Abstract
Infectious agents are likely to play a role in the pathogenesis of chronic inflammatory diseases, including abdominal aortic aneurysms (AAAs). The goal of this study was to determine if Borrelia burgdorferi sensu lato (sl), a microorganism responsible for Lyme disease, is involved in the etiology of AAAs. The presence of serum antibodies against B. burgdorferi sl was measured with enzyme-linked immunosorbent assay (ELISA) and confirmed by Western blotting in 96 AAA and 108 peripheral artery disease (PAD) patients. Polymerase chain reaction (PCR) was used for the detection of Borrelia-specific DNA in the aneurysm wall. Among AAA patients 34% and among PAD patients 16% were seropositive for B. burgdorferi sl antibodies (Fisher’s exact test, p = 0.003; odds ratio [OR] 2.79; 95% confidence interval [CI] 1.37–5.85). In the German general population, 3–17% are seropositive for Borrelia antibodies. No Borrelia DNA was detected in the aneurysm wall. Our findings suggest a relationship between AAAs and B. burgdorferi sl. We hypothesize that the underlying mechanism for B. burgdorferi sl in AAA formation is similar to that by the spirochete Treponema pallidum; alternatively, AAAs could develop due to induced autoimmunity via molecular mimicry due to similarities between some of the B. burgdorferi sl proteins and aortic proteins.
Introduction
Abdominal aortic aneurysm (AAA), defined as a dilatation greater than 3 cm of the infrarenal abdominal aorta, is a complex disease of the aging population [1]. Rupture of AAAs is associated with a high mortality rate, making aortic aneurysms the 15th leading cause of death among Caucasians over the age of 65 years in the USA [2]. The pathogenesis of AAAs is complex and poorly understood.
Characteristics of AAA pathogenesis include inflammation, vascular smooth muscle cell (VSMC) apoptosis, extracellular matrix degradation, and oxidative stress [3]. T-cells in AAA patients secrete proinflammatory cytokines [4], and natural killer cells display increased cytotoxicity [5]. Autoimmunity may also play a role in AAA development and progression [6, 7]. Several studies have examined microorganisms, including Chlamydia [8–10], Mycoplasma pneumoniae [9], Helicobacter pylori [10], human cytomegalovirus (HCMV) [11], herpes simplex virus (HSV) [12], and different oral bacteria [13–15], as possible triggers for the development of AAAs, but the data are inconclusive. A nowadays rarely seen cause, but a clinically and histologically proven etiological factor, is Treponema pallidum; the infection leads to luetic thoracic aortic aneurysm in the late stage of syphilitic disease [16]. T. pallidum is a member of the spirochete family (Treponemataceae); other members of the spirochete family are Leptospira and Borrelia. Besides B. recurrentis, the causative organism of relapsing fever, numerous Borrelia species are colonizing in humans and animals. The spirochete B. burgdorferi is known to cause human Lyme disease in Europe. To date, seven pathogenic B. burgdorferi species in humans have been described, and are collectively referred to as B. burgdorferi sensu lato (sl): B. burgdorferi sensu stricto (ss), B. afzelii, B. garinii, B. spielmanii, B. valaisiana, B. lusitaniae, and B. bissettii [17].
There have been few case reports on intracranial [18, 19] and coronary artery [20] aneurysms in patients infected with B. burgdorferi sl.
The aim of this study was to investigate the presence of antibodies against B. burgdorferi sl in patients with AAAs compared to patients with peripheral artery disease (PAD) in order to test the hypothesis that B. burgdorferi sl is an etiological agent in AAA development.
Materials and methods
Patients
In this case–control study, 96 consecutive patients diagnosed with AAA using ultrasonography or computed tomography (diameter >30 mm) were recruited at the Vascular Surgery Department, Technical University of Dresden, Germany (Table 1). The mean age of the patients was 71.3 ± 9.1 years and 89 (93%) were males. Most (80; 83%) patients had a diameter of the AAA ≥5 cm and underwent elective repair (open or endovascular) and 14 (15%) patients had a ruptured AAA.
Table 1.
Characteristics of the study groups
| AAA (n = 96) | PAD (n = 108) | p-value | |||
|---|---|---|---|---|---|
| Age, mean ± SD (years) | 72.3 | ± 9.1 | 69.1 | ± 9.6 | 0.013 |
| Men (%) | 89/96 | (93) | 94/108 | (87) | 0.249 |
| PAD (%) | 15/78 | (41) | 108/108 | (100) | <2.2 × 10−16 |
| Coronary artery disease (%) | 30/78 | (39) | 44/107 | (41) | 0.762 |
| Hypertensiona (%) | 67/77 | (87) | 97/107 | (91) | 0.477 |
| Diabetes mellitus (%) | 21/77 | (27) | 46/107 | (43) | 0.031 |
| Dyslipidemiab (%) | 50/77 | (65) | 87/107 | (81) | 0.016 |
| Aspirin (%) | 61/74 | (82) | 80/106 | (76) | 0.278 |
| Clopidogrel (%) | 3/74 | (4.1) | 36/106 | (34) | 6.9 × 10−7 |
| Calcium antagonists (%) | 24/74 | (32) | 36/106 | (34) | 0.873 |
| ACE inhibitors (%) | 45/74 | (61) | 56/106 | (53) | 0.360 |
| Diuretics (%) | 38/74 | (51) | 54/106 | (51) | 1.000 |
| Beta-blockers (%) | 56/74 | (76) | 60/106 | (57) | 0.011 |
| Statins (%) | 48/74 | (65) | 84/106 | (79) | 0.040 |
| Corticosteroids (%) | 3/74 | (4.1) | 1/106 | (0.9) | 0.307 |
| Hemoglobin (mmol/l) | 8.2 | ± 1.2 | 8.1 | ± 1.2 | 0.379 |
| Hematocrit | 0.39 | ± 0.1 | 0.39 | ± 0.1 | 0.590 |
| White blood cell count ( × 109/l) | 8.6 | ± 3.9 | 8.1 | ± 2.4 | 0.935 |
| Thrombocyte count ( × 109/l) | 211.9 | ± 67.2 | 257.6 | ± 98.1 | 0.00005 |
| HbA1c (%) | 6.6 | ± 1.3 | 6.3 | ± 0.8 | 0.553 |
| C-reactive protein (mg/l) | 21.5 | ± 41.2 | 17.4 | ± 32.8 | 0.300 |
| Cholesterol (mmol/l) | 4.7 | ± 1.3 | 4.7 | ± 1.4 | 0.363 |
| LDL (mmol/l) | 2.9 | ± 1.0 | 2.6 | ± 1.1 | 0.037 |
| HDL (mmol/l) | 1.2 | ± 0.4 | 1.2 | ± 0.4 | 0.998 |
| Triglycerides (mmol/l) | 1.9 | ± 2.0 | 13.7 | ± 56.7 | 0.143 |
| Creatinine (mmol/l) | 113.5 | ± 41.9 | 103.6 | ± 99.3 | 2.9 × 10−5 |
Comparisons between cases and controls were carried out using multivariate logistic regression. The number of individuals in each study group for whom the information was available is indicated. Laboratory parameters are shown as mean ± standard deviation (SD) and were measured preoperatively
aBlood pressure ≥ 140/90 mmHg
bIncreased LDL (male and female: ≥ 4.12 mmol/l; age > 35 years) and decreased HDL (male: ≤ 0.90 mmol/l;female ≤ 1.3 mmol/l; age > 35 years)
The control group comprised of 108 patients with PAD, with mean age of 69.1 ± 9.6 years, and 94 (87%) males. The PAD patients were approximately age- and sex-matched to the AAA cases. AAA was excluded by ultrasonography. The severity of PAD was assessed using the Fontaine classification for chronic ischemia. Altogether, 49 (45%) patients had PAD Fontaine stage IIb, 23 (21%) Fontaine stage III, and 36 (33%) had Fontaine stage IV. They all underwent endovascular or open surgery for revascularization (Table 1).
