Abstract
Background
Ehrlichiosis is a clinically important, emerging zoonosis. Only Ehrlichia chaffeensis and E. ewingii have been thought to cause ehrlichiosis in humans in the United States. Patients with suspected ehrlichiosis routinely undergo testing to ensure proper diagnosis and to ascertain the cause.
Methods
We used molecular methods, culturing, and serologic testing to diagnose and ascertain the cause of cases of ehrlichiosis.
Results
On testing, four cases of ehrlichiosis in Minnesota or Wisconsin were found not to be from E. chaffeensis or E. ewingii and instead to be caused by a newly discovered ehrlichia species. All patients had fever, malaise, headache, and lymphopenia; three had thrombocytopenia; and two had elevated liver-enzyme levels. All recovered after receiving doxycycline treatment. At least 17 of 697 Ixodes scapularis ticks collected in Minnesota or Wisconsin were positive for the same ehrlichia species on polymerase-chain-reaction testing. Genetic analyses revealed that this new ehrlichia species is closely related to E. muris.
Conclusions
We report a new ehrlichia species in Minnesota and Wisconsin and provide supportive clinical, epidemiologic, culture, DNA-sequence, and vector data. Physicians need to be aware of this newly discovered close relative of E. muris to ensure appropriate testing, treatment, and regional surveillance. (Funded by the National Institutes of Health and the Centers for Disease Control and Prevention.)
Ehrlichiosis and anaplasmosis are tickborne zoonoses caused by obligate intracellular gram-negative bacteria in the family Anaplasmataceae.1 Symptoms typically include fever, myalgia, and headache, with rash in rare instances. Severe disease may be associated with gastrointestinal, renal, respiratory, and central nervous system involvement and, in rare cases, death.
In the United States, ehrlichiosis in humans is caused primarily by infection with Ehrlichia chaffeensis, which infects monocytes, and less commonly by E. ewingii, which infects granulocytes. Anaplasma phagocytophilum is closely related to the ehrlichiae and causes human granulocytic anaplasmosis.1,2 E. ewingii and E. chaffeensis are transmitted to humans by the bite of an infected tick, Amblyomma americanum, whereas A. phagocytophilum is transmitted in the United States by the ticks Ixodes scapularis and I. pacificus.3
Ehrlichiosis is a clinically important, emerging zoonosis. E. chaffeensis, A. phagocytophilum, and E. ewingii were first recognized as human pathogens in 1991,4 1994,5 and 1999,6 respectively. Since then, E. canis and E. muris have been implicated as causes of human illness in Venezuela and Russia, respectively.7,8 However, only E. chaffeensis and E. ewingii have been thought to cause ehrlichiosis in humans in the United States.
Methods
Patients
EDTA-anticoagulated samples of whole blood obtained from patients throughout the United States with suspected ehrlichiosis or anaplasmosis were submitted for polymerase-chain-reaction (PCR) diagnostic testing for ehrlichia and anaplasma at the Mayo Clinic in Minnesota. Patients with confirmed ehrlichiosis in Minnesota and Wisconsin were interviewed by staff members of local and state health departments according to a standardized questionnaire to obtain demographic, clinical, and epidemiologic information, and medical records were reviewed.
All participants provided written informed consent for collection and testing of additional blood specimens. Research protocols were approved and monitored by the institutional review boards at the Mayo Clinic and the Centers for Disease Control and Prevention (CDC).
Real-Time PCR Assay
DNA was extracted from the blood specimens (MagNA Pure Instrument, Roche Applied Science) and tested for E. ewingii, E. chaffeensis, and A. phagocytophilum DNA with the use of a real-time PCR assay9 with primers and fluorescence resonance energy transfer–labeled probes targeting a conserved region of the GroEL heat-shock protein operon. Polymorphisms in the sequence targeted by the probes allowed for differentiation of the three species by means of analysis of melting temperature. Specimens with an atypical result (melting temperature outside the predefined ranges) were tested with the use of a SYBR Green PCR assay targeting the 16S ribosomal RNA gene (rrs) of Anaplasmataceae,10 a nested PCR assay of the GroEL gene (groEL),11 or broad-range rrs assays12 (see Table 1 in the Supplementary Appendix, available with the full text of this article at NEJM.org).
