar to those employed in acquisition studies with sucrose in sham-feeding rats and fructose in real-feeding rats (Baker et al., 2003; Yu et al., 2000a, 2000b). Following training, all seven groups were given six daily two-bottle choice sessions (2 h/day) with unlimited access to the CS+ and CS− solutions; no drugs were administered prior to these sessions. The positions of the CS+ and CS− solutions were counterbalanced across sessions.
Data analysis
In the expression study, training intakes were averaged over the five CS+/3.5% CO and five CS−/0.9% CO sessions and evaluated by t-tests. Intakes during the preference tests were averaged over the two sessions at each dose and evaluated with two-way repeated-measures analyses of variance (ANOVA, CS condition vs. Dose) for the D1 and D2 groups, respectively. Separate ANOVAs evaluated percent CS+ intakes as a function of dose for the two groups.
In the acquisition studies, training intakes were averaged over the five CS+/3.5% CO and CS−/0.9% CO sessions and were analyzed with a two-way randomized-blocks ANOVA (CS conditions x Groups). Intakes during the preference tests were averaged over sessions 1–2, 3–4, and 5–6 (referred to as Tests 1, 2, and 3) to control for side position effects. A three-way randomized-blocks ANOVA compared the CS intakes of D1, D2 and control groups (Group x CS x Test). A separate two-way ANOVA evaluated percent CS+ intakes of the groups. When main or interaction effects were found, Bonferroni corrected comparisons (p<0.05) detected significant effects.
Results
Experiment 1
CO-CFP baseline study
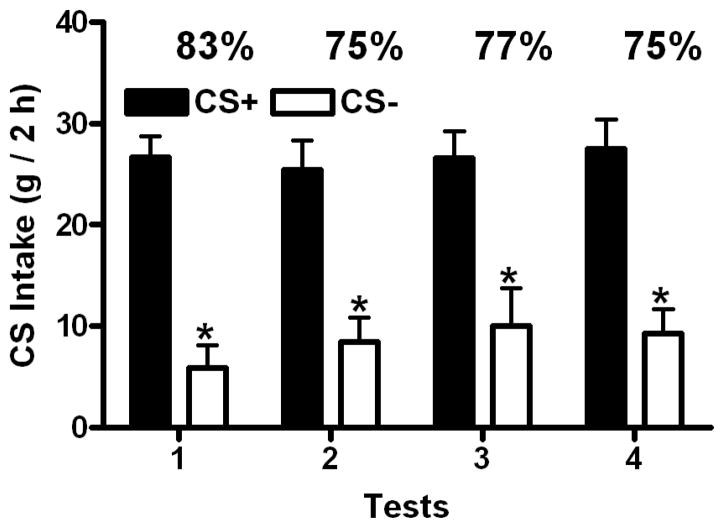
The mean 1-bottle training intake of the CS+/3 .5% CO solution (20.4 (+1.2) g) was significantly (t(1,7)= 7.77, p=0.027) higher than intake of the CS−/ 0.9% CO solution (15.7 (+1.9) g). In the two-bottle preference tests, overall, rats consumed significantly more CS+ than CS− (F(1,7)= 36.97, p<0.0001), but neither total intake or tests (F(3,21)= 1.70, ns) nor the test by CS interaction (F(3,21)= 0.44, ns) achieved significance. CS+ intakes significantly exceeded CS− intakes during all four tests, indicating a CO-CFP (Figure 1). CS+ or CS− intake failed to vary across the four tests; total intake also failed to vary across tests. Significant differences in the percent CS+/s intakes failed to be observed (F(3,21)= 0.71, ns) across the first (83%), second (75%), third (77%) and fourth (75%) tests, indicating stability of the CO-CFP preferences.
Figure 1.
(Baseline Study): Intakes (mean in g, +SEM, 2 h) of CS+ and CS− solutions in four two-bottle preference tests in untreated animals. Significant differences are denoted between CS+ and CS− intake within tests. The percentages of CS+ intake over total intake are denoted above each pair of values.
