Abstract
Aim
Oocyte cryopreservation remains largely experimental, with live birth rates of only 2–4% per thawed oocyte. In this study, we present a nanoliter droplet technology for oocyte vitrification.
Materials & methods
An ejector-based droplet vitrification system was designed to continuously cryopreserve oocytes in nanoliter droplets. Oocyte survival rates, morphologies and parthenogenetic development after each vitrification step were assessed in comparison with fresh oocytes.
Results
Oocytes were retrieved after cryoprotectant agent loading/unloading, and nanoliter droplet encapsulation showed comparable survival rates to fresh oocytes after 24 h in culture. Also, oocytes recovered after vitrification/thawing showed similar morphologies to those of fresh oocytes. Additionally, the rate of oocyte parthenogenetic activation after nanoliter droplet encapsulation was comparable with that observed for fresh oocytes. This nanoliter droplet technology enables the vitrification of oocytes at higher cooling and warming rates using lower cryoprotectant agent levels (i.e., 1.4 M ethylene glycol, 1.1 M dimethyl sulfoxide and 1 M sucrose), thus making it a potential technology to improve oocyte cryopreservation outcomes.
Keywords: cryopreservation, fertility, nanoliter droplet vitrification, oocyte
Oocyte cryopreservation has potential to impact preservation of female fertility by facilitating oocyte banking [1]. The ability to successfully freeze oocytes would offer an alternative option to embryo cryopreservation, which is particularly important for:
Women who have no male partner and who wish to bank oocytes for later use
Female cancer patients of reproductive age who risk losing ovarian function during extirpative surgery, chemotherapy or radiotherapy
Patients who have ethical, moral or religious objections to embryo preservation [2,3]
Since the first pregnancy using frozen oocytes was reported in 1986 [4], several oocyte cryopreservation technologies have been developed. However, the clinical efficiency entailed in using frozen oocytes remains low, with pregnancy and live birth rates of 2–4% [5]. In addition, according to the American Society of Reproductive Medicine, oocyte cryopreservation is currently considered experimental and should be performed only under an Institutional Review Board [6], which is mostly due to the low success rate of obtaining live births from thawed oocytes. Furthermore, most vitrification protocols involve manual sequential treatment of oocytes, which is laborious and requires expertise. Therefore, innovative technologies that could improve oocyte cryopreservation outcomes are urgently needed to advance the clinical practice and expand fertility preservation options for patients.
Currently, two methods exist for oocyte cryopreservation
The slow freezing–rapid thawing method employs relatively low levels of cryoprotectant agents (CPAs; i.e., ~1–2 M), and it freezes oocytes at slow cooling rates (typically 0.3°C/min) to prevent intracellular ice formation and minimize structural damage [10]. However, oocytes cryopreserved using slow freezing are prone to hardening of the zona pellucida [11], disruption of the chromosomes [11,12], and a substantial loss in the ability to fertilize and develop in culture [12]. These deleterious outcomes have been attributed to the formation of ice crystals, extreme hyperosmolarity and dehydration [13].
Due to the prevention of intracellular ice formation and the avoidance of increasing ionic concentrations of unfrozen solutions [14,15], vitrification has made it possible to increase post-thaw cell survivability compared with conventional slow freezing [16–18]. Vitrification technology has been successful when employed for embryonic and somatic cells [19–22]. With vitrification, high concentrations of CPAs (i.e., ~4–8 M) and rapid cooling rates (i.e., 1500°C/min) are required to ensure direct solidification of the vitrification solution without ice crystal formation. However, it has been suggested that high CPA levels lead to osmotic shock and toxicity to oocytes [23], thus resulting in cytoskeletal alterations [24–26], chromosome dispersal [27] and spindle disassembly [27].
Various methods have been developed to reduce the overall toxicity of the CPAs that are used for vitrification, including the use of a combination of CPAs [28,29] and stepwise equilibration with CPAs at room temperature [30]. The most effective recent improvement in vitrification technology has been the use of minimum volume methods, including the open pulled straws technique [31–35], electron microscopy grids [36,37], the cryoloop [38] and droplet-based vitrification methods [39–42]. The small sample size not only enables a substantial increase in cooling rates, but also ultra high warming rates, which have been reported to be more important than cooling rates determining the survival of oocytes subjected to a vitrification procedure [43]. In addition, the increase in cooling and warming rates also enables reduction in the required CPA concentrations.
Among these minimum volume methods, droplet-based vitrification offers the potential to achieve higher cooling and warming rates than carrier-based vitrification technologies. As we have shown previously [39,44], this is due to the enhanced heat transfer of a droplet in the absence of a carrier, which reduces the likelihood of ice formation and the need for high CPA concentrations. However, all current droplet vitrification approaches are constrained to generate droplets manually using pipettes [35,42,44–47]. Pipette methods require a high degree of technical skill and do not ensure consistency in droplet size. Furthermore, with pipette methods, it is very challenging to reliably obtain droplets smaller than 1 μl, thus limiting cooling and warming rates, and precluding the use of further reduced CPA levels. It has been demonstrated that minimizing sample volume during vitrification can potentially improve the cryopreservation outcome of oocytes by significantly increasing the cooling and warming rates and through the use of lower CPA concentrations. The technology presented in this study allows oocyte vitrification at droplets on a nanoliter scale, while currently available open procedures cannot provide reliable generation and control of droplet volumes at the nanoscale. In addition, the operator variability with the current procedures becomes more significant when handling smaller volumes. Heat transfer characteristics with these open procedures can vary as a function of the droplet size, thereby affecting the clinical results.
Recently, microscale technologies have been introduced to the cryogenics field [48], and droplet generation technologies with control over droplet sizes have been created to reliably encapsulate live functional cells [49–54]. Manipulating cells encapsulated in small volumes has been presented earlier for various applications, including tissue engineering [44,49–51] and diagnostics [55–57]. Furthermore, cryopreservation of large volumes of blood cells on polyethylene film containing millions of droplets using a high-throughput vitrification system was also presented for storage purposes [53]. In our prior study, we reported the dynamics of vitrification using a rapid nanoliter droplet encapsulation system developed in our laboratory, and demonstrated that the Leidenfrost effect is present during droplet vitrification [44]. Here, through a system that provides continuous generation of nanoliter droplets, we modified the ejector-based droplet vitrification system to address limitations of the current droplet vitrification approaches. As a first step to evaluate this novel technology for oocyte cryopreservation, the mouse species was chosen as a model to optimize the protocols and the design. By vitrifying oocytes encapsulated in nanoliter droplets, this system potentially enables ultra-rapid cooling rates with reductions in required CPA concentrations. This is the first application of a scalable vitrification method in oocyte cryopreservation. We have also evaluated the efficiency of this vitrification system in a step-by-step fashion. At reduced CPA concentrations, oocytes survived after vitrification and warming processes. In addition, no significant effect of droplet encapsulation on oocyte survival or parthenogenetic embryo development was observed. Results obtained with mouse oocytes in this study should provide guidance for optimization of the system for potential human oocyte vitrification in a clinical environment.