Table 1.
Results of Tests for IgM and IgG Antibodies against the New Ehrlichia Species, Ehrlichia chaffeensis, and Anaplasma phagocytophilum.*
| Patient No.† |
Days from Illness Onset to Specimen Collection |
Specimen Type |
Reciprocal IgM and IgG Titer, CDC Assay | Reciprocal IgG Titer, Mayo Clinic Assay | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| New Ehrlichia Species |
E. chaffeensis | A. phagocytophilum | E. chaffeensis | A. phagocytophilum | ||||||
| IgM | IgG | IgM | IgG | IgM | IgG | |||||
| 1 | 198 | Plasma | 256 | 128 | 256 | <16 | <16 | <16 | NA | NA |
| 2 | 2 | Serum | NA | NA | NA | <32 | NA | <32 | <64 | <64 |
| 2 | 15 | Serum | 512 | 256 | 256 | 64 | <16 | <16 | <64 | <64 |
| 2 | 188 | Plasma | 16 | 64 | <16 | 32 | <16 | <16 | NA | NA |
| 4 | 7 | Serum | NA | NA | NA | NA | NA | NA | 256 | <64 |
| 4 | 46 | Serum | NA | NA | NA | NA | NA | NA | 1024 | <64 |
| 4 | 76 | Plasma | 32 | 2048 | <16 | 2048 | <16 | <16 | NA | NA |
Antibody titers were assessed with the use of a noncommercial indirect immunofluorescence assay at the Centers for Disease Control and Prevention and with the use of a commercial indirect immunofluorescence assay (testing for IgG antibody but not IgM antibody) at the Mayo Clinic. NA denotes not available.
Specimens from Patient 3 were not available for testing.
DNA Sequencing
Amplified DNA fragments were sequenced (3730 DNA sequencer, Applied Biosystems) and analyzed (Sequencher software, version 4.2; Gene Codes). New sequences were submitted to GenBank (accession numbers HM543745 for rrs and HM543746 for groEL). New, homologous sequences of infective bacteria and related bacteria were aligned with the use of ClustalW software, and phylogenetic analysis was conducted with the use of Molecular Evolutionary Genetics Analysis software, version 4.0.13
Culture Isolation
Buffy-coat and erythrocyte fractions of the whole-blood specimens were processed and inoculated into a tick cell line (ISE6, derived from I. scapularis) and a mammalian cell line (RF/6A, derived from rhesus monkey choroid retina; American Type Culture Collection number CRL-1780), according to published protocols.14 Mammalian cell cultures were incubated in RPMI 1640 medium with 10% fetal bovine serum at 37°C in 5% carbon dioxide, whereas ISE6 cultures were incubated at 34°C in sealed flasks.15 Cells were examined microscopically for intracellular morulae (bacterial clusters) of ehrlichia and anaplasma with the use of phase-contrast or bright-field microscopy.
Serologic Testing
Serum and plasma specimens from patients with an atypical groEL PCR product were tested for IgG-class antibodies reacting to E. chaffeensis or A. phagocytophilum with the use of a commercial indirect immunofluorescence assay (Focus Diagnostics).16 Serum and plasma samples were also tested by means of noncommercial indirect immunofluorescence assays developed and used at the CDC for IgM- and IgG-class antibodies against E. chaffeensis, A. phagocytophilum, and an ehrlichia species isolated in this study17; antigens were derived from canine monocytic DH82 cultures infected with ehrlichia and human promyelocytic HL-60 cultures infected with A. phagocytophilum. A reciprocal titer of 64 or higher was considered positive for both assays.
Morphologic Examination of Peripheral-Blood Smears
Wright-stained peripheral-blood smears from each patient with an atypical groEL PCR product were screened for the presence of intracellular morulae characteristic of ehrlichia species.
Tick Collection and DNA Extraction
Ticks were collected in June and July 2009 by the Wisconsin Division of Public Health, Medical Entomology Laboratory, University of Wisconsin–Madison and the Minnesota Department of Health. Tick collection was conducted by dragging a fabric flag (1 m by 1 m) across vegetation at or near residences of patients in northwestern Wisconsin and northeastern, central, and northwestern Minnesota. DNA extraction from ticks was performed with the use of a modified version of a published protocol,18 with three to five nymphs from Wisconsin processed at a time. DNA was tested with the use of the groEL fluorescence resonance energy transfer assay and the rrs SYBR Green PCR assay.