The study was approved by the Ethics Committee of the Medical Faculty at the Technical University Dresden, Germany (EK 316122008). All patients gave informed written consent prior to enrolment into the study.
Sample collection
Venous blood samples were collected with Serum Monovette® (Sarstedt AG & Co., Nümbrecht, Germany). Samples were centrifuged immediately and stored at −80°C.
AAA wall specimens were obtained during surgery from 26 patients with aortic diameter >5 cm; of those, ten were known to be seronegative and 16 seropositive for Borrelia antibodies. In each group, four patients had ruptured AAAs. AAA wall tissue specimens were snap-frozen in liquid nitrogen immediately after harvesting and stored at −80°C.
Immunological tests
Antibodies against B. burgdorferi sl were analyzed according to the Quality Standards for the Microbiological Diagnosis of Infectious Disease (MiQ) [21] 12/2000 and DIN58969-44 by enzyme-linked immunosorbent assay (ELISA), and positive results were confirmed by Western blots. Borrelia afzelii + VlsE IgG ELISA (Pko strain) and Borrelia afzelii IgM ELISA test kits (Genzyme Virotec GmbH, Rüsselsheim, Germany) were used as screening tests in the serum of 96 AAA patients and 108 PAD patients to quantify specific IgG and IgM classes.
Quantitative results were given as arbitrary ELISA units according to the manufacturer’s instructions (positive >11 U/ml, borderline 9–11 U/ml). According to the two-stage diagnosis scheme MiQ [21], immunoblotting with Borrelia LINE test kits (Genzyme Virotec GmbH, Rüsselsheim, Germany) was carried out with samples showing borderline and positive results in ELISA. The immunoblotting kits use recombinant antigens against the four most common B. burgdorferi sl species (B. burgdorferi ss, B. afzelii, B. garinii, and B. spielmanii). Other species causing Lyme disease are not detected with the assay, but they are rare and, to date, described only in certain geographic regions [17]. Samples that gave positive results in both ELISA and Western blot were considered to be positive for B. burgdorferi sl antibodies.
Treponema pallidum hemagglutination assay (TPHA; Immunogenetics, Heiden, Germany) was performed in order to exclude infection with T. pallidum.
DNA analysis
DNA was extracted from AAA tissue specimens with a method using the TRIzol reagent (Invitrogen GmbH, Darmstadt, Germany). The nested polymerase chain reaction (PCR) method approved for clinical use for the detection of Borrelia-specific DNA and β-actin DNA as a “housekeeping” gene was used. Nested PCR was carried out first with eubacterial primers TPU1 and RT1 [22] (Eurofins MWG Operon, Ebersberg, Germany) for the screening of any bacterial DNA. In a second step, amplification with Borrelia-specific primer BB16SF and RT1 primer [22] (Eurofins MWG Operon, Ebersberg, Germany) was performed according to the manufacturer’s instructions. Positive as well as no-template controls were included in each PCR run.
Statistical analysis
Clinical data were evaluated by Fisher’s exact test or the Wilcoxon rank-sum test and multivariate logistic regression analysis. The data are expressed as mean ± SD for continuous variables. Fisher’s exact test was used for comparison of the seroprevalences. Odds ratios (ORs) and 95% confidence intervals (CIs) were also calculated. Statistical analyses were performed using SPSS for Windows version 11.0 (SPSS, Birmingham, UK), except for the CIs for binomial probabilities, which were calculated in R [23]. All reported p-values are two-sided and a p-value of <0.05 was considered to be statistically significant.
Results
The clinical data and results from the multivariate logistic regression analysis between the study groups are presented in Table 1. The AAA group of patients differed from the PAD group in preoperative creatinine levels and thrombocyte counts. A slight difference in the number of individuals with a diagnosis of dyslipidemia [24] or diabetes mellitus [25] was observed, consistent with previous studies. Also, significantly more PAD patients were treated with clopidogrel and AAA patients were treated more often with beta-blockers. The groups were slightly different in regards to the mean age (72 vs. 69 years), but both mean ages were beyond the second peak of infection rate with B. burgdorferi sl (60 to 64 years) [26].