Materials & methods
Animals & oocyte collection
Female B6D2F1 mice 6–8 weeks old (Jackson Laboratory, ME, USA) were superovulated by intraperitoneal injection of 5 IU of pregnant mare serum gonadotropin (EMD Chemicals, NJ, USA) followed by the injection of 5 IU human chorionic gonadotrophin 48 h later (EMD Chemicals). A total of 15 h after human chorionic gonadotrophin treatment, mice were exposed to CO2 until movement ceased and euthanized by cervical dislocation. A small incision was made over the midsection, the skin was reflected back and the peritoneum was entered with a sharp dissection to expose the viscera. The oviducts were immediately excised and placed into a culture dish containing 4 ml EmbryoMax® flushing hold medium (FHM) 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffered medium (Millipore Corporation, Billerica, M A, USA) supplemented with 4 mg/ml bovine serum albumin (BSA). Oviducts were then transferred to a center-well dish containing 1 ml FHM medium supplemented with 4 mg/ml BSA for cumulus–oocyte complex collection. While holding the oviduct in place with forceps, an incision was made in the swollen part of the oviduct using an insulin needle to allow cumulus–oocyte complex extrusion. Oocytes were then transferred to a center-well dish filled with 1 ml hyaluronidase solution (0.3 mg/ml; Sigma, MO, USA) in FHM medium until the cumulus cells were dispersed, followed by five washes in 50 μl of FHM medium drops. Oocytes were collected in 50 μl drops of potassium simplex optimized medium (KSOM) medium (Millipore Corporation, MA, USA), covered with mineral oil (Sigma, MO, USA) and cultured at 37°C in 5% CO2 and 95% air. Oocyte size measurements were obtained from 45 oocytes collected from eight mice.
Cell encapsulating droplet generation system
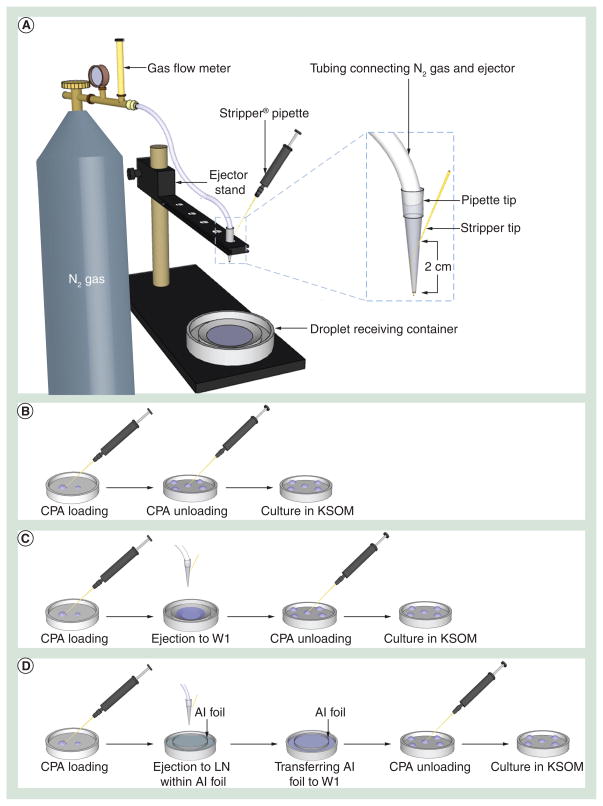
The design of the coflow droplet generation system is shown in Figure 1A. The coflow ejector was built by placing a Stripper® tip (MidAtlantic Diagnostics, NJ, USA) through the small open end of a 200-μl pipette tip to create a sheath flow. The inner diameter of the Stripper tip was 125 μm. As shown in the magnified image of Figure 1A, the two ejector components were assembled by inserting the Stripper tip across the pipette tip wall at a point 2 cm away from the small open end of the pipette tip. The Stripper tip was then pushed further inside the pipette tip until it protruded 2 mm from the small opening. The Stripper tip of the ejector was then attached to the Stripper pipette and the CPA loaded oocytes were aspirated into the Stripper tip with the aid of the Stripper pipette. After assembling the ejector onto the stand, Tygon® tubing (inner diameter = 3.2 mm; Saint-Gobain Performance Plastics, MA, USA) attached to a nitrogen gas cylinder was connected to the ejector. Nitrogen gas was flowed through the pipette tip while CPA solution loaded with oocytes flowed through the Stripper tip, creating droplets from the ejector (Figure 1A).
Figure 1. The ejector-based droplet generation setup and the overview of stepwise experimental procedures for evaluating each step for oocyte survival.
(A) The experimental setup for oocyte encapsulating droplet ejection into the droplet receiving container. (B) CPA was loaded to and unloaded from oocytes sequentially. (C) Oocytes were ejected into the thawing medium (W1) after CPA loading followed by CPA unloading. (D) After CPA loading, oocytes encapsulated in CPA droplets were ejected into LN on an Al foil (collection sieve), thawed in thawing medium (W1) followed by CPA unloading. Oocytes retrieved from each procedure (B, C & D) were transferred to the medium and cultured at 37°C before survival analysis.
Al: Aluminium; CPA: Cryoprotectant agent; KSOM: Potassium simplex optimized medium; LN: Liquid nitrogen; W1: Warming solution 1.
Vitrification & thawing
All the procedures were performed at room temperature (20–24°C). In this study, mature oocytes at the M II stage, identified by extrusion of the first polar body, were used for droplet-based vitrification. Before vitrification, oocytes were first suspended for 3 min in a 100-μl droplet of pre-equilibrium solution (V1) composed of 4% ethylene glycol (v/v; Sigma) and 4% dimethyl sulfoxide (v/v; Sigma) in FHM medium supplemented with 4 mg/ml BSA. Oocytes were then transferred to a 100-μl droplet of vitrification solution (V2), a mixture of 8% ethylene glycol (v/v [1.4 M]), 8% dimethyl sulfoxide (v/v [1.1 M]), and 1 M sucrose in FHM HEPES buffered medium supplemented with 4 mg/ml BSA and equilibrated for 30 s.
For vitrification, oocytes in vitrification solution were first aspirated into the Stripper tip. The loaded ejector was then put on the rack and the top was connected to a nitrogen gas cylinder by Tygon® tubing (Figure 1A). Aluminum foil in a bowl shape (collection sieve) was placed in a 6-mm Petri dish and then filled with liquid nitrogen to serve as a droplet receiving container. Finally, by using the Stripper pipette, CPA droplets encapsulating oocytes were generated, ejected directly into liquid nitrogen and vitrified (Figure 1A). The aluminum foil was used as a collector sieve to recover vitrified droplets for the rapid warming step.