Experiment 2A. DA D1 receptor antagonism and expression of CO-CFP
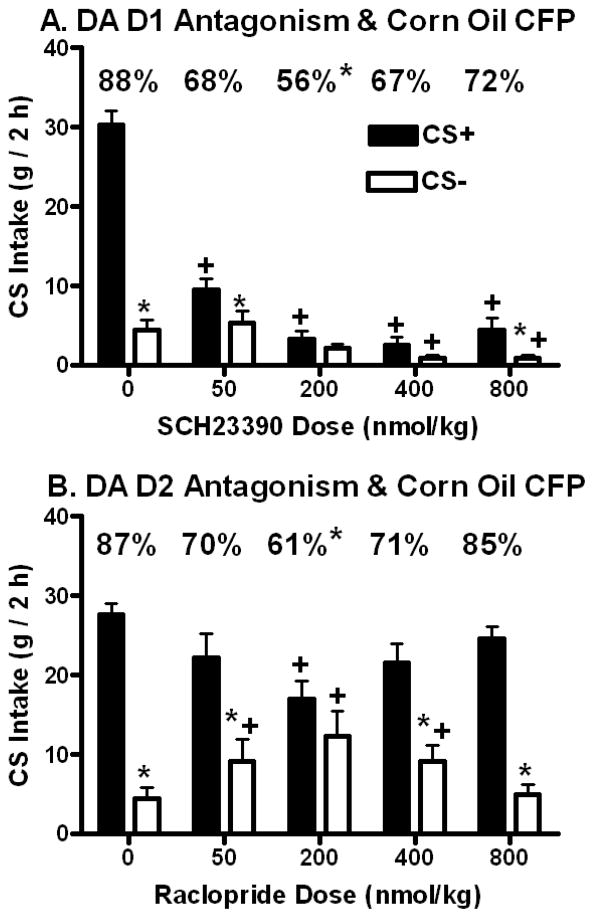
The mean 1-bottle training intakes of the CS+/3.5% CO (22.5 (+0.7) g) and CS−/0.9% CO (20.1 (+0.8) g) solutions failed to significantly (t(1,10)= 2.05, p=0.068) differ from each other. In the two-bottle choice tests, overall, rats consumed significantly more CS+ than CS− (F(1,10)= 162.95, p<0.0001); total intake significantly varied as a function of drug dose (F(4,40)= 67.78, p<0.0001), and there was a significant CS x Dose interaction (F(4,40)= 33.96, p<0.0001). CS+ intakes significantly exceeded CS− intakes at the 0 (vehicle), 50 and 800 nmol/kg doses of SCH23390, but not at the 200 or 400 nmol/kg doses (Figure 2A). Rats consumed significantly less CS+ at all SCH23390 doses as compared to vehicle, whereas CS− intakes were significantly reduced following the two highest SCH23390 doses (Figure 2A). Correspondingly, total intake significantly declined following the 50 (14.8 (+1.9) g), 200 (5.3 (+1.3) g), 400 (3.4 (+1.0) g) and 800 (5.4 (+1.6) g) nmol/kg SCH23390 doses relative to vehicle (34.7 (+2.0) g). Significant differences in the percent CS+ intakes were observed (F(4,40)= 3.13, p<0.025), and the preference (56%) at the 200 nmol/kg SCH23390 dose was significantly lower than the preference (88%) following vehicle (Figure 2A). Preferences at the 50 (68%), 400 (67%) and 800 (72%) nmol/kg SCH23390 doses were intermediate, but failed to significantly differ from the vehicle test. For these DA D1 receptor antagonist effects, issues related to low intakes might explain the failure to detect a meaningful preference.
Figure 2.
(Expression Study): Intakes (mean in g, +SEM, 2 h) of CS+ and CS− solutions in two-bottle preference tests in animals receiving systemic injections of the DA D1 antagonist, SCH23390 (Panel A) or the DA D2 antagonist, raclopride (Panel B) 30 min prior to testing. Significant differences are denoted between CS+ and CS− intake within an injection condition (*) and between CS+ or CS− intake following a drug dose relative to the corresponding vehicle treatment (+). The percentages of CS+ intake over total intake are denoted above each pair of values with significant differences relative to vehicle treatment (*) noted.