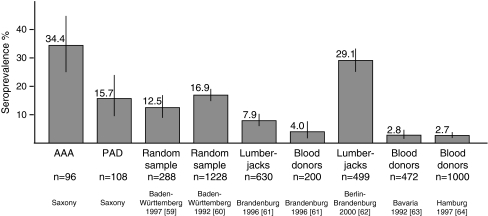
AAA cases and PAD controls were tested for the presence of IgG and IgM antibodies against Borrelia with two immunological techniques using validated reagents approved for the clinical testing of Lyme disease in Germany. The IgG antibodies detected by ELISA were present in 44 (46%) AAA patients, but in only 34 (32%) controls. The presence of IgG antibodies against B. burgdorferi sl was confirmed in 33 (34%) AAA patients and in 17 (16%) controls (p = 0.003, Fisher’s exact test; OR 2.79; 95% CI 1.37–5.85) using immunoblotting (Fig. 1). The lower number of positive samples observed in the immunoblotting compared to the ELISA is expected, as some of the samples gave only borderline positive results in ELISA. These results illustrate the need for two-step screening with ELISA and immunoblotting in order to avoid false-positives.
Fig. 1.
Seroprevalences in different risk groups in Germany [59–64]. Only studies with both enzyme-linked immunosorbent assay (ELISA) and immunoblotting data were included. The confidence intervals for binomial probabilities were calculated in R [23]
A summary of the Western blot results is provided in Table 2. Serum samples from the 33 AAA patients with positive results in the immunoblots reacted against B. afzelii (DbpA, decorin-binding protein A) in 20 cases and against B. garinii (p58, oligopeptide permease protein A-2) in eight cases; the serum from six AAA cases reacted against both species. In eight cases, only bands indicative of reaction against B. burgdorferi sl were seen (VlsE, variable major protein-like sequence E; OspC, outer surface protein C; BmpA, borrelial membrane protein A). Three patients had evidence for late-stage borreliosis by being positive for antigens against in vitro-expressed proteins BB0323, BBA36, and/or Crasp3 (complement regulator-acquiring surface protein 3).
Table 2.
Summary of immunoblotting results for abdominal aortic aneurysm (AAA) and peripheral artery disease (PAD) patients
| Borrelia classification | Immunoblota result | Number (%)b of positive samples in: | |
|---|---|---|---|
| AAA, n = 33 | PAD, n = 17 | ||
| B. burgdorferi sl | VlsE | 30 (91) | 17 (100) |
| OspC | 5 (15) | 1 (6) | |
| BmpA | 5 (15) | 2 (12) | |
| p83c | 6 (18) | 5 (29) | |
| BBO323c | 2 (6) | – | |
| BBA36c | 1 (3) | – | |
| Crasp3c | 3 (9) | – | |
| B. afzelii | DbpA-PKo | 20 (61) | 13 (76) |
| B. garinii | p58 | 8 (24) | 8 (47) |
aDescription of the bands seen in immunoblots: VlsE “vmp (variable major protein)-like-sequence E”, in vivo experiment B. burgdorferi lipoprotein with conserved highly immunogenic epitopes, which is a species-independent main marker in Borrelia diagnostics; OspC “outer surface protein C”, a highly variable surface protein of the B. burgdorferi sl group, early IgM marker of the B-cell immune response; BmpA “borrelial membrane protein A”, main marker of IgG serology for disseminated Lyme borreliosis; p83 highly conserved antigen in the B. burgdorferi sl group, an IgG marker of late-stage borreliosis; BB0323 a hypothetical protein identified in strain B31 of B. burgdorferi sl by genome analysis and verified by epitopes in vivo, marker of late-stage borreliosis, frequently positive in neuroborreliosis; BBA36 a lipoprotein of unknown function of B. burgdorferi sl strain B31, verified by epitopes in vivo, marker of late-stage borreliosis; Crasp3 “complement regulator-acquiring surface protein 3”, marker of late-stage borreliosis; DbpA-PKo “decorin-binding protein A”, highly specific marker for B. afzelii; p58 “oligopeptide permease protein A-2”, marker for B. garinii
bThe percentages indicate the proportion of samples positive for the given marker among all positive samples
cMarkers for late-stage borreliosis
The immunoblotting bands in the 17 PAD controls were similarly distributed. Thirteen PAD cases had bands for DbpA and eight for p58, five of whom had both bands. Crasp3 was not detected in any serum sample. In one case only were antibodies for OspC detected. None of the PAD cases had serological evidence for late-stage borreliosis (Table 2).
None of the samples were positive for B. burgdorferi sl IgM antibodies or showed positive TPHA test results, ruling out syphilitic aneurysms.
DNA analysis using nested PCR on ten seropositive and 16 seronegative aortic wall specimens did not detect any B. burgdorferi sl DNA. The failure to detect B. burgdorferi sl DNA was unlikely to be due to technical problems, but a true negative finding, since PCRs in which the samples were spiked with B. burgdorferi DNA, yielded a product. The results are consistent with a previous study [8].
Discussion
The underlying pathobiology of AAAs remains unknown, but several characteristic features have been recognized in the aortic wall, including chronic inflammation [7]. T-cell invasion in the adventitia and media with the secretion of cytokines [4, 27] and the detection of other inflammatory cells such as neutrophils, mast cells [28], natural killer cells [5], and plasma cells [29] raise the possibility that infectious agents trigger the inflammatory process in AAAs. Infectious agents as inducers of AAA development have been studied for more than 25 years. Bacterial cultures of thrombus and AAA wall specimens taken during open repair operations were positive in 8–43% of cases and revealed a variety of different microorganisms [30–32]. A significantly higher incidence of bacterial species was found in ruptured AAAs [33].
Using PCR-based techniques, bacterial DNA is found in more than 80% of AAA wall specimens [13, 15]. Of interest is that a high number of oral bacteria, associated with bacteremia, have been detected in patients with AAAs and other cardiovascular diseases [13, 34]. Infection with Porphyromonas gingivalis was recently shown to be associated with AAAs in Japanese [14] but not in Norwegian patients [35]. In vitro experiments demonstrated that VSMC proliferation was influenced by infection with P. gingivalis, providing a possible mechanism for the weakening of the AAA wall [36]. It is not clear, however, whether the bacteria contribute to weakening of the aortic wall by eliciting inflammation or whether they are secondary colonizers of thrombus and AAAs.
Among measured serum antigens in AAA patients, the most studied agent is C. pneumoniae [8, 10, 37, 38], which may be involved in the initiation or progression of AAAs [39, 40]. C. pneumoniae reactive T-lymphocytes were found in the mononuclear cell infiltrates of AAAs [41], but studies on detecting C. pneumonia DNA in the AAA wall were inconsistent (Table 3) [8, 10]. One of these studies screened also for Borrelia DNA in the AAA wall, with negative results [8].