Results
Real-Time PCR Assay and Sequencing
From June 1 through December 31, 2009, a total of 4247 blood specimens from residents in 45 states were tested by means of groEL PCR assay. Of the 1518 specimens obtained from Wisconsin and Minnesota residents, 163 (10.7%) were positive for A. phagocytophilum (35 from Wisconsin and 128 from Minnesota), whereas none were positive for E. chaffeensis or E. ewingii. Three additional Wisconsin residents and one Minnesota resident had positive PCR tests with a melting temperature that was outside the melting temperature range for E. chaffeensis, E. ewingii, and A. phagocytophilum (Fig. 1 in the Supplementary Appendix). This atypical result was not found for the 2729 specimens collected from the 43 other states.
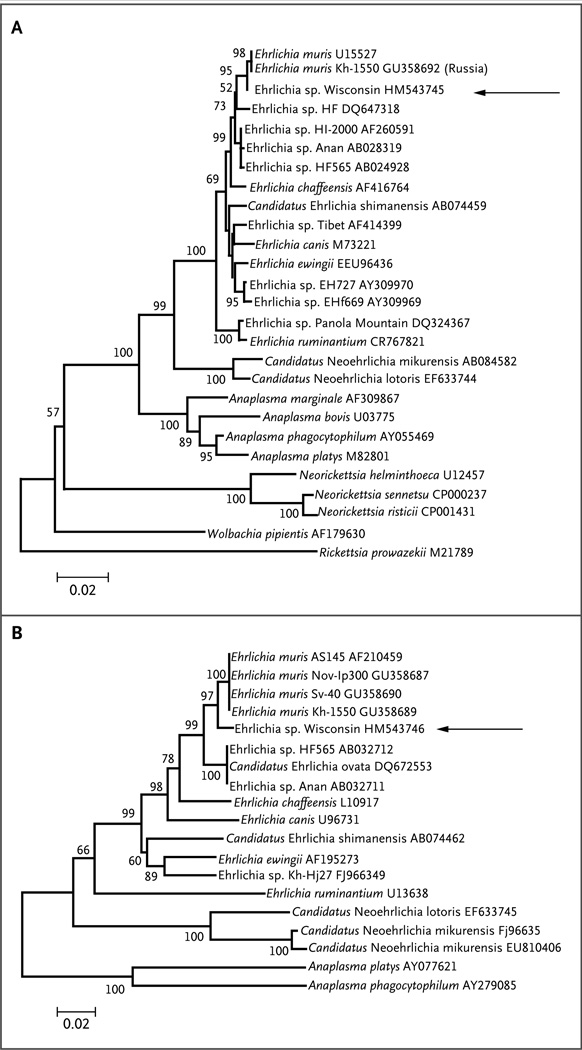
Figure 1. Genetic Relationships between the New Ehrlichia Species and Related Bacteria.
The arrow to the right of each phylogenetic tree indicates the newly discovered ehrlichia species (called “Wisconsin”). Panel A shows the phylogeny based on the 16S ribosomal RNA gene (rrs), inferred with the use of the minimum-evolution method and with distances calculated by means of the Jukes–Cantor method as the number of base substitutions per site. Panel B shows the phylogeny based on the GroEL heat-shock protein operon gene (groEL), inferred with the use of the neighbor-joining method and with distances calculated by means of the Kimura two-parameter method as the number of base substitutions per site. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (of 1000 replicates) is shown to the left of each branch. The trees are drawn to scale, with branch lengths in the same units as those of the evolutionary distances (see scale bars) used to infer the phylogenetic tree. Positions containing gaps, missing data, and primer sequences were eliminated from the data set. A total of 1160 positions for rrs and 591 positions for groEL were analyzed. Phylogenetic analyses were conducted with Molecular Evolutionary Genetics Analysis software, version 4.0.13 The GenBank accession number is listed at the end of each isolate name.
The four specimens with an atypical groEL PCR melting temperature also tested positive for Anaplasmataceae rrs with the use of the SYBR Green PCR assay. The nucleotide sequences of the amplified rrs and groEL fragments were identical among the four specimens and shared 98% sequence similarity with the homologous rrs and groEL genes of E. muris (Fig. 1).