Experiment 2B. DA D2 receptor antagonism and expression of CO-CFP
The mean 1-bottle training intake of the CS+/3.5% CO solution (22.7 (+0.6) g) was significantly (t(1,10)= 36.23, p<0.0001) higher than intake of the CS−/0.9% CO solution (18.0 (+0.6) g). In the two-bottle preference tests, overall, rats consumed significantly more CS+ than CS− (F(1,10)= 51.87, p<0.0001), and significant effects were observed for the CS x Dose interaction (F(4,40)= 3.35, p<0.019), but not across drug doses (F(4,40)= 0.51, ns). CS+ intakes significantly exceeded CS− intakes at the 0 (vehicle), 50, 400 and 800 nmol/kg doses of raclopride, but not at the 200 nmol/kg dose (Figure 2B). Rats consumed significantly less CS+ at the 200 nmol/kg raclopride dose as compared to vehicle, whereas CS− intakes were significantly increased following the 50, 200 and 400 nmol/kg raclopride doses (Figure 2B, +). Total intake failed to differ across the 0 (32.0 (+1.6) g), 50 (31.2 (+1.7) g), 200 (29.2 (+1.8) g), 400 (30.7 (+1.8) g) and 800 (29.5 (+1.9) g) nmol/kg raclopride dose range. Significant differences in the percent CS+ intakes were observed (F(4,40)= 3.31, p<0.02), and the preference (61%) at the 200 nmol/kg raclopride dose was significantly lower than the preference (87%) following vehicle (Figure 2B). Preferences at the 50 (70%), 400 (71%) and 800 (85%) nmol/kg raclopride doses were intermediate, but failed to significantly differ from the vehicle test.
Experiment 3
DA D1 and D2 receptor antagonism and acquisition of CO-CFP
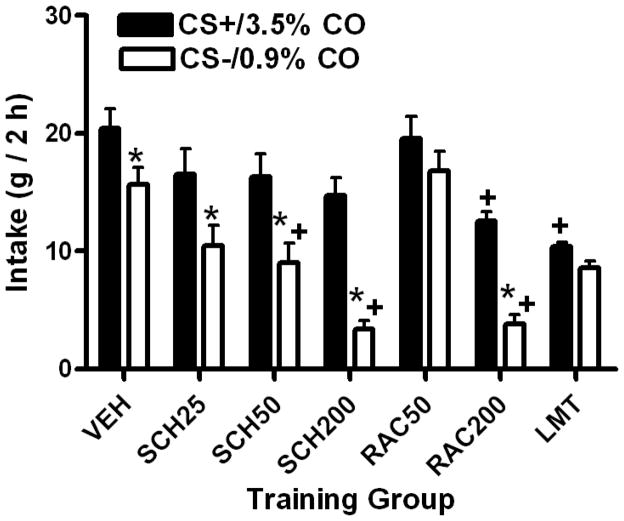
In the 1-bottle training intakes, overall CS+/3.5% CO intake (31.4 (+2.0) g) significantly (F(1,55)= 86.80, p<0.0001) exceeded CS−/0.9% CO intake (19.2 (+2.2) g), and significant differences were observed among the seven groups (F(6,55)= 12.50, p<0.0001) and for the group by CS interaction (F(6,55)= 4.20, p<0.002). Total training intakes significantly differed from VEH group (36.2 (+1.9) g) in the Limited VEH (19.0 (+0.5) g), SCH50 (25.3 (+3.3) g) and SCH200 (18.1 (+1.9) g) groups and the RAC200 (16.4 (+1.0) g) group. However in turn, these three drug groups failed to differ from the Limited VEH group in total training intakes. CS+/3.5% CO training intake was significantly greater than CS−/0.9% CO intake in VEH, SCH25, SCH50, SCH200 and RAC200 groups, but not in RAC50 or Limited VEH groups (Figure 3). The SCH groups did not differ significantly from the VEH group in their CS+/3.5% CO intakes. Animals in the RAC200 group consumed significantly less CS+/3.5% CO relative to VEH animals, but were similar to the Limited VEH rats (Figure 3). Whereas the SCH50, SCH200, and RAC200 rat consumed significantly less CS−/0.9% CO than VEH rats, they failed to differ from the Limited VEH rats (Figure 3).
Figure 3.