Table 3.
Summary of studies investigating the presence of serum antibodies against microorganisms in AAA patients
| Microorganism | Study design | Sample number | IgG serum Ab (%)a, cases/controls | DNA in the AAA wall (%)b, cases/controls | Study |
|---|---|---|---|---|---|
| C. pneumoniae | Case only | 51 | 80/– | 51/– | Blasi et al., 1996 [10] |
| Case only | 12 | 67/– | 50/– | Juvonen et al., 1997 [65] | |
| Case only | 139c | 75/– | ND | Lindholt et al., 1999 [40] | |
| Case only | 22 | 54/– | ND | Halme et al., 1999 [41] | |
| Case–control | 81/56 | 96/88 | ND | Blanchard et al., 2000 [37] | |
| Case only | 102 | 61/– | 32/– | Porqueddu et al., 2002 [66] | |
| Case–control | 119/36 | 74/72 | ND | Nyberg et al., 2007 [67] | |
| Case–control | 68/68 | 47/37 | 0/0d | Falkensammer et al., 2007 [8] | |
| Case only | 211c | 76/– | ND | Karlsson et al., 2009 [68] | |
| Case–control | 50/110 | 82/77 | ND | Vikatmaa et al., 2010 [69] | |
| Case–control | 42/100 | 82/70 | ND | Karlsson et al., 2011 [38] | |
| H. pylori | Case only | 51 | 92/– | 0/– | Blasi et al., 1996 [10] |
| B. burgdorferi sl | Case–control | 96/108 | 34/16 | 0/–e | Present study |
aThe numbers indicate the percentage of positive samples in each study group
bThe presence of microorganism-specific DNA sequences in aortic wall tissue samples was assayed by PCR-based techniques
cStudy on AAA expansion
dNew sample set of 30 cases/15 controls. These samples were also tested for the presence of Borrelia DNA and all samples were negative for Borrelia DNA
eAnalyzed in ten seropositive and 16 seronegative cases
ND not determined
To date, the only proven bacterium causing aortic aneurysm remains the spirochete T. pallidum in the manifestation of tertiary (cardiovascular) syphilis [42]. The aneurysms occur mostly in the ascending aorta and only in less than 5% of cases in the abdominal aorta [43]. Diagnosis is confirmed by serological markers or by detecting Treponema DNA using PCR [42]. Histological examination demonstrated the invasion of T. pallidum in the infected aortic wall, as well as the presence of lymphocytes and plasma cells around the vasa vasorum in the adventitia [42].
Nowadays, infections with T. pallidum are, however, rare in developed countries. Infections by spirochetes in Europe are mostly due to B. burgdorferi sl, which can cause Lyme disease in humans; the microorganisms are transmitted by tick bites (Ixodes ricinus in Europe) and these ticks are vectors of more than 100 reservoir animals [44]. Risk groups include children between the ages of 5 and 9 years and adults of ages 60 to 64 years [26].
Apart from the infection path, Lyme disease is similar to syphilis in passing through different stages with silent phases of several years between the stages and affecting skin, joints, heart, and the central nervous system [45]. Lyme disease can manifest after 1 to 10 years in a latent stage of borreliosis. It is a chronic disease with multiple symptoms, of which neuroborreliosis is the most common form in Europe [45]. The acute phase of the disease can be skipped and symptoms can vary widely. It has been estimated that 3–4% of the German population have antibodies against B. burgdorferi sl and are asymptomatic (Fig. 1). In endemic regions, up to 17% of the population is seropositive for B. burgdorferi sl IgG antibodies (Fig. 1). The epidemiological significance of seroprevalence is unknown.
Besides the known late neurological symptoms, late vascular manifestations are also possible, as suggested by multiple lines of evidence. First, in neuroborreliosis, perivascular and vascular lymphocytic inflammation is associated with the presence of B. burgdorferi sl DNA [46]. Second, 30 days after the intradermal inoculation of B. burgdorferi sl in mice, leukocyte infiltrates, including plasma cells, occurred primarily in the periaortic adventitia [47]. Third, another animal model in monkeys showed that inoculation with B. burgdorferi sl resulted in carditis and aortitis, with the infiltration of T-cells, plasma cells, and macrophages; spirochetes were found in the aortic connective tissue [48]. Activation of the complement cascade as an immune reaction against the bacterium with deposition of the membrane attack complex was also shown [48]. Fourth, in vitro, B. burgdorferi sl lipoprotein was shown to upregulate transendothelial neutrophil migration [49]. Neutrophil recruitment in the AAA wall is thought to be critical in AAA development based on studies in a mouse model [50]. Fifth, carditis is found in up to 25% of Lyme disease patients [51].
The fact that we could not detect Borrelia DNA in the AAA wall, even with sensitive PCR-based methods, makes us hypothesize that AAAs are not triggered by the direct infection of the aortic wall by Borrelia. Instead, an alternative disease mechanism could involve molecular mimicry in which similar epitopes are present in the surface of Borrelia and components of the aortic wall. In this disease model, serologically detectable infection with Borrelia leads, in the long run, to destruction of the aortic tissue through autoimmune reaction. Although concrete evidence for this hypothesis in the case of Borrelia is still lacking, it has been suggested as the mechanism in other studies. These studies identified a candidate autoantigen called aortic aneurysm-associated protein-40 (AAAP-40) in the aortic wall. Interestingly, AAAP-40 has sequence homology with T. pallidum, HSV, and HCMV [6].
Of particular interest is the activation of complement cascade by B. burgdorferi sl [48]. In vitro, in spite of activation of the alternative and classical pathway in normal human serum, B. burgdorferi sl was resistant to the bactericidal activity [52]. B. burgdorferi sl was found to bind to complement factor H (CFH) [53], a natural inhibitor in the alternative pathway of the complement cascade. A role for complement cascade in human AAA was shown recently in a microarray-based expression analysis, where CFH was found to be downregulated [54]. The combination of decreased CFH expression in AAA tissue and additional inhibition of CFH by B. burgdorferi sl may lead to over-activation of the complement cascade, which can cause tissue damage in the aortic wall.