Culture Isolation
Two ehrlichia species isolates (designated Wisconsin 1 and 2) were cultured from blood specimens obtained from one of the four patients 3 and 4 days before culturing in ISE6 and RF/6A cell lines. Sequence analysis of the PCR-amplified portions of rrs showed that they were identical to each other and to the sequences obtained from the clinical specimens with the atypical melting-temperature results.
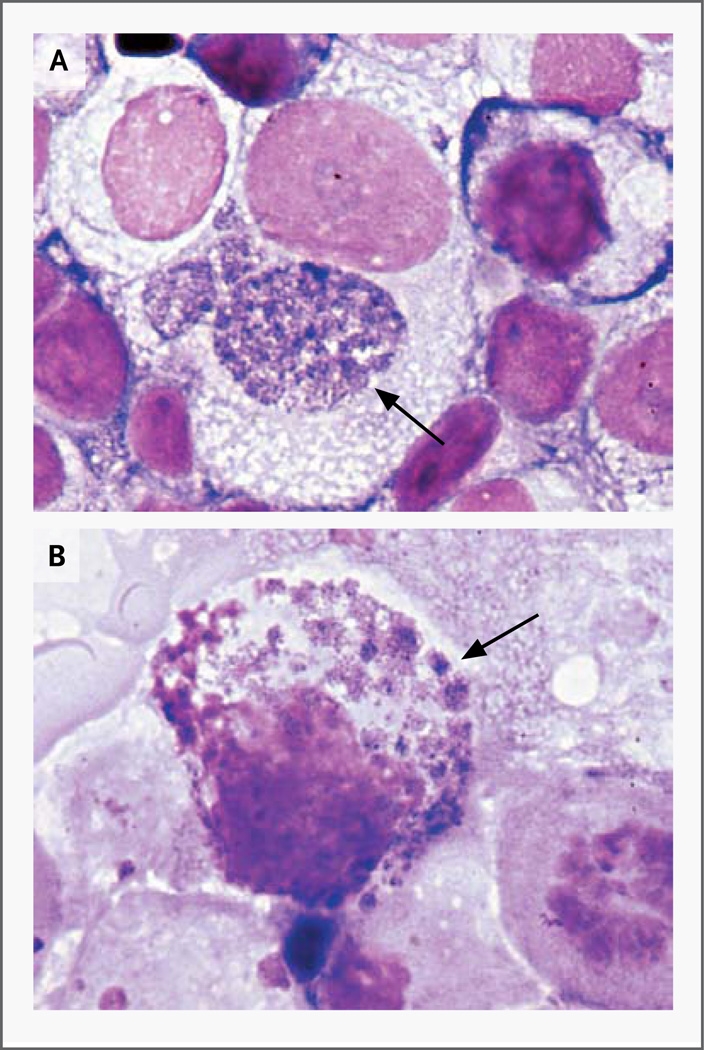
Morulae were detected with the use of phase-contrast microscopy of live RF/6A cultures 5 weeks after inoculation. Fixed and stained ISE6 cells contained one to three large morulae per cell, whereas RF/6A cells contained numerous, small morulae (Fig. 2).
Figure 2. Intracellular Morulae of the New Ehrlichia Species in the ISE6 and RF/6A Cell Cultures.
Panel A shows the ISE6 cell line, and Panel B shows the RF/6A cell line. Morulae are indicated by arrows (Giemsa stain).
Serologic Testing
Of the four patients with atypical PCR results, two (Patients 2 and 4) (Table 1) were tested by means of the commercial indirect immunofluorescence assay. Serum samples collected from Patient 2 were negative for IgG antibodies to E. chaffeensis (i.e., titer <64) on days 2 and 15 after the onset of fever, whereas serum specimens from Patient 4 were positive (i.e., titer ≥64) for IgG antibodies on day 5 (titer of 256) and day 54 (titer of 1024) after the onset of fever.