(Acquisition Study - Training): Training intakes (mean in g, +SEM, 2 h) of rats exposed to ten 1-bottle sessions of flavored corn oil solutions of 3.5% (CS+/3.5% CO, Days 1, 3, 5, 7, 9) or 0.9% (CS−/0.9% CO, Days 2, 4, 6, 8, 10) 30 min following systemic injections of vehicle (VEH), the DA D1 antagonist, SCH23390 at doses of 25 (S25), 50 (S50) or 200 (S200) nmol/kg or the DA D2 antagonist, raclopride at doses of 50 (R50) or 200 (R200) nmol/kg). A seventh group (LMT) received vehicle injections and had CS+/3.5% CO and CS−/0.9% CO intakes limited to approximate the intakes of the drug groups. Significant differences are denoted between CS+/3.5% CO and CS−/0.9% CO intake within an injection condition (*) and between CS+/3.5% CO or CS−/0.9% CO intake following a drug dose relative to VEH treatment (+).
In the two-bottle preference tests, overall, rats consumed significantly more (F(1,55)= 239.18, p<0.0001) CS+ (26.5 g) than CS− (8.3 g) solution, and significant differences were observed among the seven groups (F(6,55)= 5.15, p<0.0003) and for the interaction between tests and CS (F(2,110)= 5.06, p<0.008), but not among tests (F(2,110)= 0.52, ns) or for the interactions between groups and tests (F(12,110)= 0.98, ns), groups and CS (F(6,55)= 0.94, ns) or among groups, tests and CS (F(12,110)= 1.05, ns). Total CS intake was significantly lower in RAC200 group (23.0 (+2.9) g) relative to VEH (38.6 (+2.5) g) or Limited VEH (32.8 (+1.5) g) groups during testing; all other groups were similar to the control groups. CS+ intakes significantly exceeded CS− intakes across all three tests in VEH, Limited VEH, SCH25, SCH50, SCH200 and RAC200 during training (Figure 4). CS+ intakes significantly exceeded CS− intakes for the first and second, but not third tests in the RAC50 group (Figure 4). Finally, CS+ intake in the RAC50 group was significantly lower than that of the VEH and Limited VEH groups in test 1 (Figure 4).
Figure 4.
(Acquisition Study - Testing): Intakes (mean in g, +SEM, 2 h) of CS+ and CS− in three two-bottle preference tests in the VEH (Panel A), SCH25 (Panel B), SCH50 (Panel C), SCH200 (Panel D), RAC50 (Panel E), RAC200 (Panel F) and Limited VEH (Panel G) groups. Significant differences (*) are denoted between CS+ and CS-intake within each test and each group.
Significant differences in the percent CS+ intakes were observed among tests (F(2,110)= 6.37, p<0.002), but not among groups (F(6,55)= 0.81, ns), or for the interaction between groups and tests (F(12,110)= 1.23, ns). Thus, none of the groups significantly differed from each other in the magnitude of their corn oil-CFP across the three tests (Figure 4).
Discussion
The present study was designed to investigate the role of DA signaling in fat-CFP. Experiment 1 demonstrated that a CFP could be conditioned in male rats by mixing one novel flavor (CS+) in a higher concentration of corn oil (3.5%) and a second novel flavor (CS−) in a lower concentration (0.9%) of corn oil. In subsequent two-bottle tests with each flavor mixed in 0.9% corn oil solutions, rats displayed significant preferences (75–83%) for the CS+ flavor that persisted over 8 two-bottle test sessions. These findings extend earlier flavor preference results obtained with rats trained with CS+ flavored corn oil solutions (3.5 or 7.1%) (Elizalde et al., 1990; Mehiel & Bolles, 1988). The preferences reported in these earlier studies were measured in only one or two test sessions whereas Experiment 1 revealed a CS+ preference that persisted over at least 8 test sessions. The consistent preference over repeated tests therefore made this CFP protocol amenable to the investigation of the effects of DA D1 and D2 receptor antagonists on the expression (Experiment 2) and acquisition (Experiment 3) of fat-CFP. This was of interest in view of published studies showing that DA receptor antagonism suppresses the intake of fat emulsions and high-fat diets (Baker et al., 2001; Davis et al., 2006; Rao et al., 2008; Weatherford et al., 1988; 1990), and attenuates flavor conditioning by sugar solutions (Azzara et al., 2001; Baker et al., 2003; Yu et al., 2000a, 2000b).