For rapid warming, a thawing solution composed of 1 M sucrose and 4 mg/ml BSA in FHM medium (W1) was used. A culture dish containing 4 ml of W1 was prewarmed to 37°C. The aluminum foil, on which the vitrified CPA droplets encapsulating oocytes were collected, was first lifted above the liquid nitrogen surface. Immediately after liquid nitrogen evaporated, the aluminum foil was inverted and immersed into the warming solution (W1) in the dish (Figure 1D). This procedure allowed simultaneous removal of CPAs and rapid warming. After 2.5 min, thawed oocytes recovered under stereo microscope were sequentially transferred to graded sucrose solutions of 0.5 M (W2) and 0.2 M (W3) with 2.5 min incubation in each solution. Finally, the oocytes were transferred to FHM medium (three washes) for a total of 5 min to remove any remaining CPAs. All oocytes were then collected in 50-μl droplet of KSOM medium, covered with mineral oil and cultured in 5% CO2/95% air at 37°C.
Droplet-size measurements
The size distribution of droplets generated at different nitrogen gas flow rates (0.6, 0.7, 0.8, 0.9, 1.0 and 1.1 standard liters per min [SLPM]) was measured. At each flow condition, ejected droplets were collected on a polyethylene film (Avery, CA, USA), and then imaged under a microscope (TE 2000; Nikon, Japan). The images were taken at 4× magnification, and the droplet sizes were measured using SPOT Imaging Solutions (Diagnostic Instruments Inc., MI, USA). Finally, the acquired data was analyzed and size distribution of droplets was plotted.
Assessment of oocyte morphology & survival
To understand how each procedure affects the ultimate oocyte survival after vitrification and thawing, oocyte morphology was analyzed 30 min and 24 h after each step (i.e., CPA loading/unloading, droplet encapsulation and vitrification/thawing). Normal oocyte morphological integrity was defined by round and regular appearance of the oocyte membrane, by refringent cytoplasm with fine homogenous granularity and by absence of signs of cytoplasmic degeneration. Fresh oocytes cultured in the KSOM medium right after retrieval were used as controls. Oocytes that appeared pycnotic or showed signs of degeneration were removed from the culture medium drop to minimize detrimental effects on the rest of the cohort.
To assess the effect of CPA loading and unloading on oocytes, CPA unloading was performed immediately following CPA loading without vitrification (Figure 1B). Oocytes were initially suspended in V1 for 3 min and then transferred to V2 for 30 s. CPAs were then subsequently removed by transferring oocytes into W1, W2 and W3 for 2.5 min per step, followed by three washes in FHM medium for a total of 5 min. Oocytes were then cultured in KSOM medium in 5% CO2/95% air at 37°C for morphological analysis.
Oocyte survival was examined after nanoliter droplet encapsulation. The oocytes were first loaded with V1 and V2 as described above and then ejected into W1 in a center well culture dish (BD, NJ, USA) using the droplet generator. The ejected oocytes were then retrieved and transferred into unloading solutions (W1, W2 and W3), followed by three washes in FHM medium to completely remove CPAs. Oocytes were then cultured in KSOM medium in 5% CO2/95% air at 37°C for further analysis (Figure 1C).
To further evaluate oocyte survival after vitrification and thawing, oocytes were first loaded with V1 and V2 as detailed above, and then ejected into a culture dish filled with liquid nitrogen. The droplets in the liquid nitrogen were transferred to warming solution as described in the ‘vitrification and thawing’ section. The CPAs were subsequently removed by transferring oocytes into W1, W2 and W3 followed by three washes in FHM as described above. The oocytes were finally cultured in KSOM medium in 5% CO2/95% air at 37°C for morphological analysis (Figure 1D).
Parthenogenetic activation of oocytes
Prior to parthenogenetic activation, oocytes were cultured in KSOM medium supplemented with 4 mg/ml BSA for 2 h to allow oocytes to recover from the previous experimental procedure. For parthenogenetic activation, oocytes were incubated in SrCl2 solution in KSOM medium at 37°C in 5% CO2/95% air for 2 h and then washed three-times with FHM medium. Oocytes were then transferred to KSOM medium and cultured at 37°C in 5% CO2/95% air.
To determine the optimal SrCl2 concentration to parthenogenetically activate fresh mouse oocytes, we tested four SrCl2 concentrations (10 mM, 20 mM, 50 mM and 100 mM). Over the following 6 days, treated oocytes were examined microscopically every day for embryo development to the cleavage and blastocyst stages. Day 1 is defined as 24 h after treatment. Oocytes incubated in the regular KSOM medium without SrCl2 were used as controls.
To determine whether droplet encapsulation has effect on oocyte parthenogenetic activation, oocytes in FHM medium were ejected into a center well culture dish containing 400 μl of FHM medium. To minimize the effects of shear stress generated at the ejector tip when using medium instead of CPAs, the flow rate of nitrogen gas was reduced to 0.6 SLPM to obtain droplet sizes similar to those obtained at 0.8 SLPM using CPAs. Oocytes were retrieved and cultured in KSOM medium at 37°C in 5% CO2/95% air for 2 h before parthenogenetic activation. SrCl2 solution at the concentration of 50 mM, which gave the highest cleavage and blastocyst rates with control oocytes, was used for the activation of oocytes following ejection into the medium.
Statistics
All experiments were repeated at least four-times. Experimental data were analyzed statistically by nonparametric Mann–Whitney U test with pairwise comparisons (Minitab Release 14, Minitab Inc., PA, USA). The statistical significance threshold was set at 0.05 (p < 0.05).
Results
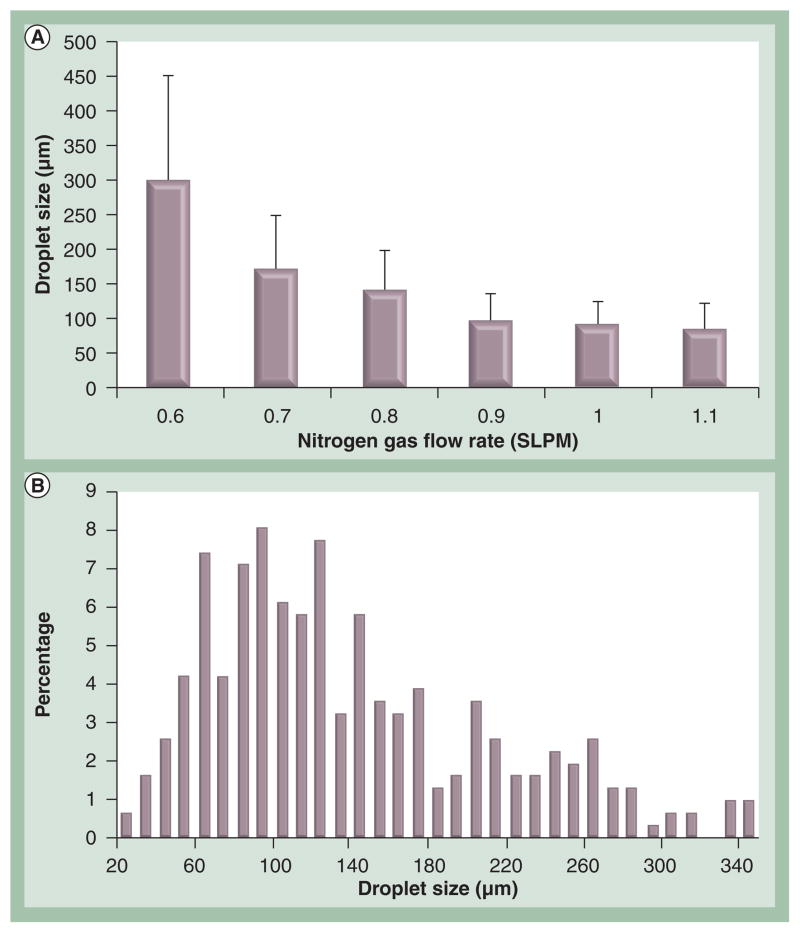
In this study, we modified the ejection system to continuously generate oocyte encapsulated nanoliter droplets, which were vitrified upon ejection into liquid nitrogen (Figure 1A). Since the degree of crystallization during ultra-rapid cooling increases with an increase in droplet diameter [44], the ejection conditions were initially experimentally optimized to generate desirable droplet sizes for vitrification. The ideal size of droplets would allow oocytes to be vitrified at potentially the highest cooling and warming rates under the constraint of minimal volume. The minimal volume has to be large enough to satisfactorily encapsulate mouse oocytes, which are on average 92.6 ± 4.4 μm (82–107 μm) in diameter. Droplets generated at different nitrogen flow rates (0.6–1.1 SLPM) were collected on polyethylene films and the average droplet sizes and distributions were analyzed. As shown in Figure 2A, an increase in droplet size was observed when the nitrogen gas flow rate was reduced, and ejection at a nitrogen gas flow rate of 0.8 SLPM gave the optimal droplet sizes (140 ± 58 μm). The size distribution of droplets generated at nitrogen gas flow rate of 0.8 SLPM is shown in Figure 2B.