In addition, serum and plasma specimens from three of these four patients were tested by means of the CDC indirect immunofluorescence assays. At least one specimen from each patient tested was positive for IgM or IgG antibodies reacting to E. chaffeensis, and the titers were even higher in response to the new ehrlichia species. A specimen obtained 15 days after the onset of illness from Patient 2 had high titers of IgM and IgG antibodies against the new ehrlichia species; in a specimen obtained 188 days after onset, IgM and IgG antibody titers were substantially reduced. Patient 4 had a strong seroconversion with a high IgG antibody titer 76 days after infection. No antibodies reacting to A. phagocytophilum antigens were detected (i.e., titer <64) in three patients with the use of the commercial or noncommercial assay. Specimens from Patient 3 were not available.
Patients and Clinical Presentation
The four patients had an onset of illness between June 8 and October 27, 2009. Their ages ranged from 23 to 51 years; two were men (Table 2). All four patients whose specimens were positive for the newly discovered ehrlichia species reported fever, fatigue, and headache. Patient 2 also reported nausea and vomiting. The interval between the onset of illness and the physician visit was 1 to 4 days. Laboratory findings included lymphopenia (in all four patients), thrombocytopenia (in three), elevated hepatic aminotransferase levels (in one of the three patients tested), and mildly elevated alkaline phosphatase levels (in one of the two patients tested). No morulae or other blood parasites were detected in peripheral-blood smears.
Table 2.
Laboratory Test Results in the Four Patients Infected with the New Ehrlichia Species, Shortly after Presentation.*
| Patient No. | Age | Sex | White-Cell Count |
Lymphocyte Count |
Platelet Count |
AST | ALT | Alkaline Phosphatase |
|---|---|---|---|---|---|---|---|---|
| yr | ×10−9/liter | U/liter | ||||||
| 1 | 51 | M | 3.4 | 0.48 | 87 | 76 | 75 | NA |
| 2 | 23 | M | 3.6 | 0.41 | 104 | 42 | NA | 134 |
| 3 | 50 | F | 5.0 | 0.84 | 132 | NA | NA | NA |
| 4 | 50 | F | 3.6 | 0.54 | 212 | 16 | NA | 88 |
| Normal range | 3.5–10.5 | 0.9–2.9 | 150–450 | 8–48 | 7–55 | 45–115 | ||
ALT denotes alanine aminotransferase, AST aspartate aminotransferase, and NA not available (not performed or not reported).
Two patients had previously received solid-organ transplants and were taking immuno-suppressive drugs at the time of diagnosis. One patient had cystic fibrosis and had undergone bilateral lung transplantation 2 years before the onset of illness; medications received included mycophenolate mofetil, cyclosporine, and prednisone. This patient was hospitalized for 3 days during the acute illness for management of an infiltrate in the left lung and pleural effusion on the left side for which a specific cause was not determined. The symptoms improved after administration of ceftazidime and doxycycline. The second patient had received a renal allograft 4 years before the onset of symptoms and was receiving mycophenolate mofetil, tacrolimus, and prednisone. This patient had acute kidney injury (serum creatinine level, 2.2 mg per deciliter [194.5 µmol per liter] vs. a baseline of 1.2 mg per deciliter [106.1 µmol per liter]) and was successfully treated with doxycycline. The two immuno-competent patients had relatively mild illnesses and were successfully treated with doxycycline. The patient from Wisconsin received doxycycline at 100 mg twice daily for 21 days, and the patient from Minnesota received the same regimen for 10 days.
Epidemiologic Investigation
All patients reported peridomestic (e.g., from mowing the lawn) or recreational exposure to ticks or wooded areas (Fig. 2 in the Supplementary Appendix). The three Wisconsin patients resided in Eau Claire or Burnett County, and one reported traveling to Bayfield County in northwest Wisconsin 1 week before the onset of illness. The Minnesota patient resided in Rice County and had traveled to a wooded area in Pine County, Minnesota, within 30 days before the onset of symptoms.
Tick Collection and PCR Assay
A total of 697 ticks were tested. DNA from the newly discovered ehrlichia species was detected in 16 of 534 I. scapularis ticks (7 nymphs and 9 adults) from Minnesota, as well as in 1 group of 5 nymphs (of 154 total) from Wisconsin (where the minimum infective rate is 6.5 infected nymphs per 1000 nymphs tested) (Table 2 in the Supplementary Appendix). No DNA from the newly discovered ehrlichia species was detected in 9 I. scapularis adults from Wisconsin or 88 Dermacentor variabilis adults from Wisconsin.