DA D1/D2 Antagonism and Expression of CO-CFP
In the two-bottle tests of Experiment 2, the rats consumed significantly more CS+ than CS− following vehicle treatment but not following SCH23390 at the 200 and 400 nmol/kg doses. All drug doses significantly reduced CS+ intake compared to vehicle, but at the 50 nmol/kg dose and, unexpectedly at the 800 nmol/kg dose, CS+ intake exceeded CS− intake. With respect to the percent CS+ measure, there was a significant reduction at the 200 nmol/kg dose (to 56%) but only intermediate reductions at the other SCH23390 doses relative to vehicle (88%). This dose-limited reduction in expression of corn oil-CFP stands in contrast to SCH23390-induced eliminations of the expression of sucrose-CFP in sham-feeding rats (Yu et al., 2000a, 2000b) and fructose-CFP in real-feeding rats (Baker et al., 2003). Moreover, total CS intakes were greatly suppressed by the three highest doses, which makes the interpretation difficult. Thus, it appears that DA D1 receptor antagonism resulted in at best a modest attenuation of the expression of previously learned corn oil-CFP that was accompanied by overall fat intake suppression. Such DA D1 antagonist effects upon the expression of fat-CFP is not unique given that systemic DA D1 antagonism suppressed the expression of a preference conditioned by IG sucrose only at doses that were accompanied by strong sugar intake suppression (Azzara et al., 2001). A similar pattern of effects were also observed for the expression of preferences conditioned by IG glucose following administration of SCH23390 directly into the nucleus accumbens or amygdala (see review: Touzani et al., 2010b).
Treatment with the DA D2 antagonist raclopride blocked the CS+ preference only at the 200 nmol/kg dose which also significantly reduced the percent CS+ intake relative to the vehicle (61% vs. 87%). In contrast to SCH23390, raclopride did not reduce total CS intakes during the two-bottle tests. Why D2 receptor antagonism decreased CS+ preferences at intermediate doses is not clear. With the ascending/descending dose sequences used here, the tests with the intermediate doses occurred after other drug doses in the non-reinforced sequence. This U-shaped dose-response curve was also observed with the raclopride effect on the expression of fructose-CFP (Baker et al., 2003). Further study is needed to evaluate the independent effects of these raclopride doses in separate groups of animals on the expression of fat-CFP.
DA D1/D2 Antagonism and Acquisition of CO-CFP
Our previous studies (Azzara et al., 2001; Baker et al., 2003; Yu et al., 2000a, 2000b) demonstrated that DA D1 and D2 receptor signaling was essential for the acquisition of flavor-flavor conditioning produced by the sweet taste of sugars, whereas only D1 receptor signaling was essential for the acquisition of flavor-nutrient conditioning by IG sugar. In these studies, the 75–90% preference observed in rats receiving vehicle during training was reduced to ~50% (i.e., indifference) by the effective antagonist treatments. In the present study, SCH23390 treatment at 25, 50 or 200 nmol/kg doses during training failed to significantly reduce the CS+ preference during two-bottle testing. The drug significantly reduced total CS intake during training, but not the intake of the CS+/3.5% CO solution. This is of particular interest because the 200 nmol/kg dose used in the prior sugar conditioning studies reduced the intake of the CS+ as well as CS− solutions during training (Baker et al., 2003, Yu et al., 2000b). Hence, it is possible that the failure of SCH23390 to attenuate fat-CFP is due to the failure of the antagonist to reduce CS+ training intakes. Why SCH23390 was effective in reducing CS+/sugar but not CS+/fat is not clear. It is possible that the 3.5% corn oil solution (which has both orosensory and post-oral positive consequences) induces more DA release in the reward areas involved in flavor preference learning than does a 8% fructose solution in real-fed rats or a 16% sucrose solution in sham-fed rats. Further experiments are needed to address this issue.
With raclopride, the only significant drug acquisition effect was that the 50 nmol/kg dose resulted in a hastening of extinction of the CS+ preference. Corn oil-CFP has both orosensory and viscerosensory positive reinforcing signals and the failure of raclopride to affect the acquisition of this preference was probably due to the postingestive consequences of corn oil. We previously reported that raclopride has no effect on flavor preference learning induced by IG sugar infusions (Azzara et al., 2001). Thus, compared to DA antagonist effects observed in sugar-conditioned animals (Azzara et al., 2001; Baker et al., 2003; Yu et al., 2000a, 2000b), DA D1 and D2 antagonism is much less effective in attenuating the acquisition of fat-conditioned flavor preferences. In contrast to these findings, other studies suggest that DA receptor antagonism is more effective in blocking the conditioning of place preferences by fat than sugar, but procedural and species (rat vs. mouse) differences may account for the discrepant results (Ågmo et al., 1995; Imaizumi et al., 2000). Additional studies are needed to clarify the effects of DA receptor antagonism on fat vs. sucrose conditioned preferences and to differentiate between the orosensory vs. post-ingestive reinforcing actions of these nutrients. This can be accomplished by comparing DA drug effects on flavor conditioning by IG nutrient infusions as compared to sham-fed nutrient intakes.