Another hypothesis is that positive Borrelia serology is only epiphenomena of the tick bites and that other pathological ingredients in tick saliva contribute to aneurysm development. In vitro experiments, using human microvascular endothelial cells, demonstrated that tick saliva is a negative modulator of angiogenesis-dependent wound healing and tissue repair [55]. It can induce the apoptosis of endothelial cells by degrading ITGA5β1. ITGA5, a major fibronectin receptor which is essential for cell growth and development, was shown to be significantly decreased in AAA tissue [56, 57].
One of the limitations of the current study is that no data were available on tick bites and that Lyme disease symptoms are treated medically in patients with previous history of Lyme disease. Additionally, we did not analyze differences in leisure activities, such as gardening, hiking, or hunting, which could expose the individuals to tick bites, or take into account the place of living (near forest areas or in more urban settings) between the study groups. We have no reason to assume that PAD patients were less active at the time, many years ago, when they were exposed to B. burgdorferi sl. It is interesting to note that the seroprevalence was higher in AAA patients than in groups such as forestry workers considered to be at high risk for Lyme disease (Fig. 1).
Another limitation of the study was the relatively small number of AAA (n = 96) and PAD (n = 108) patients studied. These results should, therefore, be considered as preliminary until they are confirmed in larger studies. Also, we studied only patients from a small geographic area; thus, it will be important to investigate other populations in the future. Finally, the results might have been different if we compared the AAA patients to so-called “supercontrols” without any evidence of cardiovascular diseases, rather than the PAD patients studied here. Our rationale for choosing PAD patients as the control group was to minimize false-positive findings, since both of these conditions have an underlying inflammatory component (as shown by the elevated C-reactive protein levels), but multiple lines of evidence strongly suggest that, although AAA and occlusive diseases share some common risk factors, they have different pathophysiology [58].
In conclusion, in this case–control study, significantly more patients with AAAs had B. burgdorferi sl IgG antibodies than an age- and sex-matched control group of patients with PAD from the same geographic region of Germany. No Borrelia-specific DNA was detected in seropositive AAA specimens using a sensitive PCR-based assay. These observations led us to speculate that B. burgdorferi sl acts through autoimmunity and molecular mimicry in AAAs and that B. burgdorferi sl is one component in the complex disease process of AAAs. Patients who have antibodies against B. burgdorferi sl and develop AAAs might have genetic susceptibility to AAAs and the microorganism acts as an environmental trigger in the disease process.
Acknowledgments
I.H. is a recipient of Research Fellowships from Deutsche Forschungsgemeinschaft (Hi 1479/2-1) and from the Technical University of Dresden (“Frauenhabilitationsstipendium der Medizinischen Fakultät Dresden”), Germany.
Conflict of interest
All authors declare no conflicts of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
I. Hinterseher and G. Gäbel contributed equally to the work.
References
- 1.Sakalihasan N, Limet R, Defawe OD. Abdominal aortic aneurysm. Lancet. 2005;365:1577–1589. doi: 10.1016/S0140-6736(05)66459-8. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC), National Center for Injury Prevention and Control (NCIPC) (2005) Web-based Injury Statistics Query and Reporting System (WISQARS™). http://www.cdc.gov/injury/wisqars/index.html. Accessed 4 July 2011
- 3.Boddy AM, Lenk GM, Lillvis JH, Nischan J, Kyo Y, Kuivaniemi H. Basic research studies to understand aneurysm disease. Drug News Perspect. 2008;21:142–148. [PubMed] [Google Scholar]
- 4.Forester ND, Cruickshank SM, Scott DJ, Carding SR. Functional characterization of T cells in abdominal aortic aneurysms. Immunology. 2005;115:262–270. doi: 10.1111/j.1365-2567.2005.02157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forester ND, Cruickshank SM, Scott DJ, Carding SR. Increased natural killer cell activity in patients with an abdominal aortic aneurysm. Br J Surg. 2006;93:46–54. doi: 10.1002/bjs.5215. [DOI] [PubMed] [Google Scholar]
- 6.Jagadesham VP, Scott DJ, Carding SR. Abdominal aortic aneurysms: an autoimmune disease? Trends Mol Med. 2008;14:522–529. doi: 10.1016/j.molmed.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 7.Kuivaniemi H, Platsoucas CD, Tilson MD., 3rd Aortic aneurysms: an immune disease with a strong genetic component. Circulation. 2008;117:242–252. doi: 10.1161/CIRCULATIONAHA.107.690982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falkensammer B, Duftner C, Seiler R, Pavlic M, Walder G, Wilflingseder D, Stoiber H, Klein-Weigel P, Dierich M, Fraedrich G, Würzner R, Schirmer M. Lack of microbial DNA in tissue specimens of patients with abdominal aortic aneurysms and positive Chlamydiales serology. Eur J Clin Microbiol Infect Dis. 2007;26:141–145. doi: 10.1007/s10096-006-0245-5. [DOI] [PubMed] [Google Scholar]
- 9.Pires LJ, Gutierrez PS. Morphometrical quantification of Chlamydia pneumoniae and Mycoplasma pneumoniae in human atherosclerotic abdominal aortic aneurysms. Rev Bras Cir Cardiovasc. 2007;22:322–331. doi: 10.1590/S0102-76382007000300009. [DOI] [PubMed] [Google Scholar]