Discussion
We have identified a new ehrlichia species (subsequently referred to as ehrlichia species Wisconsin) in blood from four patients living in Wisconsin or Minnesota, by using molecular, culture, and serologic methods. The presence of ehrlichia species Wisconsin DNA in blood specimens from these patients collected during the period of acute illness suggests that this organism was the etiologic agent of their infection. This is supported by the results of serologic testing with whole-cell antigens of the Wisconsin isolate: IgM and IgG antibody responses against the species were positive in the three patients tested, with consistently higher titers than those to E. chaffeensis. All four patients recovered after administration of doxycycline, which is the antibiotic of choice for the treatment of ehrlichiosis.
The identification of ehrlichia species Wisconsin in humans has important clinical and epidemiologic implications. Ehrlichiosis was not previously thought to be endemic in Minnesota and Wisconsin and would not be routinely tested for among patients from these areas. Also, commercial tests for ehrlichiosis may fail to provide an accurate identification of this organism. The considerable serologic cross-reactivity of the Wisconsin isolate with E. chaffeensis could confound diagnostic and epidemiologic studies and may explain the recent increase in the numbers of cases attributed to E. chaffeensis infection in Wisconsin and Minnesota on the basis of serologic testing only. In addition, PCR assays for E. chaffeensis and E. ewingii may not detect ehrlichia species Wisconsin because of lack of primer and probe homology. The ehrlichia–anaplasma real-time groEL PCR assay used in our investigation has the advantage of providing differential detection of ehrlichia species Wisconsin from E. chaffeensis, E. ewingii, and A. phagocytophilum on the basis of differences in DNA composition of the amplified fragment.9 Finally, detection of morulae in peripheral-blood samples from infected persons is an unreliable means of diagnosing infection with ehrlichia species Wisconsin. Morulae are detected infrequently in blood from patients infected with ehrlichia species19 and were not found in blood from our four patients.
Ehrlichia infections in the United States are commonly transmitted by A. americanum. However, the northern range for A. americanum is not thought to extend into Wisconsin and Minnesota, and public submissions of A. americanum ticks to the University of Wisconsin–Madison or the Minnesota Department of Health are uncommon. In contrast, both D. variabilis and I. scapularis are abundant, human-biting species in northwestern Wisconsin and Minnesota. The presence of ehrlichia species Wisconsin DNA in at least 17 I. scapularis nymphs and adults, as well as the absence of ehrlichia DNA in the D. variabilis ticks tested, suggests that I. scapularis is a vector for ehrlichia species Wisconsin. Extended investigation and tick surveillance are required to understand the distribution of this agent in Wisconsin and Minnesota and to definitively implicate a specific tick vector.
The new ehrlichia species reported in this study is closely related to E. muris (with approximately 98% sequence homology), but its exact taxonomic placement cannot yet be determined, because only a few isolates and limited genetic data are available. E. muris is considered to be an Old World pathogen found in different ticks of the I. persulcatus complex ranging from Eastern Europe to Japan.10,20,21 E. muris DNA has been detected in the blood of small rodents and deer from these areas,22 suggesting that these animals may be reservoirs of E. muris and related organisms. We are also aware of at least 2 PCR-confirmed and 84 serologically diagnosed cases in humans attributed to E. muris infection in the Perm region of Russia.12 Similarly, Japanese investigators reported a 1.1% seroprevalence of antibodies against E. muris among 1893 Tokyo residents, with an even higher seroprevalence among rodents (6 to 63%)23; however, it is difficult to ascertain whether these antibodies in mice and humans are related to E. muris or to other antigenically related organisms, because multiple ehrlichia agents have been reported from the same region.10,21,24
In summary, we have characterized a newly discovered ehrlichia species with supportive clinical, epidemiologic, culture, DNA-sequence, and vector data. Further assessment of the ecologic, epidemiologic, and clinical features of the infection caused by this species is required to facilitate its distinction from other known tickborne infections in this region. To guide diagnostic testing and treatment, physicians should be aware that a novel pathogenic ehrlichia agent is present in Minnesota and Wisconsin and that organism-specific PCR and serologic testing can be used to identify the cause of suspected infections.