Figure 2. Droplet sizes obtained at various nitrogen gas flow rates and the droplet size distribution at 0.8 SLPM.
(A) Average droplet size was observed to decrease as nitrogen gas flow rate increased up to 0.9 SPLM. For nitrogen gas flow rate values higher than 0.9 SLPM, the droplet size mean values were similar. Error bars indicate standard deviations. (B) Droplet size distribution ejected at 0.8 SLPM displayed a normal distribution with left hand skew.
SLPM: Standard liter per min.
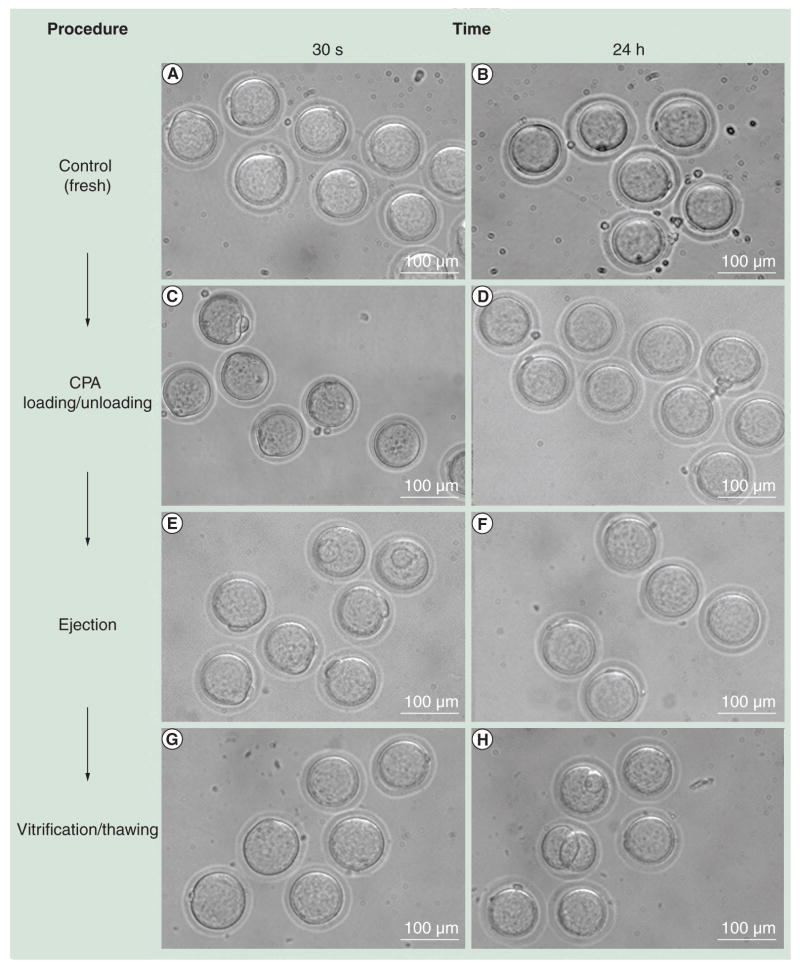
To assess the overall vitrification process using the droplet ejection system, we evaluated the effect of each step (CPA loading/unloading, droplet encapsulation and vitrification/thawing) on oocyte survival rates morphologically. As shown in Figure 3, oocytes survived after CPA loading and unloading, encapsulation in CPA droplets, and vitrification and thawing steps. They also showed a similar morphology as the control oocytes. There was no statistically significant difference in survival rates among the oocytes ejected in CPA droplets (89.9%), loaded and unloaded with CPAs (92.2%), and fresh control oocytes (94.9%) (Table 1). The oocyte recovery from ejection into warming medium was 83.2% (99 recovered from 119 oocytes ejected).
Figure 3. Morphological observations of oocytes at each procedure step.
Surviving oocytes from each procedure showed no difference compared with the controls in morphology. (A & B) Fresh oocytes (control); (C & D) oocytes recovered after CPA loading and unloading; (E & F) oocytes after ejection with CPA; and (G & H) oocytes after vitrification and thawing steps. Images were taken (A, C, E & G) 30 min and (B, D, F & H) 24 h after each procedure.
CPA: Cryoprotectant agent.
Table 1.
Oocyte survival after cryoprotectant agents loading/unloading and ejection with cryoprotectant agents.
| Experimental groups | Number of oocytes initiated | Number of surviving oocytes after 24 h | Survival rate after 24 h (%) |
|---|---|---|---|
| Control (fresh) | 99 | 94 | 94.9 |
| CPA loading/unloading | 102 | 94 | 92.2 |
| Ejection with CPA | 99 | 89 | 89.9 |
CPA: Cryoprotectant agent.