- 10.Blasi F, Denti F, Erba M, Cosentini R, Raccanelli R, Rinaldi A, Fagetti L, Esposito G, Ruberti U, Allegra L. Detection of Chlamydia pneumoniae but not Helicobacter pylori in atherosclerotic plaques of aortic aneurysms. J Clin Microbiol. 1996;34:2766–2769. doi: 10.1128/jcm.34.11.2766-2769.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gredmark-Russ S, Dzabic M, Rahbar A, Wanhainen A, Björck M, Larsson E, Michel JB, Söderberg-Nauclér C. Active cytomegalovirus infection in aortic smooth muscle cells from patients with abdominal aortic aneurysm. J Mol Med (Berl) 2009;87:347–356. doi: 10.1007/s00109-008-0413-4. [DOI] [PubMed] [Google Scholar]
- 12.Ozsvath KJ, Hirose H, Xia S, Tilson MD. Molecular mimicry in human aortic aneurysmal diseases. Ann N Y Acad Sci. 1996;800:288–293. doi: 10.1111/j.1749-6632.1996.tb33335.x. [DOI] [PubMed] [Google Scholar]
- 13.Kurihara N, Inoue Y, Iwai T, Umeda M, Huang Y, Ishikawa I. Detection and localization of periodontopathic bacteria in abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2004;28:553–558. doi: 10.1016/j.ejvs.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 14.Wada K, Kamisaki Y. Roles of oral bacteria in cardiovascular diseases—from molecular mechanisms to clinical cases: involvement of Porphyromonas gingivalis in the development of human aortic aneurysm. J Pharmacol Sci. 2010;113:115–119. doi: 10.1254/jphs.09R22FM. [DOI] [PubMed] [Google Scholar]
- 15.Marques da Silva R, Caugant DA, Eribe ER, Aas JA, Lingaas PS, Geiran O, Tronstad L, Olsen I. Bacterial diversity in aortic aneurysms determined by 16S ribosomal RNA gene analysis. J Vasc Surg. 2006;44:1055–1060. doi: 10.1016/j.jvs.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 16.Cruz RP, Marrone LC, Marrone AC. Chronic syphilitic aortic aneurysm complicated with chronic aortic dissection. Am J Surg. 2010;200:e64–e66. doi: 10.1016/j.amjsurg.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 17.Rudenko N, Golovchenko M, Růzek D, Piskunova N, Mallátová N, Grubhoffer L. Molecular detection of Borrelia bissettii DNA in serum samples from patients in the Czech Republic with suspected borreliosis. FEMS Microbiol Lett. 2009;292:274–281. doi: 10.1111/j.1574-6968.2009.01498.x. [DOI] [PubMed] [Google Scholar]
- 18.Oksi J, Kalimo H, Marttila RJ, Marjamäki M, Sonninen P, Nikoskelainen J, Viljanen MK. Intracranial aneurysms in three patients with disseminated Lyme borreliosis: cause or chance association? J Neurol Neurosurg Psychiatry. 1998;64:636–642. doi: 10.1136/jnnp.64.5.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Polet JD, Weinstein HC. Lyme borreliosis and intracranial aneurysm. J Neurol Neurosurg Psychiatry. 1999;66:806–807. doi: 10.1136/jnnp.66.6.806a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gasser R, Watzinger N, Eber B, Luha O, Reisinger E, Seinost G, Klein W. Coronary artery aneurysm in two patients with long-standing Lyme borreliosis. Borreliosis Study Group. Lancet. 1994;344:1300–1301. doi: 10.1016/S0140-6736(94)90789-7. [DOI] [PubMed] [Google Scholar]
- 21.Wilske B, Zöller L, Brade V, Eiffert M, Göbel UB, Stanek G, in cooperation with Pfister H-W . MiQ-12 Lyme-Borreliose. In: Mauch H, Lütticken R, Gatermann S, editors. Qualitätsstandards in der mikrobiologisch-infektiologischen Diagnostik. München Jena: Urban & Fischer Verlag; 2000. [Google Scholar]
- 22.Wang G, van Dam AP, Le Fleche A, Postic D, Peter O, Baranton G, de Boer R, Spanjaard L, Dankert J. Genetic and phenotypic analysis of Borrelia valaisiana sp. nov. (Borrelia genomic groups VS116 and M19) Int J Syst Bacteriol. 1997;47:926–932. doi: 10.1099/00207713-47-4-926. [DOI] [PubMed] [Google Scholar]
- 23.R Development Core Team . R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2009. [Google Scholar]
- 24.Golledge J, van Bockxmeer F, Jamrozik K, McCann M, Norman PE. Association between serum lipoproteins and abdominal aortic aneurysm. Am J Cardiol. 2010;105:1480–1484. doi: 10.1016/j.amjcard.2009.12.076. [DOI] [PubMed] [Google Scholar]
- 25.Le MT, Jamrozik K, Davis TM, Norman PE. Negative association between infra-renal aortic diameter and glycaemia: the Health in Men Study. Eur J Vasc Endovasc Surg. 2007;33:599–604. doi: 10.1016/j.ejvs.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 26.Robert Koch Institut (RKI) Lyme-Borreliose: Analyse der gemeldeten Erkrankungsfälle der Jahre 2007 bis 2009 aus den sechs östlichen Bundesländern. Epid Bull. 2010;12:101–110. [Google Scholar]
- 27.Patel A, Jagadesham VP, Porter KE, Scott DJ, Carding SR. Characterisation of fractalkine/CX3CL1 and fractalkine receptor (CX3CR1) expression in abdominal aortic aneurysm disease. Eur J Vasc Endovasc Surg. 2008;36:20–27. doi: 10.1016/j.ejvs.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 28.Mäyränpää MI, Trosien JA, Fontaine V, Folkesson M, Kazi M, Eriksson P, Swedenborg J, Hedin U. Mast cells associate with neovessels in the media and adventitia of abdominal aortic aneurysms. J Vasc Surg. 2009;50:388–395. doi: 10.1016/j.jvs.2009.03.055. [DOI] [PubMed] [Google Scholar]
- 29.Beckman EN. Plasma cell infiltrates in atherosclerotic abdominal aortic aneurysms. Am J Clin Pathol. 1986;85:21–24. doi: 10.1093/ajcp/85.1.21. [DOI] [PubMed] [Google Scholar]