Supplementary Material
Acknowledgments
Supported in part by a grant from the National Institutes of Health (R01 AI042792, to Dr. Munderloh) and a cooperative agreement with the CDC (5U50C1000483-03, to Mr. Neitzel).
We thank Richard Thoune, Denise Wirth, and Paulette Magur from the Eau Claire City County Health Department; Carol Larson from the Burnett County Department of Health and Human Services; Sue Shea from the Mayo Clinic Health System–Eau Claire; Richard Heffernan, Tom Haupt, and Kristin Hardy from the Wisconsin Division of Public Health; and Gregory A. Dasch, Aubree Roche, and Arianna Salazar from the Rickettsial Zoonoses Branch, CDC.
Footnotes
Note added in proof: After this article was submitted, Telford et al. reported findings of an E. muris–like bacterium in Wisconsin I. scapularis ticks collected during the 1990s.25
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Presented in part at the annual meeting of the American Society of Tropical Medicine and Hygiene, Washington, DC, November 18–22, 2009; the annual conference of the Epidemic Intelligence Service, Atlanta, April 19–23, 2010; the International Conference on Emerging Infectious Diseases, Atlanta, July 11–14, 2010; the 50th Interscience Conference on Antimicrobial Agents and Chemotherapy, Boston, September 12–15, 2010; the annual meeting of the American Society of Tropical Medicine and Hygiene, Atlanta, November 3–7, 2010; and the 6th International Meeting on Rickettsiae and Rickettsial Diseases, Heraklion, Greece, June 5–7, 2011. The findings reported here were also described in a Health Alert Network public health announcement by the Minnesota Department of Health and the Wisconsin Division of Public Health.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Dumler JS, Barbet AF, Bekker CP, et al. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int J Syst Evol Microbiol. 2001;51:2145–2165. doi: 10.1099/00207713-51-6-2145. [DOI] [PubMed] [Google Scholar]
- 2.Demma LJ, Holman RC, McQuiston JH, Krebs JW, Swerdlow DL. Epidemiology of human ehrlichiosis and anaplasmosis in the United States, 2001–2002. Am J Trop Med Hyg. 2005;73:400–409. [PubMed] [Google Scholar]
- 3.Childs JE, Paddock CD. The ascendancy of Amblyomma americanum as a vector of pathogens affecting humans in the United States. Annu Rev Entomol. 2003;48:307–337. doi: 10.1146/annurev.ento.48.091801.112728. [DOI] [PubMed] [Google Scholar]
- 4.Anderson BE, Dawson JE, Jones DC, Wilson KH. Ehrlichia chaffeensis, a new species associated with human ehrlichiosis. J Clin Microbiol. 1991;29:2838–2842. doi: 10.1128/jcm.29.12.2838-2842.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen SM, Dumler JS, Bakken JS, Walker DH. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J Clin Microbiol. 1994;32:589–595. doi: 10.1128/jcm.32.3.589-595.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buller RS, Arens M, Hmiel SP, et al. Ehrlichia ewingii, a newly recognized agent of human ehrlichiosis. N Engl J Med. 1999;341:148–155. doi: 10.1056/NEJM199907153410303. [DOI] [PubMed] [Google Scholar]
- 7.Perez M, Rikihisa Y, Wen B. Ehrlichia canis-like agent isolated from a man in Venezuela: antigenic and genetic characterization. J Clin Microbiol. 1996;34:2133–2139. doi: 10.1128/jcm.34.9.2133-2139.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nefedova VV, Korenberg EI, Kovalevski IuV, Gorelova NB, Vorob’eva NN. Microorganisms of the order Rickettsiales in taiga tick (Ixodes persulcatus Sch.) from the Pre-Ural region. Vestn Ross Akad Med Nauk. 2008;7:47–50. (In Russian.) [PubMed] [Google Scholar]
- 9.Bell CA, Patel R. A real-time combined polymerase chain reaction assay for the rapid detection and differentiation of Anaplasma phagocytophilum, Ehrlichia chaffeensis, and Ehrlichia ewingii. Diagn Microbiol Infect Dis. 2005;53:301–306. doi: 10.1016/j.diagmicrobio.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 10.Eremeeva ME, Oliveira A, Moriarity J, et al. Detection and identification of bacterial agents in Ixodes persulcatus Schulze ticks from the north western region of Russia. Vector Borne Zoonotic Dis. 2007;7:426–436. doi: 10.1089/vbz.2007.0112. [DOI] [PubMed] [Google Scholar]