Highlights.
Rats display robust sugar and fat conditioned flavor preferences (CFP).
Dopamine (DA) D1 receptors mediate CFP conditioned by intragastric sugar.
DA D1 and D2 receptors mediate CFP conditioned by orosensory fructose.
DA D1 and D2 receptors produce limited effects on expression of corn oil CFP.
DA D2 receptors produce limited effects on acquisition of corn oil CFP.
Acknowledgments
This research was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK071761. We thank Heather Henry, Matthew Yagudayev, Salomon Kandov, Ester Illyayeva and Gregory Fitzgerald for technical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackerman SH, Albert M, Shindledecker RD, Gayle C, Smith GP. Intake of different concentrations of sucrose and corn oil in preweanling rats. American Journal of Physiology. 1992;262:R624–R627. doi: 10.1152/ajpregu.1992.262.4.R624. [DOI] [PubMed] [Google Scholar]
- Ackroff K, Sclafani A. Oral and post-oral determinants of dietary fat appetite. In: Montmayeur J-P, le Coutre J, editors. Fat Detection: Taste, Texture, and Post Ingestive Effects. Taylor & Francis; Boca Raton: 2009. pp. 295–321. [Google Scholar]
- Ågmo A, Galvan A, Talamantes B. Reward and reinforcement produced by drinking sucrose: Two processes that may depend on different neurotransmitters. Pharmacology Biochemistry and Behavior. 1995;52:403–414. doi: 10.1016/0091-3057(95)00128-j. [DOI] [PubMed] [Google Scholar]
- Azzara AV, Bodnar RJ, Delamater AR, Sclafani A. Naltrexone fails to block the acquisition or expression of a flavor preference conditioned by intragastric carbohydrate infusions. Pharmacology Biochemistry and Behavior. 2000;67:545–557. doi: 10.1016/s0091-3057(00)00395-6. [DOI] [PubMed] [Google Scholar]
- Azzara AV, Bodnar RJ, Delamater AR, Sclafani A. D-1 but not D-2 dopamine receptor antagonism blocks the acquisition of a flavor preference conditioned by intragastric carbohydrate infusions. Pharmacology Biochemistry and Behavior. 2001;68:709–720. doi: 10.1016/s0091-3057(01)00484-1. [DOI] [PubMed] [Google Scholar]
- Baker RW, Osman J, Bodnar RJ. Differential actions of dopamine receptor antagonism in rats upon food intake elicited by mercaptoacetate or exposure to a palatable high-fat diet. Pharmacology Biochemistry and Behavior. 2001;69:201–208. doi: 10.1016/s0091-3057(01)00528-7. [DOI] [PubMed] [Google Scholar]
- Baker RW, Shah MJ, Sclafani A, Bodnar RJ. Dopamine D1 and D2 antagonists reduce the acquisition and expression of flavor-preferences conditioned by fructose in rats. Pharmacology Biochemistry and Behavior. 2003;75:55–65. doi: 10.1016/s0091-3057(03)00039-x. [DOI] [PubMed] [Google Scholar]
- Baker RW, Li Y, Lee MG, Sclafani A, Bodnar RJ. Naltrexone does not prevent acquisition or expression of flavor preferences conditioned by fructose in rats. Pharmacology Biochemistry and Behavior. 2004;78:239–246. doi: 10.1016/j.pbb.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Bernal S, Dostova I, Kest A, Abayev Y, Kandova E, Touzani K, Sclafani A, Bodnar RJ. Role of dopamine D1 and D2 receptors in the nucleus accumbens shell on the acquisition and expression of fructose-conditioned flavor-flavor preferences in rats. Behavioural Brain Research. 2008;190:59–66. doi: 10.1016/j.bbr.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal S, Miner P, Abayev Y, Kandova E, Gerges M, Touzani K, Sclafani A, Bodnar RJ. Role of amygdala dopamine D1 and D2 receptors in the acquisition and expression of fructose-conditioned flavor preferences in rats. Behavioural Brain Research. 2009;205:183–190. doi: 10.1016/j.bbr.2009.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal S, Touzani K, Gerges M, Abayev Y, Sclafani A, Bodnar RJ. Opioid receptor antagonism in the nucleus accumberns fails to block the expression of sugar-conditioned flavor preferences in rats. Pharmacology Biochemistry and Behavior. 2010;95:56–62. doi: 10.1016/j.pbb.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch LL, Fisher JO. Development of eating behaviors among children and adolescents. Pediatrics. 1998;101:539–549. [PubMed] [Google Scholar]