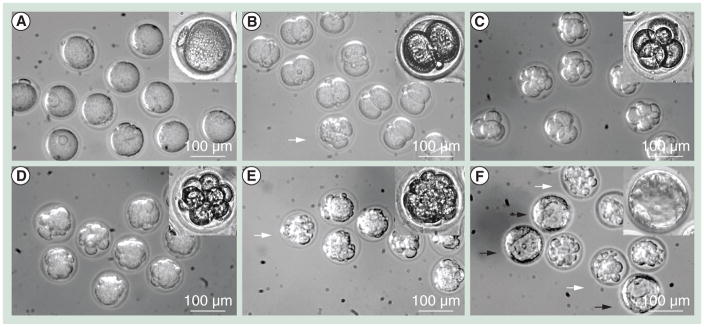
To further examine oocyte function after the oocytes were encapsulated and ejected into droplets of oocyte culture medium, we performed parthenogenetic activation of oocytes using SrCl2 and compared embryo development (cleavage and blastocyst formation) to that observed for control oocytes. To identify the concentration for optimal SrCl2 mouse oocyte parthenogenesis under culture conditions, we first tested fresh control oocytes at different SrCl2 concentrations (10, 20, 50 and 100 mM). We observed that 50 mM SrCl2 resulted in the highest cleavage rate (88.9%) and blastocyst rate (49.4%) with fresh oocytes (Table 2). SrCl2 at 50 mM was then used to parthenogenetically activate oocytes retrieved after ejection into the medium (Figure 4). We observed a statistically higher (p < 0.01) cleavage rate (96%) compared with the control group (88.6%). The blastocyst rate of ejected oocytes (26.0%) was lower (p < 0.01) than that of the control group (49.4%).
Table 2.
Parthenogenetic activation using SrCl2 solution for oocytes retrieved after harvesting (control) and after encapsulation in medium droplets followed by ejection into medium.
| Experimental groups | SrCl2 concentration (mM) | Number of oocytes initiated | Number of oocytes cleaved (%) | Blastocytes developed (%) |
|---|---|---|---|---|
| Control | 10 | 81 | 3 (3.7) | 0 (0) |
| 20 | 82 | 38 (46.3) | 13 (15.9) | |
| 50 | 79 | 70 (88.6)† | 33 (49.4)‡ | |
| 100 | 88 | 43 (48.9) | 2 (2.3) | |
|
| ||||
| Ejection with medium | 50 | 100 | 96 (97.0)† | 26 (26.0)‡ |
Values are significantly different (p < 0.01).
Values are significantly different (p < 0.01).
Figure 4. Embryo development of parthenogenetically activated murine oocytes using 50 mM SrCl2.
Embryo cleavage was evaluated daily. (A) Oocytes before activation on day 0; (B) two-cell embryos on day 1; (C) four-cell to six-cell embryos on day 2; (D) eight-cell to 12-cell embryos on day 3; (E) morula on day 4 and (F) blastocysts on day 5. The images were taken with a 10× objective and the insets were taken with a 20× objective. White arrows indicate abnormal embryos. Black arrows indicate blastocysts.
Discussion
We have demonstrated for the first time that a simple droplet ejection system can be used to vitrify oocytes encapsulated in nanoliter droplets. Oocytes cryopreserved using this system retain survivability and morphology after ejection, vitrification and thawing.
Vitrification for oocyte cryopreservation has been demonstrated to provide improved pregnancy and live birth rates; this has been attributed to effects including the elimination of mechanical injury caused by intracellular and extracellular ice crystal formation [8,58]. However, as the attainable cooling and warming rates are limited due to the presence of carriers and heat transfer inefficiencies, high CPA levels are usually required during vitrification. The droplet vitrification method described here has the potential to overcome this limitation by minimizing the vitrification sample volume and by direct contact with liquid nitrogen.
In existing oocyte vitrification studies, droplet sizes were mostly greater than 1 μl, which reduced the potential for reduced CPA levels due to limited cooling and warming rates associated with large sample volume. For instance, Seki and Mazur showed that if the samples were warmed at a rate of 2950°C/min, more than 80% of the oocytes survived at the cooling rates of 187–1827°C/min [43]. However, if the samples were warmed at a low rate, survival was as low as 0% regardless of the cooling rate. These results indicated the importance of achieving high warming rates for oocyte survival, which is a challenge in current technologies due to limited control over sample size. Using the ejector-based droplet generation system described here, smaller droplet sizes down to the nanoliter scale can be achieved by controlling parameters such as the rate of nitrogen gas flow at a given Stripper tip size. Thus, for the application of oocyte cryopreservation, an average droplet volume of 1.4 nl was used for vitrification. This significant reduction in droplet volume allowed oocytes to be vitrified using CPA concentrations as low as 2.5 M (8% ethylene glycol [v/v, 1.4 M], 8% dimethyl sulfoxide [v/v, 1.1 M]), and 1 M sucrose, much less than the CPA concentration used in previous studies [30,32,37,59].
In addition, prior droplet vitrification studies prepared droplets manually using pipettes, which lead to inconsistent cryopreservation results due to variation in operator skills and experimental conditions. Also, these approaches are both time and labor intensive, which makes the entire vitrification process inefficient and costly. The ejection system demonstrated here enables droplet generation in a continuous manner with minimal manual involvement, thus significantly improving the vitrification efficiency and repeatability. Moreover, oocytes can be introduced into the ejector using a Stripper that is commonly used in in vitro fertilization clinics, making the system gentle for oocyte handling and simple for embryologists. We evaluated oocyte survival and parthenogenetic development postejection to understand how the droplet generation and encapsulation processes affect the oocytes. While we did not observe any damage to the zona pellucida or a considerable difference in oocyte survivability, a reduction in the rate of blastocyst formation was noted for ejected oocytes compared with control oocytes. The increase in cleavage rate could be partially due to mechanical stress posed on the ejector tip during ejection, which could in principle be reduced by decreasing the nitrogen air-flow rate. Furthermore, although the recovery of oocytes was high when ejected into cell media or CPA, we observed that the recovery rate became more challenging when oocytes were ejected into liquid nitrogen. This could be attributed to the Leidenfrost effect that levitate the droplets to the liquid nitrogen surface and to the side of the receiving container [44]. Recovery in liquid nitrogen can be improved by designing more efficient ejector and collector systems.
Pertinent to future clinical applications, sterilization of each component of the ejection system and performing the vitrification experiments in a sterile hood are required elements of the protocol. The ejector, Tygon tubing and aluminium foil can be sterilized using an autoclave. The nitrogen gas from the gas cylinder can be sterilized by passing it through a 0.22-μm filter before reaching the ejector, while the sterilization of liquid nitrogen can be accomplished by using sterile polytetrafluoroethylene cartridge filters [60], or by UV radiation [61]. The use of sterile liquid nitrogen would minimize potential contamination [62] during the vitrification process in which oocyte encapsulating CPA droplets come into direct contact with liquid nitrogen.
The advent of vitrification technologies was enabled by the ability to decrease the freezing volumes and increase the heat transfer rates. The recent work already presents advancements in clinical outcomes enabled by using vitrification over slow-freezing methods. The technology presented here overcomes a significant problem with vitrification technologies, which is the inability to control the freezing volume. Here, we presented a system that can limit and control the droplet volumes encapsulating vitrified oocytes. This controllable process minimizes the variability in the procedures. Furthermore, the automation capabilities introduced by this technology can be useful for repeatable and reliable operations in an embryology laboratory.
Conclusion
We have introduced a nanoliter-scale, ultra-rapid vitrification method for oocyte cryopreservation that employs continuously generated droplets vitrified at low CPA concentrations. We envision that this methodology has the potential to substantially improve the efficiency of oocyte cryopreservation and potentially the success rate of artificial reproductive techniques that use post-thawed oocytes.