- 30.Farkas JC, Fichelle JM, Laurian C, Jean-Baptiste A, Gigou F, Marzelle J, Goldstein FW, Cormier JM. Long-term follow-up of positive cultures in 500 abdominal aortic aneurysms. Arch Surg. 1993;128:284–288. doi: 10.1001/archsurg.1993.01420150038007. [DOI] [PubMed] [Google Scholar]
- 31.Brandimarte C, Santini C, Venditti M, Baiocchi P, Serra P, Gallo P, d’Amati G, Rizzo L, Speziale F, Fiorani P. Clinical significance of intraoperative cultures of aneurysm walls and contents in elective abdominal aortic aneurysmectomy. Eur J Epidemiol. 1989;5:521–525. doi: 10.1007/BF00140150. [DOI] [PubMed] [Google Scholar]
- 32.Marques da Silva R, Lingaas PS, Geiran O, Tronstad L, Olsen I. Multiple bacteria in aortic aneurysms. J Vasc Surg. 2003;38:1384–1389. doi: 10.1016/S0741-5214(03)00926-1. [DOI] [PubMed] [Google Scholar]
- 33.Buckels JA, Fielding JW, Black J, Ashton F, Slaney G. Significance of positive bacterial cultures from aortic aneurysm contents. Br J Surg. 1985;72:440–442. doi: 10.1002/bjs.1800720610. [DOI] [PubMed] [Google Scholar]
- 34.Nakano K, Nemoto H, Nomura R, Inaba H, Yoshioka H, Taniguchi K, Amano A, Ooshima T. Detection of oral bacteria in cardiovascular specimens. Oral Microbiol Immunol. 2009;24:64–68. doi: 10.1111/j.1399-302X.2008.00479.x. [DOI] [PubMed] [Google Scholar]
- 35.Marques da Silva R, Caugant DA, Lingaas PS, Geiran O, Tronstad L, Olsen I. Detection of Actinobacillus actinomycetemcomitans but not bacteria of the red complex in aortic aneurysms by multiplex polymerase chain reaction. J Periodontol. 2005;76:590–594. doi: 10.1902/jop.2005.76.4.590. [DOI] [PubMed] [Google Scholar]
- 36.Inaba H, Hokamura K, Nakano K, Nomura R, Katayama K, Nakajima A, Yoshioka H, Taniguchi K, Kamisaki Y, Ooshima T, Umemura K, Murad F, Wada K, Amano A. Upregulation of S100 calcium-binding protein A9 is required for induction of smooth muscle cell proliferation by a periodontal pathogen. FEBS Lett. 2009;583:128–134. doi: 10.1016/j.febslet.2008.11.036. [DOI] [PubMed] [Google Scholar]
- 37.Blanchard JF, Armenian HK, Peeling R, Friesen PP, Shen C, Brunham RC. The relation between Chlamydia pneumoniae infection and abdominal aortic aneurysm: case–control study. Clin Infect Dis. 2000;30:946–947. doi: 10.1086/313806. [DOI] [PubMed] [Google Scholar]
- 38.Karlsson L, Björck M, Pärsson H, Wanhainen A. The association between serological markers for Chlamydophila pneumoniae and the development of abdominal aortic aneurysm. Ann Vasc Surg. 2011;25:322–326. doi: 10.1016/j.avsg.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 39.Cheuk BL, Ting AC, Cheng SW. Detection of C. pneumoniae by polymerase chain reaction–enzyme immunoassay in abdominal aortic aneurysm walls and its association with rupture. Eur J Vasc Endovasc Surg. 2005;29:150–155. doi: 10.1016/j.ejvs.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 40.Lindholt JS, Juul S, Vammen S, Lind I, Fasting H, Henneberg EW. Immunoglobulin A antibodies against Chlamydia pneumoniae are associated with expansion of abdominal aortic aneurysm. Br J Surg. 1999;86:634–638. doi: 10.1046/j.1365-2168.1999.01126.x. [DOI] [PubMed] [Google Scholar]
- 41.Halme S, Juvonen T, Laurila A, Juvonen J, Mosorin M, Saikku P, Surcel HM. Chlamydia pneumoniae reactive T lymphocytes in the walls of abdominal aortic aneurysms. Eur J Clin Invest. 1999;29:546–552. doi: 10.1046/j.1365-2362.1999.00463.x. [DOI] [PubMed] [Google Scholar]
- 42.Singh AE, Romanowski B. Syphilis: review with emphasis on clinical, epidemiologic, and some biologic features. Clin Microbiol Rev. 1999;12:187–209. doi: 10.1128/cmr.12.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jackman JD, Jr, Radolf JD. Cardiovascular syphilis. Am J Med. 1989;87:425–433. doi: 10.1016/S0002-9343(89)80826-5. [DOI] [PubMed] [Google Scholar]
- 44.Richter D, Klug B, Spielman A, Matuschka FR. Adaptation of diverse lyme disease spirochetes in a natural rodent reservoir host. Infect Immun. 2004;72:2442–2444. doi: 10.1128/IAI.72.4.2442-2444.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aguero-Rosenfeld ME, Wang G, Schwartz I, Wormser GP. Diagnosis of lyme borreliosis. Clin Microbiol Rev. 2005;18:484–509. doi: 10.1128/CMR.18.3.484-509.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lidar M, Lipschitz N, Langevitz P, Shoenfeld Y. The infectious etiology of vasculitis. Autoimmunity. 2009;42:432–438. doi: 10.1080/08916930802613210. [DOI] [PubMed] [Google Scholar]
- 47.Barthold SW, Persing DH, Armstrong AL, Peeples RA. Kinetics of Borrelia burgdorferi dissemination and evolution of disease after intradermal inoculation of mice. Am J Pathol. 1991;139:263–273. [PMC free article] [PubMed] [Google Scholar]
- 48.Cadavid D, Bai Y, Hodzic E, Narayan K, Barthold SW, Pachner AR. Cardiac involvement in non-human primates infected with the Lyme disease spirochete Borrelia burgdorferi. Lab Invest. 2004;84:1439–1450. doi: 10.1038/labinvest.3700177. [DOI] [PubMed] [Google Scholar]