- Davis JF, McQuade JA, Drazen DL, Woods SC, Seeley RJ, Benoit SC. Role for dopamine-3 receptor in the hyperphagia of an unanticipated high-fat meal in rats. Pharmacology Biochemistry and Behavior. 2006;85:190–197. doi: 10.1016/j.pbb.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Dym CT, Bae V, Kraft T, Yakubov Y, Winn A, Sclafani A, Bodnar RJ. Genetic variance contributes to dopamine and opioid receptor antagonist-induced inhibition of Intralipid (Fat) intake in inbred and outbred mouse strains. Brain Research. 2010;1316:51–61. doi: 10.1016/j.brainres.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elizalde G, Sclafani A. Fat appetite in rats: Flavor preferences conditioned by nutritive and non-nutritive oil emulsions. Appetite. 1990;15:189–197. doi: 10.1016/0195-6663(90)90019-5. [DOI] [PubMed] [Google Scholar]
- Imaizumi M, Takeda M, Fushiki T. Effects of oil intake in the conditioned place preference test in mice. Brain Research. 2000;870:150–156. doi: 10.1016/s0006-8993(00)02416-1. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Baskshi VP, Haber SN, Steiniger TL, Will MJ, Zhang M. Opioid modulation of taste hedonics within the ventral striatum. Physiology and Behavior. 2002;73:389–395. doi: 10.1016/s0031-9384(02)00751-5. [DOI] [PubMed] [Google Scholar]
- Levine AS, Kotz CM, Gosnell BA. Sugars and fats: the neurobiology of preference. American Society of Nutritional Science. 2003;133:831S–834S. doi: 10.1093/jn/133.3.831S. [DOI] [PubMed] [Google Scholar]
- Liang NC, Hajnal A, Norgren R. Sham feeding corn oil increases accumbens dopamine in the rat. American Journal of Physiology. 2006;291:R1236–R1239. doi: 10.1152/ajpregu.00226.2006. [DOI] [PubMed] [Google Scholar]
- Mehiel R, Bolles RC. Learned flavor preferences based on calories are independent of initial hedonic value. Animal Learning and Behavior. 1988;16:383–387. [Google Scholar]
- Malkusz DC, Banakos T, Vongwattanakit T, Mohammed A, Bohn T, Mahmud F, Martinez S, Saeed S, Orr J, Touzani K, Sclafani A, Bodnar RJ. Acquisition of fructose-conditioned flavor preference is blocked by dopamine D1 and D2 receptor antagonists in the medial prefrontal cortex in rats. Society for Neuroscience Abstracts. 2010:36. [Google Scholar]
- Passilly-Degrace P, Gaillard D, Besnard P. Orosensory perception of dietary lipids in mammals. Results Problems in Cell Differentiation. 2009;47:221–238. doi: 10.1007/400_2008_7. [DOI] [PubMed] [Google Scholar]
- Rao RE, Wojnicki FH, Coupland J, Ghosh S, Corwin RL. Baclofen, raclopride and naltrexone differentially reduce solid fat emulsion intake under limited access conditions. Pharmacology Biochemistry and Behavior. 2008;89:581–590. doi: 10.1016/j.pbb.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A. Macronutrient-conditioned flavor preferences. In: Berthoud H-R, Seeley R, editors. Neural and metabolic control of macronutrient intake. CRC Press; Boca Raton, FL: 1999. pp. 93–107. [Google Scholar]