Future perspective
In the light of ever increasing survival rates in women of childbearing age with cancer, recommendation for fertility preservation at the initial treatment stage arises, as the cancer treatment can lead to infertility. Oocyte cryopreservation presents as a viable and preferred fertility preservation option for young females and patients who have ethical or religious objections to embryo freezing. The advances in clinical cryopreservation methods have been accelerated in the recent years with the emerging nano/microscale technologies. These technologies enable manipulation of cells in nanoscale volumes reliably and repeatably. Using these technological advances to address oocyte biopreservation, which is still considered challenging and experimental, may lead to automated routine clinical procedures increasing successful biopreservation outcomes. The future directions of this work could enable new vitrification strategies and improved protocols. This research can have a significant impact on the long-term storage for both clinical and research applications by combining the low CPA toxicity of slow freezing with diminished ice crystal formation of vitrification to address the challenging demands of cryopreservation technologies. Furthermore, this approach creates new pathways to biopreserve other biological materials, including stem cells and human tissues, for example, blood leading to potential broad medical applications.
Executive summary.
Ejector-based droplet generation system was demonstrated for the first time to continuously vitrify oocytes encapsulated in nanoliter droplets.
The nitrogen gas flow rates were optimized to generate droplets at an average size of 140 ± 58 μm, which was equivalent to 1.4 nl for oocyte vitrification.
Oocytes retrieved from cryoprotectant agents loading/unloading, ejection and vitrification/thawing were evaluated for survival rates, and similar oocyte morphology were observed compared with fresh oocytes.
Oocytes recovered from ejection were assessed for embyo development resulting from parthenogenetical activation using SrCl2, where 96% of cleavage rate and 26% of blastocyst rate were achieved.
The significant reduction in droplet volume allowed oocytes to be vitrified using cryoprotectant agent concentrations as low as 2.5 M (8% ethylene glycol [v/v], 8% dimethyl sulfoxide [v/v] and 1 M sucrose).
The ejector-based nanodroplet generation system enables to create droplets in a continuous manner with minimal manual involvement, thus improving the vitrification efficiency and repeatability.
Acknowledgments
X Zhang, E Kayaalp, A Nureddin, RM Anchan, RL Maas and U Demirci designed the experiments. X Zhang, I Khimji, L Shao, H Safaee, K Desai and HO Keles performed the experiments. X Zhang, UA Gurkan, E Kayaalp, A Nureddin, RM Anchan, RL Maas and U Demirci analyzed data. X Zhang, UA Gurkan, A Nureddin, RM Anchan, RL Maas and U Demirci prepared the manuscript.
We would like to thank YS Song for helping with the initial training.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Financial & competing interests disclosure
This work was partially supported by NIH Common Fund grants UL1DE019581 and RL1DE019021 (RLM, RMA and UD) and by NIH grants R21HL095960, R21EB007707, R01AI081534, R21AI087107, the W.H. Coulter Foundation Young Investigator Award, and by the Center for Integration of Medicine and Innovative Technology (CIMIT) under the US Army Medical Research Acquisition Activity Cooperative Agreement (UD). RM Anchan would like to acknowledge the following funding sources: Presidential New Investigator Award established by the Klarman Family Foundation and NIH grant (K12HD001255) from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Gidoni Y, Holzer H, Tulandi T, Tan SL. Fertility preservation in patients with non-oncological conditions. Reprod Biomed Online. 2008;16(6):792–800. doi: 10.1016/s1472-6483(10)60144-7. [DOI] [PubMed] [Google Scholar]
- 2.Vogelzang NJ. Treatment options in metastatic renal carcinoma: an embarrassment of riches. J Clin Oncol. 2006;24(1):1–3. doi: 10.1200/JCO.2005.03.7234. [DOI] [PubMed] [Google Scholar]
- 3.Ethics Committee of the American Society of Reproductive Medicine. Fertility preservation and reproduction in cancer patients. Fertil Steril. 2005;83(6):1622–1628. doi: 10.1016/j.fertnstert.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 4.Chen C. Pregnancy after human oocyte cryopreservation. Lancet. 1986;1(8486):884–886. doi: 10.1016/s0140-6736(86)90989-x. [DOI] [PubMed] [Google Scholar]
- 5.Oktay K, Cil AP, Bang H. Efficiency of oocyte cryopreservation: a meta-analysis. Fertil Steril. 2006;86(1):70–80. doi: 10.1016/j.fertnstert.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 6.Practice Committee of Society for Assisted Reproductive Technology, Practice Committee of American Society for Reproductive Medicine. Essential elements of informed consent for elective oocyte cryopreservation: a Practice Committee opinion. Fertil Steril. 2008;90(5 Suppl):S134–S135. doi: 10.1016/j.fertnstert.2008.08.061. [DOI] [PubMed] [Google Scholar]
- 7▪.Tao T, Zhang W, Del Valle A. Human oocyte cryopreservation. Curr Opin Obstet Gynecol. 2009;21(3):247–252. doi: 10.1097/GCO.0b013e328329c2d2. Summarizes the clinical breakthroughs in human oocyte cryopreservation with an emphasis on vitrification methods. [DOI] [PubMed] [Google Scholar]
- 8.Fadini R, Brambillasca F, Renzini MM, et al. Human oocyte cryopreservation: comparison between slow and ultrarapid methods. Reprod Biomed Online. 2009;19(2):171–180. doi: 10.1016/s1472-6483(10)60069-7. [DOI] [PubMed] [Google Scholar]
- 9.Agca Y. Cryopreservation of oocyte and ovarian tissue. ILAR J. 2000;41(4):207–220. doi: 10.1093/ilar.41.4.207. [DOI] [PubMed] [Google Scholar]
- 10.Fabbri R, Porcu E, Marsella T, Rocchetta G, Venturoli S, Flamigni C. Human oocyte cryopreservation: new perspectives regarding oocyte survival. Hum Reprod. 2001;16(3):411–416. doi: 10.1093/humrep/16.3.411. [DOI] [PubMed] [Google Scholar]
- 11.Lucena E, Bernal DP, Lucena C, Rojas A, Moran A, Lucena A. Successful ongoing pregnancies after vitrification of oocytes. Fertil Steril. 2006;85(1):108–111. doi: 10.1016/j.fertnstert.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 12.Bernard A, Fuller BJ. Cryopreservation of human oocytes: a review of current problems and perspectives. Hum Reprod Update. 1996;2(3):193–207. doi: 10.1093/humupd/2.3.193. [DOI] [PubMed] [Google Scholar]