- 11.Takano A, Ando S, Kishimoto T, et al. Presence of a novel Ehrlichia sp. in Ixodes granulatus found in Okinawa, Japan. Microbiol Immunol. 2009;53:101–106. doi: 10.1111/j.1348-0421.2008.00093.x. [DOI] [PubMed] [Google Scholar]
- 12.Eremeeva ME, Gerns HL, Lydy SL, et al. Bacteremia, fever, and splenomegaly caused by a newly recognized bartonella species. N Engl J Med. 2007;356:2381–2387. doi: 10.1056/NEJMoa065987. [DOI] [PubMed] [Google Scholar]
- 13.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 14.Munderloh UG, Silverman DJ, MacNamara KC, Ahlstrand GG, Chatterjee M, Winslow GM. Ixodes ovatus Ehrlichia exhibits unique ultrastructural characteristics in mammalian endothelial and tick-derived cells. Ann N Y Acad Sci. 2009;1166:112–119. doi: 10.1111/j.1749-6632.2009.04520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelson CM, Herron MJ, Felsheim RF, et al. Whole genome transcription profiling of Anaplasma phagocytophilum in human and tick host cells by tiling array analysis. BMC Genomics. 2008;9:364. doi: 10.1186/1471-2164-9-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olano JP, Hogrefe W, Seaton B, Walker DH. Clinical manifestations, epidemiology, and laboratory diagnosis of human monocytotropic ehrlichiosis in a commercial laboratory setting. Clin Diagn Lab Immunol. 2003;10:891–896. doi: 10.1128/CDLI.10.5.891-896.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicholson WL, Comer JA, Sumner JW, et al. An indirect immunofluorescence assay using a cell culture-derived antigen for detection of antibodies to the agent of human granulocytic ehrlichiosis. J Clin Microbiol. 1997;35:1510–1516. doi: 10.1128/jcm.35.6.1510-1516.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao WC, Gao YM, Zhang PH, et al. Identification of Ehrlichia chaffeensis by nested PCR in ticks from Southern China. J Clin Microbiol. 2000;38:2778–2880. doi: 10.1128/jcm.38.7.2778-2780.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ismail N, Bloch KC, McBride JW. Human ehrlichiosis and anaplasmosis. Clin Lab Med. 2010;30:261–292. doi: 10.1016/j.cll.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spitalská E, Boldis V, Kostanová Z, Kocianová E, Stefanidesová K. Incidence of various tick-borne microorganisms in rodents and ticks of central Slovakia. Acta Virol. 2008;52:175–179. [PubMed] [Google Scholar]
- 21.Rar VA, Fomenko NV, Dobrotvorsky AK, et al. Tickborne pathogen detection, Western Siberia, Russia. Emerg Infect Dis. 2005;11:1708–1715. doi: 10.3201/eid1111.041195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tamamoto C, Seino N, Suzuki M, Kaji K, Takahashi H, Inokuma H. Detection of Ehrlichia muris DNA from sika deer (Cervus nippon yesoensis) in Hokkaido, Japan. Vet Parasitol. 2007;150:370–373. doi: 10.1016/j.vetpar.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 23.Kawahara M, Ito T, Suto C, et al. Comparison of Ehrlichia muris strains isolated from wild mice and ticks and serologic survey of humans and animals with E. muris as antigen. J Clin Microbiol. 1999;37:1123–1129. doi: 10.1128/jcm.37.4.1123-1129.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alekseev AN, Dubinina HV, Van De Pol I, Schouls LM. Identification of Ehrlichia spp. and Borrelia burgdorferi in Ixodes ticks in the Baltic regions of Russia. J Clin Microbiol. 2001;39:2237–2242. doi: 10.1128/JCM.39.6.2237-2242.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Telford SR, III, Goethert HK, Cunningham JA. Prevalence of Ehrlichia muris in Wisconsin deer ticks collected during the mid 1990s. Open Microbiol J. 2011;5:18–20. doi: 10.2174/1874285801105010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.