- Sclafani A, Ackroff K. Glucose- and fructose-conditioned flavor preferences in rats: Taste versus postingestive conditioning. Physiology and Behavior. 1994;56:399–405. doi: 10.1016/0031-9384(94)90213-5. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Cardieri C, Tucker K, Blusk D, Ackroff K. Intragastric glucose but not fructose conditions robust flavor preferences in rats. American Journal of Physiology. 1993;265:R320–R325. doi: 10.1152/ajpregu.1993.265.2.R320. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Fanizza LJ, Azzara AV. Conditioned flavor avoidance, preference and indifference produced by intragastric infusions of galactose, glucose and fructose in rats. Physiology and Behavior. 1999;67:227–234. doi: 10.1016/s0031-9384(99)00053-0. [DOI] [PubMed] [Google Scholar]
- Touzani K, Bodnar RJ, Sclafani A. Activation of dopamine D1 receptors in nucleus accumbens is critical for the acquisition, but not the expression, of glucose-conditioned flavor preference in rats. European Journal of Neuroscience. 2008;27:1525–1533. doi: 10.1111/j.1460-9568.2008.06127.x. [DOI] [PubMed] [Google Scholar]
- Touzani K, Bodnar RJ, Sclafani A. Dopamine D1-like receptor antagonism in amygdala impairs the acquisition of glucose-conditioned flavor preferences in rats. European Journal of Neuroscience. 2009a;30:289–298. doi: 10.1111/j.1460-9568.2009.06829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touzani K, Bodnar RJ, Sclafani A. A role of the lateral hypothalamic dopamine D1 receptors in intragastric glucose-conditioned flavor preferences in rats. Neurobiology of Learning and Memory. 2009b;92:464–467. doi: 10.1016/j.nlm.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touzani K, Bodnar RJ, Sclafani A. Acquisition of glucose-conditioned flavor preference requires the activation of dopamine D1-like receptors within the medial prefrontal cortex in rats. Neurobiology of Learning and Memory. 2010a;94:214–219. doi: 10.1016/j.nlm.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touzani K, Bodnar RJ, Sclafani A. Pharmacology of food learning preferences. Pharmacology Biochemistry and Behavior. 2010b;97:55–62. doi: 10.1016/j.pbb.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DJ, Volkow VD, Logan J, Pappas NR, Wong CT, Zhu W, Netusil N, Fowler JS. Brain dopamine and obesity. The Lancet. 2001;357:354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- Weatherford SC, Greenberg D, Gibbs J, Smith GP. The potency of D-1 and D-2 receptor antagonists is inversely related to the reward value of sham-fed corn oil and sucrose in rats. Pharmacology Biochemistry and Behavior. 1990;37:317–323. doi: 10.1016/0091-3057(90)90341-e. [DOI] [PubMed] [Google Scholar]
- Weatherford SC, Smith GP, Melville LD. D-1 and D-2 receptor antagonists decrease corn oil sham feeding in rats. Physiology and Behavior. 1988;44:569–572. doi: 10.1016/0031-9384(88)90320-4. [DOI] [PubMed] [Google Scholar]
- Yoneda T, Taka Y, Okamura M, Mizushige T, Matsumura S, Manabe Y, Tsuzuki S, Inoue K, Fushiki T. Reinforcing effect for corn oil stimulus was concentration dependent in an operant task in mice. Life Sciences. 2007;81:1585–1592. doi: 10.1016/j.lfs.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Yu WZ, Sclafani A, Delamater AR, Bodnar RJ. Pharmacology of flavor preference conditioning in sham-feeding rats: effects of naltrexone. Pharmacology Biochemistry and Behavior. 1999;64:573–584. doi: 10.1016/s0091-3057(99)00124-0. [DOI] [PubMed] [Google Scholar]
- Yu WZ, Silva RM, Sclafani A, Delamater AR, Bodnar RJ. Pharmacology of flavor preference conditioning in sham-feeding rats: effects of dopamine receptor antagonists. Pharmacology Biochemistry and Behavior. 2000a;65:635–647. doi: 10.1016/s0091-3057(99)00239-7. [DOI] [PubMed] [Google Scholar]
- Yu WZ, Silva RM, Sclafani A, Delamater AR, Bodnar RJ. Role of D1 and D2 dopamine receptors in the acquisition and expression of flavor preference conditioning in sham-feeding rats. Pharmacology Biochemistry and Behavior. 2000b;67:537–544. doi: 10.1016/s0091-3057(00)00396-8. [DOI] [PubMed] [Google Scholar]