- 13.Gratwohl A. Thomas’ hematopoietic cell transplantation. Eur J Haematol. 2010;84(1):95. doi: 10.1111/j.1600-0609.2009.01360.x. [DOI] [PubMed] [Google Scholar]
- 14.Fahy GM, MacFarlane DR, Angell CA, Meryman HT. Vitrification as an approach to cryopreservation. Cryobiology. 1984;21(4):407–426. doi: 10.1016/0011-2240(84)90079-8. [DOI] [PubMed] [Google Scholar]
- 15.Rayos AA, Takahashi Y, Hishinuma M, Kanagawa H. Quick freezing of unfertilized mouse oocytes using ethylene glycol with sucrose or trehalose. J Reprod Fertil. 1994;100(1):123–129. doi: 10.1530/jrf.0.1000123. [DOI] [PubMed] [Google Scholar]
- 16.Lin TK, Su JT, Lee FK, Lin YR, Lo HC. Cryotop vitrification as compared to conventional slow freezing for human embryos at the cleavage stage: survival and outcomes. Taiwan J Obstet Gynecol. 2010;49(3):272–278. doi: 10.1016/S1028-4559(10)60060-5. [DOI] [PubMed] [Google Scholar]
- 17.Desai N, Abdelhafez F, Ali MY, et al. Mouse ovarian follicle cryopreservation using vitrification or slow programmed cooling: assessment of in vitro development, maturation, ultra-structure and meiotic spindle organization. J Obstet Gynaecol Res. 2010:1–12. doi: 10.1111/j.1447-0756.2010.01215.x. [DOI] [PubMed] [Google Scholar]
- 18.Keskintepe L, Agca Y, Sher G, Keskintepe M, Maassarani G. High survival rate of metaphase II human oocytes after first polar body biopsy and vitrification: determining the effect of previtrification conditions. Fertil Steril. 2009;92(5):1706–1715. doi: 10.1016/j.fertnstert.2008.08.114. [DOI] [PubMed] [Google Scholar]
- 19.Mukaida T, Takahashi K, Kasai M. Blastocyst cryopreservation: ultrarapid vitrification using cryoloop technique. Reprod Biomed Online. 2003;6(2):221–225. doi: 10.1016/s1472-6483(10)61713-0. [DOI] [PubMed] [Google Scholar]
- 20.Liebermann J, Tucker MJ, Graham JR, Han T, Davis A, Levy MJ. Blastocyst development after vitrification of multipronuclear zygotes using the Flexipet denuding pipette. Reprod Biomed Online. 2002;4(2):146–150. doi: 10.1016/s1472-6483(10)61932-3. [DOI] [PubMed] [Google Scholar]
- 21.Abdelhafez FF, Desai N, Abou-Setta AM, Falcone T, Goldfarb J. Slow freezing, vitrification and ultra-rapid freezing of human embryos: a systematic review and meta-analysis. Reprod Biomed Online. 2010;20(2):209–222. doi: 10.1016/j.rbmo.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 22.Magalhaes R, Wang XW, Gouk SS, et al. Vitrification successfully preserves hepatocyte spheroids. Cell Transplant. 2008;17(7):813–828. doi: 10.3727/096368908786516765. [DOI] [PubMed] [Google Scholar]
- 23.Arav A, Shehu D, Mattioli M. Osmotic and cytotoxic study of vitrification of immature bovine oocytes. J Reprod Fertil. 1993;99(2):353–358. doi: 10.1530/jrf.0.0990353. [DOI] [PubMed] [Google Scholar]
- 24.Vincent C, Johnson MH. Cooling, cryoprotectants, and the cytoskeleton of the mammalian oocyte. Oxf Rev Reprod Biol. 1992;14:73–100. [PubMed] [Google Scholar]
- 25.Vincent C, Garnier V, Heyman Y, Renard JP. Solvent effects on cytoskeletal organization and in-vivo survival after freezing of rabbit oocytes. J Reprod Fertil. 1989;87(2):809–820. doi: 10.1530/jrf.0.0870809. [DOI] [PubMed] [Google Scholar]
- 26.Joly C, Bchini O, Boulekbache H, Testart J, Maro B. Effects of 1,2-propanediol on the cytoskeletal organization of the mouse oocyte. Hum Reprod. 1992;7(3):374–378. doi: 10.1093/oxfordjournals.humrep.a137654. [DOI] [PubMed] [Google Scholar]
- 27.Saunders KM, Parks JE. Effects of cryopreservation procedures on the cytology and fertilization rate of in vitro-matured bovine oocytes. Biol Reprod. 1999;61(1):178–187. doi: 10.1095/biolreprod61.1.178. [DOI] [PubMed] [Google Scholar]
- 28.Fahy GM, Wowk B, Wu J, Paynter S. Improved vitrification solutions based on the predictability of vitrification solution toxicity. Cryobiology. 2004;48(1):22–35. doi: 10.1016/j.cryobiol.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 29.Eroglu A. Cryopreservation of mammalian oocytes by using sugars: intra- and extracellular raffinose with small amounts of dimethylsulfoxide yields high cryosurvival, fertilization, and development rates. Cryobiology. 2010;60(3 Suppl):S54–S59. doi: 10.1016/j.cryobiol.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Z, Sun Z, Chen Y, He F. A modified cryoloop vitrification protocol in the cryopreservation of mature mouse oocytes. Zygote. 2009;17(3):217–224. doi: 10.1017/S0967199409005309. [DOI] [PubMed] [Google Scholar]
- 31.Vajta G, Holm P, Kuwayama M, et al. Open pulled straw (OPS) vitrification: a new way to reduce cryoinjuries of bovine ova and embryos. Mol Reprod Dev. 1998;51(1):53–58. doi: 10.1002/(SICI)1098-2795(199809)51:1<53::AID-MRD6>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 32.Suo L, Zhou GB, Meng QG, et al. OPS vitrification of mouse immature oocytes before or after meiosis: the effect on cumulus cells maintenance and subsequent development. Zygote. 2009;17(1):71–77. doi: 10.1017/S0967199408005091. [DOI] [PubMed] [Google Scholar]
- 33.Chen SU, Lien YR, Chen HF, Chao KH, Ho HN, Yang YS. Open pulled straws for vitrification of mature mouse oocytes preserve patterns of meiotic spindles and chromosomes better than conventional straws. Hum Reprod. 2000;15(12):2598–2603. doi: 10.1093/humrep/15.12.2598. [DOI] [PubMed] [Google Scholar]
- 34.Chen SU, Lien YR, Cheng YY, Chen HF, Ho HN, Yang YS. Vitrification of mouse oocytes using closed pulled straws (CPS) achieves a high survival and preserves good patterns of meiotic spindles, compared with conventional straws, open pulled straws (OPS) and grids. Hum Reprod. 2001;16(11):2350–2356. doi: 10.1093/humrep/16.11.2350. [DOI] [PubMed] [Google Scholar]
- 35▪.Zhang X, Catalano PN, Gurkan UA, Khimji I, Demirci U. Emerging technologies in medical applications of minimum volume vitrification. Nanomedicine. 2011;6(6):1115–1129. doi: 10.2217/nnm.11.71. Comprehensive review on broad applications of minimum volume vitrification. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martino A, Songsasen N, Leibo SP. Development into blastocysts of bovine oocytes cryopreserved by ultra-rapid cooling. Biol Reprod. 1996;54(5):1059–1069. doi: 10.1095/biolreprod54.5.1059. [DOI] [PubMed] [Google Scholar]