- 49.Sellati TJ, Burns MJ, Ficazzola MA, Furie MB. Borrelia burgdorferi upregulates expression of adhesion molecules on endothelial cells and promotes transendothelial migration of neutrophils in vitro. Infect Immun. 1995;63:4439–4447. doi: 10.1128/iai.63.11.4439-4447.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pagano MB, Zhou HF, Ennis TL, Wu X, Lambris JD, Atkinson JP, Thompson RW, Hourcade DE, Pham CT. Complement-dependent neutrophil recruitment is critical for the development of elastase-induced abdominal aortic aneurysm. Circulation. 2009;119:1805–1813. doi: 10.1161/CIRCULATIONAHA.108.832972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cary NR, Fox B, Wright DJ, Cutler SJ, Shapiro LM, Grace AA. Fatal Lyme carditis and endodermal heterotopia of the atrioventricular node. Postgrad Med J. 1990;66:134–136. doi: 10.1136/pgmj.66.772.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kochi SK, Johnson RC. Role of immunoglobulin G in killing of Borrelia burgdorferi by the classical complement pathway. Infect Immun. 1988;56:314–321. doi: 10.1128/iai.56.2.314-321.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rogers EA, Abdunnur SV, McDowell JV, Marconi RT. Comparative analysis of the properties and ligand binding characteristics of CspZ, a factor H binding protein, derived from Borrelia burgdorferi isolates of human origin. Infect Immun. 2009;77:4396–4405. doi: 10.1128/IAI.00393-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hinterseher I, Erdman R, Donoso LA, Vrabec TR, Schworer CM, Lillvis JH, Boddy AM, Derr K, Golden A, Bowen WD, Gatalica Z, Tapinos N, Elmore JR, Franklin DP, Gray JL, Garvin RP, Gerhard GS, Carey DJ, Tromp G, Kuivaniemi H. Role of complement cascade in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2011;31:1653–1660. doi: 10.1161/ATVBAHA.111.227652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Francischetti IM, Mather TN, Ribeiro JM. Tick saliva is a potent inhibitor of endothelial cell proliferation and angiogenesis. Thromb Haemost. 2005;94:167–174. doi: 10.1267/THRO05010167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheuk BL, Cheng SW. Differential expression of integrin alpha5beta1 in human abdominal aortic aneurysm and healthy aortic tissues and its significance in pathogenesis. J Surg Res. 2004;118:176–182. doi: 10.1016/S0022-4804(03)00351-2. [DOI] [PubMed] [Google Scholar]
- 57.Armstrong PJ, Johanning JM, Calton WC, Jr, Delatore JR, Franklin DP, Han DC, Carey DJ, Elmore JR. Differential gene expression in human abdominal aorta: aneurysmal versus occlusive disease. J Vasc Surg. 2002;35:346–355. doi: 10.1067/mva.2002.121071. [DOI] [PubMed] [Google Scholar]
- 58.Johnsen SH, Forsdahl SH, Singh K, Jacobsen BK. Atherosclerosis in abdominal aortic aneurysms: a causal event or a process running in parallel? The Tromsø study. Arterioscler Thromb Vasc Biol. 2010;30:1263–1268. doi: 10.1161/ATVBAHA.110.203588. [DOI] [PubMed] [Google Scholar]
- 59.Böhme M, Schwenecke S, Fuchs E, Wiebecke D, Karch H. Screening of blood donors and recipients for Borrelia burgdorferi antibodies: no evidence of B. burgdorferi infection transmitted by transfusion. Infusionsther Transfusionsmed. 1992;19:204–207. doi: 10.1159/000222625. [DOI] [PubMed] [Google Scholar]
- 60.Hassler D, Zoller L, Haude M, Hufnagel HD, Sonntag HG. Lyme borreliosis in an endemic region in Europe. Prevalence of antibodies and clinical spectrum. Dtsch Med Wochenschr. 1992;117:767–774. doi: 10.1055/s-2008-1062374. [DOI] [PubMed] [Google Scholar]
- 61.Kaiser R, Kern A, Kampa D, Neumann-Haefelin D. Prevalence of antibodies to Borrelia burgdorferi and tick-borne encephalitis virus in an endemic region in southern Germany. Zentralbl Bakteriol. 1997;286:534–541. doi: 10.1016/S0934-8840(97)80057-6. [DOI] [PubMed] [Google Scholar]
- 62.Rath PM, Ibershoff B, Mohnhaupt A, Albig J, Eljaschewitsch B, Jurgens D, Horbach I, Fehrenbach FJ. Seroprevalence of Lyme borreliosis in forestry workers from Brandenburg, Germany. Eur J Clin Microbiol Infect Dis. 1996;15:372–377. doi: 10.1007/BF01690092. [DOI] [PubMed] [Google Scholar]
- 63.Robert Koch Institut (RKI) Waldarbeiter-Studie Berlin-Brandenburg 2000 zu zeckenübertragenden und anderen Zoonosen. Epid Bull. 2001;16:109–110. [Google Scholar]
- 64.Weiland T, Kühnl P, Laufs R, Heesemann J. Prävalenz von Borrelia-burgdorferi-Antikörpern bei Hamburger Blutspendern. In: Kretschmer V, Stangel W, Wiebecke D, editors. Transfusionsmedizin 1991/92 Beitrag Infusionstherapie. Basel: Karger; 1992. pp. 92–95. [PubMed] [Google Scholar]
- 65.Juvonen J, Juvonen T, Laurila A, Alakärppä H, Lounatmaa K, Surcel HM, Leinonen M, Kairaluoma MI, Saikku P. Demonstration of Chlamydia pneumoniae in the walls of abdominal aortic aneurysms. J Vasc Surg. 1997;25:499–505. doi: 10.1016/S0741-5214(97)70260-X. [DOI] [PubMed] [Google Scholar]
- 66.Porqueddu M, Spirito R, Parolari A, Zanobini M, Pompilio G, Polvani G, Alamanni F, Stangalini D, Tremoli E, Biglioli P. Lack of association between serum immunoreactivity and Chlamydia pneumoniae detection in the human aortic wall. Circulation. 2002;106:2647–2648. doi: 10.1161/01.CIR.0000041626.38101.DB. [DOI] [PubMed] [Google Scholar]
- 67.Nyberg A, Skagius E, Nilsson I, Ljungh A, Henriksson AE. Lack of association between Chlamydophila pneumoniae seropositivity and abdominal aortic aneurysm. Vasc Endovascular Surg. 2007;41:246–248. doi: 10.1177/1538574407301429. [DOI] [PubMed] [Google Scholar]
- 68.Karlsson L, Gnarpe J, Bergqvist D, Lindbäck J, Pärsson H. The effect of azithromycin and Chlamydophilia pneumonia infection on expansion of small abdominal aortic aneurysms—a prospective randomized double-blind trial. J Vasc Surg. 2009;50:23–29. doi: 10.1016/j.jvs.2008.12.048. [DOI] [PubMed] [Google Scholar]
- 69.Vikatmaa P, Lajunen T, Ikonen TS, Pussinen PJ, Lepäntalo M, Leinonen M, Saikku P. Chlamydial lipopolysaccharide (cLPS) is present in atherosclerotic and aneurysmal arterial wall—cLPS levels depend on disease manifestation. Cardiovasc Pathol. 2010;19:48–54. doi: 10.1016/j.carpath.2008.10.012. [DOI] [PubMed] [Google Scholar]