- 37.Kim SH, Ku SY, Sung KC, et al. Simplified EM grid vitrification is a convenient and efficient method for mouse mature oocyte cryopreservation. Yonsei Med J. 2006;47(3):399–404. doi: 10.3349/ymj.2006.47.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38▪▪.Lane M, Schoolcraft WB, Gardner DK. Vitrification of mouse and human blastocysts using a novel cryoloop container-less technique. Fertil Steril. 1999;72(6):1073–1078. doi: 10.1016/s0015-0282(99)00418-5. Cryoloop was for the first time successfully used in the cryopreservation of both mouse and human embryos. [DOI] [PubMed] [Google Scholar]
- 39.Xu F, Moon S, Zhang X, Shao L, Song YS, Demirci U. Multi-scale heat and mass transfer modelling of cell and tissue cryopreservation. Philos Transact A Math Phys Eng Sci. 2010;368(1912):561–583. doi: 10.1098/rsta.2009.0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Landa V, Tepla O. Cryopreservation of mouse 8-cell embryos in microdrops. Folia Biol (Praha) 1990;36(3–4):153–158. [PubMed] [Google Scholar]
- 41.Kuwayama M. Highly efficient vitrification for cryopreservation of human oocytes and embryos: the Cryotop method. Theriogenology. 2007;67(1):73–80. doi: 10.1016/j.theriogenology.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 42.Papis K, Shimizu M, Izaike Y. Factors affecting the survivability of bovine oocytes vitrified in droplets. Theriogenology. 2000;54(5):651–658. doi: 10.1016/S0093-691X(00)00380-0. [DOI] [PubMed] [Google Scholar]
- 43.Seki S, Mazur P. The dominance of warming rate over cooling rate in the survival of mouse oocytes subjected to a vitrification procedure. Cryobiology. 2009;59(1):75–82. doi: 10.1016/j.cryobiol.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44▪▪.Song YS, Adler D, Xu F, et al. Vitrification and levitation of a liquid droplet on liquid nitrogen. Proc Natl Acad Sci USA. 2010;107(10):4596–4600. doi: 10.1073/pnas.0914059107. Simultaneous occurrence of levitation of droplets on liquid nitrogen (Leidenfrost phenomenon) and vitrification was theoretically and experimentally demonstrated for the first time. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bagis H, Sagirkaya H, Mercan HO, Dinnyes A. Vitrification of pronuclear-stage mouse embryos on solid surface (SSV) versus in cryotube: comparison of the effect of equilibration time and different sugars in the vitrification solution. Mol Reprod Dev. 2004;67(2):186–192. doi: 10.1002/mrd.10388. [DOI] [PubMed] [Google Scholar]
- 46.Dhali A, Anchamparuthy VM, Butler SP, Pearson RE, Mullarky IK, Gwazdauskas FC. Gene expression and development of mouse zygotes following droplet vitrification. Theriogenology. 2007;68(9):1292–1298. doi: 10.1016/j.theriogenology.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 47.Dinnyes A, Dai Y, Jiang S, Yang X. High developmental rates of vitrified bovine oocytes following parthenogenetic activation, in vitro fertilization, and somatic cell nuclear transfer. Biol Reprod. 2000;63(2):513–518. doi: 10.1095/biolreprod63.2.513. [DOI] [PubMed] [Google Scholar]
- 48.Song YS, Moon S, Hulli L, Hasan SK, Kayaalp E, Demirci U. Microfluidics for cryopreservation. Lab Chip. 2009;9(13):1874–1881. doi: 10.1039/b823062e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49▪▪.Demirci U, Montesano G. Cell encapsulating droplet vitrification. Lab Chip. 2007;7(11):1428–1433. doi: 10.1039/b705809h. Highlights for the first time that vitrification of droplets can be achieved at high throughput. [DOI] [PubMed] [Google Scholar]
- 50.Demirci U, Montesano G. Single cell epitaxy by acoustic picolitre droplets. Lab Chip. 2007;7(9):1139–1145. doi: 10.1039/b704965j. [DOI] [PubMed] [Google Scholar]
- 51.Moon S, Hasan SK, Song YS, et al. Layer by layer three-dimensional tissue epitaxy by cell-laden hydrogel droplets. Tissue Eng Part C Methods. 2010;16(1):157–166. doi: 10.1089/ten.TEC.2009.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu F, Moon SJ, Emre AE, et al. A droplet-based building block approach for bladder smooth muscle cell (SMC) proliferation. Biofabrication. 2010;2(1):014105. doi: 10.1088/1758-5082/2/1/014105. [DOI] [PubMed] [Google Scholar]
- 53▪▪.Samot J, Moon S, Shao L, et al. Blood banking in living droplets. PLoS ONE. 2011;6(3):e17530. doi: 10.1371/journal.pone.0017530. First demonstration of a high-throughput blood vitrification platform based on nanoliter droplets. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moon S, Kim YG, Dong L, et al. Drop-on-demand single cell isolation and total RNA analysis. PLoS ONE. 2011;6(3):e17455. doi: 10.1371/journal.pone.0017455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim YG, Moon S, Kuritzkes DR, Demirci U. Quantum dot-based HIV capture imaging in a microfluidic channel. Biosens Bioelectron. 2009;25(1):253–258. doi: 10.1016/j.bios.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moon S, Keles HO, Ozcan A, et al. Integrating microfluidics and lensless imaging for point-of-care testing. Biosens Bioelectron. 2009;24(11):3208–3214. doi: 10.1016/j.bios.2009.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang X, Khimji I, Gurkan UA, et al. Lensless imaging for simultaneous microfluidic sperm monitoring and sorting. Lab Chip. 2011;11:2535–2540. doi: 10.1039/c1lc20236g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen SU, Yang YS. Slow freezing or vitrification of oocytes: their effects on survival and meiotic spindles, and the time schedule for clinical practice. Taiwan J Obstet Gynecol. 2009;48(1):15–22. doi: 10.1016/S1028-4559(09)60030-9. [DOI] [PubMed] [Google Scholar]
- 59.Lane M, Gardner DK. Vitrification of mouse oocytes using a nylon loop. Mol Reprod Dev. 2001;58(3):342–347. doi: 10.1002/1098-2795(200103)58:3<342::AID-MRD13>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 60▪▪.McBurnie LD, Bardo B. Validation of sterile filtration of liquid nitrogen. Pharm Technol. 2002;26:9. A paper describing how liquid nitrogen can be sterilized through filtration technique. [Google Scholar]
- 61.Parmegiani L, Cognigni GE, Filicori M. Ultra-violet sterilization of liquid nitrogen prior to vitrification. Hum Reprod. 2009;24(11):2969. doi: 10.1093/humrep/dep329. [DOI] [PubMed] [Google Scholar]
- 62.Bielanski A, Vajta G. Risk of contamination of germplasm during cryopreservation and cryobanking in IVF units. Hum Reprod. 2009;24(10):2457–2467. doi: 10.1093/humrep/dep117. [DOI] [PubMed] [Google Scholar]